Anti-Inflammatory, Thrombolytic and Hair-Growth Promoting Activity of the n-Hexane Fraction of the Methanol Extract of Leea indica Leaves
Abstract
1. Introduction
2. Results
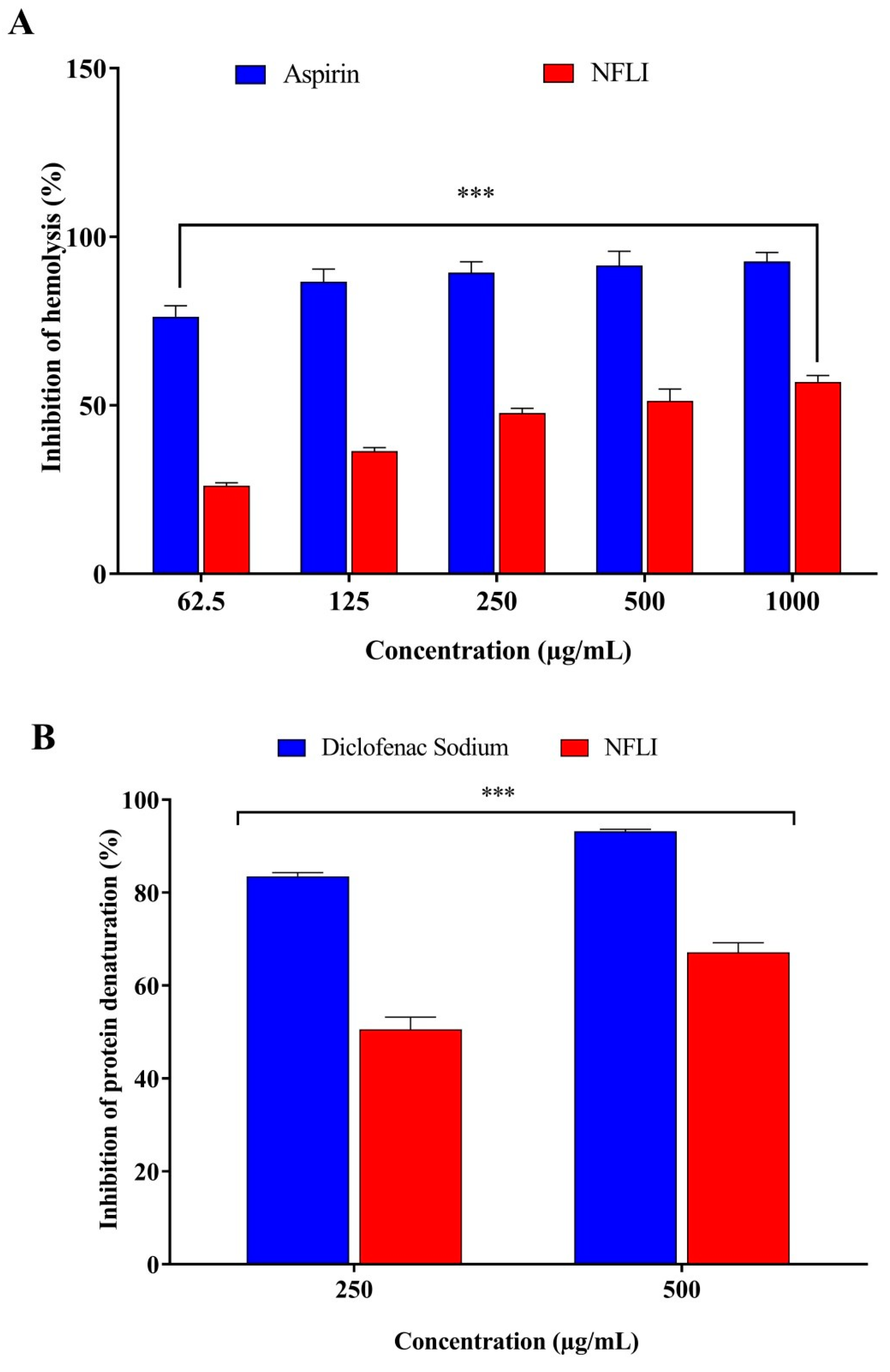
2.1. Anti-Inflammatory Activity
2.1.1. Membrane Stabilisation Assay
2.1.2. Inhibition of Protein Denaturation
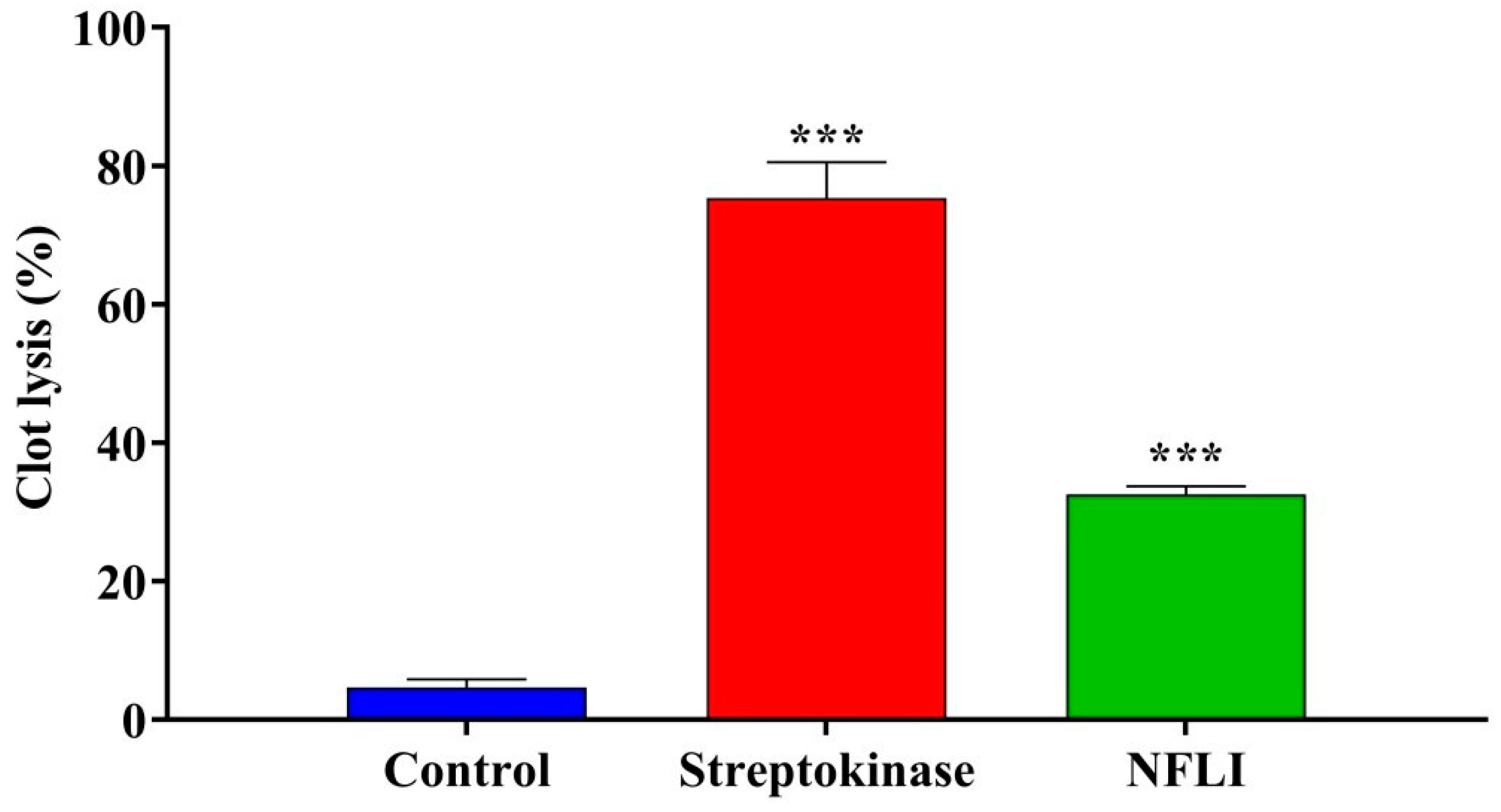
2.2. Thrombolytic Activity
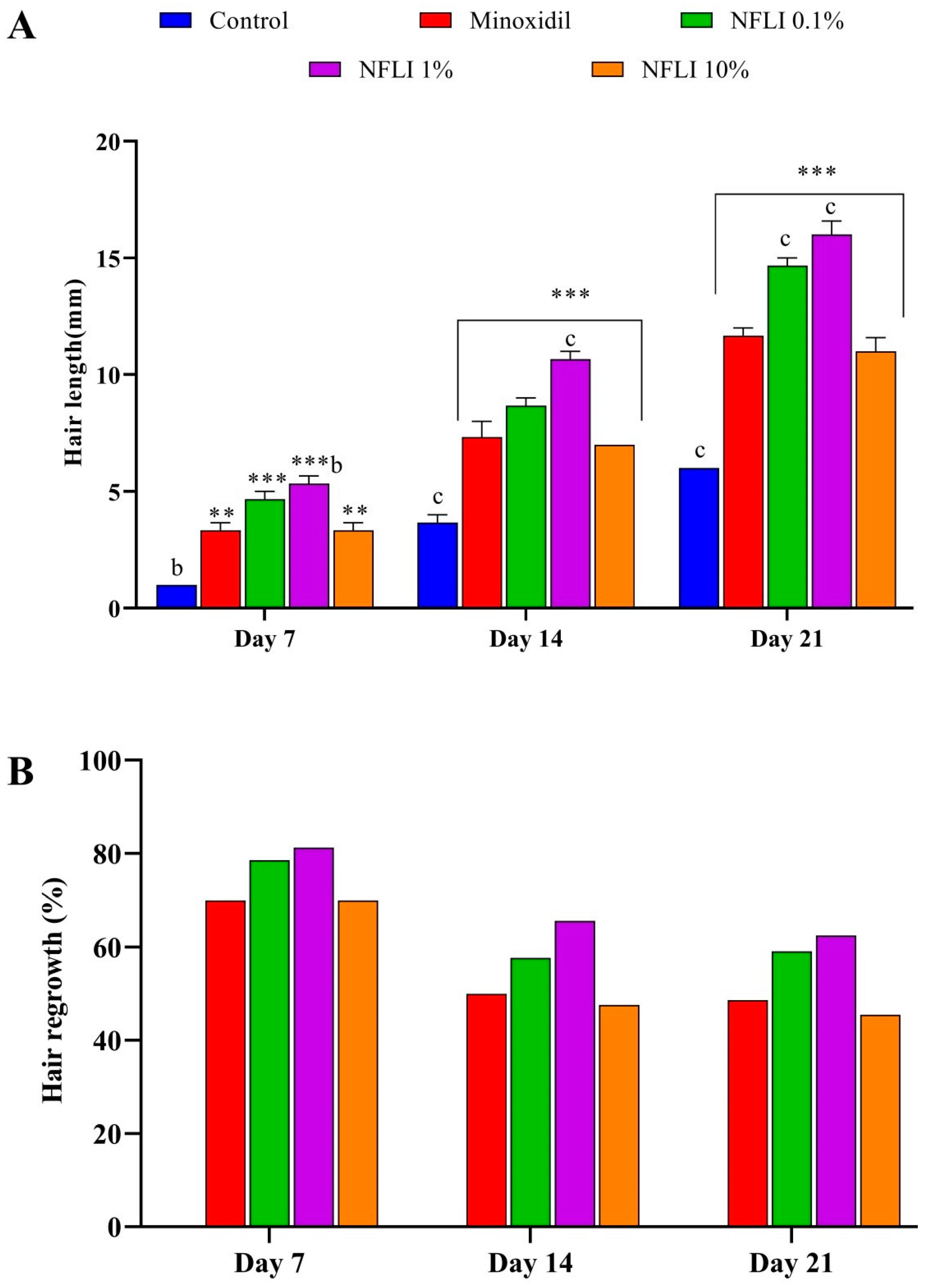
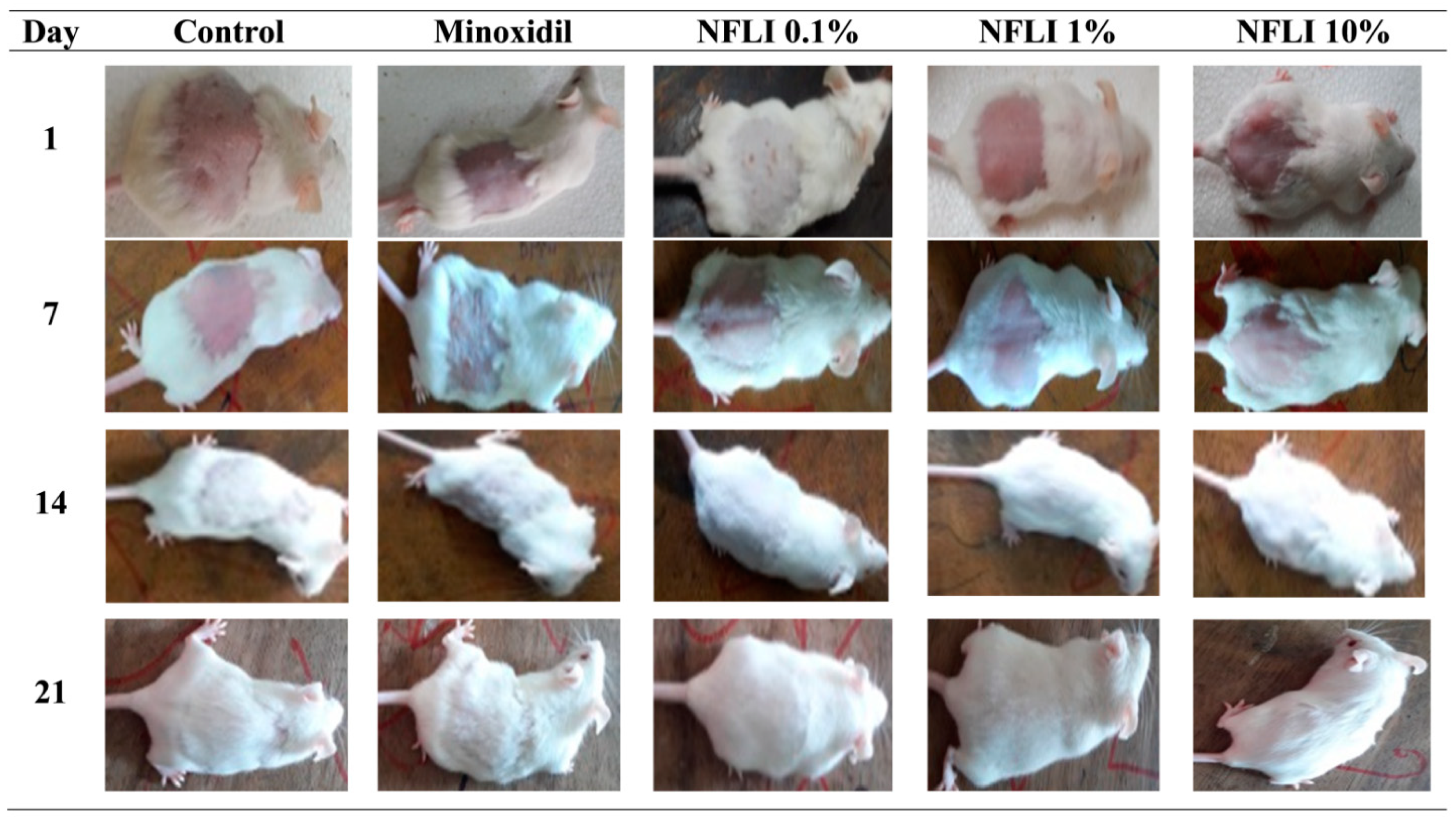
2.3. Hair-Growth Promoting Activity
2.4. Molecular Docking of 16 Phytochemicals Previously Reported in L. indica against PGD2 Synthase
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Extraction of Plant Material
4.3. Experimental Animals
4.4. Anti-Inflammatory Activity
4.4.1. Membrane Stabilisation Assay
Preparation of Erythrocyte Suspension
Hypotonicity-Induced Human Red Blood Cell Hemolysis
4.4.2. Protein Denaturation Assay
4.5. Thrombolytic Activity
4.6. Hair Growth-Promoting Activity
4.6.1. Preparation of Samples for Topical Application
4.6.2. Hair Growth-Promoting Assay
4.7. Statistical Analysis
4.8. Molecular Docking Study
4.8.1. Protein Preparation
4.8.2. Ligand Preparation
4.8.3. Grid Generation and Standard Precision (SP) Ligand Docking
4.8.4. Ligand Efficiencies and Protein–Ligand Interactions Prediction
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burkill, I.H. A Dictionary of the Economic Products of the Malay Peninsula, 2nd ed.; Ministry of Agriculture and Co-Operatices: Kuala Lumpur, Malaysia, 1966; Volume 2. [Google Scholar]
- Lattif, A.G.; Omar, I.M.; Said, I.M.; Kadri, A. A multi-variate approach to the study of medicinal plants in Malaysia. J. Singap. Natl. Acad. Sci. 1984, 13, 101–105. [Google Scholar]
- Chatterjee, A.; Pakrashi, S.C. Treatise on Indian Medicinal Plants; Publications & Information Directorate: New Delhi, India, 1991. [Google Scholar]
- Bourdy, G.; Walter, A. Maternity and medicinal plants in Vanuatu. I. The cycle of reproduction. J. Ethnopharmacol. 1992, 37, 179–196. [Google Scholar] [CrossRef]
- Prajapati, N.D.; Purohit, S.S.; Sharma, A.K.; Kumar, T. A Handbook of Medicinal Plants: A Complete Source Book; Agrobios: Jodhpur, India, 2003; p. 554. [Google Scholar]
