Development of a New DNA Marker for Fusarium Yellows Resistance in Brassica rapa Vegetables
Abstract
1. Introduction
2. Results
2.1. Screening of Lines for Resistance to F. oxysporum f. sp. Rapae
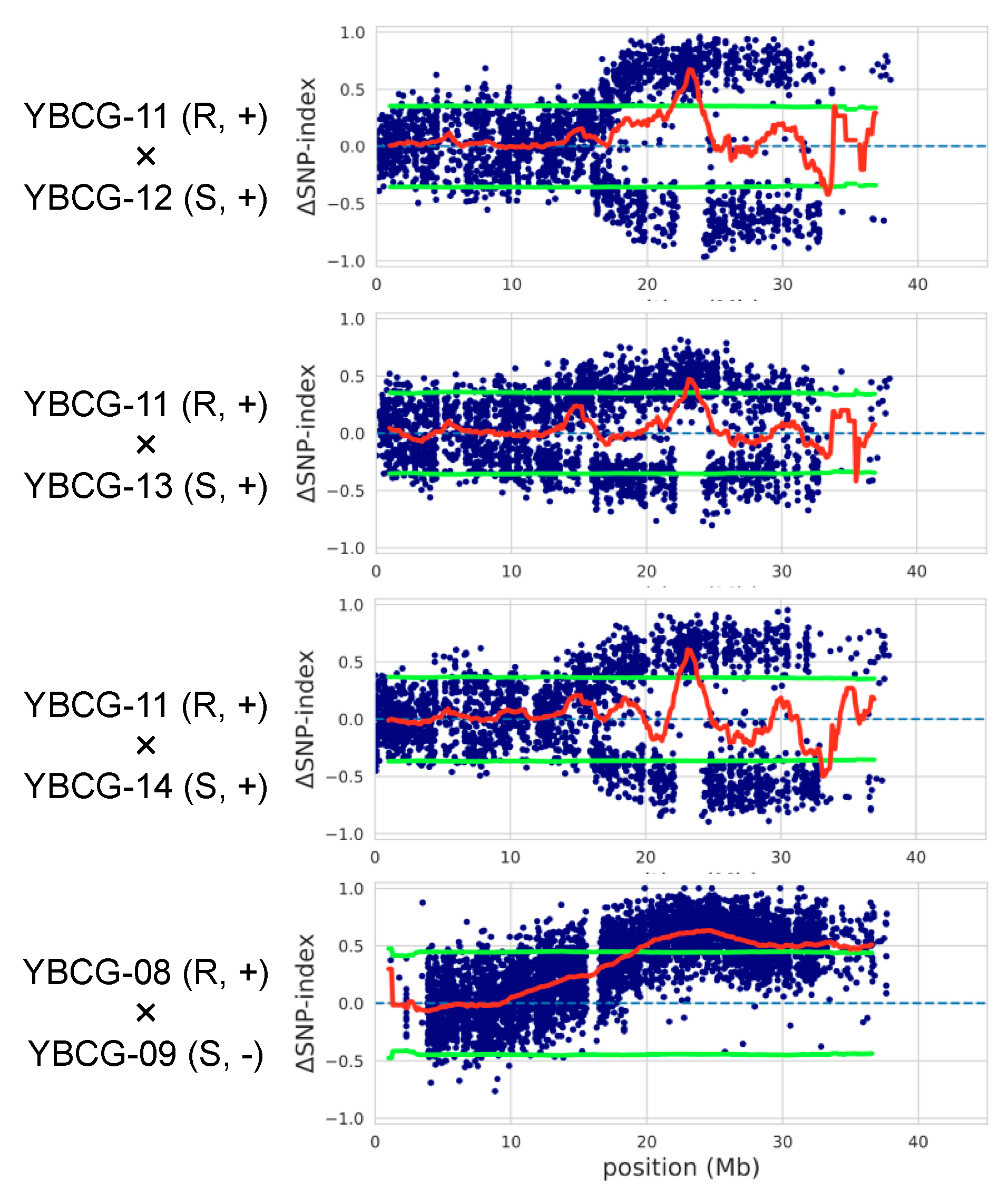
2.2. Identification of the Causative Region of Resistance for F. oxysporum f. sp. Rapae
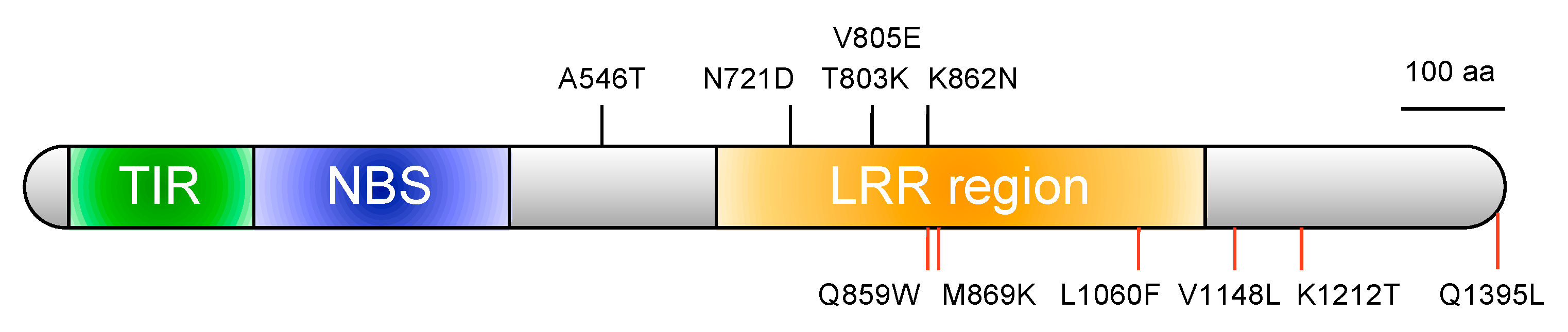
2.3. A New Susceptible Allele of FocBr1 Was Identified
2.4. Development of a New DNA Marker for Fusarium Yellows Resistance
2.5. Prediction of Fusarium Yellows Resistance in Commercial B. rapa Vegetables by DNA Marker
3. Discussion
4. Materials and Methods
4.1. Plant Materials and DNA and RNA Extraction
4.2. Inoculation Test
4.3. QTL-Seq
4.4. Prediction of Fusarium Yellows Resistance by DNA Markers
4.5. Gene Expression Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, F.; Sun, R.; Hou, X.; Zheng, H.; Zhang, F.; Zhang, Y.; Liu, B.; Liang, J.; Zhuang, M.; Liu, Y.; et al. Subgenome parallel selection is associated with morphotype diversification and convergent crop domestication in Brassica rapa and Brassica oleracea. Nat. Genet. 2016, 48, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Miyaji, N.; Osabe, K.; Akter, A.; Mehraj, H.; Shea, D.J.; Fujimoto, R. The importance of genetic and epigenetic research in the Brassica vegetables in the face of climate change. In Genomic Designing of Climate-Smart Vegetable Crops; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 161–255. [Google Scholar]
- Fujimoto, R.; Uezono, K.; Ishikura, S.; Osabe, K.; Peacock, W.J.; Dennis, E.S. Recent research on the mechanism of heterosis is important for crop and vegetable breeding systems. Breed. Sci. 2018, 68, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Akter, A.; Itabashi, E.; Kakizaki, T.; Okazaki, K.; Dennis, E.S.; Fujimoto, R. Genome triplication leads to transcriptional divergence of FLOWERING LOCUS C genes during vernalization in the genus Brassica. Front. Plant Sci. 2021, 11, 619417. [Google Scholar] [CrossRef] [PubMed]
