Subcellular Alterations Induced by Cyanotoxins in Vascular Plants—A Review
Abstract
1. Introduction
- (i)
- Microcystins (MCs, Figure 1) are cyclic heptapeptides with inhibitory activity of eukaryotic protein phosphatases PP1 and PP2A and the minor phosphatases PP4 and PP5. They are also known to induce oxidative stress in eukaryotes. At least 279 variants (congeners) of MC have been identified from different cyanobacterial genera such as Microcystis, Anabaena, Nostoc, Planktothrix, and Gleotrichia [8]. The well-known MC-LR and MC-RR congeners are the most common cyanotoxins responsible for several toxic effects caused by cyanobacteria [5,6,7,9]. Nodularin (NOD) is a pentapeptide with similar effects on MCs [10].
- (ii)
- Cylindrospermopsins (CYNs, Figure 1) are tricyclic guanidine alkaloids identified among others from Cylindrospermopsis, Anabaena, and Aphanizomenon species. Their toxicity is characterized by their action on multiple organs, and neurotoxic and genotoxic activity was noted [11]. Their protein synthesis inhibitory activity was published for various organisms [6,7].
- (iii)
- The acetylcholine receptor blocker bicyclic alkaloid anatoxin-a (ANA), the acetylcholine esterase inhibitor phosphorylated cyclic N-hydroxyguanine anatoxin-a (s), and the sodium channel blocker alkaloid saxitoxins are neurotoxins produced mainly by filamentous genera [5]. The amino acid type toxin β-N-methylamino-L-alanine (BMAA) is also defined as a neurotoxin [12].
2. Alterations Induced by Cyanotoxins on Peculiar Plant Structures
2.1. The Plastid System
2.2. The Organization of Plant Cytoskeleton and Mitotic Chromatin
- (i)
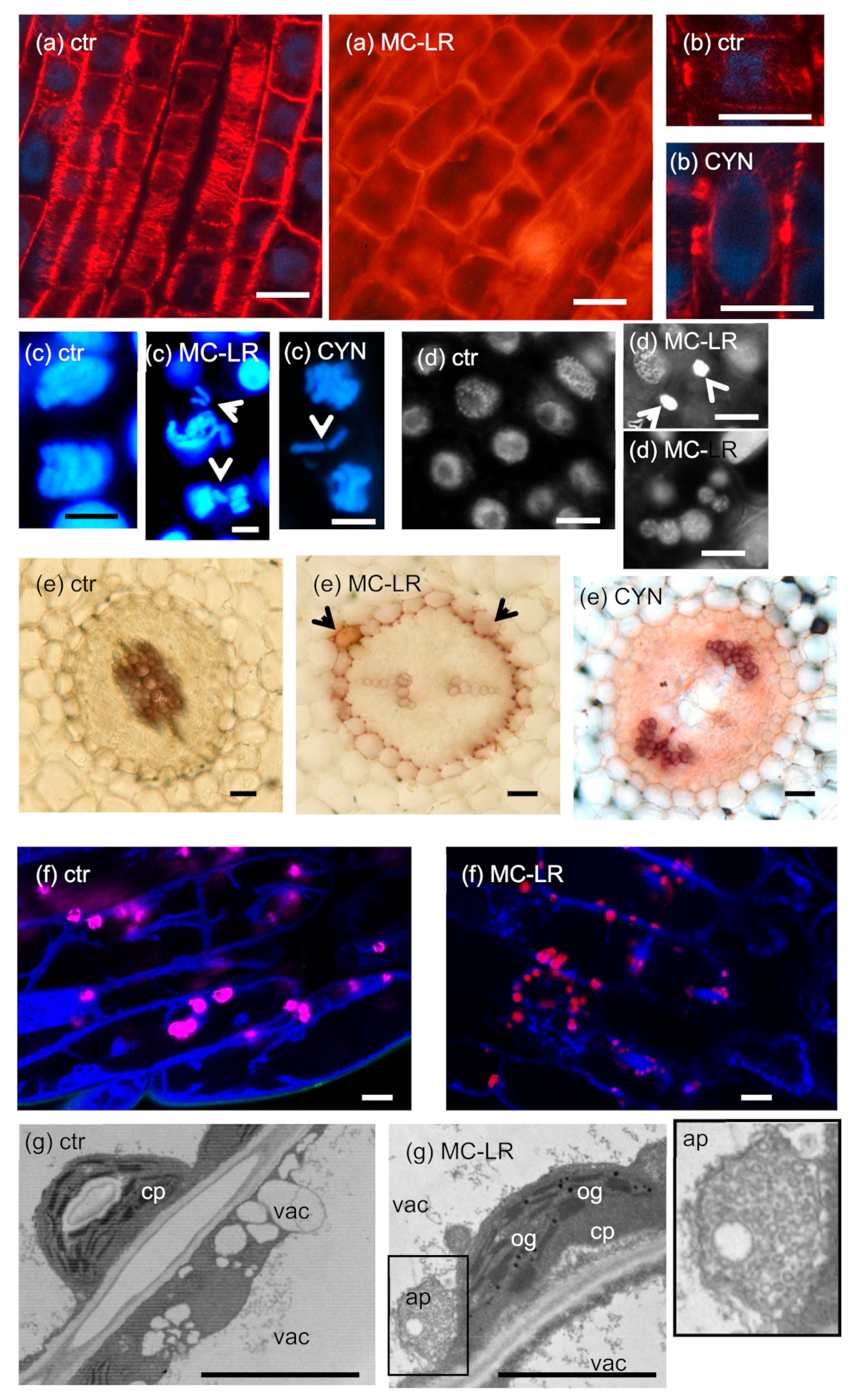
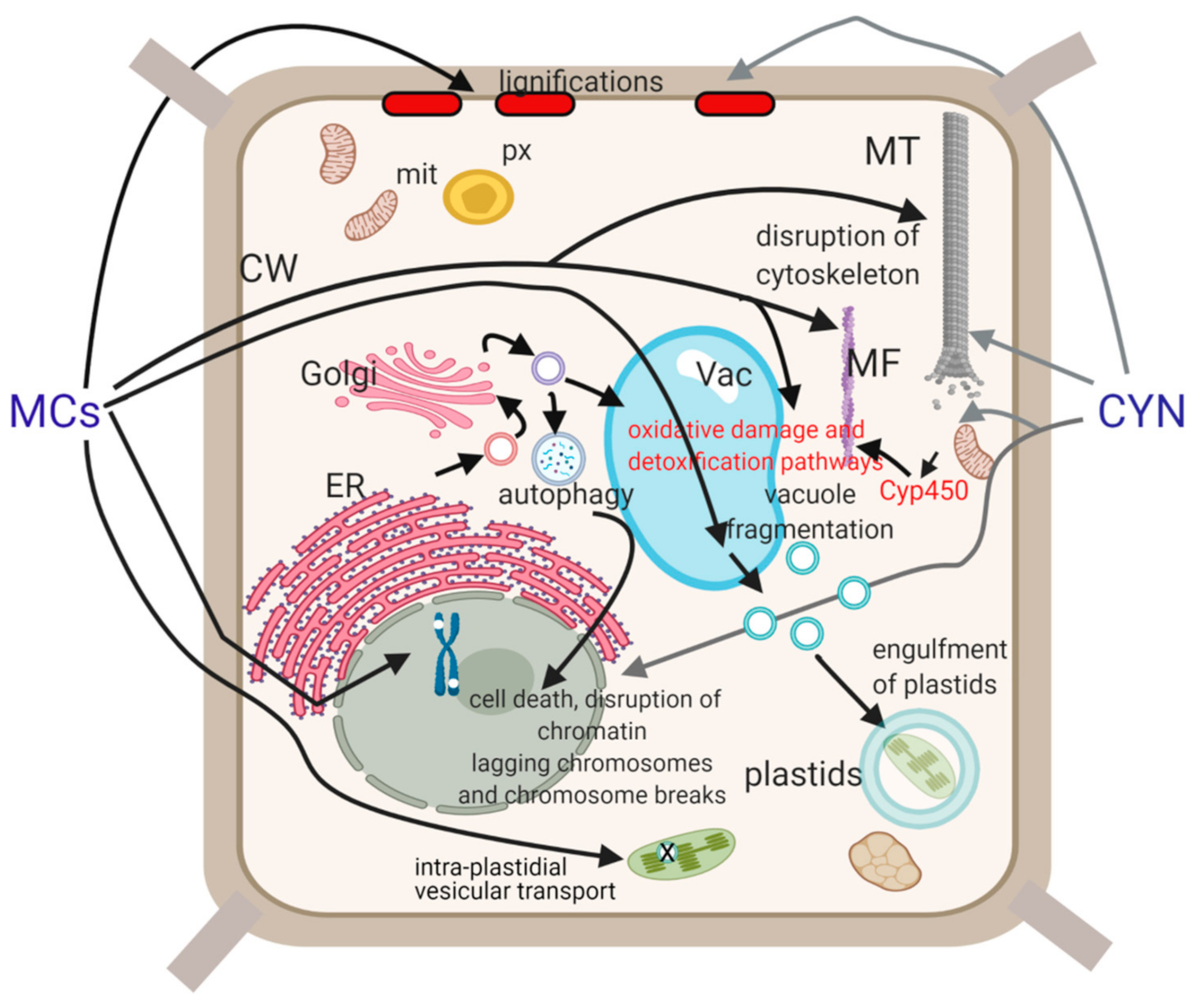
