Metabolomic Analysis of Cannabinoid and Essential Oil Profiles in Different Hemp (Cannabis sativa L.) Phenotypes
Abstract
1. Introduction
2. Results and Discussion
2.1. Differentiation of Phenotypes according to Visual Traits
2.2. Chemical Analysis of the Essential Oil of Hemp (Cannabis sativa L.)
2.3. Chemical Analysis of Cannabinoids in Hemp (Cannabis sativa L.) Phenotypes
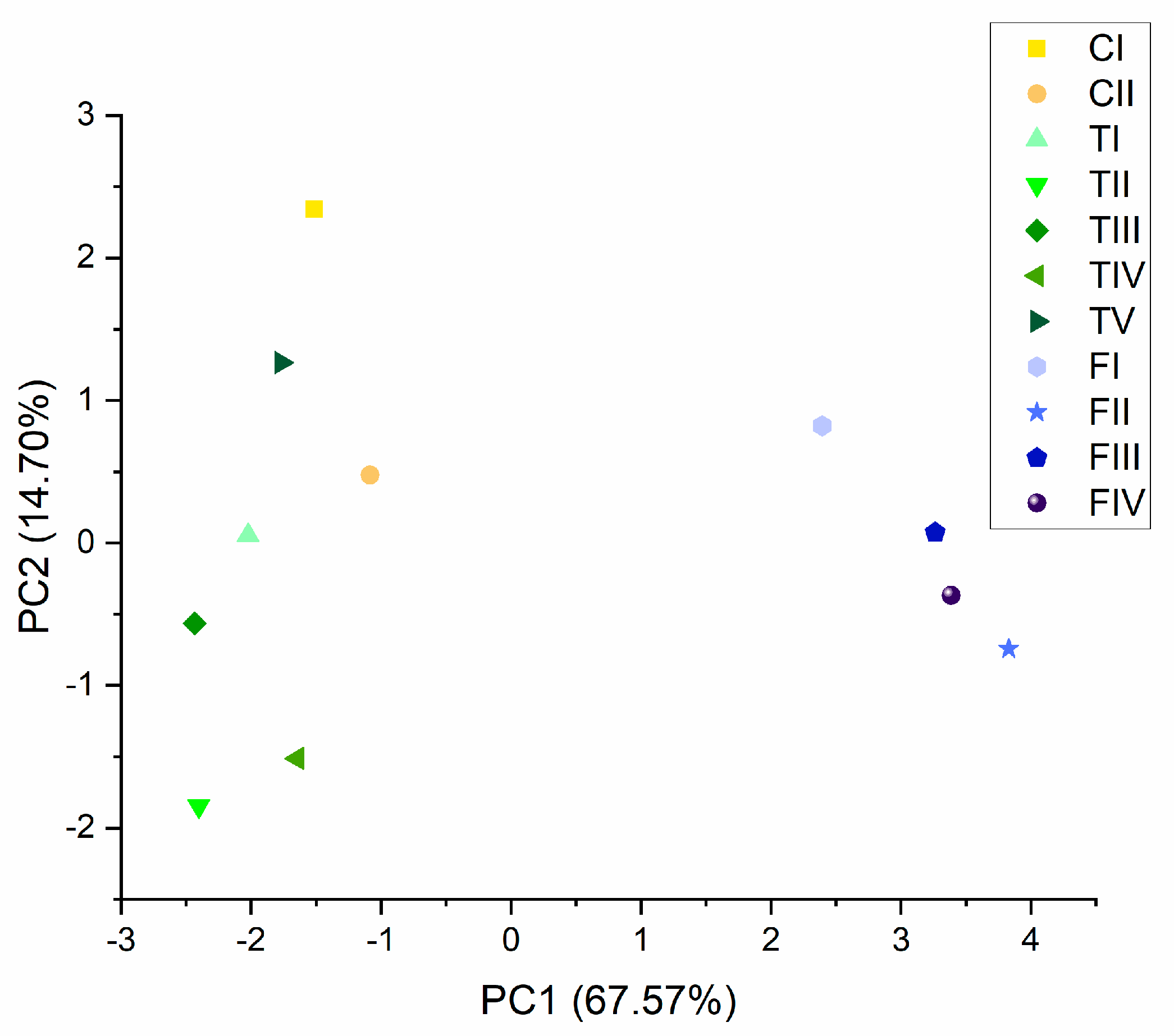
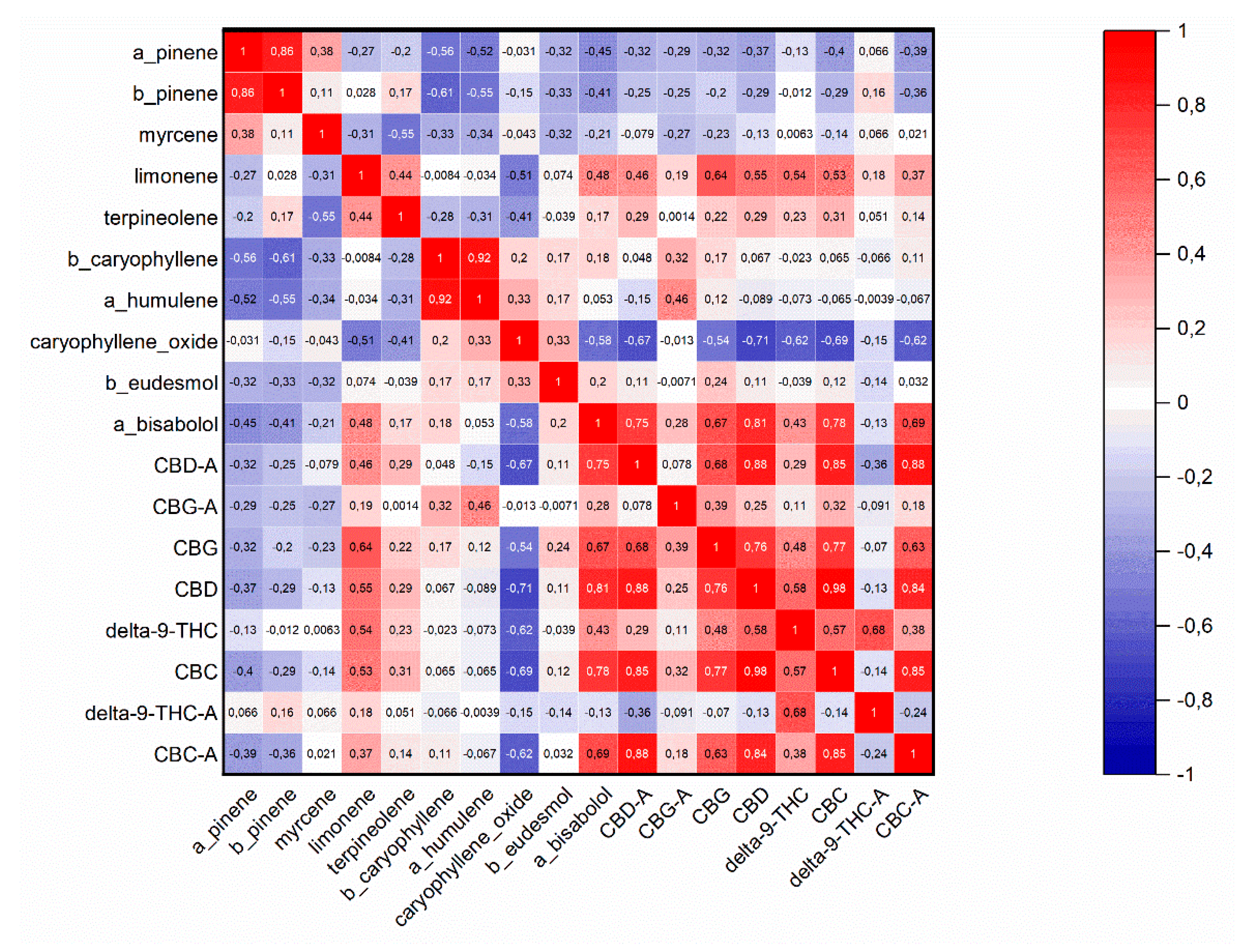
2.4. Chemical Analysis of Both Cannabinoids and Essential Oil in Hemp (Cannabis sativa L.) Phenotypes
3. Materials and Methods
3.1. Plant Material
3.2. Standards and Reagents
3.3. Sample Preparation
3.4. GC–FID Analysis
3.5. HPLC Analysis
3.6. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hillig, K.W. Genetic evidence for speciation in Cannabis (Cannabaceae). Genet. Resour. Crop. Evol. 2005, 52, 161–180. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: A review. Environ. Chem. Lett. 2020, 18, 1–26. [Google Scholar] [CrossRef]
- Namdar, D.; Mazuz, M.; Ion, A.; Koltai, H. Variation in the compositions of cannabinoid and terpenoids in Cannabis sativa derived from inflorescence position along the stem and extraction methods. Ind. Crop. Prod. 2018, 113, 376–382. [Google Scholar] [CrossRef]
