Metabolites Profiling, In Vitro, In Vivo, Computational Pharmacokinetics and Biological Predictions of Aloe perryi Resins Methanolic Extract
Abstract
1. Introduction
2. Results
2.1. Botanical Material Sampling and Extract Preparation
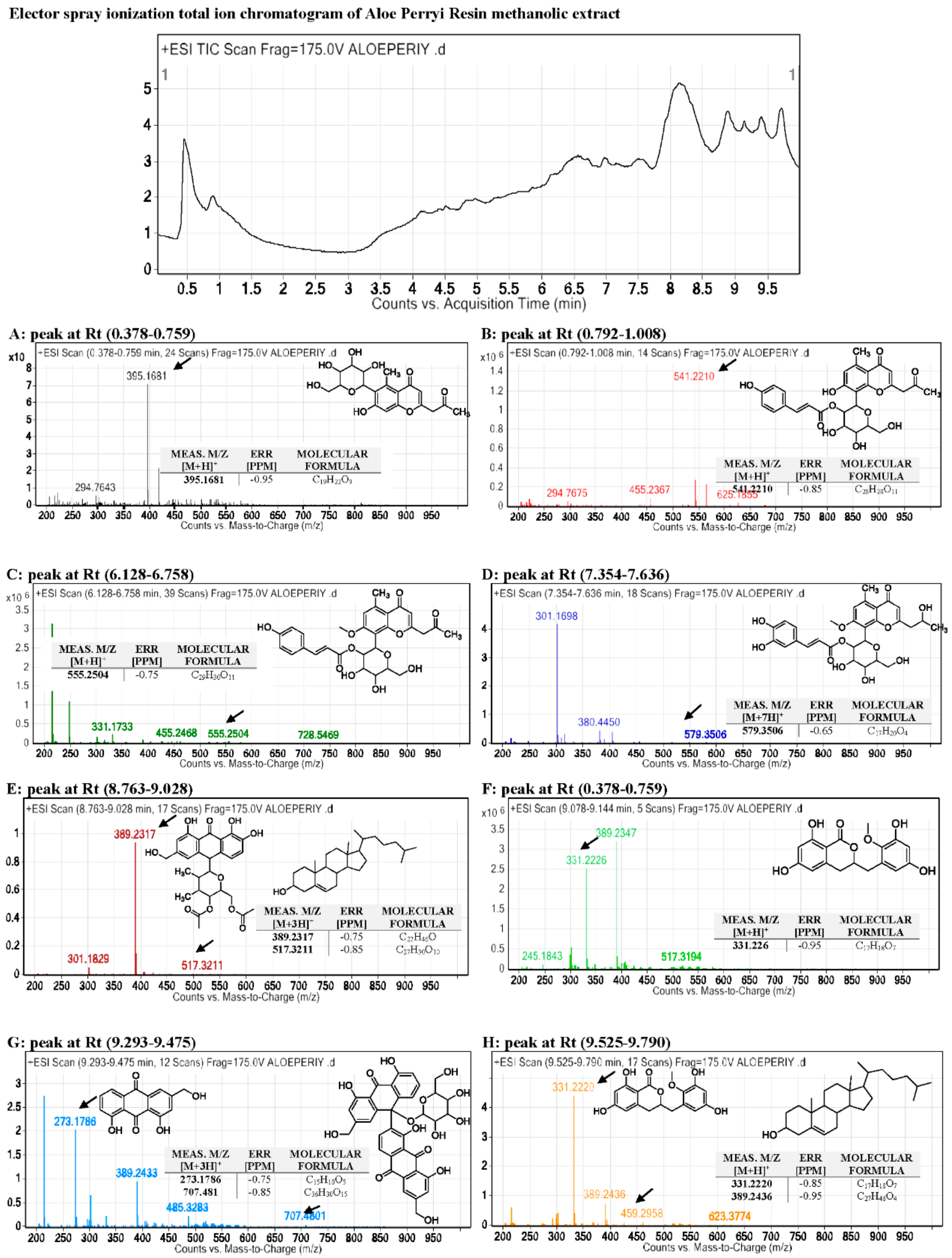
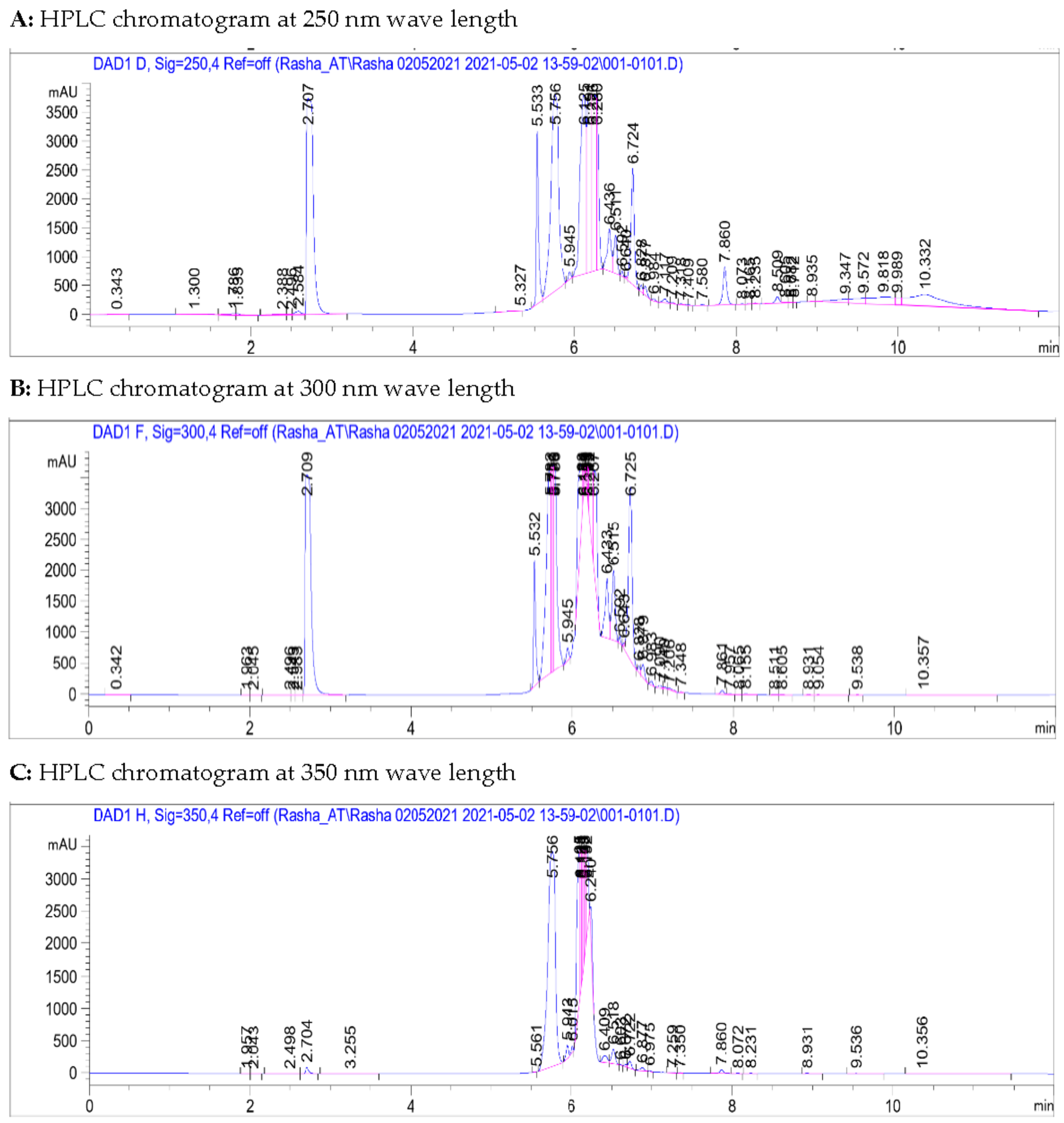
2.2. Metabolites Profiling Using HPLC and QTOF/LCMS
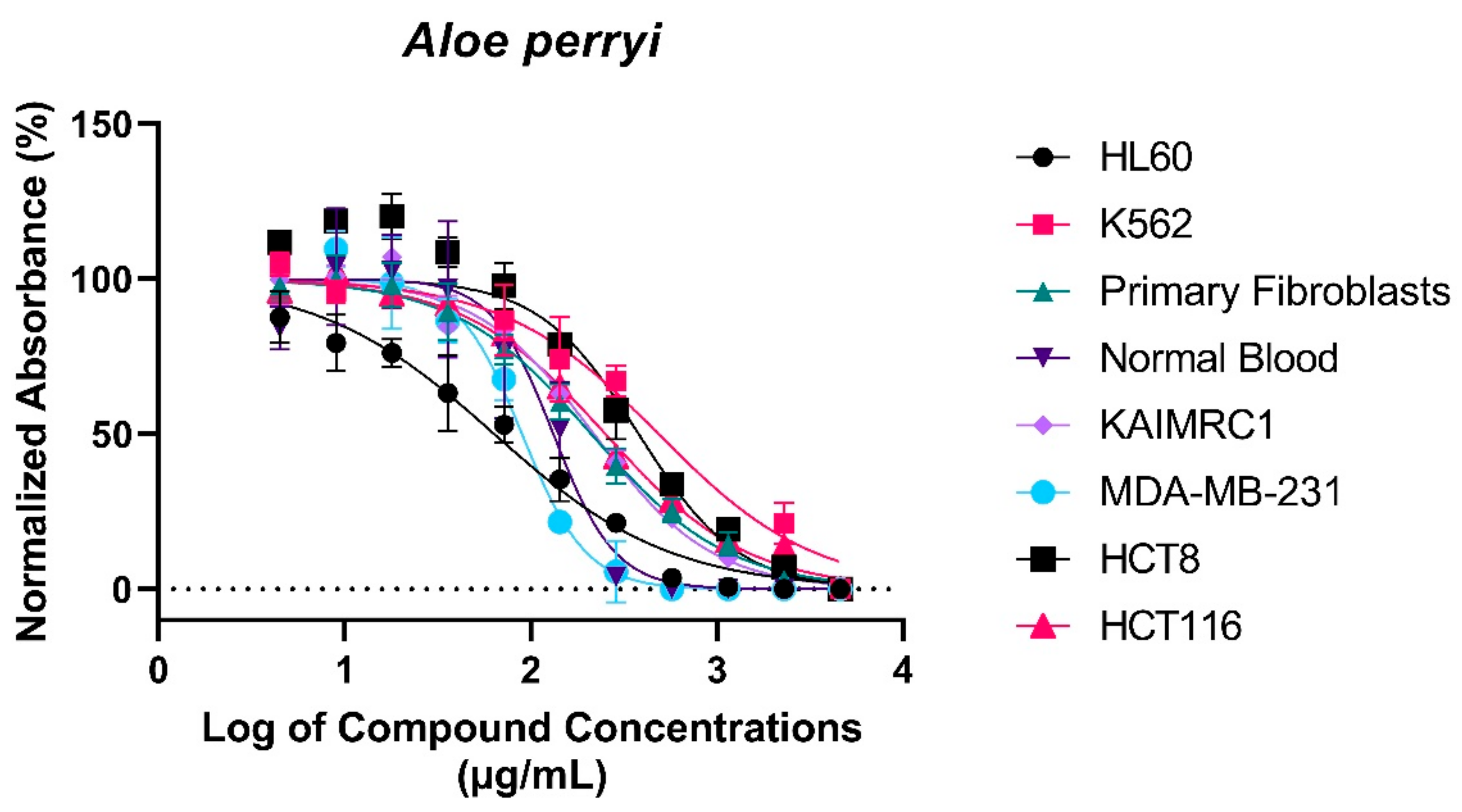
2.3. Treatment with Aloe perryi Extract Demonstrates Cytotoxic Properties against Several Cancer Cells
2.3.1. Prediction of Biological Activity Using PASS Online
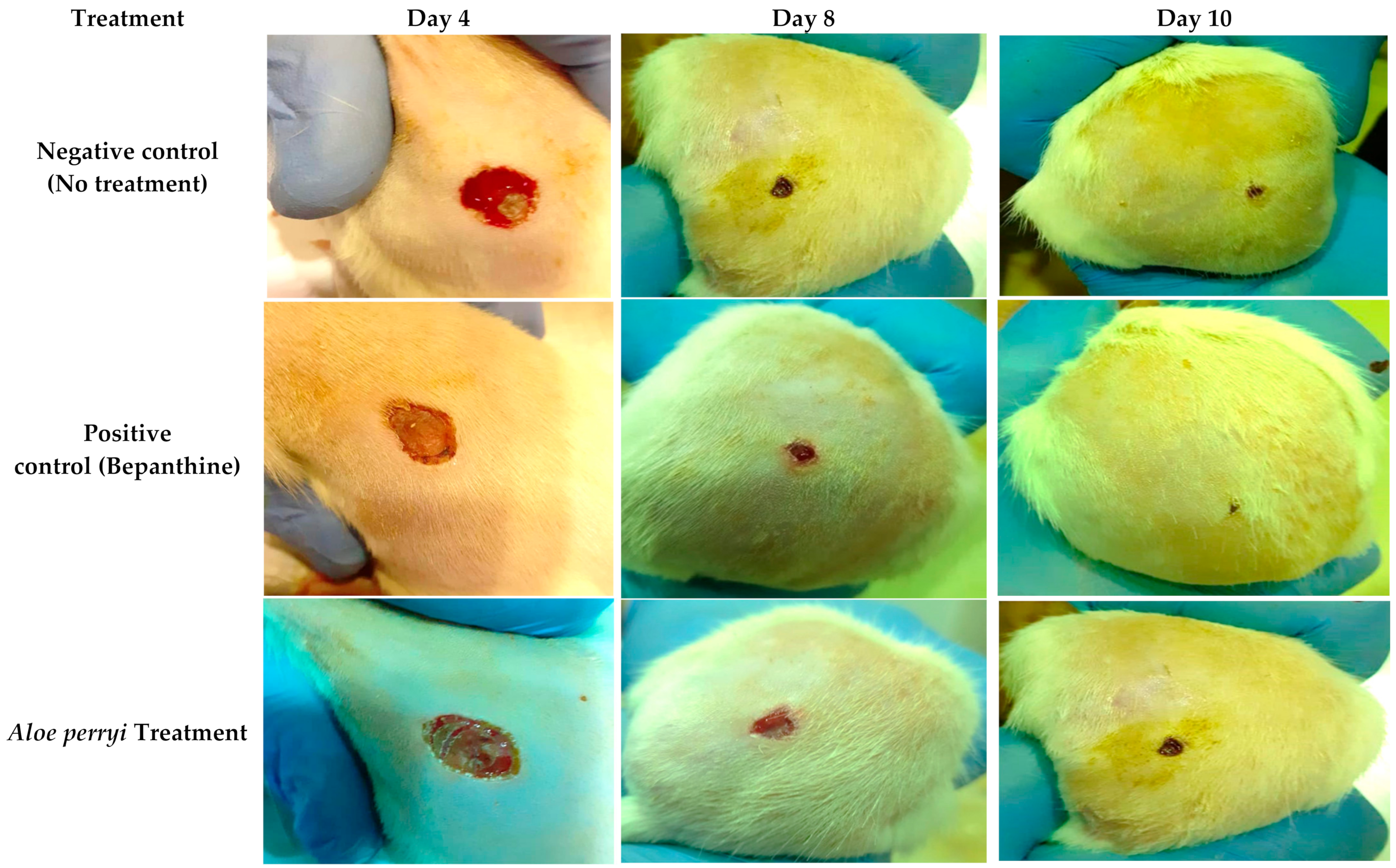
2.3.2. Topical Treatment with Aloe perryi Extract Increases Wound Closure in the Skin of Healthy Rats
2.4. Topical Treatment with Aloe perryi Extract Enhances the Complete Re-Epithelialization in the Skin of Healthy Rats
2.5. Pharmacokinetics ADME Predictions of the Bioactive Constituents of Aloe perryi Extract
2.5.1. Molecular Weight (MW)
2.5.2. Lipophilicity (Log P) and Gastrointestinal (GI) Absorption
2.5.3. Solubility (Log S)
2.5.4. Blood–Brain Barrier (BBB) Permeability
2.5.5. Hydrogen Bond Donor and Acceptor (HBD/HBA)
2.6. Target Prediction of the Bioactive Constituents of Aloe perryi Extract
3. Discussion
3.1. Metabolites Profiling Using HPLC and QTOF/LCMS
3.2. Cytotoxic Properties of Aloe perryi Extracts against Six Cancer Cell Lines
3.3. Anti-Inflammatory Properties of Aloe perryi Extract
3.4. Histological Assessment of Skin Wound after Aloe perryi Extract Treatment
3.5. Pharmacokinetics ADME Properties
3.6. Target Prediction
4. Materials and Methods
4.1. Botanical Material Sampling and Extract Preparation
4.2. Chemical Identification
4.2.1. Chemicals
4.2.2. LC–QTOF-MS Method
4.3. Cell Viability Assay
4.4. In Vivo Anti-Inflammatory Testing
4.5. Histology
4.5.1. Tissue Processing
4.5.2. Sectioning
4.5.3. Haematoxylin and Eosin (H&E) Staining
4.6. Pharmacokinetics ADME and Target Predictions
4.7. Target Predictions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Enna, S. Phenotypic drug screening. J. Peripher. Nerv. Syst. 2014, 19, S4–S5. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, C.; Kanjilal, S.; Gupta, A.; Katiyar, S. Drug discovery from plant sources: An integrated approach. AYU Int. Q. J. Res. Ayurveda 2012, 33, 10–19. [Google Scholar] [CrossRef]