- CMTs are important in determining plant cell shape and direction of cell growth by regulating the orientation of cellulose microfibrils in the cell wall [49]. The effects of MCs are largely species- and organ-dependent. In primary roots of the aquatic macrophyte Phragmites australis (common reed), both low and high MC-LR concentrations induced CMT disruption (Figure 2a), while in Ceratophyllum demersum (coontail) shoots, CMT was not depolymerized, but reoriented. Both changes were attributed to changes in the phosphorylation state and thus functioning of microtubule-associated proteins (MAPs) [23,50]. Differences in the effects of MCs in different species/organs may be related to different tubulin isoforms and MAPs affected. Both types of alterations induced a shift from longitudinal to radial expansion of cells, inducing deformed shapes of whole organs [23,50].For CYN, a study on P. australis roots showed reorientation and decreased density of CMTs that led to the cessation of cell growth [33]. Protein synthesis inhibition was likely to play a role in this, since the amount of MAPs that regulate MT stability decreased [33]. On the other hand, it is surprising that Western-blots of protein extracts from Phragmites roots proved that CYN increased the amount of β-tubulin protein in roots [33]. This was not the case for G-actin; CYN decreased its amount in lettuce leaves [18].
- (ii)
- PPB is important in the determination of the orientation of the mitotic spindle and thus regulation of the division plane position [49]. No effects of MCs on PPB MT organization in dicot plants have been detected to date. However, Pappas et al. [28] detected disruptions in PPB assembly in rice root cells under short-term exposure to a very high concentration of MC-LR. This alteration resembled preprophase anomalies in the fass mutants of Arabidopsis, defective in the B” subunit of PP2A [51].CYN induced the formation of double and split PPBs in roots of the dicot Vicia faba (broad bean) and the monocot P. australis [27,33] (Figure 2b). For broad bean, this led to misorientation of mitotic divisions in root tip meristems [27]. Such PPB anomalies were observed in the presence of the well-known protein synthesis inhibitor cycloheximide [52].
