Microcystin-LR, a Cyanobacterial Toxin, Induces DNA Strand Breaks Correlated with Changes in Specific Nuclease and Protease Activities in White Mustard (Sinapis alba) Seedlings
Abstract
:1. Introduction
2. Results
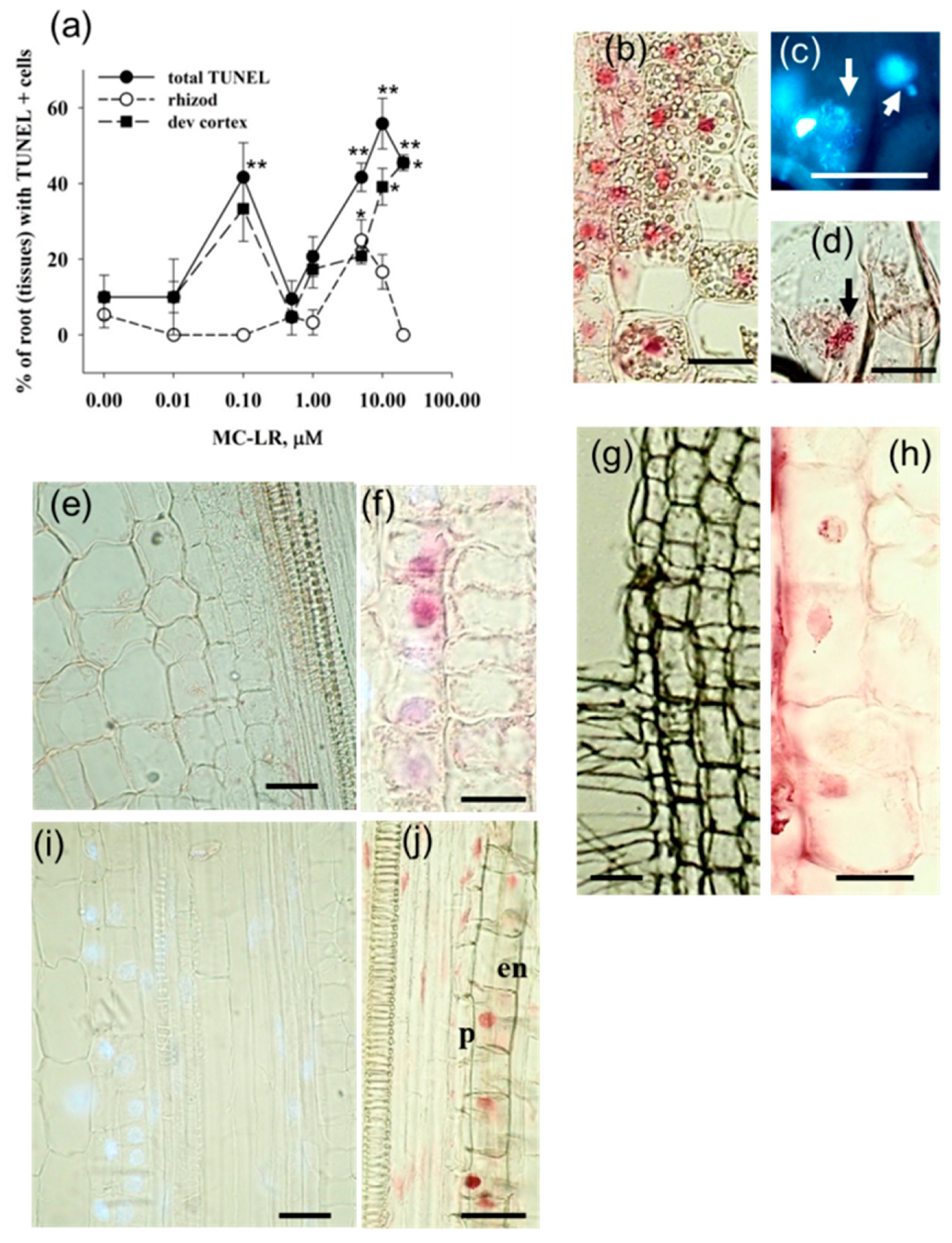
2.1. MC-LR Induces DNA Strand Breaks in Mustard Root Cells as Revealed by the TUNEL Reaction
2.2. MC-LR Induces Changes in Single-Stranded DNase Activities in Mustard Seedlings
2.3. MC-LR Induces Changes in Protease Activities in Mustard Seedlings
3. Discussion
4. Materials and Methods
4.1. The Purification of MC-LR
4.2. Plant Material and MC-LR Treatments
4.3. TUNEL Assay
4.4. Spectrophotometric and In-Gel Activity Assays of Single-Strand Preferring DNase (SSP Nuclease) Activities
4.5. In-Gel Activity Assays of Protease Activities
4.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rastogi, R.P.; Sinha, R.P.; Incharoensakdi, A. The cyanotoxin-microcystins: Current overview. Rev. Environ. Sci. Bio/Technol. 2014, 13, 215–249. [Google Scholar] [CrossRef]
- Campos, A.; Vasconcelos, V. Molecular mechanisms of microcystin toxicity in animal cells. Int. J. Mol. Sci. 2010, 11, 268–287. [Google Scholar] [CrossRef] [Green Version]
- Campos, A.; Redouane, E.M.; Freitas, M.; Amaral, S.; Azevedo, T.; Loss, L.; Máthé, C.; Mohamed, Z.A.; Oudra, B.; Vasconcelos, V. Impacts of microcystins on morphological and physiological parameters of agricultural plants: A review. Plants 2021, 10, 639. [Google Scholar] [CrossRef] [PubMed]
- Máthé, C.; M-Hamvas, M.; Vasas, G.; Garda, T.; Freytag, C. Subcellular alterations induced by cyanotoxins in vascular plants—A review. Plants 2021, 10, 984. [Google Scholar] [CrossRef]
- MacKintosh, C.; Diplexcito, J. Naturally occurring inhibitors of serine/threonine phosphatases. In Handbook of Cell Signaling; Bradshaw, R.A., Dennis, E.A., Eds.; Elsevier: San Diego, CA, USA, 2003; pp. 607–611. [Google Scholar]
- Žegura, B. An overview of the mechanisms of microcystin-LR genotoxicity and potential carcinogenicity. Mini-Rev. Med. Chem. 2016, 16, 1042–1062. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Huang, J.; Huang, W.; Li, D.; Wang, G.; Liu, Y. Microcystin-RR-induced accumulation of reactive oxygen species and alteration of antioxidant systems in tobacco BY-2 cells. Toxicon 2005, 46, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Jámbrik, K.; Máthé, C.; Vasas, G.; Beyer, D.; Molnár, E.; Borbély, G.; M-Hamvas, M. Microcystin-LR induces chromatin alterations and modulates neutral single-strand-preferring nuclease activity in Phragmites australis. J. Plant Physiol. 2011, 168, 678–686. [Google Scholar] [CrossRef]
- Máthé, C.; M-Hamvas, M.; Vasas, G. Microcystin-LR and cylindrospermopsin induced alterations in chromatin organization of plant cells. Mar. Drugs 2013, 11, 3689–3717. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, I.E.W.; Reutelingsoerger, C.P.M.; Holdaway, K.M. Annexin-V and TUNEL use in monitoring the progression of apoptosis in plants. Cytometry 1997, 29, 28–33. [Google Scholar] [CrossRef]