- Mehraj, H.; Akter, A.; Miyaji, N.; Miyazaki, J.; Shea, D.J.; Fujimoto, R.; Doullah, M.A.U. Genetics of clubroot and Fusarium wilt disease resistance in Brassica vegetables: The application of marker assisted breeding for disease resistance. Plants 2020, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Fang, Z.; Yang, L.; Zhang, Y.; Wang, Y. An update on the arsenal: Mining resistance genes for disease management of Brassica crops in the genomic era. Hortic. Res. 2020, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Enya, J.; Togawa, M.; Takeuchi, T.; Yoshida, S.; Tsushima, S.; Arie, T.; Sakai, T. Biological and phylogenetic characterization of Fusarium oxysporum complex, which causes yellows on Brassica spp., and proposal of F. oxysporum f. sp. rapae, a novel forma specialis pathogenic on B. rapa in Japan. Phytopathology 2008, 98, 475–483. [Google Scholar] [CrossRef]
- Pu, Z.; Shimizu, M.; Zhang, Y.; Nagaoka, T.; Hayashi, T.; Hori, H.; Matsumoto, S.; Fujimoto, R.; Okazaki, K. Genetic mapping of a fusarium wilt resistance gene in Brassica oleracea. Mol. Breed. 2012, 30, 809–818. [Google Scholar] [CrossRef]
- Lv, H.; Fang, Z.; Yang, L.; Zhang, Y.; Wang, Q.; Liu, Y.; Zhuang, M.; Yang, Y.; Xie, B.; Liu, B.; et al. Mapping and analysis of a novel candidate Fusarium wilt resistance gene FOC1 in Brassica oleracea. BMC Genom. 2014, 15, 1094. [Google Scholar] [CrossRef]
- Shimizu, M.; Fujimoto, R.; Ying, H.; Pu, Z.; Ebe, Y.; Kawanabe, T.; Saeki, N.; Taylor, J.M.; Kaji, M.; Dennis, E.S.; et al. Identification of candidate genes for fusarium yellows resistance in Chinese cabbage by differential expression analysis. Plant Mol. Biol. 2014, 85, 247–257. [Google Scholar] [CrossRef]
- Shimizu, M.; Pu, Z.; Kawanabe, T.; Kitashiba, H.; Matsumoto, S.; Ebe, Y.; Sano, M.; Funaki, T.; Fukai, E.; Fujimoto, R.; et al. Map-based cloning of a candidate gene conferring Fusarium yellows resistance in Brassica oleracea. Theor. Appl. Genet. 2015, 128, 119–130. [Google Scholar] [CrossRef]
- Akter, M.A.; Mehraj, H.; Itabashi, T.; Shindo, T.; Osaka, M.; Akter, A.; Miyaji, N.; Chiba, N.; Miyazaki, J.; Fujimoto, R. Breeding for disease resistance in Brassica vegetables using DNA marker selection. In Brassica Breeding and Biotechnology; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Kawamura, K.; Kawanabe, T.; Shimizu, M.; Okazaki, K.; Kaji, M.; Dennis, E.S.; Osabe, K.; Fujimoto, R. Genetic characterization of inbred lines of Chinese cabbage by DNA markers: Towards the application of DNA markers to breeding of F1 hybrid cultivars. Data Brief 2016, 6, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Shimizu, M.; Kawanabe, T.; Pu, Z.; Kodama, T.; Kaji, M.; Osabe, K.; Fujimoto, R.; Okazaki, K. Assessment of DNA markers for seed contamination testing and selection of disease resistance in cabbage. Euphytica 2017, 213, 28. [Google Scholar] [CrossRef]