- (iii)
- As for all eukaryotic cells, the acentriolar plant mitotic spindle is normally bipolar. MC-LR induces spindle disruptions in a wide variety of species, both dicots and monocots, with the formation of tripolar spindles as a common feature [22,23,25,29]. A wide variety of spindle anomalies (multi-and monopolar, C-and S-shaped, asymmetric, and completely disrupted spindles) were observed in root tip meristems of Vicia faba [25]. All these alterations were attributed to an altered phosphorylation state of proteins (e.g., MAPs) that regulate spindle assembly and lead to abnormal sister chromatid segregation during mitosis.Spindle disruptions were observed for CYN as well, and in P. australis root tip meristematic cells, they were accompanied by lagging chromosomes [29,33]. Although it is likely that protein synthesis inhibition played a role, it should be noted that CYN inhibits protein phosphatase activities in vivo to some extent in Sinapis alba (white mustard) seedlings as confirmed by Máthé et al. [29] Meanwhile, CYN has no such effect in vitro [29].
- (iv)
- As we have seen in the introductory section, the phragmoplast is crucial for building up the new cell wall during cytokinesis. Both MC-LR (for P. australis and V. faba) and CYN (for P. australis) induce phragmoplast disruptions in dividing root cells [22,23,33]. There is no evidence for abnormal cell plate formation after these disruptions.
- (v)
- microfilaments (MFs) of non-dividing cells. A very high (45 µM) concentration of MC-LR induced reorientation and then depolymerization of MFs in root protodermal and differentiated cells of Oryza sativa (rice) in very short-term (maximum 1 h) treatments [28]. There are no data on cyanotoxin-induced MF alterations in the mitotic apparatus of plant cells.
2.3. Plant Cell Wall and Plasmodesmata
- (i)
- lignifications. This was observed in the endodermis and stele of MC-LR treated S. alba and cortical parenchyma of P. australis primary roots. CYN induced partial lignifications in S. alba endodermis [29,35] (Figure 2e). Strong lignifications were observed in lateral buds of tissue culture-regenerated P. australis stems treated with MC-LR [35]. What is the physiological consequence of such alterations? Lignifications usually occur during secondary wall thickening in dicots and accelerated during thickening in monocots (gramineous plants have a Type II primary cell wall that contains phenylpropanoids ab ovo) [57]. The formation of phenylpropanoid polymers is frequently accompanied by obturation of plasmodesmata that finally leads to cell death [57]. These alterations induced by cyanotoxins seem to be non-specific stress reactions (“side-effects”) that occur under long-term exposure to high concentrations (5 µM and above for MC-LR; 24 µM and above for CYN) of cyanotoxins [24,29,35]. The lignification of endodermal cell walls blocks the symplastic pathway of nutrient uptake by roots. This can be considered as a defense response to inhibit the transport of toxins towards shoots.
- (ii)
- callose formation and deposition in tracheary elements. MC-LR induce sporadic deposition of cell wall callose materials in the vascular tissue of P. australis roots. Callose obturates tracheary elements that impede the transport of water and minerals (Máthé et al., unpublished data). This is a non-specific stress response. Vascular blockages are observed during organic acid contamination of roots at reed die-back sites as well [58].
2.4. The Plant Vacuolar System and Other Endomembranes
2.5. Plant Cell Death
3. Conclusions
- (i)
- Endomembrane systems such as the ER are as important as the cytoskeleton for the integrated functioning of the plant cell. There is still a lack of knowledge on the relevant effects of cyanotoxins. For example, do cyanotoxins induce ER stress as related to plant cell death?
- (ii)
- Although CYN is considered to be a protein synthesis inhibitory toxin in eukaryotes, we still do not know much on its particular molecular targets. Studies on plant cells might be essential in this issue.
- (iii)
- Most knowledge on the effects of cyanotoxins on plant cells involves MCs and CYNs. What about the other cyanotoxins? Non-MC peptides such as aeruginosins, microginins, etc., are of particular interest because many of them are protein phosphatase inhibitors, proteases or protease inhibitors and as such, they are likely to induce subcellular alterations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitton, B.A.; Potts, M. Introduction to the Cyanobacteria. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 1–13. ISBN 978-94-007-3855-3. [Google Scholar]
- Padisák, J.; Vasas, G.; Borics, G. Phycogeography of freshwater phytoplankton: Traditional knowledge and new molecular tools. Hydrobiologia 2016, 764, 3–27. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. CLIMATE: Blooms Like It Hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef]
