Abstract
Due to increasing resistance of pathogenic fungi to antifungal treatments, new types of drugs are needed. For this purpose, active substances with antifungal properties occurring in natural compounds should be considered. The herb Psephellus bellus shows strong antifungal activity and is characterized by unique guaianolides, which have an ester on C-2. Thus, a specialized method of isolation and testing was applied to assess the pharmacological effects of these guaianolides. After phytochemical analysis (chromatography and spectral methods), selected lipophilic compounds and the herb extract of this species containing 26 sesquiterpene lactones were tested. The antifungal effect of the herbal compounds was determined on clinical strains of fungi Candida, Rhodotorula, Trichophyton, Microsporum, and Scopulariopsis using a diffusion test. The MTT assay was employed to study the cytotoxic effects of the extract against human fibroblasts. Statistical analysis was performed. All analyzed compounds exhibited antifungal activity in cultivations suitable for assessment. Most lipophilic cebellins from Psephellus bellus prevent the growth of most fungal strains.
1. Introduction
Fungal infections affect about 40% of the world’s population and may be viewed as an epidemiological, therapeutic, and social problem. Commonly used antibiotics change people’s microbial flora, which has caused an increase in the number of people with impaired immunity. Factors such as industrial development, agriculture, technology, and the increase in the duration of people’s lives have increased the susceptibility of the population to infections. The number and variety of fungi causing diseases are increasing globally. In different parts of the world, sometimes even within one country, differences exist in fungal flora regarding the frequency of occurrence of individual species [1,2,3]. Moreover, pathogenic fungi are becoming resistant to commonly used and widely known antifungal treatments. Hence, novel active pharmaceutical ingredients are needed, including those with novel mechanisms of action or that act on new receptors. Several natural products exhibit promising antifungal properties, and these compounds might provide new active substances with the desired activity. In this context, compounds such as sesquiterpene lactones and coumarins, known for their strong antimicrobial effects, have often been studied for their antifungal properties [4,5,6]. Numerous investigations showed that plant extracts combined with antibiotics increase their activity, and decrease the required doses of antibiotics and their side effects. These positive interactions are considered a potential strategy to combat bacterial resistance [7].
It has been proven that these properties of sesquiterpene lactones are enabled by their specific skeletal structure: a lactone ring coupled with an exo-methylene, which results in the inhibition of cellular enzymes through Michael nucleophilic addition [8]. The substituents, mainly esters and hydroxyl groups, and their conformation, are responsible for compound potency and bioavailability by making them either lipophilic or hydrophilic. The lipophilicity of compounds allows them to penetrate cell walls more efficiently; this phenomenon is described as the lipophilicity rule [4].
Sesquiterpene lactones are the most characteristic compounds occurring in the plants of the Centaureinae subtribe (Compositae). Guaianolides are the most commonly appearing type of lactones [9]. Either in isolated form or extracts, they show broad biological properties, including anti-inflammatory, antibacterial, antiviral (including against SARS-CoV-2) [10], antihelminthic [11], and antimigraine [12] activities.
There are at least 26 different sesquiterpene lactones (1–26) in Psephellus bellus (Figure 1) [13,14], among which 10 guaianolides occur only in this species and those related to it (cebellins: L, O, K, N, A, B, G, H, I, and J) [15].
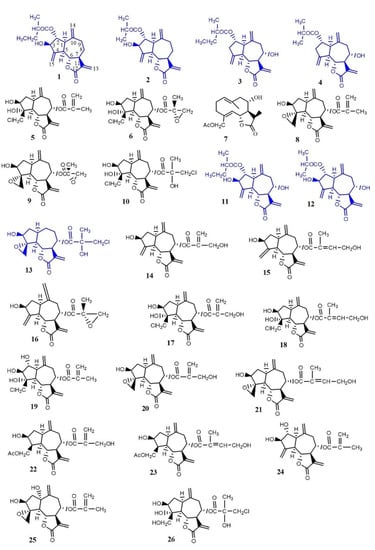
Figure 1.