- Strohl, W.R. The role of natural products in a modern drug discovery program. Drug Discov. Today 2000, 5, 39–41. [Google Scholar] [CrossRef]
- Wali, A.F.; Majid, S.; Rasool, S.; Shehada, S.B.; Abdulkareem, S.K.; Firdous, A.; Beigh, S.; Shakeel, S.; Mushtaq, S.; Akbar, I.; et al. Natural products against cancer: Review on phytochemicals from marine sources in preventing cancer. Saudi Pharm. J. 2019, 27, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Miele, M.; Mumot, A.M.; Zappa, A.; Romano, P.; Ottaggio, L. Hazel and other sources of paclitaxel and related compounds. Phytochem. Rev. 2012, 11, 211–225. [Google Scholar] [CrossRef]
- Chopra, R.; Nayar, S.; Chopra, I.C. Plants as a source of anticancer agents. In Glossary of Indianmedicinal Plants; National Institute of Science Communication: New Delhi, India, 1996; Volume 100, pp. 72–79. [Google Scholar]
- Hazafa, A.; Rehman, K.-U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Muñiz, E.; Guerrero-Palomo, G.; Vega, L.J.T. Natural products: New anti-cancer agents derived from plants. Curr. Top. Toxicol. 2006, 8, 1. [Google Scholar]
- Hausman, D.M. What Is Cancer? Perspect. Biol. Med. 2019, 62, 778–784. [Google Scholar] [CrossRef]
- Nagai, H.; Kim, Y.H. Cancer prevention from the perspective of global cancer burden patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef]
- Carbone, A. Cancer classification at the crossroads. Cancers 2020, 12, 980. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Cancer treatment: Present and future. In Molecular Biology of the Cell, 4th ed.; Garland Science: London, UK, 2002. [Google Scholar]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update. Int. J. Mol. Sci. 2020, 21, 5920. [Google Scholar] [CrossRef]
- Ali, I.; Nadeem Lone, M.; Al-Othman, Z.A.; Al-Warthan, Z.A.; Marsin Sanagi, M. Heterocyclic scaffolds: Centrality in anticancer drug development. Curr. Drug Targets 2015, 16, 711–734. [Google Scholar] [CrossRef]
- Al-Oqail, M.M.; El-Shaibany, A.; Al-Jassas, E.; Al-Sheddi, E.S.; Al-Massarani, S.M.; Farshori, N.N. In vitro anti-proliferative activities of Aloe perryi flowers extract on human liver, colon, breast, lung, prostate and epithelial cancer cell lines. Pak. J. Pharm. Sci. 2016, 29, 723–729. [Google Scholar]