- Demay, J.; Bernard, C.; Reinhardt, A.; Marie, B. Natural products from cyanobacteria: Focus on feneficial activities. Mar. Drugs 2019, 17, 320. [Google Scholar] [CrossRef]
- Meriluoto, J.; Spoof, L.; Codd, G.A. Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; John Wiley Sons: Chichester, UK, 2017; ISBN 978-1-119-06868-6. [Google Scholar]
- Fastner, J.; Humpage, A. Cylindrospermopsins: Chemical structures. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Chorus, I., Welker, M., Eds.; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-0-367-53332-8. [Google Scholar]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Bouaïcha, N.; Miles, C.O.; Beach, D.G.; Labidi, Z.; Djabri, A.; Benayache, N.Y.; Nguyen-Quang, T. Structural diversity, characterization and toxicology of microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Pouria, S.; de Andrade, A.; Barbosa, J.; Cavalcanti, R.L.; Barreto, V.T.S.; Ward, C.J.; Preiser, W.; Poon, G.K.; Neild, G.H.; Codd, G.A. Fatal microcystin intoxication in haemodialysis unit in Caruaru, Brazil. Lancet 1998, 352, 21–26. [Google Scholar] [CrossRef]
- Svirčev, Z.; Lujić, J.; Marinović, Z.; Drobac, D.; Tokodi, N.; Stojiljković, B.; Meriluoto, J. Toxicopathology induced by microcystins and nodularin: A histopathological review. J. Environ. Sci. Health C 2015, 33, 125–167. [Google Scholar] [CrossRef]
- De la Cruz, A.A.; Hiskia, A.; Kaloudis, T.; Chernoff, N.; Hill, D.; Antoniou, M.G.; He, X.; Loftin, K.; O’Shea, K.; Zhao, C.; et al. A review on cylindrospermopsin: The global occurrence, detection, toxicity and degradation of a potent cyanotoxin. Environ. Sci. Process. Impacts 2013, 15, 1979–2003. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce β-N-methylamino-l-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef]
- Kós, P.; Gorzó, G.; Surányi, G.; Borbely, G. Simple and efficient method for isolation and measurement of cyanobacterial hepatotoxins by plant tests (Sinapis alba L.). Anal. Biochem. 1995, 225, 49–53. [Google Scholar] [CrossRef]
- Campos, A.; Redouane, E.M.; Freitas, M.; Amaral, S.; Azevedo, T.; Loss, L.; Máthé, C.; Mohamed, Z.A.; Oudra, B.; Vasconcelos, V. Impacts of microcystins on orphological and physiological parameters of agricultural plants: A review. Plants 2021, 10, 639. [Google Scholar] [CrossRef]
- Machado, J.; Campos, A.; Vasconcelos, V.; Freitas, M. Effects of microcystin-LR and cylindrospermopsin on plant-soil systems: A review of their relevance for agricultural plant quality and public health. Environ. Res. 2017, 153, 191–204. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. (Eds.) Biochemistry and Molecular Biology of Plants, 2nd ed.; John Wiley Sons: Chichester, UK, 2015; ISBN 978-0-470-71422-5. [Google Scholar]
- Margulis, L. Origin of Eukaryotic Cells: Evidence and Research Implications for a Theory of the Origin and Evolution of Microbial, Plant and Animal Cells on the Precambrian Earth; Yale University Press: New Haven, CT, USA, 1970; ISBN 978-0-300-01353-5. [Google Scholar]
- Freitas, M.; Campos, A.; Azevedo, J.; Barreiro, A.; Planchon, S.; Renaut, J.; Vasconcelos, V. Lettuce (Lactuca sativa L.) leaf-proteome profiles after exposure to cylindrospermopsin and a microcystin-LR/cylindrospermopsin mixture: A concentration-dependent response. Phytochemistry 2015, 110, 91–103. [Google Scholar] [CrossRef]
- Li, Q.; Gu, P.; Zhang, H.; Luo, X.; Zhang, J.; Zheng, Z. Response of submerged macrophytes and leaf biofilms to the decline phase of Microcystis aeruginosa: Antioxidant response, ultrastructure, microbial properties, and potential mechanism. Sci. Total Environ. 2020, 699, 134325. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gu, P.; Zhang, C.; Luo, X.; Zhang, H.; Zhang, J.; Zheng, Z. Combined toxic effects of anatoxin-a and microcystin-LR on submerged macrophytes and biofilms. J. Hazard. Mater. 2020, 389, 122053. [Google Scholar] [CrossRef] [PubMed]
- Westphal, S.; Soll, J.; Vothknecht, U.C. A vesicle transport system inside chloroplasts. Febs Lett. 2001, 506, 257–261. [Google Scholar] [CrossRef]
- Beyer, D.; Tándor, I.; Kónya, Z.; Bátori, R.; Roszik, J.; Vereb, G.; Erdődi, F.; Vasas, G.; M-Hamvas, M.; Jambrovics, K.; et al. Microcystin-LR, a protein phosphatase inhibitor, induces alterations in mitotic chromatin and microtubule organization leading to the formation of micronuclei in Vicia faba. Ann. Bot. 2012, 110, 797–808. [Google Scholar] [CrossRef]
- Máthé, C.; Beyer, D.; Erdődi, F.; Serfőző, Z.; Székvölgyi, L.; Vasas, G.; M-Hamvas, M.; Jámbrik, K.; Gonda, S.; Kiss, A.; et al. Microcystin-LR induces abnormal root development by altering microtubule organization in tissue-cultured common reed (Phragmites australis) plantlets. Aquat. Toxicol. 2009, 92, 122–130. [Google Scholar] [CrossRef]
- Máthé, C.; M-Hamvas, M.; Vasas, G. Microcystin-LR and cylindrospermopsin induced alterations in chromatin organization of plant cells. Mar. Drugs 2013, 11, 3689–3717. [Google Scholar] [CrossRef] [PubMed]