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Srinivasan, G.V.; Ranjith, C.; Vijayan, K.K. Identification of chemical compounds from the leaves of Leea indica. Acta Pharm. 2008, 58, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Siew, Y.-Y.; Chong, T.-I.; Yew, H.-C.; Ho, S.S.; Lim, C.S.; Tan, W.-X.; Neo, S.-Y.; Koh, H.-L. Identification of Phytoconstituents in Leea indica (Burm. F.) Merr. Leaves by High Performance Liquid Chromatography Micro Time-of-Flight Mass Spectrometry. Molecules 2019, 24, 714. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.H.; Abdul Kadir, H.; Ling, S.K. Bioassay-Guided Isolation of Cytotoxic Cycloartane Triterpenoid Glycosides from the Traditionally Used Medicinal Plant Leea indica. Evid. Based Complement Altern. Med. 2012, 2012, 164689. [Google Scholar] [CrossRef] [PubMed]
- Raihan, M.O.; Habib, M.R.; Brishti, A.; Rahman, M.M.; Saleheen, M.M.; Manna, M. Sedative and anxiolytic effects of the methanolic extract of Leea indica (Burm. f.) Merr. leaf. Drug Discov. 2011, 5, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Mahboob, T.; Nawaz, M.; de Lourdes Pereira, M.; Tian-Chye, T.; Samudi, C.; Sekaran, S.D.; Wiart, C.; Nissapatorn, V. PLGA nanoparticles loaded with Gallic acid- a constituent of Leea indica against Acanthamoeba triangularis. Sci. Rep. 2020, 10, 8954. [Google Scholar] [CrossRef] [PubMed]
- Ado, M.A.; Abas, F.; Mohammed, A.S.; Ghazali, H.M. Anti-and pro-lipase activity of selected medicinal, herbal and aquatic plants, and structure elucidation of an anti-lipase compound. Molecules 2013, 18, 14651–14669. [Google Scholar] [CrossRef]
- Saha, K.; Lajis, N.H.; Israf, D.A.; Hamzah, A.S.; Khozirah, S.; Khamis, S.; Syahida, A. Evaluation of antioxidant and nitric oxide inhibitory activities of selected Malaysian medicinal plants. J. Ethnopharmacol. 2004, 92, 263–267. [Google Scholar] [CrossRef]
- Nurhanan, M.Y.; Asiah, O.; Ilham, M.A.M.; Syarifah, M.M.S.; Norhayati, I.; Sahira, H.L. Anti-proliferative activities of 32 Malaysian plant species in breast cancer cell lines. J. Trop. Sci. 2008, 20, 77–81. [Google Scholar]