- Turner, J.C.; Hemphill, J.K.; Mahlberg, P.G. Quantitative Determination of Cannabinoids in Individual Glandular Trichomes of Cannabis sativa L. (Cannabaceae). Am. J. Bot. 1978, 65, 1103–1106. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef]
- Bertoli, A.; Tozzi, S.; Pistelli, L.; Angelini, L.G. Fibre hemp inflorescences: From crop-residues to essential oil production. Ind. Crop. Prod. 2010, 32, 329–337. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Da Silva, A.C.R.; Lopes, P.M.; De Azevedo, M.M.B.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological Activities of a-Pinene and β-Pinene Enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial Cannabinoids from Cannabis sativa: A Structure−Activity Study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef]
- Small, E.; Beckstead, H.D. Common cannabinoid phenotypes in 350 stocks of Cannabis. Lloydia 1973, 36, 144–165. [Google Scholar]
- Fournier, G.; Richez-Dumanois, C.; Duvezin, J.; Mathieu, J.-P.; Paris, M. Identification of a New Chemotype inCannabis sativa: Cannabigerol—Dominant Plants, Biogenetic and Agronomic Prospects. Planta Med. 1987, 53, 277–280. [Google Scholar] [CrossRef]
- Pollio, A. The Name ofCannabis: A Short Guide for Nonbotanists. Cannabis Cannabinoid Res. 2016, 1, 234–238. [Google Scholar] [CrossRef]
- Schilling, S.; Melzer, R.; McCabe, P.F. Cannabis sativa. Curr. Biol. 2020, 30, R8–R9. [Google Scholar] [CrossRef]