- Garda, T.; Kónya, Z.; Tándor, I.; Beyer, D.; Vasas, G.; Erdődi, F.; Vereb, G.; Papp, G.; Riba, M.; M-Hamvas, M.; et al. Microcystin-LR induces mitotic spindle assembly disorders in Vicia faba by protein phosphatase inhibition and not reactive oxygen species induction. J. Plant Physiol. 2016, 199, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pappas, D.; Panou, M.; Adamakis, I.-D.S.; Gkelis, S.; Panteris, E. Beyond microcystins: Cyanobacterial extracts induce cytoskeletal alterations in rice root cells. Int. J. Mol. Sci. 2020, 21, 9649. [Google Scholar] [CrossRef]
- Garda, T.; Riba, M.; Vasas, G.; Beyer, D.; M-Hamvas, M.; Hajdu, G.; Tándor, I.; Máthé, C. Cytotoxic effects of cylindrospermopsin in mitotic and non-mitotic Vicia faba cells. Chemosphere 2015, 120, 145–153. [Google Scholar] [CrossRef]
- Pappas, D.; Gkelis, S.; Panteris, E. The effects of microcystin-LR in Oryza sativa root cells: F-actin as a new target of cyanobacterial toxicity. Plant Biol. J. 2020, 22, 839–849. [Google Scholar] [CrossRef]
- Máthé, C.; Vasas, G.; Borbély, G.; Erdődi, F.; Beyer, D.; Kiss, A.; Surányi, G.; Gonda, S.; Jámbrik, K.; M-Hamvas, M. Histological, cytological and biochemical alterations induced by microcystin-LR and cylindrospermopsin in white mustard (Sinapis alba L.) seedlings. Acta Biol. Hung. 2013, 64, 71–85. [Google Scholar] [CrossRef]
- Garda, T.; Kónya, Z.; Freytag, C.; Erdődi, F.; Gonda, S.; Vasas, G.; Szücs, B.; M-Hamvas, M.; Kiss-Szikszai, A.; Vámosi, G.; et al. Allyl-isothiocyanate and microcystin-LR reveal the protein phosphatase mediated regulation of metaphase-anaphase transition in Vicia faba. Front. Plant. Sci. 2018, 9. [Google Scholar] [CrossRef]
- Pamplona-Silva, M.T.; Gonçalves, L.C.; Marin-Morales, M.A. Genetic toxicity of water contaminated by microcystins collected during a cyanobacteria bloom. Ecotoxicol. Environ. Saf. 2018, 166, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Pamplona-Silva, M.T.; Gonçalves, L.C.; do Carmo Bittencourt-Oliveira, M.; Marin-Morales, M.A. DNA damages induced by both endotoxin and exotoxin produced by cyanobacteria. Chemosphere 2020, 254, 126716. [Google Scholar] [CrossRef] [PubMed]
- Beyer, D.; Surányi, G.; Vasas, G.; Roszik, J.; Erdődi, F.; M-Hamvas, M.; Bácsi, I.; Bátori, R.; Serfőző, Z.; Szigeti, Z.M.; et al. Cylindrospermopsin induces alterations of root histology and microtubule organization in common reed (Phragmites australis) plantlets cultured in vitro. Toxicon 2009, 54, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Laughinghouse, H.D.; Prá, D.; Silva-Stenico, M.E.; Rieger, A.; Frescura, V.D.-S.; Fiore, M.F.; Tedesco, S.B. Biomonitoring genotoxicity and cytotoxicity of Microcystis aeruginosa (Chroococcales, Cyanobacteria) using the Allium cepa test. Sci. Total Environ. 2012, 432, 180–188. [Google Scholar] [CrossRef]
- Máthé, C.; M-Hamvas, M.; Vasas, G.; Surányi, G.; Bácsi, I.; Beyer, D.; Tóth, S.; Tímár, M.; Borbély, G. Microcystin-LR, a cyanobacterial toxin, induces growth inhibition and histological alterations in common reed (Phragmites australis) plants regenerated from embryogenic calli. New Phytol. 2007, 176, 824–835. [Google Scholar] [CrossRef]
- Nagy, M.; Kéki, S.; Rácz, D.; Mathur, J.; Vereb, G.; Garda, T.; M-Hamvas, M.; Chaumont, F.; Bóka, K.; Böddi, B.; et al. Novel fluorochromes label tonoplast in living plant cells and reveal changes in vacuolar organization after treatment with protein phosphatase inhibitors. Protoplasma 2018, 255, 829–839. [Google Scholar] [CrossRef]
- M-Hamvas, M.; Máthé, C.; Molnár, E.; Vasas, G.; Grigorszky, I.; Borbely, G. Microcystin-LR alters the growth, anthocyanin content and single-stranded DNase enzyme activities in Sinapis alba L. seedlings. Aquat. Toxicol. 2003, 62, 1–9. [Google Scholar] [CrossRef]
- M.-Hamvas, M.; Máthé, C.; Vasas, G.; Jámbrik, K.; Papp, M.; Beyer, D.; Mészáros, I.; Borbély, G. Cylindrospermopsin and microcystin-LR alter the growth, development and peroxidase enzyme activity of white mustard (Sinapis alba L.) seedlings, a comparative analysis. Acta Biol. Hung. 2010, 61, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Ujvárosi, A.Z.; Riba, M.; Garda, T.; Gyémánt, G.; Vereb, G.; M-Hamvas, M.; Vasas, G.; Máthé, C. Attack of Microcystis aeruginosa bloom on a Ceratophyllum submersum field: Ecotoxicological measurements in real environment with real microcystin exposure. Sci. Total Environ. 2019, 662, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Jámbrik, K.; Máthé, C.; Vasas, G.; Beyer, D.; Molnár, E.; Borbély, G.; M-Hamvas, M. Microcystin-LR induces chromatin alterations and modulates neutral single-strand-preferring nuclease activity in Phragmites australis. J. Plant Physiol. 2011, 168, 678–686. [Google Scholar] [CrossRef] [PubMed]
- M-Hamvas, M.; Ajtay, K.; Beyer, D.; Jámbrik, K.; Vasas, G.; Surányi, G.; Máthé, C. Cylindrospermopsin induces biochemical changes leading to programmed cell death in plants. Apoptosis 2017, 22, 254–264. [Google Scholar] [CrossRef]