- Rahman, M.A.; Sultana, R.; Bin Emran, T.; Islam, M.S.; Rahman, M.A.; Chakma, J.S.; Rashid, H.-u.; Hasan, C.M.M. Effects of organic extracts of six Bangladeshi plants on in vitro thrombolysis and cytotoxicity. BMC Complement Altern. Med. 2013, 13, 25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reddy, N.S.; Navanesan, S.; Sinniah, S.K.; Wahab, N.A.; Sim, K.S. Phenolic content, antioxidant effect and cytotoxic activity of Leea indica leaves. BMC Complement Altern. Med. 2012, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.H.; Abdul Kadir, H. Leea indica Ethyl Acetate Fraction Induces Growth-Inhibitory Effect in Various Cancer Cell Lines and Apoptosis in Ca Ski Human Cervical Epidermoid Carcinoma Cells. Evid. Based Complement Altern. Med. 2011, 2011, 293060. [Google Scholar]
- Wong, Y.H.; Kadir, H.A. Induction of Mitochondria-Mediated Apoptosis in Ca Ski Human Cervical Cancer Cells Triggered by Mollic Acid Arabinoside Isolated from Leea indica. Evid. Based Complement Altern. Med. 2012, 2012, 684740. [Google Scholar] [CrossRef] [PubMed]
- Ghagane, S.C.; Puranik, S.I.; Kumbar, V.M.; Nerli, R.B.; Jalalpure, S.S.; Hiremath, M.B.; Neelagund, S.; Aladakatti, R. In vitro antioxidant and anticancer activity of Leea indica leaf extracts on human prostate cancer cell lines. Integr. Med. Res. 2017, 6, 79–87. [Google Scholar] [CrossRef]
- Kekuda, P.T.R.; Raghavendra, H.L.; BharadwajNa, A.S. Traditional uses, chemistry and pharmacological activities of Leea indica (Burm. f.) Merr. (Vitaceae): A comprehensive review. Int. J. Green Pharm. 2018, 12 (Suppl. 1), S71–S80. [Google Scholar]
- Dalu, D.; Duggirala, S.; Akarapu, S. Anti-hyperglycemic and hypolipidemic activity of Leea indica. Int. J. Bioassay 2014, 3, 3155–3159. [Google Scholar]
- Siew, Y.-Y.; Yew, H.-C.; Neo, S.-Y.; Seow, S.-V.; Lew, S.-M.; Lim, S.-W.; Lim, C.S.E.-S.; Ng, Y.-C.; Seetoh, W.-G.; Ali, A. Evaluation of anti-proliferative activity of medicinal plants used in Asian Traditional Medicine to treat cancer. J. Ethnopharmacol. 2019, 235, 75–87. [Google Scholar] [CrossRef]
- Tareq, S.; Ibrahim, M.; Shahadat, S.; Chowdhury, M.U.; Jakaria, M. Comparative anti-dirrhoeal and antimicrobial activities of methanol extract of Leea indica (Burm. F.) Merr. and Leea macrophylla Roxb. Ex. Hornem (Fam. Vitaceae) and four Bangladeshi market preparations. Der. Pharma. Chem. 2017, 9, 27–34. [Google Scholar]
- Patel, B.; Patel, J.; Shah, S. Effect of single and combinational herbal formulation in alloxan induced hyperglycemia. Der. Pharm. Lett. 2016, 8, 114–120. [Google Scholar]