- López-Fernandez, M.P.; Maldonado, S. Programmed cell death during quinoa perisperm development. J. Exp. Bot. 2013, 64, 3313–3325. [Google Scholar] [CrossRef] [Green Version]
- Jámbrik, K.; Máthé, C.; Vasas, G.; Bácsi, I.; Surányi, G.; Gonda, S.; Borbély, G.; M-Hamvas, M. Cylindrospermopsin inhibits growth and modulates protease activity in the aquatic plants Lemna minor L. and Wolffia arrhiza (L.) Horkel. Acta Biol. Hung. 2010, 61, 77–94. [Google Scholar] [CrossRef]
- Máthé, C.; Vasas, G.; Borbély, G.; Erdődi, F.; Beyer, D.; Kiss, A.; Surányi, G.; Gonda, S.; Jámbrik, K.; M-Hamvas, M. Histological, cytological and biochemical alterations induced by microcystin-LR and cylindrospermopsin in white mustard (Sinapis alba L.) seedlings. Acta Biol. Hung. 2013, 64, 71–85. [Google Scholar] [CrossRef] [PubMed]
- M-Hamvas, M.; Ajtay, K.; Beyer, D.; Jámbrik, K.; Vasas, G.; Surányi, G.; Máthé, C. Cylindrospermopsin induces biochemical changes leading to programmed cell death in plants. Apoptosis 2017, 22, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.I.; Abe, K.; Endo, M.; Ohtsuki, N.; Nishizawa-Yokoi, A.; Tagari, A.; Saika, H.; Toki, S. DNA replication arrest leads to enhanced homologous recombination and cell death in meristems of rice OsRecQ14 mutants. BMC Plant Biol. 2013, 13, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- M-Hamvas, M.; Máthé, C.; Molnár, E.; Vasas, G.; Grigorszky, I.; Borbely, G. Microcystin-LR alters the growth, anthocyanin content and single-stranded DNase enzyme activities in Sinapis alba L. seedlings. Aquat. Toxicol. 2003, 62, 1–9. [Google Scholar] [CrossRef]
- Štraser, A.; Filipič, M.; Gorenc, I.; Žegura, B. The influence of cylindrospermopsin on oxidative DNA damage and apoptosis induction in HepG2 cells. Chemosphere 2013, 92, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrushkina, N.I.; Vanyushin, B.F. Endonucleases and their involvement in plant apoptosis. Russ. J. Plant Physiol. 2009, 56, 291–305. [Google Scholar] [CrossRef]
- Pérez-Amador, M.A.; Abler, M.L.; De Rocher, J.; Thompson, D.M.; van Holol, A.; LeBrasseur, N.D.; Lers, A.; Green, P.J. Identification of BFN1, a bifunctional nuclease induced during leaf andstem senescence in Arabidopsis. Plant Physiol. 2000, 122, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Tada, Y.; Hata, S.; Takata, Y.; Nakayashiki, H.; Tosa, Y.; Mayama, S. Induction and signaling of an apoptotic response typified by DNA laddering in the defense response of oats to infection and elicitors. Mol. Plant Microb. Interact. 2001, 14, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Desai, N.A.; Shankar, V. Single strand-specific nucleases. FEMS Microbiol. Rev. 2003, 26, 457–491. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.H.; Varner, J.E. Induction of cysteine and serine proteases during xylogenesis in Zinnia elegans. Plant Mol. Biol. 1996, 30, 1233–1246. [Google Scholar] [CrossRef]