- Sato, M.; Shimizu, M.; Shea, D.J.; Hoque, M.; Kawanabe, T.; Miyaji, N.; Fujimoto, R.; Fukai, E.; Okazaki, K. Allele specific DNA marker for fusarium resistance gene FocBo1 in Brassica oleracea. Breed. Sci. 2019, 69, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Narusaka, M.; Shirasu, K.; Noutoshi, Y.; Kubo, Y.; Shiraishi, T.; Iwabuchi, M.; Narusaka, Y. RRS1 and RPS4 provide a dual Resistance-gene system against fungal and bacterial pathogens. Plant J. 2009, 60, 218–226. [Google Scholar] [CrossRef]
- Huh, S.U.; Cevik, V.; Ding, P.; Duxbury, Z.; Ma, Y.; Tomlinson, L.; Sarris, P.F.; Jones, J.D.G. Protein-protein interactions in the RPS4/RRS1 immune receptor complex. PLoS Pathog. 2017, 13, e1006376. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, H.; Hu, L.; Martinez, P.P.; Moschou, P.N.; Cevik, V.; Ding, P.; Duxbury, Z.; Sarris, P.F.; Jones, J.D.G. Distinct modes of derepression of an Arabidopsis immune receptor complex by two different bacterial effectors. Proc. Natl. Acad. Sci. USA 2018, 115, 10218–10227. [Google Scholar] [CrossRef]
- Suwabe, K.; Tsukazaki, H.; Iketani, H.; Hatakeyama, K.; Fujimura, M.; Nunome, T.; Fukuoka, H.; Matsumoto, S.; Hirai, M. Identification of two loci for resistance to clubroot (Plasmodiophora brassicae Woronin) in Brassica rapa L. Theor. Appl. Genet. 2003, 107, 997–1002. [Google Scholar] [CrossRef]
- Nomura, K.; Minegishi, Y.; Kimizuka-Takagi, C.; Fujioka, T.; Moriguchi, K.; Shishido, R.; Ikehashi, H. Evaluation of F2 and F3 plants introgressed with QTLs for clubroot resistance in cabbage developed by using SCAR markers. Plant Breed. 2005, 124, 371–375. [Google Scholar] [CrossRef]
- Nagaoka, T.; Doullah, M.A.U.; Matsumoto, S.; Kawasaki, S.; Ishikawa, T.; Hori, H.; Okazaki, K. Identification of QTLs that control clubroot resistance in Brassica oleracea and comparative analysis of clubroot resistance genes between B. rapa and B. oleracea. Theor. Appl. Genet. 2010, 120, 1335–1346. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Abe, A.; Yoshida, K.; Kosugi, S.; Natsume, S.; Mitsuoka, C.; Uemura, A.; Utsushi, H.; Tamiru, M.; Takuno, S.; et al. QTL-seq: Rapid mapping of quantitative trait loci in rice by whole genome resequencing of DNA from two bulked populations. Plant J. 2013, 74, 174–183. [Google Scholar] [CrossRef] [PubMed]