- Sulistyaningsih, E.; Amalia, T.Y.; Kartikasari, R. Antioxidant and antimalarial activity of Leea indica leaf extract against malaria-mice model. J. Appl. Pharm. Sci. 2017, 7, 163–168. [Google Scholar]
- Sreedhanya, S.; Athira, A.; Pushpalatha, E. Larvicidal and repellent efficacy of some of the weed plant extracts against Culex quinquefasciatus Say. J. Adv. Lab. Res. Biol. 2017, 8, 6–11. [Google Scholar]
- Emran, T.B.; Rahman, M.A.; Hosen, S.Z.; Rahman, M.M.; Islam, A.M.T.; Chowdhury, M.A.U.; Uddin, M.E. Analgesic activity of Leea indica (Burm.f.) Merr. Phytopharmacology 2012, 3, 150–157. [Google Scholar]
- Fong, P.; Tong, H.H.Y.; Ng, K.H.; Lao, C.K.; Chong, C.I.; Chao, C.M. In silico prediction of prostaglandin D2 synthase inhibitors from herbal constituents for the treatment of hair loss. J. Ethnopharmacol. 2015, 175, 470–480. [Google Scholar] [CrossRef]
- Yamamoto, J.; Yamada, K.; Naemura, A.; Yamashita, T.; Arai, R. Testing various herbs for antithrombotic effect. Nutrition 2005, 21, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Rambwawasvika, H.; Dzomba, P.; Gwatidzo, L. Hair Growth Promoting Effect of Dicerocaryum senecioides Phytochemicals. Int. J. Med. Chem. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Blume-Peytavi, U.; Whiting, D.A.; Trüeb, R.M. Hair Growth and Disorders; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Geyfman, M.; Plikus, M.V.; Treffeisen, E.; Andersen, B.; Paus, R. Resting no more: Re-defining telogen, the maintenance stage of the hair-growth cycle. Biol. Rev. 2015, 90, 1179–1196. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Li, L.; Fuchs, E. Emerging interactions between skin stem cells and their niches. Nat. Med. 2014, 20, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Lee, J.S.; Kim, Y.C. Hair growth-promoting effects of lavender oil in C57BL/6 mice. Toxicol. Res. 2016, 32, 103–108. [Google Scholar] [CrossRef]
- Husain, A.; Khan, M.S.; Hasan, S.M.; Alam, M.M. 2-Arylidene-4-(4-phenoxy-phenyl)but-3-en-4-olides: Synthesis, reactions and biological activity. Eur. J. Med. Chem. 2005, 40, 1394–1404. [Google Scholar] [CrossRef]
- Meidan, V.M.; Touitou, E. Treatments for androgenetic alopecia and alopecia areata. Drugs 2001, 61, 53–69. [Google Scholar] [CrossRef]
- Bergstrom, K.G. What’s new in androgenetic alopecia: Approvals, long-term safety data, cancer risk and treatment options for women. J. Drugs Derm. 2011, 10, 98–101. [Google Scholar]