- Beers, E.P.; Freeman, T.B. Proteinase activity during tracheary element differentiation in Zinnia mesophyll cultures. Plant Physiol. 1997, 113, 873–880. [Google Scholar] [CrossRef] [Green Version]
- Khanna-Chopra, R.; Srivalli, B.; Ahlawat, Y.S. Drought induces many forms of cysteine proteases not observed during natural senescence. Biochem. Biophys. Res. Commun. 1999, 255, 324–327. [Google Scholar] [CrossRef]
- Sychta, K.; Dubas, E.; Yamada, K.; Słomka, A.; Krzewska, M.; Kuta, E. Papain-like cysteine proteases are involved in programmed cell death in plants under heavy metal stress. Environ. Exp. Bot. 2020, 174, 104041. [Google Scholar] [CrossRef]
- Kós, P.; Gorzó, G.; Surányi, G.; Borbely, G. Simple and efficient method for isolation and measurement of cyanobacterial hepatotoxins by plant tests (Sinapis alba L.). Anal. Biochem. 1995, 225, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Vasas, G.; Gáspár, A.; Páger, C.; Surányi, G.; Máthé, C.; Hamvas, M.M.; Borbely, G. Analysis of cyanobacterial toxins (anatoxin-a, cylindrospermopsin, microcystin-LR) by capillary electrophoresis. Electrophoresis 2004, 25, 108–115. [Google Scholar] [CrossRef]
- M-Hamvas, M.; Máthé, C.; Vasas, G.; Jámbrik, K.; Papp, M.; Beyer, D.; Mészáros, I.; Borbély, G. Cylindrospermopsin and microcystin-LR alter the growth, development and peroxidase enzyme activity of white mustard (Sinapis alba L.) seedlings, a comparative analysis. Acta Biol. Hung. 2010, 61, 35–48. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Schlereth, A.; Becker, C.; Horstmann, C.; Tiedemann, J.; Müntz, K. Comparison of globulin mobilization and cysteine proteinases in embryogenic axes and cotyledons during germination and seedling growth of vetch (Vicia sativa L.). J. Exp. Bot. 2000, 51, 1423–1433. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
M-Hamvas, M.; Vasas, G.; Beyer, D.; Nagylaki, E.; Máthé, C. Microcystin-LR, a Cyanobacterial Toxin, Induces DNA Strand Breaks Correlated with Changes in Specific Nuclease and Protease Activities in White Mustard (Sinapis alba) Seedlings. Plants 2021, 10, 2045. https://doi.org/10.3390/plants10102045
M-Hamvas M, Vasas G, Beyer D, Nagylaki E, Máthé C. Microcystin-LR, a Cyanobacterial Toxin, Induces DNA Strand Breaks Correlated with Changes in Specific Nuclease and Protease Activities in White Mustard (Sinapis alba) Seedlings. Plants. 2021; 10(10):2045. https://doi.org/10.3390/plants10102045
Chicago/Turabian StyleM-Hamvas, Márta, Gábor Vasas, Dániel Beyer, Eszter Nagylaki, and Csaba Máthé. 2021. "Microcystin-LR, a Cyanobacterial Toxin, Induces DNA Strand Breaks Correlated with Changes in Specific Nuclease and Protease Activities in White Mustard (Sinapis alba) Seedlings" Plants 10, no. 10: 2045. https://doi.org/10.3390/plants10102045