- Sawler, J.; Stout, J.M.; Gardner, K.M.; Hudson, D.; Vidmar, J.; Butler, L.; Page, J.E.; Myles, S. The Genetic Structure of Marijuana and Hemp. PLoS ONE 2015, 10, e0133292. [Google Scholar] [CrossRef]
- Janatová, A.; Fraňková, A.; Tlustoš, P.; Hamouz, K.; Božik, M.; Klouček, P. Yield and cannabinoids contents in different cannabis (Cannabis sativa L.) genotypes for medical use. Ind. Crop. Prod. 2018, 112, 363–367. [Google Scholar] [CrossRef]
- Aguilar, S.; Gutiérrez, V.; Sánchez, L. Medicinal cannabis policies and practices around the world. Int. Drug Policy Consort 2018, 1–32. Available online: https://idpc.net/publications/2018/04/medicinal-cannabis-policies-and-practices-around-the-world (accessed on 19 April 2021).
- Ranalli, P. Current status and future scenarios of hemp breeding. Euphytica 2004, 140, 121–131. [Google Scholar] [CrossRef]
- Centro di Ricerca per le Coulture Industriali CRA. Carmagnola CS Description. Available online: https://www.crea.gov.it/web/cerealicoltura-e-colture-industriali (accessed on 20 April 2021).
- National Food Chain Safety Office (NEBIH). Tiborszallasi Description. Available online: https://www.era-learn.eu/network-information/organisations/national-food-chain-safety-office-1 (accessed on 20 April 2021).
- The Finnish Food Safety Authority (Evira). Finola Description. Available online: https://www.ruokavirasto.fi/en/ (accessed on 20 April 2021).