- Varothai, S.; Bergfeld, W.F. Androgenetic alopecia: An evidence-based treatment update. Am. J. Clin. Derm. 2014, 15, 217–230. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders: A review. Drug Des. Devel. 2019, 13, 2777–2786. [Google Scholar] [CrossRef]
- Rossi, A.; Cantisani, C.; Melis, L.; Iorio, A.; Scali, E.; Calvieri, S. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm. Allergy Drug Discov. 2012, 6, 130–136. [Google Scholar] [CrossRef]
- Randolph, M.; Tosti, A. Oral minoxidil treatment for hair loss: A review of efficacy and safety. J. Am. Acad. Derm. 2020, 84, 737–746. [Google Scholar] [CrossRef]
- Garza, L.A.; Liu, Y.; Yang, Z.; Alagesan, B.; Lawson, J.A.; Norberg, S.M.; Loy, D.E.; Zhao, T.; Blatt, H.B.; Stanton, D.C.; et al. Prostaglandin D2 Inhibits Hair Growth and Is Elevated in Bald Scalp of Men with Androgenetic Alopecia. Sci. Transl. Med. 2012, 4, 126ra34. [Google Scholar] [CrossRef] [PubMed]
- Nieves, A.; Garza, L.A. Does prostaglandin D2 hold the cure to male pattern baldness? Exp. Derm. 2014, 23, 224–227. [Google Scholar] [CrossRef]
- Hohwy, M.; Spadola, L.; Lundquist, B.; Hawtin, P.; Dahmén, J.; Groth-Clausen, I.; Nilsson, E.; Persdotter, S.; von Wachenfeldt, K.; Folmer, R.H.A.; et al. Novel Prostaglandin D Synthase Inhibitors Generated by Fragment-Based Drug Design. J. Med. Chem. 2008, 51, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Kanaoka, Y.; Ago, H.; Inagaki, E.; Nanayama, T.; Miyano, M.; Kikuno, R.; Fujii, Y.; Eguchi, N.; Toh, H.; Urade, Y. Cloning and crystal structure of hematopoietic prostaglandin D synthase. Cell 1997, 90, 1085–1095. [Google Scholar] [CrossRef]
- Takaya, D.; Inaka, K.; Omura, A.; Takenuki, K.; Kawanishi, M.; Yabuki, Y.; Nakagawa, Y.; Tsuganezawa, K.; Ogawa, N.; Watanabe, C. Characterization of crystal water molecules in a high-affinity inhibitor and hematopoietic prostaglandin D synthase complex by interaction energy studies. Bioorg. Med. Chem. 2018, 26, 4726–4734. [Google Scholar] [CrossRef]
- Lee, I.K.; Lee, B.-M. Toxicological characterization of phthalic acid. Toxicol. Res. 2011, 27, 191–203. [Google Scholar]
- Emran, T.B.; Rahman, M.A.; Hosen, S.M.; Khanam, U.H.; Saha, D. Antioxidant, cytotoxic and phytochemical properties of the ethanol extract of Leea indica leaf. J. Pharm. Res. 2012, 5, 2938–2941. [Google Scholar]