| Name | Inoculation Test | Prediction by DNA Marker | |
|---|---|---|---|
| For | Foc | Bra012688m | |
| Chinese cabbage (var. pekinensis) | |||
| “W77” | R | R | + |
| RJKB-T23 | R | R | + |
| RJKB-T24 | S | S | - |
| Turnip (var. rapa) | |||
| “CR-Yukiakari” | R * | R * | + |
| “Hekiju” | R | R | + |
| NSI-01 | R * | R * | + |
| Komatsuna (var. perviridis) | |||
| “CR-Taiga” | R * | R * | + |
| “Manaka” | R | R | + |
| “Nanami” | R | R | + |
| “Natsurakuten” | R * | R * | + |
| “Zaoh” | S | S | + |
| YBCG-08 | R | R | + |
| YBCG-09 | S | S | - |
| YBCG-10 | S | S | - |
| YBCG-11 | R | R | + |
| YBCG-12 | S | S | + |
| YBCG-13 | S | S | + |
| YBCG-14 | S | S | + |
| YBCG-15 | S | S | + |
| YBCG-16 | R | R | + |
| YBCG-17 | R | R | + |
| YBCG-18 | R * | R * | + |
| YBCG-TC01 | R | R | + |
| YBCG-TC02 | S | S | + |
| YBCG-TC03 | R | R | + |
| YBCG-TC04 | R * | R * | + |
| YBCG-TC05 | S | S | + |
| YBCG-TC06 | R | R | + |
| F2 Population | χ2 (R:S = 3:1) | Resistant Parent | Bra012688m | Susceptible Parent | Bra012688m | ||
|---|---|---|---|---|---|---|---|
| Resistant | Susceptible | ||||||
| 1 | 169 | 31 | p < 0.05 | YBCG-11 | + | YBCG-12 | + |
| 2 | 171 | 29 | p < 0.001 | YBCG-11 | + | YBCG-13 | + |
| 3 | 171 | 29 | p < 0.001 | YBCG-11 | + | YBCG-14 | + |
| 4 | 160 | 40 | p > 0.05 | YBCG-08 | + | YBCG-09 | - |
| 5 | 149 | 51 | p > 0.05 | YBCG-TC01 | + | YBCG-10 | - |
| 6 | 156 | 44 | p > 0.05 | YBCG-11 | + | YBCG-10 | - |
| Name | Inoculation Test | Prediction by DNA Markers | |
|---|---|---|---|
| For | Bra012688m | focbr1-2m | |
| Chinese cabbage (var. pekinensis) | |||
| “W77” | R | + | A |
| RJKB-T23 | R | + | A |
| RJKB-T24 | S | - | D |
| RJKB-T36 | R | + | A |
| RJKB-T37 | R | + | A |
| RJKB-T38 | R | + | A |
| RJKB-T39 | R | + | A |
| RJKB-T40 | S | - | D |
| Turnip (var. rapa) | |||
| “CR-Yukiakari” | R * | + | A |
| “Hekiju” | R | + | A |
| “Yukibotan” | R | + | A |
| NSI-01 | R * | + | A |
| Pak choi (var. chinensis) | |||
| “Entei” | R | + | C |
| “Ryoutou” | R | + | A |
| Komatsuna (var. perviridis) | |||
| “Chijimikomatsuna” | S | + | B |
| “CR-Taiga” | R * | + | C |
| “Kahoku” | R | + | C |
| “Manaka” | R | + | C |