- Grace, O. Current perspectives on the economic botany of the genus Aloe L. (Xanthorrhoeaceae). S. Afr. J. Bot. 2011, 77, 980–987. [Google Scholar] [CrossRef]
- Cock, I.E. The genus aloe: Phytochemistry and therapeutic uses including treatments for gastrointestinal conditions and chronic inflammation. Prog. Drug Res. 2015, 70, 179–235. [Google Scholar]
- Al-Fatimi, M.; Wurster, M.; Schröder, G.; Lindequist, U. Antioxidant, antimicrobial and cytotoxic activities of selected medicinal plants from Yemen. J. Ethnopharmacol. 2007, 111, 657–666. [Google Scholar] [CrossRef]
- Aldayel, T.S.; Grace, M.H.; Lila, M.A.; Yahya, M.A.; Omar, U.M.; Alshammary, G. LC-MS characterization of bioactive metabolites from two Yemeni Aloe spp. with antioxidant and antidiabetic properties. Arab. J. Chem. 2020, 13, 5040–5049. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A. Health benefits of chromones: Common ingredients of our daily diet. Phytochem. Rev. 2020, 19, 1–25. [Google Scholar] [CrossRef]
- Mothana, R.A.; Al-Musayeib, N.M.; Matheeussen, A.; Cos, P.; Maes, L. Assessment of the in Vitro Antiprotozoal and Cytotoxic Potential of 20 Selected Medicinal Plants from the Island of Soqotra. Molecules 2012, 17, 14349–14360. [Google Scholar] [CrossRef] [PubMed]
- Mosby’s Handbook of Herbs & Natural Supplements, 4th ed.; Mosby: St. Louis, MO, USA, 2010; ISBN 9780323057417. Available online: https://evolve.elsevier.com/cs/product/9780323057417?role=student (accessed on 2 April 2021).
- Borodina, Y.V.; Filimonov, D.A.; Poroikov, V.V. Computer-aided prediction of prodrug activity using the PASS system. Pharm. Chem. J. 1996, 30, 760–763. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Arnott, J.A.; Planey, S.L. The influence of lipophilicity in drug discovery and design. Expert Opin. Drug Discov. 2012, 7, 863–875. [Google Scholar] [CrossRef]
- Tahlan, S.; Kumar, S.; Ramasamy, K.; Lim, S.M.; Shah, S.A.A.; Mani, V.; Narasimhan, B. In-silico molecular design of heterocyclic benzimidazole scaffolds as prospective anticancer agents. BMC Chem. 2019, 13, 1–22. [Google Scholar] [CrossRef]