- Park, W.-S.; Lee, C.-H.; Lee, B.-G.; Chang, I.-S. The extract of Thujae occidentalis semen inhibited 5α-reductase and androchronogenetic alopecia of B6CBAF1/j hybrid mouse. J. Derm. Sci. 2003, 31, 91–98. [Google Scholar] [CrossRef]
- Shen, Y.L.; Li, X.Q.; Pan, R.R.; Yue, W.; Zhang, L.J.; Zhang, H. Medicinal Plants for the Treatment of Hair Loss and the Suggested Mechanisms. Curr. Pharm. Des. 2018, 24, 3090–3100. [Google Scholar] [CrossRef] [PubMed]
- Dhariwala, M.Y.; Ravikumar, P. An overview of herbal alternatives in androgenetic alopecia. J. Cosmet. Derm. 2019, 18, 966–975. [Google Scholar] [CrossRef]
- Emran, T.B.; Rahman, M.A.; Uddin, M.M.N.; Rahman, M.M.; Uddin, M.Z.; Dash, R.; Layzu, C. Effects of organic extracts and their different fractions of five Bangladeshi plants on in vitro thrombolysis. BMC Complement Altern. Med. 2015, 15, 128. [Google Scholar] [CrossRef] [PubMed]
- Babar, Z.M.; Jaswir, I.; Tareq, A.M.; Ali Reza, A.S.M.; Azizi, W.M.; Hafidz, M.; Ahfter, F.; Hasan, M.; Farhad, S.; Uddin, M.M.R.; et al. In vivo anxiolytic and in vitro anti-inflammatory activities of water-soluble extract (WSE) of Nigella sativa (L.) seeds. Nat. Prod. Res. 2019, 1–6. [Google Scholar] [CrossRef]
- Uddin, M.J.; Ansari, P.; Rahman, M.M.; Mamun, A.; Islam, M.; Hazrat, M.; Reza, A.S.M.A. Anti-inflammatory, anti-diarrheal, thrombolytic and cytotoxic activities of an ornamental medicinal plant: Persicaria orientalis. J. Basic Clin. Physiol. Pharm. 2016, 28, 51–58. [Google Scholar]
- Prasad, S.; Kashyap, R.S.; Deopujari, J.Y.; Purohit, H.J.; Taori, G.M.; Daginawala, H.F. Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thromb. J. 2006, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.I.; Al-Reza, S.M.; Kang, S.C. Hair growth-promoting effect of Zizyphus jujuba essential oil. Food Chem. Toxicol. 2010, 48, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.-N.; Park, D.K.; Park, H.-J. Hair growth-promoting activity of hot water extract of Thuja Orient. BMC Complement Altern. Med. 2013, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Lionta, E.; Spyrou, G.; Vassilatis, D.K.; Cournia, Z. Structure-based virtual screening for drug discovery: Principles, applications and recent advances. Curr. Top. Med. Chem. 2014, 14, 1923–1938. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, H.; Sun, H.; Pan, P.; Li, Y.; Li, D.; Hou, T. Assessing the performance of the MM/PBSA and MM/GBSA methods. 6. Capability to predict protein–protein binding free energies and re-rank binding poses generated by protein–protein docking. Phys. Chem. Chem. Phys. 2016, 18, 22129–22139. [Google Scholar] [CrossRef]
- García-Sosa, A.T.; Hetényi, C.; Maran, U. Drug efficiency indices for improvement of molecular docking scoring functions. J. Comput. Chem. 2010, 31, 174–184. [Google Scholar] [CrossRef] [PubMed]
| Compound | Docking Score (kcal/mol) | ΔG (kcal/mol) | Ligand Efficiency (kcal/mol) |
|---|---|---|---|
| Phthalic acid | −6.078 | −41.8901 | 3.49 |
| Farnesol | −2.640 | −49.3485 | 3.08 |
| Palmitic acid | −0.801 | −47.325 | 2.63 |
| n-octadecane | +0.634 | −44.7694 | 2.49 |
| n-eicosane | +0.571 | −49.127 | 2.46 |
| n-heptadecane | +1.125 | −40.9013 | 2.41 |
| n-tricosane | −3.022 | −51.5811 | 2.24 |
| 1-eicosanol | +0.473 | −42.9662 | 2.05 |
| n-tetracosane | −2.625 | −47.9138 | 1.99 |
| n-heptacosane | −2.645 | −51.3352 | 1.90 |
| β-sitosterol | −6.191 | −46.952 | 1.57 |
| n-tetratriacontane | −2.283 | −52.2674 | 1.54 |
| Lycopersen | −5.672 | −49.1584 | 1.23 |
| Solanesol | −7.133 | −47.2383 | 1.03 |
| 17-Pentatriacontene | −0.787 | −32.9982 | 0.94 |
| Lupeol | −3.992 | −22.024 | 0.71 |
| Glutathione (positive control) | −5.278 | −34.732 | 1.74 |
| Ligand | Docking Score | Ligand Efficiency | Interacting Residues | Distance (Å) | Category | Type |
|---|---|---|---|---|---|---|
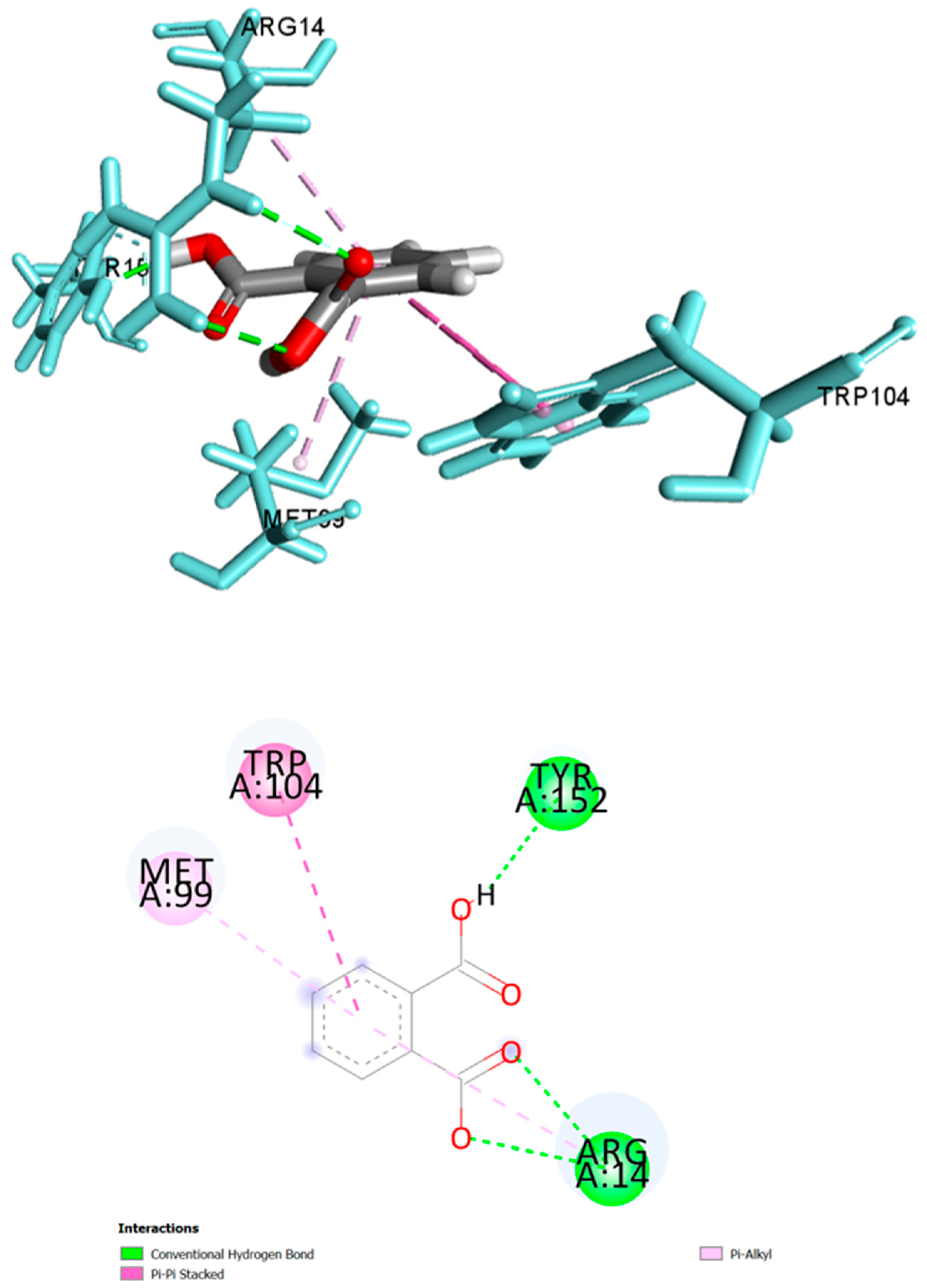
| Phthalic acid | −6.078 | 3.49 | Arg14 | 2.48, 2.73 | H-Bond | Conventional |
| Tyr152 | 1.63 | H-Bond | Conventional | |||
| Trp104 | 4.88, 5.06 | Hydrophobic | Pi-Pi Stacked | |||
| Met99 | 4.70 | Hydrophobic | Pi-Alkyl | |||
| Arg14 | 5.16 | Hydrophobic | Pi-Alkyl | |||
| Farnesol | −2.640 | 3.08 | Thr159 | 2.00 | H-Bond | Conventional |
| Gly13 | 2.13 | H-Bond | Conventional | |||
| Ile155 | 3.08 | H-Bond | C-H bond | |||
| Tyr152 | 4.76 | Hydrophobic | Pi-Alkyl | |||
| Cys156 | 4.91 | Hydrophobic | Alkyl | |||
| Arg14 | 4.55 | Hydrophobic | Alkyl | |||
| Tyr8 | 4.54 | Hydrophobic | Pi-Alkyl | |||
| Phe9 | 5.11, 4.52 | Hydrophobic | Pi-Alkyl | |||
| Met11 | 4.64 | Hydrophobic | Alkyl | |||