| “Nakamachi” | R | + | A |
| “Nanami” | R | + | A |
| “Nanane” | R | + | A |
| “Natsurakuten” | R * | + | C |
| “Norichan” | R | + | A |
| “Tsunashima” | S | + | B |
| “Zaoh” | S | + | B |
| YBCG-08 | R | + | A |
| YBCG-09 | S | - | D |
| YBCG-10 | S | - | D |
| YBCG-11 | R | + | A |
| YBCG-12 | S | + | B |
| YBCG-13 | S | + | B |
| YBCG-14 | S | + | B |
| YBCG-15 | S | + | B |
| YBCG-16 | R | + | A |
| YBCG-17 | R | + | A |
| YBCG-18 | R * | + | A |
| YBCG-TC01 | R | + | A |
| YBCG-TC02 | S | + | B |
| YBCG-TC03 | R | + | A |
| YBCG-TC04 | R * | + | C |
| YBCG-TC05 | S | + | B |
| YBCG-TC06 | R | + | A |
| Chijimina (var. narinosa) | |||
| “Hirose” | S | + | B |
| Chinese Cabbage | Turnip | Pak Choi | Komatsuna | |
|---|---|---|---|---|
| (var. pekinensis) | (var. rapa) | (var. chinensis) | (var. perviridis) | |
| FocBr1/FocBr1 or FocBr1/focbr1-1 | 145 | 30 | 24 | 44 |
| FocBr1/focbr1-2 | 6 | 5 | 16 | 21 |
| focbr1-1/focbr1-1 | 6 | 0 | 0 | 3 |
| focbr1-2/focbr1-2 or focbr1-2/focbr1-1 | 0 | 0 | 0 | 5 |
| Total | 157 | 35 | 40 | 73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyaji, N.; Akter, M.A.; Suzukamo, C.; Mehraj, H.; Shindo, T.; Itabashi, T.; Okazaki, K.; Shimizu, M.; Kaji, M.; Katsumata, M.; et al. Development of a New DNA Marker for Fusarium Yellows Resistance in Brassica rapa Vegetables. Plants 2021, 10, 1082. https://doi.org/10.3390/plants10061082
Miyaji N, Akter MA, Suzukamo C, Mehraj H, Shindo T, Itabashi T, Okazaki K, Shimizu M, Kaji M, Katsumata M, et al. Development of a New DNA Marker for Fusarium Yellows Resistance in Brassica rapa Vegetables. Plants. 2021; 10(6):1082. https://doi.org/10.3390/plants10061082
Chicago/Turabian StyleMiyaji, Naomi, Mst Arjina Akter, Chizuko Suzukamo, Hasan Mehraj, Tomoe Shindo, Takeru Itabashi, Keiichi Okazaki, Motoki Shimizu, Makoto Kaji, Masahiko Katsumata, and et al. 2021. "Development of a New DNA Marker for Fusarium Yellows Resistance in Brassica rapa Vegetables" Plants 10, no. 6: 1082. https://doi.org/10.3390/plants10061082
APA StyleMiyaji, N., Akter, M. A., Suzukamo, C., Mehraj, H., Shindo, T., Itabashi, T., Okazaki, K., Shimizu, M., Kaji, M., Katsumata, M., Dennis, E. S., & Fujimoto, R. (2021). Development of a New DNA Marker for Fusarium Yellows Resistance in Brassica rapa Vegetables. Plants, 10(6), 1082. https://doi.org/10.3390/plants10061082