Chemical structures of compounds with features responsible for their antifungal properties: 1. cebellin L, 2. cebellin O, 3. cebellin K, 4. cebellin N, 5. 19-deoxychlorojanerin, 6. 17,18-epoxy-19-deoxychlorojanerin, 7. cebellin M, 8. 8-desacylo-8α-(2′-methyl-acryloxy)-subluteolide, 9. repin, 10. centaurepensin, 11. cebellin A, 12. cebellin B, 13. acroptilin, 14. cynaropicrin, 15. cebellin F, 16. 15-deoxyrepin, 17. cebellin C, 18. cebellin D, 19. cebellin E, 20. janerin, 21. 8α-4′-tiglinate-8-desacetyl-subluteolide, 22. cebellin G, 23. cebellin H, 24. cebellin I, 25. repdiolide, 26. cebellin J. Compounds chosen for antifungal study.
Cebellins L (1), O (2), K (3), N (4), A (11), and B (12) all have an ester on C-2. The characteristic features in the structure of these four guaianolides distinguish them from compounds occurring in the other Centaureinae plants. They either lack the hydroxyl group on C-3 (compounds 3 and 4) or the substituent on C-8 (compounds 1 and 2). These features are responsible for the lipophilicity of compounds 1–4. Moreover, the presence of ester groups on C-2 with a methylene substituent also increases their lipophilicity.
The other nine sesquiterpene lactones from P. bellus are also lipophilic: 19-deoxychlorojanerin (5), 17,18-epoxy-19-deoxychlorojanerin (6), cebellin M (7), 8-desacyloxy-8α-(2′-methyloaryloxy)-subluteolide (8), repin (9), centaurepensin (10), cebellin A (11), cebellin B (12), and acroptilin (13).
The sesquiterpenoids showing interesting antifungal activities are reported in the literature [16]. Their mechanism of action is not fully understood, although it was suggested to involve membrane disruption due to their lipophilic nature [17].
Antifungal studies of various sesquiterpene lactones showed that the most effective compounds are those that contain an α-methyleno-γ-lactone group but lack bulky sterically inhibitory groups, which limit access to the α-methyleno-γ-lactone. Nonpolar or weakly polar compounds were found to be more bioactive, and sesquiterpene lactones of a guaianolide structure had the greatest antifungal potency [18,19]. As such, there was reason to think that the lipophilic compounds from the herb P. bellus may have antifungal properties, which was our motivation to conduct the appropriate studies, as presented in this paper.
For this study, compounds 1 and 11–13 were chosen, in addition to the mixture of compounds 2–4 and the extract of the herb of P. bellus containing 26 sesquiterpene lactones. Their antifungal effect was tested on four clinical strains of yeast-like fungi, four clinical strains of dermatophytes, and one clinical strain of mold fungus.
2. Results and Discussion
2.1. Isolation and Identification of Compounds from P. bellus for Antifungal Studies
Four guaianolides were isolated from a methylene chloride (CH2Cl2) extract of P. bellus and identified using their 1H NMR spectra (Table 1) to study their antifungal properties. Compound 1 contains an additional proton at C-8 (δ = 2.24 ppm). Compounds 1, 11, and 12 with a substituent at C-2 are characterized by signals of proton H-3α and H-2β (dd 4.94–4.98 ppm). The H-8 signal indicates the presence of free hydroxyl in compounds 11 and 12 at C-8 (δ = 4.01 ppm).

Table 1.
1H NMR (600 MHz) spectroscopic data (δH in ppm, mult; J in Hz) of compounds: 1 and 11–13.
The OH group signal at C-8 of compounds 11 and 12 was observed at 1.85 ppm. The characteristic signals indicated the presence of the 4α,15-epoxy group in compound 13 of oxirane protons (doublets of H-15 and H-15’ at δ = 3.34 and 3.07 ppm with J (15, 15’) = 4.2 Hz) [12,13,14,16,17,20]. The mixture of compounds 2–4 was identified using the TLC method (Figure 2).