| Trp104 | 4.33, 4.12 | Hydrophobic | Pi-Alkyl | |||
| Leu199 | 4.44 | Hydrophobic | Alkyl | |||
| n-tricosane | −3.022 | 2.24 | Ile51 | 5.11 | Hydrophobic | Alkyl |
| Tyr8 | 5.32 | Hydrophobic | Pi-Alkyl | |||
| Phe9 | 5.42 | Hydrophobic | Pi-Alkyl | |||
| Cys156 | 4.28 | Hydrophobic | Alkyl | |||
| Ile155 | 4.72 | Hydrophobic | Alkyl | |||
| Tyr152 | 4.16 | Hydrophobic | Pi-Alkyl | |||
| n-tetracosane | −2.625 | 1.99 | Lys43 | 4.63 | Hydrophobic | Alkyl |
| Tyr152 | 4.02 | Hydrophobic | Pi-Alkyl | |||
| Ile155 | 4.93 | Hydrophobic | Alkyl | |||
| Cys156 | 3.84 | Hydrophobic | Alkyl | |||
| Trp104 | 2.79 | Hydrophobic | Pi-Sigma | |||
| n-heptacosane | −2.645 | 1.90 | Lys43 | 4.01 | Hydrophobic | Alkyl |
| Ile155 | 5.23 | Hydrophobic | Alkyl | |||
| Trp104 | 2.80 | Hydrophobic | Pi-Sigma | |||
| Met99 | 4.50 | Hydrophobic | Alkyl | |||
| Cys156 | 4.05 | Hydrophobic | Alkyl |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakib, S.A.; Tareq, A.M.; Islam, A.; Rakib, A.; Islam, M.N.; Uddin, M.A.; Rahman, M.M.; Seidel, V.; Emran, T.B. Anti-Inflammatory, Thrombolytic and Hair-Growth Promoting Activity of the n-Hexane Fraction of the Methanol Extract of Leea indica Leaves. Plants 2021, 10, 1081. https://doi.org/10.3390/plants10061081
Sakib SA, Tareq AM, Islam A, Rakib A, Islam MN, Uddin MA, Rahman MM, Seidel V, Emran TB. Anti-Inflammatory, Thrombolytic and Hair-Growth Promoting Activity of the n-Hexane Fraction of the Methanol Extract of Leea indica Leaves. Plants. 2021; 10(6):1081. https://doi.org/10.3390/plants10061081
Chicago/Turabian StyleSakib, Shahenur Alam, Abu Montakim Tareq, Ameerul Islam, Ahmed Rakib, Mohammad Nazmul Islam, Mohammad Arafat Uddin, Md. Masudur Rahman, Veronique Seidel, and Talha Bin Emran. 2021. "Anti-Inflammatory, Thrombolytic and Hair-Growth Promoting Activity of the n-Hexane Fraction of the Methanol Extract of Leea indica Leaves" Plants 10, no. 6: 1081. https://doi.org/10.3390/plants10061081
APA StyleSakib, S. A., Tareq, A. M., Islam, A., Rakib, A., Islam, M. N., Uddin, M. A., Rahman, M. M., Seidel, V., & Emran, T. B. (2021). Anti-Inflammatory, Thrombolytic and Hair-Growth Promoting Activity of the n-Hexane Fraction of the Methanol Extract of Leea indica Leaves. Plants, 10(6), 1081. https://doi.org/10.3390/plants10061081