- Gomes, A.; Neuwirth, O.; Freitas, M.; Couto, D.; Ribeiro, D.; Figueiredo, A.G.; Silva, A.M.; Seixas, R.S.; Pinto, D.C.; Tomé, A.C.; et al. Synthesis and antioxidant properties of new chromone derivatives. Bioorganic Med. Chem. 2009, 17, 7218–7226. [Google Scholar] [CrossRef]
- Dagne, E.; Bisrat, D.; Viljoen, A.; Van Wyk, B.-E. Chemistry of Aloe Species. Curr. Org. Chem. 2005, 4, 1055–1078. [Google Scholar] [CrossRef]
- Maurya, D.K.; Devasagayam, T.P.A. Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food Chem. Toxicol. 2010, 48, 3369–3373. [Google Scholar] [CrossRef]
- Moon, E.-J.; Lee, Y.M.; Lee, O.-H.; Lee, M.-J.; Lee, S.-K.; Chung, M.-H.; Park, Y.-I.; Sung, C.-K.; Choi, J.-S.; Kim, K.-W. A ncovel angiogenic factor derived from Aloe vera gel: β-sitosterol, a plant sterol. Angiogenesis 1999, 3, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Speranza, G.; Manitto, P.; Cassara’, P.; Monti, D. Feralolide, a dihydroisocoumarin from cape aloe. Phytochemistry 1993, 33, 175–178. [Google Scholar] [CrossRef]
- Cheang, M.C.; Voduc, D.; Bajdik, C.; Leung, S.; McKinney, S.; Chia, S.K.; Perou, C.M.; Nielsen, T.O. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin. Cancer Res. 2008, 14, 13681376. [Google Scholar] [CrossRef]
- Rahman, M.; Davis, S.R.; Pumphrey, J.G.; Bao, J.; Nau, M.M.; Meltzer, P.S.; Lipkowitz, S. TRAIL induces apoptosis in triple-negative breast cancer cells with a mesenchymal phenotype. Breast Cancer Res. Treat. 2009, 113, 217–230. [Google Scholar] [CrossRef]
- Corkery, B.; Crown, J.; Clynes, M.; O’Donovan, N. Epidermal growth factor receptor as a potential therapeutic target in triple-negative breast cancer. Ann. Oncol. 2009, 20, 862–867. [Google Scholar] [CrossRef]
- Hwang, S.-Y.; Park, S.; Kwon, Y. Recent therapeutic trends and promising targets in triple negative breast cancer. Pharmacol. Ther. 2019, 199, 30–57. [Google Scholar] [CrossRef] [PubMed]
- Grace, O.M.; Simmonds, M.S.J.; Smith, G.; van Wyk, A.E. Documented Utility and Biocultural Value of Aloe L. (Asphodelaceae): A Review. Econ. Bot. 2009, 63, 167–178. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bartolo, P.J.D.S. Traditional Therapies for Skin Wound Healing. Adv. Wound Care 2016, 5, 208–229. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhao, G.; Jia, J. Preliminary evaluation: The effects of Aloe ferox Miller and Aloe arborescens Miller on wound healing. J. Ethnopharmacol. 2008, 120, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.T.; Mazumder, A.; Dwivedi, A.; Gerber, M.; du Plessis, J.; Hamman, J.H. In vitro wound healing and cytotoxic activity of the gel and whole-leaf materials from selected aloe species. J. Ethnopharmacol. 2017, 200, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hęś, M.; Dziedzic, K.; Górecka, D.; Jędrusek-Golińska, A.; Gujska, E. Aloe vera (L.) Webb.: Natural Sources of Antioxidants—A Review. Plant Foods Hum. Nutr. 2019, 74, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Kerns, E.H.; Di, L. Drug-like Properties: Concepts, Structure Design and Methods; Academic Press: Berkeley, CA, USA, 2008. [Google Scholar]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Sharom, F.J. ABC multidrug transporters: Structure, function and role in chemoresistance. Pharmacogenomics 2008, 9, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Shaik, N.A.; Al-Kreathy, H.M.; Ajabnoor, G.M.; Verma, P.K.; Banaganapalli, B. Molecular designing, virtual screening and docking study of novel curcumin analogue as mutation (S769L and K846R) selective inhibitor for EGFR. Saudi J. Biol. Sci. 2019, 26, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, S.; Gustafsson, J.-Å. Nuclear receptors: Recent drug discovery for cancer therapies. Endocr. Rev. 2019, 40, 1207–1249. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Samman, N.; Al Zahrani, H.; Nehdi, A.; Rahman, S.; Khan, A.L.; Al Balwi, M.; Alriyees, L.A.; Alzaid, M.; Al Askar, A.; et al. Isolation and characterization of a new naturally immortalized human breast carcinoma cell line, KAIMRC1. BMC Cancer 2017, 17, 1–13. [Google Scholar] [CrossRef]
- Nehdi, A.; Ali, R.; Alhallaj, A.; Alzahrani, H.; Samman, N.; Mashhour, A.; Baz, O.; Barhoumi, T.; Alghanem, B.; Khan, A.; et al. Nuclear Receptors Are Differentially Expressed and Activated in KAIMRC1 Compared to MCF7 and MDA-MB231 Breast Cancer Cells. Molecules 2019, 24, 2028. [Google Scholar] [CrossRef]