- Small, E.; Antle, T. A Preliminary Study of Pollen Dispersal inCannabis sativain Relation to Wind Direction. J. Ind. Hemp 2003, 8, 37–50. [Google Scholar] [CrossRef]
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419. [Google Scholar] [CrossRef]
- Novak, J.; Zitterl-Eglseer, K.; Deans, S.G.; Franz, C.M. Essential oils of different cultivars of Cannabis sativa L. and their antimicrobial activity. Flavour Fragr. J. 2001, 16, 259–262. [Google Scholar] [CrossRef]
- Novak, J.; Franz, C. Composition of the Essential Oils and Extracts of Two Populations ofCannabis sativaL. ssp.spontaneafrom Austria. J. Essent. Oil Res. 2003, 15, 158–160. [Google Scholar] [CrossRef]
- Russo, E.B.; Marcu, J. Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. Adv Pharmacol. 2017, 80, 67–134. [Google Scholar] [CrossRef] [PubMed]
- Nuutinen, T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur. J. Med. Chem. 2018, 157, 198–228. [Google Scholar] [CrossRef] [PubMed]
- Mudge, E.M.; Brown, P.N.; Murch, S.J. The Terroir of Cannabis: Terpene Metabolomics as a Tool to Understand Cannabis sativa Selections. Planta Med. 2019, 85, 781–796. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Soydaner, U.; Öztürk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes. J. Nat. Prod. 2016, 79, 324–331. [Google Scholar] [CrossRef]
- Glivar, T.; Eržen, J.; Kreft, S.; Zagožen, M.; Čerenak, A.; Čeh, B.; Benković, E.T. Cannabinoid content in industrial hemp (Cannabis sativa L.) varieties grown in Slovenia. Ind. Crop. Prod. 2020, 145, 112082. [Google Scholar] [CrossRef]
- Hillig, K.W. A chemotaxonomic analysis of terpenoid variation in Cannabis. Biochem. Syst. Ecol. 2004, 32, 875–891. [Google Scholar] [CrossRef]
- Ware, M.; Adams, H.; Guy, G. The medicinal use of cannabis in the UK: Results of a nationwide survey. Int. J. Clin. Pr. 2004, 59, 291–295. [Google Scholar] [CrossRef]
- Callaway, J.C. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Lewis, M.A.; Russo, E.B.; Smith, K.M. Pharmacological Foundations of Cannabis Chemovars. Planta Med. 2017, 84, 225–233. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309–3314. [Google Scholar] [CrossRef]
- Chandra, S.; Lata, H.; ElSohly, M.A.; Walker, L.A.; Potter, D. Cannabis cultivation: Methodological issues for obtaining medical-grade product. Epilepsy Behav. 2017, 70, 302–312. [Google Scholar] [CrossRef]
- MedChemExpress (MCE): Inhibitors, Agonists & Screening Libraies/Master of Small Molecules. Available online: https://www.medchemexpress.com/ (accessed on 19 April 2021).
- ChemSpider/Search and Share Chemistry. Available online: http://www.chemspider.com/ (accessed on 19 April 2021).
- Cheméo—Chemical & Physical Properties by Cheméo. Available online: https://www.chemeo.com/ (accessed on 19 April 2021).
- Mudge, E.M.; Murch, S.J.; Brown, P.N. Leaner and greener analysis of cannabinoids. Anal. Bioanal. Chem. 2017, 409, 3153–3163. [Google Scholar] [CrossRef]