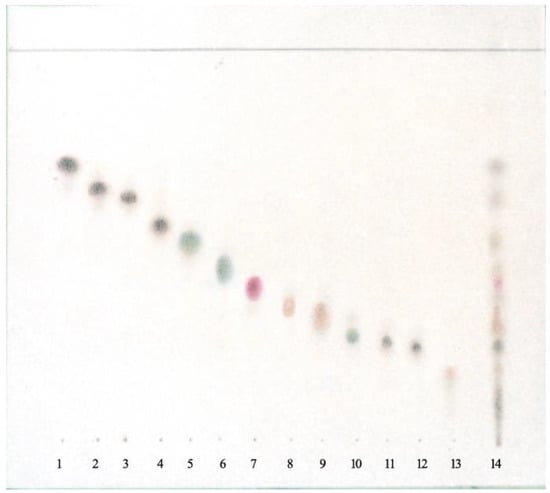
Figure 2.
TLC of lipophilic compounds from Psephellus bellus. Adsorbent: silica gel. Mobile phase: n-hexane–CH2Cl2–AcOEt 4:2:5. 1. cebellin L, 2. cebellin O, 3. cebellin K, 4. cebellin N, 5. 19-deoxychlorojanerin, 6. 17,18-epoxy-19-deoxychlorojanerin, 7. cebellin M, 8. 8-desacylo-8α-(2′-methyl-acryloxy)-subluteolide, 9. repin, 10. centaurepensin, 11. cebellin A, 12. cebellin B, 13. acroptilin, and 14. extract from Psephellus bellus herb.
2.2. Antifungal Activity of the Guaianolides from P. bellus
No inhibition zone was observed in the negative controls. The results (diameter of growth inhibition zone) of controls with traditional drugs (C. albicans) were as follows (Figure 3): (i) amphotericin B, exhibiting susceptibility >17 mm, resistance <12 mm; (ii) 5-fluorocytosine, exhibiting susceptibility >21 mm, resistance <16 mm; and (iii) nystatin, exhibiting susceptibility >19 mm, resistance <14 mm.
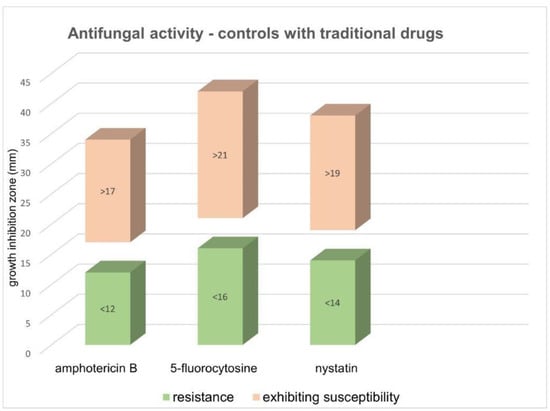
Figure 3.
Antifungal activity of controls with traditional drugs for C. albicans. The results are expressed as growth inhibition for three antifungal drugs.
The analysis of all suitable-for-assessment fungi cultures showed that all of the studied compounds have antifungal properties. Because of the limited amount of some compounds, only 0.39% solutions of the compounds and extract were used during the study.
The diameters of the inhibition zones were measured to assess the susceptibility of the fungi to the activity of the tested compounds. The analyzed fungi were classified into the following categories: (i) susceptible, with an inhibition zone diameter over 10 mm; (ii) moderate-susceptible, with an inhibition zone diameter between 1 and 10 mm; and (iii) resistant, with no inhibition zone.
The antifungal activity investigation of the examined compounds from P. bellus was performed in duplicate and the inhibition zones were measured 7–10 times each. Detailed statistical analysis results and a comparison of the antifungal activity P. bellus compounds in relation to fungal strains are presented in Table 2 and Table 3. The measurements were recorded over 3 days from Candida cultures, and 14 days from the dermatophytes and mold fungus (Scopulariopsis brevicaulis).

Table 2.
The results of the antifungal activity of the examined compounds from P. bellus (growth inhibition zone (mm) ± standard deviation).

Table 3.
The results of the difference test between averages for the examined compounds in relation to various fungal strains.