- Yin, L.; Huang, J.; Huang, W.; Li, D.; Wang, G.; Liu, Y. Microcystin-RR-induced accumulation of reactive oxygen species and alteration of antioxidant systems in tobacco BY-2 cells. Toxicon 2005, 46, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Huang, J.; Li, W.; Liu, Y. Microcystin-RR-induced apoptosis in tobacco BY-2 cells. Toxicon 2006, 48, 204–210. [Google Scholar] [CrossRef]
- Abe, T.; Lawson, T.; Weyers, J.D.B.; Codd, G.A. Microcystin-LR inhibits photosynthesis of Phaseolus vulgaris primary leaves: Implications for current spray irrigation practice. New Phytol. 1996, 133, 651–658. [Google Scholar] [CrossRef]
- Rahikainen, M.; Pascual, J.; Alegre, S.; Durian, G.; Kangasjärvi, S. PP2A phosphatase as a regulator of ROS signaling in plants. Antioxidants 2016, 5, 8. [Google Scholar] [CrossRef]
- Saqrane, S.; El ghazali, I.; Oudra, B.; Bouarab, L.; Dekayir, S.; Mandi, L.; Ouazzani, N.; Vasconcelos, V.M. Detection of microcystin contamination by the measurement of the variability of the in vivo chlorophyll fluorescence in aquatic plant Lemna gibba. Toxicon 2009, 53, 9–14. [Google Scholar] [CrossRef]
- Westphal, S.; Soll, J.; Vothknecht, U.C. Evolution of chloroplast vesicle transport. Plant Cell Physiol. 2003, 44, 217–222. [Google Scholar] [CrossRef]
- Gutiérrez-Praena, D.; Campos, A.; Azevedo, J.; Neves, J.; Freitas, M.; Guzmán-Guillén, R.; Cameán, A.M.; Renaut, J.; Vasconcelos, V. Exposure of Lycopersicon esculentum to microcystin-LR: Effects in the leaf proteome and toxin translocation from water to leaves and fruits. Toxins 2014, 6, 1837–1854. [Google Scholar] [CrossRef]
- Baskin, T. The cytoskeleton. In Biochemistry and Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; John Wiley Sons: Chichester, UK, 2015; pp. 191–238. ISBN 978-0-470-71422-5. [Google Scholar]
- Szigeti, Z.M.; Jámbrik, K.; Roszik, J.; M-Hamvas, M.; Tándor, I.; Beyer, D.; Vasas, G.; Vereb, G.; Surányi, G.; Máthé, C. Cytoskeletal and developmental alterations in Ceratophyllum demersum induced by microcystin-LR, a cyanobacterial toxin. Aquat. Bot. 2010, 92, 179–184. [Google Scholar] [CrossRef]
- Spinner, L.; Gadeyne, A.; Belcram, K.; Goussot, M.; Moison, M.; Duroc, Y.; Eeckhout, D.; Winne, N.D.; Schaefer, E.; Slijke, E.V.D.; et al. A protein phosphatase 2A complex spatially controls plant cell division. Nat. Commun. 2013, 4, 1863. [Google Scholar] [CrossRef] [PubMed]
- Mineyuki, Y. The Preprophase Band of Microtubules: Its Function as a Cytokinetic Apparatus in Higher Plants. In International Review of Cytology; Jeon, K.W., Ed.; Academic Cambridge: Cambridge, UK, 1999; Volume 187, pp. 1–49. [Google Scholar]
- Houben, A.; Demidov, D.; Caperta, A.D.; Karimi, R.; Agueci, F.; Vlasenko, L. Phosphorylation of histone H3 in plants—A dynamic affair. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 2007, 1769, 308–315. [Google Scholar] [CrossRef]
- Manzanero, S.; Rutten, T.; Kotseruba, V.; Houben, A. Alterations in the distribution of histone H3 phosphorylation in mitotic plant chromosomes in response to cold treatment and the protein phosphatase inhibitor cantharidin. Chromosome Res. 2002, 10, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Kaszás, É.; Cande, W.Z. Phosphorylation of histone H3 is correlated with changes in the maintenance of sister chromatid cohesion during meiosis in maize, rather than the condensation of the chromatin. J. Cell Sci. 2000, 113, 3217–3226. [Google Scholar] [CrossRef]
- Zegura, B. An overview of the mechanisms of microcystin-LR genotoxicity and potential carcinogenicity. Mini-Rev. Med. Chem. 2016, 16, 1042–1062. [Google Scholar] [CrossRef] [PubMed]
- Carpita, N.C.; Ralph, J.; McCann, M.C. The cell wall. In Biochemistry and Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; John Wiley Sons: Chichester, UK, 2015; pp. 191–238. ISBN 978-0-470-71422-5. [Google Scholar]
- Armstrong, J.; Afreen-Zobayed, F.; Armstrong, W. Phragmites die-back: Sulphide- and acetic acid-induced bud and root death, lignifications, and blockages within aeration and vascular systems. New Phytol. 1996, 134, 601–614. [Google Scholar] [CrossRef]
- Jürgens, G. Membrane trafficking in plants. Annu. Rev. Cell Dev. Biol. 2004, 20, 481–504. [Google Scholar] [CrossRef] [PubMed]
- Michaillat, L.; Baars, T.L.; Mayer, A. Cell-free reconstitution of vacuole membrane fragmentation reveals regulation of vacuole size and number by TORC1. Mol. Biol. Cell 2012, 23, 881–895. [Google Scholar] [CrossRef]
- Pflugmacher, S. Possible allelopathic effects of cyanotoxins, with reference to microcystin-LR, in aquatic ecosystems. Environ. Toxicol. 2002, 17, 407–413. [Google Scholar] [CrossRef]
- Chen, J.; Song, L.; Dai, J.; Gan, N.; Liu, Z. Effects of microcystins on the growth and the activity of superoxide dismutase and peroxidase of rape (Brassica napus L.) and rice (Oryza sativa L.). Toxicon 2004, 43, 393–400. [Google Scholar] [CrossRef]
- Kurki-Helasmo, K.; Meriluoto, J. Microcystin uptake inhibits growth and protein phosphatase activity in mustard (Sinapis alba L.) seedlings. Toxicon 1926, 36, 1921–1926. [Google Scholar] [CrossRef]