- Alghanem, B.; Ali, R.; Nehdi, A.; Al Zahrani, H.; Altolayyan, A.; Shaibah, H.; Baz, O.; Alhallaj, A.; Moresco, J.J.; Diedrich, J.K.; et al. Proteomics Profiling of KAIMRC1 in Comparison to MDA-MB231 and MCF-7. Int. J. Mol. Sci. 2020, 21, 4328. [Google Scholar] [CrossRef]

| Extract Type | Cell Line | Activity IC50 (μg) |
|---|---|---|
| Petroleum Ether extract | HepG-2 HCT-116 MCF-7 A-549 PC-3 HEp-2 HeLa | 8.2 5.61 7.88 7.54 9.51 10.1 5.83 |
| Chloroform Extract | HepG-2 HCT-116 MCF-7 A-549 PC-3 HEp-2 HeLa | 17.3 14.1 22.9 13.0 21.7 18.7 12.1 |
| Ethyl Acetate Extract | HepG-2 HCT-116 MCF-7 A-549 PC-3 HEp-2 HeLa | 18.5 19.8 >50 18.8 >50 29.1 >50 |
| Butanol Extract | HepG-2 HCT-116 MCF-7 A-549 PC-3 HEp-2 HeLa | 16.9 10.3 12.7 18.8 19.7 26.7 11.1 |
| Aqueous Extract | HepG-2 HCT-116 MCF-7 A-549 PC-3 HEp-2 HeLa | 21.5 17.5 27.4 22.6 29.4 40.1 >50 |
| Cell lines | Treatment with Aloe perryi Extract | |||
|---|---|---|---|---|
| IC50 (µg/mL) | Se | R2 | ||
| Leukemia | HL60 | 63.81 | 6.896 | 0.960 |
| K562 | 486.9 | 8.587 | 0.951 | |
| Normal primary fibroblasts | P1 | 211.6 | 5.048 | 0.983 |
| Normal blood sample | N1 | 132.5 | 12.30 | 0.931 |
| Breast cancer | KAIMRC1 | 218.5 | 6.059 | 0.979 |
| MDA-MB-231 | 89.85 | 6.947 | 0.977 | |
| Colorectal cancer | HCT8 | 380.3 | 11.24 | 0.950 |
| HCT116 | 252.2 | 4.136 | 0.988 | |
| Compound Number | Predicted Activity | Pa | Pi |
|---|---|---|---|
| 1 | Anticarcinogenic Antineoplastic | 0.716 0.50 | 0.008 0.035 |
| 2 | Anticarcinogenic Antineoplastic | 0.820 0.814 | 0.005 0.010 |
| 3 | Anticarcinogenic Antineoplastic | 0.806 0.802 | 0.005 0.012 |
| 4 | Anticarcinogenic Antineoplastic | 0.569 0.474 | 0.014 0.079 |
| 5 | Anticarcinogenic Antineoplastic | 0.627 0.821 | 0.011 0.009 |
| 6 | Anticarcinogenic Antineoplastic | 0.382 0.666 | 0.034 0.032 |
| 7 | Anticarcinogenic Antineoplastic | 0.510 0.722 | 0.018 0.022 |
| 8 | Anticarcinogenic Antineoplastic | 0.785 0.770 | 0.006 0.016 |
| 9 | Anticarcinogenic Antineoplastic | 0.382 0.666 | 0.034 0.032 |
| 10 | Anticarcinogenic Antineoplastic | 0.569 0.474 | 0.014 0.079 |
| Compound Codes | Molecular Weight | HB Donor | HB Acceptor | Log Po/w (WLOGP) | Log S (SILICO S-IT) | BBB Permeability | GI Absorption | Rule of Five (ROF). |
|---|---|---|---|---|---|---|---|---|
| Aloe perryi Active Constituents | ||||||||