The examined compounds and P. bellus extract inhibited the growth of Candida albicans fungi less than Candida non-albicans. However, due to the lack of growth of C. albicans, the antifungal activity of this strain could only be assessed for the two substances. C. albicans was sensitive only to the mixture of K + N + O (2–4) cebellins (the zone of growth inhibition was 12 mm). Cebellin A (11) showed a significantly weaker inhibitory effect on the growth of C. albicans (the zone of growth inhibition was 2 mm).
In comparison to C. albicans, the strains were easier to grow, which is why the antifungal activity of all the studied compounds was observed.
Most fungi (i.e., Candida albicans, Microsporum canis, and Rhodotorula rubra) strains were susceptible to cebellins 1–4 from P. bellus. These compounds are the most lipophilic of the studied lactones. Their lipophilicity enables those cebellins to penetrate fungus cell walls and destroy them more easily [4].
Of the yeast-like fungi, Candida famata and C. glabrata, in addition to dermatophytes from Trichophyton genus T. rubrum and T. mentagrophytes var. interdigitale, were the most susceptible to the analyzed compounds. The multiple comparison test showed a statistically significant difference in the susceptibility to the analyzed compounds between Scopulariopsis brevicaulis and T. mentagrophytes var. interdigitale, and between Scopulariopsis brevicaulis and C. glabrata. The highest potency of the P. bellus herb extract was shown by an inhibition zone diameter that reached 34 mm.
The findings suggest that lipophilic sesquiterpene lactones (compounds 1–13), mostly those with an ester on C-2 (compounds 1–4, 11, 12), are responsible for the antifungal effect of the extract from P. bellus. The antifungal studies of various sesquiterpene lactones showed that sesquiterpene lactones of a guaianolide structure have the strongest antifungal potency. Moreover, the highest potency of the studied extract suggested that guaianolides present in the extract show a synergistic effect.
Because all examined compounds and full extract were analyzed in relation to clinical fungal strains (especially dermatophytes), we decided to evaluate biosafety (toxicity) on human skin cells using the MTT test. The cell viability determined by the MTT assay was 94% for 0.028 mg/mL and 29% for 4.2 mg/mL of P. bellus extract. The IC50 value was calculated as 0.139 mg/mL. The obtained cell viability results for each P. bellus extract concentration are shown in Figure 4. However, we observed that the extract precipitates crush the cells, causing mechanical damage. Furthermore, phase contrast microscopy of cultured cells exposed to the P. bellus extract suggested that the cells did not retain a confluent appearance and were damaged, probably due to the weight of the sample. As such, we could not directly reach conclusions about the biosafety of P. bellus extract; further investigation with micronized extract is needed. Moreover, the full extract of P. bellus may be relatively more toxic than the single test compounds. Overall, the findings show that the combined compounds in full P. bellus extract do not act in synergy to protect or rejuvenate the cells. Thus, in the next step, the cytotoxicity effect of the individual compound should be determined.
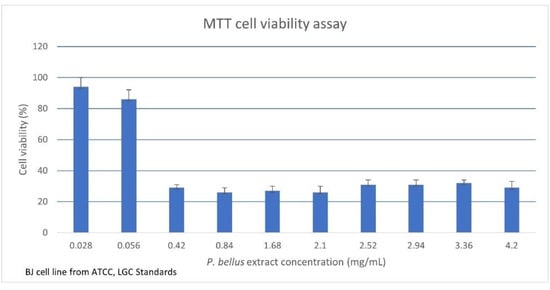
Figure 4.
The MTT cell viability assay on a human skin fibroblast cell line. The results are expressed as cell viability with different P. bellus extract concentrations. Values are given as mean + standard error.
In our study, the biological activities of natural compounds of P. bellus were investigated in relation to the antifungal properties, revealing novel potential application in therapy. These novel inhibitors—sesquiterpene lactones, guaianolides, and coumarins—may be potentially therapeutic against fungal infections. Notably, the frequency of fungal infections is still increasing [21] while resistance of pathogenic fungi to classical antifungal agents is increasing and the number of available drugs is decreasing [22]. As such, identifying and commercializing alternative, active substances from natural sources as antifungal remedies are required [23,24,25]. To the best of our knowledge, this is the first report on the antifungal activity of various compounds of P. bellus.