- Neubauer, D.; Benedik, M.P.; Osredkar, D. Cannabidiol for treatment of refractory childhood epilepsies: Experience from a single tertiary epilepsy center in Slovenia. Epilepsy Behav. 2018, 81, 79–85. [Google Scholar] [CrossRef]



| Phenotype | Size | Color | Leaf Size | Inflorescences | Anthocyanin Coloration of Leaf Petiole | Branching | Remarks |
|---|---|---|---|---|---|---|---|
| ‘Carmagnola selected’ | |||||||
| CI | Tall | Light | Large | Small | No | *** | |
| CII | Tall | Dark | Small | Small | Yes | ***** | |
| Reference type | Tall | Dark | Medium | - | Medium | - | |
| ‘Tiborszallasi’ | |||||||
| TI | Tall | Medium | Medium | Small | No | ***** | |
| TII | Medium | Dark | Medium | Medium | Yes | *** | |
| TIII | Small | Dark | Small | Medium | Yes | ** | |
| TIV | Medium | Dark | Large | Big | No | *** | Compact flowers |
| TV | Small | Medium | Small | Medium | Yes | * | Strong anthocyanin coloration of the whole plant |
| Reference type | Tall | Dark | - | - | - | ***** | |
| ‘Finola selection’ | |||||||
| FI | Tall | Dark | Medium | Big | No | ***** | |
| FII | Medium | Medium | Medium | Big | No | *** | |
| FIII | Medium | Light | Medium | Medium | Yes | ***** | |
| FIV | Medium | Dark | Medium | Big | Yes | ***** | |
| Reference type | Small | Medium | Small-medium | - | No | *** |
| Phenotype | CI | SD | CII | SD | TI | SD | TII | SD | TIII | SD | TIV | SD | TV | SD | FI | SD | FII | SD | FIII | SD | FIV | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average EO content | 0.23 | 0.10 | 0.53 | 0.16 | 0.58 | 0.23 | 0.39 | 0.04 | 0.52 | 0.15 | 0.64 | 0.22 | 0.56 | 0.17 | 2.75 | 0.33 | 3.11 | 0.23 | 2.82 | 0.41 | 2.59 | 0.38 |
| α- Pinene | 2.5 a,b | 1.7 | 11.6 c,d | 4.6 | 10.3 b,c,d | 3.3 | 7.4 a,b,c,d | 3.7 | 8.3 a,b,c,d | 5.2 | 11.9 d | 6.3 | 11.5 c,d | 12.2 | 3.7 a,b,c | 4.1 | 6.0 a,b,c,d | 3.4 | 3.5 a,b | 3.4 | 0.7 a | 0.4 |
| β-Pinene | 2.1 a,b | 0.9 | 7.0 c | 3.3 | 3.9 a,b | 0.4 | 3.3 a,b | 1.3 | 3.5 a,b | 1.9 | 4.5 a,b | 2.1 | 4.5 a,b | 3.6 | 2.3 a,b | 1.5 | 3.6 a,b | 0.9 | 2.8 a,b | 1.0 | 1.3 a | 0.7 |
| Myrcene | 10.5 a | 7.0 | 20.9 a,b,c | 13.0 | 29.9 c | 4.4 | 21.4 a,b,c | 8.9 | 25.2 b,c | 8.1 | 26.7 b,c | 7.2 | 24.9 b,c | 9.3 | 19.2 a,b,c | 12.3 | 21.7 a,b,c | 10.7 | 16.0 a,b | 7.4 | 16.1 a,b | 7.6 |
| Limonene | 2.6 a,b,c | 2.5 | 3.3 a,b,c | 2.6 | 0.6 a | 0.4 | 1.8 a,b | 3.1 | 1.0 a | 1.4 | 1.7 a,b | 3.0 | 1.2 a | 1.1 | 4.1 b,c | 0.9 | 4.4 c | 0.6 | 4.5 c | 1.6 | 5.2 c | 0.7 |
| Terpinolene | 2.9 a | 1.8 | 13.4 b,c | 8.3 | 2.7 a | 4.1 | 2.8 a | 3.7 | 2.6 a | 3.1 | 3.8 a,b | 5.1 | 4.2 a,b | 4.9 | 5.5 a,b | 7.1 | 10.6 a,b,c | 9.6 | 16.0 c | 9.1 | 7.9 a,b,c | 10.2 |
| β-Caryophyllene | 21.3 c | 6.9 | 10.5 a | 3.0 | 10.8 a | 4.4 | 16.1 a,b,c | 8.1 | 16.3 a,b,c | 4.4 | 11.9 a | 7.7 | 16.1 a,b,c | 7.9 | 19.7 b,c | 5.6 | 12.5 a,b | 3.6 | 13.6 a,b | 3.1 | 19.7 b,c | 6.9 |
| α-Humulene | 8.8 b | 2.8 | 4.5 a | 1.4 | 4.0 a | 2.2 | 5.6 a | 2.6 | 5.7 a | 1.8 | 4.1 a | 2.5 | 5.0 a | 2.4 | 6.4 a,b | 2.1 | 3.9 a | 1.1 | 4.2 a | 1.0 | 6.7 a,b | 2.7 |