- McElhiney, J.; Lawton, L.A.; Leifert, C. Investigations into the inhibitory effects of microcystins on plant growth, and the toxicity of plant tissues following exposure. Toxicon 2001, 39, 1411–1420. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Jung, K.; Lundvall, L.; Neumann, S.; Peuthert, A. Effects of cyanobacterial toxins and cyanobacterial cell-free crude extract on germination of alfalfa (Medicago sativa) and induction of oxidative stress. Environ. Toxicol. Chem. 2006, 25, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Sinha, R.P.; Incharoensakdi, A. The cyanotoxin-microcystins: Current overview. Rev. Environ. Sci. Biotechnol. 2014, 13, 215–249. [Google Scholar] [CrossRef]
- Redouane, E.M.; El Amrani Zerrifi, S.; El Khalloufi, F.; Oufdou, K.; Oudra, B.; Lahrouni, M.; Campos, A.; Vasconcelos, V. Mode of action and fate of microcystins in the complex soil-plant ecosystems. Chemosphere 2019, 225, 270–281. [Google Scholar] [CrossRef]
- Rymuszka, A. Microcystin-LR induces cytotoxicity and affects carp immune cells by impairment of their phagocytosis and the organization of the cytoskeleton. J. Appl. Toxicol. 2013, 33, 1294–1302. [Google Scholar] [CrossRef]
- Reape, T.J.; McCabe, P.F. Apoptotic-like regulation of programmed cell death in plants. Apoptosis 2010, 15, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Máthé, C.; Beyer, D.; M-Hamvas, M.; Vasas, G. The effects of microcystins (cyanobacterial heptapeptides) on the eukaryotic cytoskeletal system. Mini-Rev. Med. Chem. 2016, 1063–1077. [Google Scholar] [CrossRef]
- Babica, P.; Bláha, L.; Maršálek, B. Exploring the natural role of microcystins—A review of effects on photoautotrophic organisms1. J. Phycol. 2006, 42, 9–20. [Google Scholar] [CrossRef]
- Huang, W.; Xing, W.; Li, D.; Liu, Y. Microcystin-RR induced apoptosis in tobacco BY-2 suspension cells is mediated by reactive oxygen species and mitochondrial permeability transition pore status. Toxicol. In Vitro 2008, 22, 328–337. [Google Scholar] [CrossRef]
- Jiang, J.; Gu, X.; Song, R.; Wang, X.; Yang, L. Microcystin-LR induced oxidative stress and ultrastructural alterations in mesophyll cells of submerged macrophyte Vallisneria natans (Lour.) Hara. J. Hazard. Mater. 2011, 190, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Ye, J.Y.; Zhang, H.Y.; Jiang, X.J.; Zhang, Y.X.; Liu, Z.L. Freshwater toxic cyanobacteria induced DNA damage in apple (Malus pumila), rape (Brassica napus) and rice (Oryza sativa). J. Hazard. Mater. 2011, 190, 240–244. [Google Scholar] [CrossRef]
- Thomas, H. Senescence and cell death. In Biochemistry and Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; John Wiley Sons: Chichester, UK, 2015; pp. 191–238. ISBN 978-0-470-71422-5. [Google Scholar]
- Flores-Rojas, N.C.; Esterhuizen-Londt, M.; Pflugmacher, S. Antioxidative stress responses in the floating macrophyte Lemna minor L. with cylindrospermopsin exposure. Aquat. Toxicol. 2015, 169, 188–195. [Google Scholar] [CrossRef]
- Jámbrik, K.; Máthé, C.; Vasas, G.; Bácsi, I.; Surányi, G.; Gonda, S.; Borbély, G.; M.-Hamvas, M. Cylindrospermopsin inhibits growth and modulates protease activity in the aquatic plants Lemna minor L. and Wolffia arrhiza (L.) Horkel. Acta Biol. Hung. 2010, 61, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Prieto, A.; Campos, A.; Cameán, A.; Vasconcelos, V. Effects on growth and oxidative stress status of rice plants (Oryza sativa) exposed to two extracts of toxin-producing cyanobacteria (Aphanizomenon ovalisporum and Microcystis aeruginosa). Ecotoxicol. Environ. Saf. 2011, 74, 1973–1980. [Google Scholar] [CrossRef]
- Mathe, C.; M-Hamvas, M.; Garda, T.; Beyer, D.; Vasas, G. Cellular effects of cylindrospermopsin (cyanobacterial alkaloid toxin) and its potential medical consequences. Curr. Med. Chem. 2017, 24, 91–109. [Google Scholar] [CrossRef]
- Freytag, C.; Máthé, C.; Rigó, G.; Nodzyński, T.; Kónya, Z.; Erdődi, F.; Cséplő, Á.; Pózer, E.; Szabados, L.; Kelemen, A.; et al. Microcystin-LR, a cyanobacterial toxin affects root development by changing levels of PIN proteins and auxin response in Arabidopsis roots. Chemosphere 2021, 276, 130183. [Google Scholar] [CrossRef]

| Cell Compartment/Phenomenon | Effects of MCs Including MC Type and Concentration | Effects of CYNs Including CYN Concentration | Mechanisms |
|---|---|---|---|
| Plastids | 5 nM MC-LR a + 30.26 µM ANA/14 d MC-LR containing Microcystis aeruginosa cultures: accumulation of osmiophilic granules in chloroplasts of Vallisneria natans [19,20] | n.d. | Generation of ROS by MC-LR |
| 100 µM MC-LR a isolated chloroplasts of pea, inhibition of vesicle traffic in plastids [21] | PP1/PP2A inhibition | ||