3. Materials and Methods
The bioethics committee approved the study protocol under Act 1188/17, based on Polish legislation and Good Clinical Practices at the Poznań University of Medical Sciences.
3.1. Plant Material
Herbs of Psephellus bellus (Trautv.) Wagenitz (syn. Centaurea bella Trautv.) were collected from the Botanical Garden of the Department and Division of Practical Cosmetology and Skin Diseases Prophylaxis, University of Medical Sciences (Poznań, Poland) (Figure 5), where voucher specimens (voucher numbers 21/1990 and 55/2014) are deposited. Seeds of P. bellus were provided by the Botanical Garden in Karaganda (Kazachstan) in accordance with the Convention on Biological Diversity from Rio de Janeiro 1992. The seeds were all gathered in the same year from their natural habitat on the Caucasus Mountains.

Figure 5.
Psephellus bellus in the Garden of Department and Division of Practical Cosmetology and Skin Diseases Prophylaxis.
The dried herbs of P. bellus (758 g) were crushed and soaked in 4.5 L MeOH 3 times at room temperature. The MeOH extract was evaporated under reduced pressure and lyophilized to obtain 5.5852 g (0.74%). We used 25.21 mg of extract for the antifungal studies, and the remaining was dissolved in 0.5 L H2O. The aqua solution was re-extracted 3 times with 0.25 L CH2Cl2. The CH2Cl2 extract was dried with anhydrous Na2SO4, filtrated, and evaporated, producing a residue (3.8 g). It was used as the material for phytochemical studies.
The compounds were separated by column chromatography (CC) on silica gel (particle size: 0.063–0.200 mm; Merck, Darmstadt, Germany; Art. 7733). The column was run by gravity flow with CH2Cl2, and a mixture of CH2Cl2 and (CH3)2CO (ratio 35:1) as the eluent. The polarity was gradually increased by adding (CH3)2CO. Selected fractions were further rechromatographed on silica gel (particle sizes of <0.063 mm; Merck, Darmstadt, Germany; Art. 7729) with CH2Cl2–n-hexane–(CH3)2CO–AcOEt (ratio 8:3; 1:1) and n-hexane–AcOEt (1:2; 1:5, and 1:8). The NMR spectra were run on a Bruker Avance 600 instrument using 600 and 150 MHz frequencies for hydrogen nuclei (1H) and carbon nuclei (13C), respectively, and tetramethylsilane (TMS) was used as the internal standard. The spectra were obtained for CDCl3, CD3OD, or DMSO-d6 solutions at 298 K.
3.2. Antifungal Susceptibility Study
All strains were isolated from the infected skin and nails of patients of the Department of Dermatology, Poznań University of Medical Sciences (Microbiology Laboratory). The fungal isolates were identified according to microscopic examination and visual observation on different cultivation media. Strains were correctly identified using an API ID 32® (bioMerieux, Lyon, France).
The Kirby–Bauer disk diffusion susceptibility test was used to determine the antifungal activity of the examined substances or drugs and the growth inhibition zone [17]. The diameter of the inhibition zones was measured to assess the antifungal properties of the studied substances. The inhibition zones were measured in millimeters.
Clinical strains of Candida albicans, C. famata, C. glabrata, C. parapsilosis, Rhodotorula rubra, Trichophyton rubrum, T. mentagrophytes var. interdigitale, Microsporum canis, and Scopulariopsis brevicaulis were suspended in 0.9% NaCl solution. Then, 10 mL of the suspension of all examined fungal strains was adjusted to 1 McFarland unit by a densitometer and were poured onto 9 cm agar plates with the following media: (i) 0.5% yeast nitrogen base (YNB; Difco, 3% glucose, and 1.8% agar; pH 7) for yeast strains; (ii) RPMI (bioMèrieux, Marcy l’Etoile, France) for dermatophytes and mold fungi.
The microorganism suspension was poured onto the plate containing YNB or RPMI agar and then small (0.5 mm), sterile paper discs impregnated with 10 µg of antifungal agent (0.39%) were placed on the plates. The measurement was made in duplicate, thus two plates were used for a single investigation. The substance then diffused from the filter into the medium and inhibited the growth of the surrounding microorganisms. The inhibition zone was measured visually under a stereomicroscope (Nikon SMZ800, Tokyo, Japan). All examinations were conducted simultaneously by two independent observers with microbiology experience (especially medical mycology), who were blinded to the previous results.