| 1 | 394.37 g/mol | 5 | 9 | −0.87 | −2.17 soluble | No | Low | Yes; 0 violation |
| 2 | 540.52 g/mol | 5 | 11 | 0.99 | −4.32 Moderately soluble | No | Low | No; 2 violations: MW > 500, NorO > 10 |
| 3 | 554.54 g/mol | 4 | 11 | 1.29 | −5.00 Moderately soluble | No | Low | No; 2 violations: MW > 500, NorO > 10 |
| 4 | 386.65 g/mol | 1 | 1 | 7.39 | −5.78 Moderately soluble | No | Low | Yes; 0 violation |
| 5 | 514.52 g/mol | 4 | 10 | 2.35 | −4.34 Moderately soluble | No | Low | Yes; 1 violation: MW > 500 |
| 6 | 332.30 g/mol | 4 | 7 | 1.84 | −3.35 Soluble | No | High | Yes; 0 violation |
| 7 | 270.24 g/mol | 3 | 5 | 1.21 | −3.92 Soluble | No | High | Yes; 0 violation |
| 8 | 702.61 g/mol | 10 | 15 | −0.49 | −5.44 Moderately soluble | No | Low | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 |
| 9 | 332.30 g/mol | 4 | 7 | 1.84 | −3.35 soluble | No | High | Yes; 0 violation |
| 10 | 386.65 g/mol | 1 | 1 | 7.39 | −5.78 Moderately soluble | No | Low | Yes; 0 violation |
| Compounds Codes | GPCR Ligand | Ion Channel Modulator | Kinase Inhibitor | Nuclear Receptor Ligand | Protease Inhibitor | Enzyme Inhibitor |
|---|---|---|---|---|---|---|
| Aloe perryi Bioactive Constituents | ||||||
| 1 | −0.16 | −0.44 | −0.40 | 0.00 | −0.18 | 0.31 |
| 2 | −0.09 | −0.56 | −0.34 | 0.08 | −0.16 | 0.21 |
| 3 | −0.11 | −0.64 | −0.38 | 0.02 | −0.19 | 0.15 |
| 4 | 0.13 | 0.02 | −0.51 | 0.75 | 0.04 | 0.54 |
| 5 | 0.13 | 0.04 | −0.03 | 0.35 | 0.06 | 0.35 |
| 6 | 0.03 | −0.10 | −0.08 | 0.26 | −0.12 | 0.06 |
| 7 | −0.02 | 0.02 | 0.12 | 0.24 | 0.04 | 0.38 |
| 8 | −0.71 | −1.64 | −1.05 | −1.02 | −0.29 | −0.63 |
| 9 | 0.03 | −0.10 | −0.08 | 0.26 | −0.12 | 0.06 |
| 10 | 0.13 | 0.02 | −0.51 | 0.75 | 0.04 | 0.54 |
| Reagents | Duration (Time) | Temperature (°C) |
|---|---|---|
| 70% ethanol | 30 min | 37 °C |
| 80% ethanol | 30 min | 37 °C |
| 90% ethanol | 30 min | 37 °C |
| 95% ethanol | 30 min | 37 °C |
| 100% ethanol | 1:00 h | 37 °C |
| 100% ethanol | 1:00 h | 37 °C |
| 100% ethanol | 1:30 h | 37 °C |
| xylene | 1:00 h | 37 °C |
| xylene | 1:30 h | 37 °C |
| xylene | 1:30 h | 37 °C |
| paraffin wax | 1:00 h | 65 °C |
| paraffin wax | 1:00 h | 65 °C |
| paraffin wax | 1:00 h | 65 °C |
| SMILES | |
|---|---|