| Cytoskeleton | MTs: CMT—0.01–40 µM MC-LR a—disruption in Phragmites australis, reorientation in Ceratophyllum demersum PPB—45 µM MC-LR a, short-term exposure—PPB disruption in rice root meristem spindle—0.05–40 µM MC-LR a—disruptions, deformations in Sinapis alba, Vicia faba, P. australis phragmoplast—0.5–40 µM MC-LR a—disruptions in roots of P. australis, V. faba [22,23,24,25,26] | MTs: CMT—12–96 µM CYN a—reorientation, decrease in their density in P. australis PPB—2.4–24 µM CYN a—double and split PPBs in roots of P. australis, V. faba spindle—2.4–12 µM CYN a—disruptions, deformations of metaphase and anaphase spindle in roots of S. alba and P. australis phragmoplast—1.2–12 µM CYN a—disruptions in roots of P. australis [24,27] | PP1/PP2A inhibition for MCs; protein synthesis inhibition for CYN |
| MFs—45 µM MC-LR a and MCs short-term treatment: misorientation of cortical MFs in rice roots [28] | MFs: n.d. | PP1/PP2A inhibition? | |
| Mitotic chromatin, mitotic index | 0.5–40 µM MC-LR a-mis-segregation of sister chromatids including lagging chromosomes in telophase/cytokinesis, micronucleus: S. alba, V. faba, P. australis 0.001–0.002 µM MC-LR a and MCs c: chromosome aberrations and micronuclei in Allium cepa roots 1–10 µM MC-LR a: alterations in the timing of metaphase–anaphase transition MCs b—blocking of cells in early mitosis, rice roots [22,23,25,26,29,30,31,32] | 1.2–12 µM CYN a—lagging chromosomes in root tips of P. australis 2.4, 6 µM CYN a—blocking of cells in early mitosis in P. australis roots 12 µM CYN a—delay of mitosis in synchronized V. faba roots 0.24–12 µM CYN a—chromosome breaks in roots of V. faba [24,27,33] | disruptions in the mitotic MT cytoskeleton; for MCs, hyperphosphorylation of histone H3 related to PP1 (PP2A) inhibition For CYN, inhibition of protein synthesis? |
| MC-LR a—inhibition of mitosis: S. alba (≥10 μM), P. australis (≥0.5 μM); stimulation of mitosis at lower concentrations (1 µM): S. alba, V. faba MC b: stimulation of mitosis, A. cepa roots [23,24,34] | 0.024–0.24 µM CYN a—stimulation and 6–48 µM CYN a—inhibition of mitosis in roots of V. faba [24,29] | probably related to the direct biochemical targets of cyanotoxins | |
| Cell wall | 5–40 µM MC-LR—lignification of cell walls in root cortex and stele of S. alba and P. australis [29,35] | 24–48 µM CYN a—lignification of endodermis and pericycle cells of S. alba roots [24] | non-specific stress reactions? |
| Vacuoles and other endomembranes | MCs b, aggregations of ER and Golgi membranes in rice root cells 1 µM MC-LR a, short-term exposure: vacuole fragmentation, engulfment of plastids in tonoplast-coated vesicles in Arabidopsis hypocotyl cells [8,17] | n.d. | n.d. for ER/Golgi; PP2A/PP1 inhibition for vacuole fragmentation [28,36] |
| Cell death | 5–100 µM MC-LR a: cotyledon, leaf and/or root necrosis, in Phaseolus vulgaris, S. alba, Brassica napus, P. australis, Ceratophyllum submersum −5–10 µM/2–20 d: CMT reorganization caused crown root formation and radial expansion of cells 1 µM: plasmolysis, swollen chloroplasts and mitochondria with destroyed inner membrane structures in V. natans [23,29,35,37,38,39,40,41,42] | -root necrosis in P. australis (≥24 μM CYN) and V. faba (2.4–48 μM CYN, but not in S. alba −12–24 µM/2–20 d: swelling of cells and formation of a callus-like tissue S. alba, P. australis without early formation of aerenchyma [27,29,33] | generation of ROS induced by MCs and CYN; alterations in nuclease (ssDNase and dsDNase) and protease activities [37,38,39,40,41] |
| apoptosis/AL-PCD: MC-RR a 60 μM/5 d and ≥1 μM/8 d: TobaccoBY-2 cells, 5 µM/4 d S. alba seedlings: perinuclear chromatin margination, condensation of nuclear chromatin, shrinking, blebbing, fragmentation of nucleus, formation of apoptotic-like bodies, the loss of mitochondrial membrane potential (DWm) 1–2 μM MC-LR a/72 h, autophagosome formation in Arabidopsis hypocotyl cells [24,36,43,44,45] | In V. faba 12–48 µM/3–6 d CYN a induced nucleus fragmentation, blebbing and chromosomal breaks, and increased the ratio of TUNEL-positive cells in 1.2–48 µM/10 d CYN a treated P. australis and in 0.024–24 µM/4 d CYN a treated S. alba roots, in P. australis chromatin fragmentation was detected as well [24,27,41] | ||
| 50 μM MC-LR a/72–144 h reduced cell viability of TobaccoBY-2 cells (Evans blue, PI, staining) Significantly higher cell death index compared with control meristematic A. cepa root tip cells [31,42,43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Máthé, C.; M-Hamvas, M.; Vasas, G.; Garda, T.; Freytag, C. Subcellular Alterations Induced by Cyanotoxins in Vascular Plants—A Review. Plants 2021, 10, 984. https://doi.org/10.3390/plants10050984
Máthé C, M-Hamvas M, Vasas G, Garda T, Freytag C. Subcellular Alterations Induced by Cyanotoxins in Vascular Plants—A Review. Plants. 2021; 10(5):984. https://doi.org/10.3390/plants10050984
Chicago/Turabian StyleMáthé, Csaba, Márta M-Hamvas, Gábor Vasas, Tamás Garda, and Csongor Freytag. 2021. "Subcellular Alterations Induced by Cyanotoxins in Vascular Plants—A Review" Plants 10, no. 5: 984. https://doi.org/10.3390/plants10050984
APA StyleMáthé, C., M-Hamvas, M., Vasas, G., Garda, T., & Freytag, C. (2021). Subcellular Alterations Induced by Cyanotoxins in Vascular Plants—A Review. Plants, 10(5), 984. https://doi.org/10.3390/plants10050984