After 15 min, the Petri dishes were incubated. Candida cultures were incubated for 72 h at 36 °C. Dermatophytes (Trichophyton sp., Rhodotorula sp., and Microsporum sp.) and mold fungus (Scopulariopsis brevicaulis) were incubated at 27 °C for two weeks.
Two culture media were used: RPMI agar (bioMèrieux, Marcy l’Etoile, France) and 0.5%, YNB agar (Difco; glucose 3%, agar 1.8%, pH 7) obtained from DHN (Cracow, Poland). Then 15 mL of YNB/RPMI was poured into a 9 cm Petri dish. The sterilization was conducted under 1.5 atm pressure for 15 min.
We impregnated 5 mm filter paper disks with the examined compounds. Sterile NaCl 0.9% was used as the negative control.
As positive controls, we determined the sensitivity of C. albicans strains to amphotericin B (2 µg/mL), 5-fluorocytosine (16 µg/mL), and nystatin (1.25 µg/mL) through the disk diffusion susceptibility test using 5 mm filter paper disks impregnated with the mentioned commercially available drugs obtained from DHN (Cracow, Poland).
We decided not to establish the minimum inhibitory concentration (MIC) of each substance (no references were available to determine the MIC of the examined compounds). Due to the lack of standards of the used experimental materials, we could not assess the MIC through in vitro trials. Moreover, reported data indicated [18] that the MIC is of little value without the corresponding ability to interpret its clinical meaning. Certain authors [26] suggested that for some clinical isolates, MIC reading is complicated by the occurrence of MIC phenomena. It is known that the observed trailing and paradoxical effects (PXE), e.g., in C. albicans, complicate the unambiguous and reproducible determination of MICs. MICs are not a physical measurement. Moreover, host factors play a critical role in determining clinical outcomes. As such, dermatological clinicians decided not to conduct MIC testing.
3.3. Cytotoxicity Experiments
The MTT assay was employed to measure the cytotoxic effects of P. bellus extract on human dermal fibroblast (the BJ cell line from ATCC, LGC Standards; ATCC® CRL 2522™). Human dermal fibroblasts were cultured in EMEM supplemented with 5% fetal bovine serum, 4 mM L-glutamine, and 1% penicillin–streptomycin. Briefly, cells were seeded into flat-bottomed 96-well cell culture plates. The cells were then treated with varying concentrations of P. bellus extract. Dimethyl sulfoxide (DMSO) was used as a solvent. Appropriate controls (1% SDS and incubation only in culture medium) were used. The stocks of different dilutions were prepared (0.028–4.2 mg/mL) and each concentration of 100 µL was added in six repetitions to the respective wells. The plate was incubated at 37 °C in a humidified 5% CO2 incubator. Non-treated control cells were also maintained for comparing growth inhibition. The entire plate was observed after 24 h of treatment using a contrast tissue culture microscope to identify any detectable variations in the morphology of the cells.
The sample content in the wells was removed after 24 h of incubation and rinsed with phosphate-buffered saline (PBS) with calcium and magnesium ions. Subsequently, 100 µL of reconstituted MTT solution (0.5 mg/mL) was placed in all test and control wells. The plate was incubated for 3 h at 37 °C in a CO2 incubator.
The MTT was removed and 100 µL of isopropanol was added. Then, the wells were mixed for 15 min to solubilize the insoluble formazan crystals.
The absorbance values were measured at a wavelength of 570 nm with a microplate reader (TECAN Spark 10M, Zürich, Switzerland). The IC50 value was calculated.
3.4. Statistical Analysis
Statistical analysis was performed using STATISTICA version 13 (StatSoft, Inc., Tulsa, OK, USA). One-way ANOVA with a post-test (Tukey test) was used to identify possible significant differences between the compound effect on growth inhibition of examined fungal strains. The mean value of growth inhibition with the standard deviation was calculated. A value of p < 0.05 was considered statistically significant.