| Compound 1 | CC(=O)CC1=CC(=O)C2=C(C)C(C3OC(CO)C(O)C(O)C3O)=C(O)C=C2O1 |
| Compound 2 | CC(=O)CC1=CC(=O)C2=C(C)C=C(O)C(C3OC(CO)C(O)C(O)C3OC(=O)\C=C\C3=CC=C(O)C=C3)=C2O1 |
| Compound 3 | COC1=CC(C)=C2C(=O)C=C(CC(C)=O)OC2=C1C1OC(CO)C(O)C(O)C1OC(=O)\C=C\C1=CC=C(O)C=C1 |
| Compound 4 | CC(C)CCCC(C)C1CCC2C3CC=C4CC(O)CCC4(C)C3CCC12C |
| Compound 5 | CC1C(C)C(OC(COC(C)=O)C1OC(C)=O)C1C2=C(C(O)=C(O)C=C2)C(=O)C2=C1C=C(CO)C=C2O |
| Compound 6 | COC1=C(O)C=C(O)C=C1CC1CC2=CC(O)=CC(O)=C2C(=O)O1 |
| Compound 7 | OCC1=CC(O)=C2C(=O)C3=C(O)C=CC=C3C(=O)C2=C1 |
| Compound 8 | OCC1OC(OC2(C3=CC=C4C(=O)C5=C(C(O)=CC(CO)=C5)C(=O)C4=C3O)C3=C(C(O)=CC=C3)C(=O)C3=C2C=C(CO)C=C3O)C(O)C(O)C1O |
| Compound 9 | COC1=C(O)C=C(O)C=C1CC1CC2=CC(O)=CC(O)=C2C(=O)O1 |
| Compound 10 | CC(C)CCCC(C)C1CCC2C3CC=C4CC(O)CCC4(C)C3CCC12C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suliman, R.S.; Alghamdi, S.S.; Ali, R.; Aljatli, D.A.; Huwaizi, S.; Suliman, R.; Albadrani, G.M.; Tolayyan, A.A.; Alghanem, B. Metabolites Profiling, In Vitro, In Vivo, Computational Pharmacokinetics and Biological Predictions of Aloe perryi Resins Methanolic Extract. Plants 2021, 10, 1106. https://doi.org/10.3390/plants10061106
Suliman RS, Alghamdi SS, Ali R, Aljatli DA, Huwaizi S, Suliman R, Albadrani GM, Tolayyan AA, Alghanem B. Metabolites Profiling, In Vitro, In Vivo, Computational Pharmacokinetics and Biological Predictions of Aloe perryi Resins Methanolic Extract. Plants. 2021; 10(6):1106. https://doi.org/10.3390/plants10061106
Chicago/Turabian StyleSuliman, Rasha Saad, Sahar Saleh Alghamdi, Rizwan Ali, Dimah A. Aljatli, Sarah Huwaizi, Rania Suliman, Ghadeer M. Albadrani, Abdulellah Al Tolayyan, and Bandar Alghanem. 2021. "Metabolites Profiling, In Vitro, In Vivo, Computational Pharmacokinetics and Biological Predictions of Aloe perryi Resins Methanolic Extract" Plants 10, no. 6: 1106. https://doi.org/10.3390/plants10061106
APA StyleSuliman, R. S., Alghamdi, S. S., Ali, R., Aljatli, D. A., Huwaizi, S., Suliman, R., Albadrani, G. M., Tolayyan, A. A., & Alghanem, B. (2021). Metabolites Profiling, In Vitro, In Vivo, Computational Pharmacokinetics and Biological Predictions of Aloe perryi Resins Methanolic Extract. Plants, 10(6), 1106. https://doi.org/10.3390/plants10061106