4. Conclusions
Containing compounds with possible antifungal properties, P. bellus is a plant with significant chemical and pharmacological potential.
The findings of the biological study described in this paper suggest that C-2 ester guaianolides from P. bellus are responsible for the potent antifungal activity of the extract from this plant. Pathogenic fungi are becoming resistant to commonly used and widely known antifungal treatments; thus, compounds from the studied material from P. bellus might provide a promising new source of compounds to be further evaluated in future studies for use as new antifungal agents in the clinic. Such a drug may be used for the treatment of infections caused by T. mentagrophytes var. interdigitale. It is possible that the drug would also affect infectious fungi strains that are resilient to the already available antifungal preparations.
Natural plant products need to be standardized and preliminary studies conducted to evaluate the possible risks, such as toxicity, of these compounds.
Author Contributions
J.N., G.N., and J.G.-P. conceived and initiated the study, and executed the experiments; J.N. and G.N. undertook phytochemistry of the compounds for the antifungal study; B.K.-K., H.K.-R., A.K., and Z.A. conceived, initiated, and executed the antifungal activity study; G.N., Z.A., J.N., and J.G.-P. supervised experiments; G.N., writing—original draft preparation; J.G.-P. and J.N., writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Poznan University of Medical Sciences, Poland (protocol code 1188/17; 7 December 2017).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Perfect, J.R. The antifungal pipeline: A reality check. Nature reviews. Drug Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Badiee, P.; Hashenmizadeh, Z. Opportunistic invasive fungal infections: Diagnosis & clinical management. Indian J. Med. Res. 2014, 139, 195–204. [Google Scholar] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Barrero, A.F.; Oltra, J.E.; Alvarez, M.; Raslan, D.S.; Saude, D.A.; Aksira, M. New sources and antifungal activity of sesquiterpene lactone. Fitoterapia 2000, 71, 60–64. [Google Scholar] [CrossRef]
- Bruno, M.; Bancheva, S.; Roselli, S.; Maggio, A. Sesquiterpenoids in subtribe Centaureinae (Cass) Dumort (tribe Cardueae, Asteraceae): Distribution, 13C NMR spectra data and biological properties. Phytochemistry 2013, 95, 19–93. [Google Scholar] [CrossRef]
- Nawrot, J.; Gornowicz-Porowska, J.; Nowak, G. Phytotherapy Perspectives for Treating Fungal Infections, Migraine, Sebohrreic Dermatitis and Hyperpigmentations with the Plants of the Centaureinae Subtribe (Asteraceae). Molecules 2020, 25, 5329. [Google Scholar] [CrossRef]
- Stefanović, O.D. Synergistic Activity of Antibiotics and Bioactive Plant Extracts: A Study Against Gram-Positive and GramNegative Bacteria. Bact. Pathog. Antibact. Control 2017, 23. [Google Scholar] [CrossRef]
- Heptinstall, S.; Groenwegen, W.A.; Spangenberg, P.; Loesche, W. Extracts feverfew may inhibit behavior via naturalization of sulphydryl groups. J. Pharm. Pharmacol. 1987, 39, 459–465. [Google Scholar] [CrossRef]
- Nowak, G.; Drozdz, B.; Holub, M. Sesquiterpene lactones of the Cardueae, subtribe Centaureinae. In Compositae: Systematics, Proceedings of the International Compositae Conference, Kew, Richmond, UK, 1994; Hind, D.J.N., Beentje, Eds.; Royal Botanic Gardens, Kew: Richmond, UK, 1996; Volume 1, pp. 219–227. [Google Scholar]
- Wyganowska-Swiatkowska, M.; Nohawica, M.; Grocholewicz, K.; Nowak, G. Influence of herbal medicines, on HMCBG1 release SARS-CoV-2 viral attachment, acute respiratory failure and sepsis. A literature review. Int. J. Mol. Sci. 2020, 21, 4639. [Google Scholar] [CrossRef]
- Hadas, E.; Derda, M.; Nawrot, J.; Nowak, G.; Thiem, B. Evaluation of the amoebicidal activities of Centaurea bella, Centaurea daghestanica, Rhaponticum pulchrum and Tanacetum vulgarae against pathogenic Acanthamoeba spp. Acta Pol. Pharm. 2017, 74, 1827–1832. [Google Scholar]
- Nawrot, J.; Napierała, M.; Kaczerowska-Pietrzak, K.; Florek, E.; Gornowicz-Porowska, J.; Pelant, E.; Nowak, G. The antiserotonin effect of parthenolide derivatives and standardised extract from the leaves of Stizolophus balsamita. Molecules 2019, 24, 4131. [Google Scholar] [CrossRef]
- Budesinsky, M.; Nowak, G.; Rychlewska, U.; Hodgson, D.J.; Saman, D.; Daniewski, W.M.; Holub, M. Structure of sesquiterpenic lactones of some species of subtribe Centaureinae. Collect. Czech. Chem. Commun. 1994, 59, 1175–1201. [Google Scholar] [CrossRef]
- Daniewski, W.M.; Nowak, G. Further sesquiterpene lactones of Centaurea bella. Phytochemistry 1993, 32, 204–205. [Google Scholar] [CrossRef]
- Nowak, G. A chemotaxonomy study of sesquiterpene lactones from subtribe Centaureinae of the Compositae. Phytochemistry 1992, 31, 2363–2368. [Google Scholar] [CrossRef]
- Abad, M.J.; Ansuategui, M.; Bermejo, P. Active antifungal substances from natural sources. Arkivoc 2007, 7, 116–145. [Google Scholar]
- Arif, T.; Bhosale, J.B.; Kumar, N.; Mandal, T.K.; Bendre, R.S.; Lavekar, G.S.; Dabur, R. Natural products-antifungal agents derived from plants. J. Asian Nat. Prod. Res. 2009, 11, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene Lactones from Artemisia Genus: Biological Activities and Methods of Analysis. J. Anal. Methods Chem. 2015, 247685. [Google Scholar] [CrossRef]
- Wedge, D.E.; Galindo, J.C.; Macías, F.A. Fungicidal activity of natural and synthetic sesquiterpene lactone analogs. Phytochemistry 2000, 53, 747–757. [Google Scholar] [CrossRef]
- Nowak, G. Chromatography of twenty six sesquiterpene lactones from Centaurea bella. Chromatographia 1993, 35, 325–328. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Guarner, J. Emerging and reemerging fungal infections. Semin. Diagn. Pathol. 2019, 36, 177–181. [Google Scholar] [CrossRef]
- Mehra, T.; Köberle, M.; Braunsdorf, C.; Mailänder-Sanchez, D.; Borelli, C.; Schaller, M. Alternative approaches to antifungal therapies. Exp. Dermatol. 2012, 21, 778–782. [Google Scholar] [CrossRef]
- Magaldi, S.; Mata-Essayag, S.; Hartung de Capriles, C.; Perez, C.; Colella, M.T.; Olaizola, C.; Ontiveros, Y. Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis. 2004, 8, 39–45. [Google Scholar] [CrossRef]
- Rex, J.H.; Pfaller, M.A.; Walsh, T.J.; Chaturvedi, V.; Espinel-Ingroff, A.; Ghannoum, M.A.; Gosey, L.L.; Odds, F.C.; Rinaldi, M.G.; Sheehan, D.J.; et al. Antifungal susceptibility testing: Practical aspects and current challenges. Clin. Microbiol. Rev. 2001, 14, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Shirani, M.; Samimi, A.; Kalantari, H.; Madani, M.; Kord-Zanganeh, A. Chemical composition and antifungal effect of hydroalcoholic extract of Allium tripedale Trautv. against Candida species. Curr. Med. Mycol. 2017, 3, 6–12. [Google Scholar] [PubMed]
- Binder, U.; Aigner, M.; Risslegger, B.; Hörtnagl, C.; Lass-Flörl, C.; Lackner, M. Minimal Inhibitory Concentration (MIC)-Phenomena in Candida albicans and Their Impact on the Diagnosis of Antifungal Resistance. J. Fungi 2019, 5, 83. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).