| Caryophyllene oxide | 2.9 d | 0.8 | 1.1 b | 0.9 | 1.5 b,c | 0.4 | 2.6 d | 0.9 | 2.2 c,d | 0.8 | 1.6 b,c | 0.5 | 1.3 b | 0.5 | 0.3 a | 0.2 | 0.3 a | 0.1 | 0.3 a | 0.1 | 0.4 a | 0.2 |
| β-Eudesmol | 2.8 b | 1.9 | 0.9 a | 1.5 | 0.5 a | 0.6 | 0.8 a | 0.8 | 1.0 a | 1.5 | 0.8 a | 0.8 | 0.4 a | 0.4 | 1.3 a | 0.22 | 1.31 a | 0.24 | 1.09 a | 0.47 | 1.41 a | 0.24 |
| α-Bisabolol | 0.9 a | 0.3 | 0.4 a | 0.4 | 0.7 a | 0.6 | 0.4 a | 0.3 | 0.8 a | 0.8 | 0.6 a | 0.4 | 0.2 a | 0.1 | 4.8 d | 1.15 | 3.03 b | 1.67 | 3.37 b,c | 0.90 | 4.31 c,d | 0.26 |
| CI | SD | CII | SD | TI | SD | TII | SD | TIII | SD | TIV | SD | TV | SD | FI | SD | FII | SD | FIII | SD | FIV | SD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CBD | 0.05 a | 0.04 | 0.10 a | 0.04 | 0.07 a | 0.03 | 0.04 a | 0.02 | 0.05 a | 0.02 | 0.07 a | 0.04 | 0.06 a | 0.03 | 0.60 b | 0.08 | 0.78 c | 0.12 | 0.61 b | 0.12 | 0.64 b | 0.19 |
| CBD-A | 1.70 a | 1.53 | 3.78 b | 0.94 | 3.22 b | 1.11 | 3.11 b | 0.56 | 2.65 a,b | 1.06 | 3.78 b | 0.62 | 2.93 b | 1.37 | 6.48 c | 0.35 | 6.41 c | 0.35 | 6.36 c | 0.25 | 6.59 c | 0.24 |
| CBG | 0.04 b,c | 0.02 | 0.04 b,c | 0.01 | 0.01 a | 0.01 | 0.01 a | 0.01 | 0.01 a | 0.01 | 0.02 a,b | 0.02 | 0.02 a | 0.01 | 0.05 c,d | 0.01 | 0.05 c,d | 0.01 | 0.06 d | 0.01 | 0.06 d | 0.02 |
| CBG-A | 1.62 b | 2.39 | 0.44 a | 0.24 | 0.25 a | 0.08 | 0.16 a | 0.05 | 0.19 a | 0.06 | 0.22 a | 0.07 | 0.18 a | 0.07 | 1.00 a,b | 0.37 | 0.63 a,b | 0.16 | 0.91 a,b | 0.23 | 1.11 a,b | 0.26 |
| Δ-9-THC | 0.05 a,b,c | 0.09 | 0.04 a,b,c | 0.05 | 0.04 a,b,c | 0.03 | 0.01 a | 0.01 | 0.02 a,b | 0.02 | 0.01 a | 0.01 | 0.06 b,c,d | 0.05 | 0.08 c,d | 0.02 | 0.11 d | 0.02 | 0.08 c,d | 0.01 | 0.08 c,d | 0.02 |
| Δ-9-THC-A | 0.91 a,b | 1.81 | 0.75 a,b | 1.26 | 0.71 a,b | 0.72 | 0.14 a | 0.03 | 0.53 a,b | 0.54 | 0.18 a | 0.04 | 1.39 b | 1.23 | 0.50 a,b | 0.08 | 0.46 a,b | 0.07 | 0.47 a,b | 0.06 | 0.51 a,b | 0.05 |
| CBC | 0.01 a,b | 0.00 | 0.02 a | 0.01 | 0.01 a,b | 0.01 | 0.00 a | 0.00 | 0.01 a,b | 0.01 | 0.01 a,b | 0.01 | 0.01 a,b | 0.00 | 0.05 c | 0.01 | 0.06 d | 0.01 | 0.05 c | 0.01 | 0.05 c | 0.01 |
| CBC-A | 0.20 a | 0.06 | 0.27 a | 0.09 | 0.34 a | 0.21 | 0.24 a | 0.10 | 0.25 a | 0.07 | 0.34 a | 0.16 | 0.30 a | 0.16 | 0.62 b | 0.10 | 0.59 b | 0.09 | 0.64 b | 0.10 | 0.64 b | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eržen, M.; Košir, I.J.; Ocvirk, M.; Kreft, S.; Čerenak, A. Metabolomic Analysis of Cannabinoid and Essential Oil Profiles in Different Hemp (Cannabis sativa L.) Phenotypes. Plants 2021, 10, 966. https://doi.org/10.3390/plants10050966
Eržen M, Košir IJ, Ocvirk M, Kreft S, Čerenak A. Metabolomic Analysis of Cannabinoid and Essential Oil Profiles in Different Hemp (Cannabis sativa L.) Phenotypes. Plants. 2021; 10(5):966. https://doi.org/10.3390/plants10050966
Chicago/Turabian StyleEržen, Marjeta, Iztok J. Košir, Miha Ocvirk, Samo Kreft, and Andreja Čerenak. 2021. "Metabolomic Analysis of Cannabinoid and Essential Oil Profiles in Different Hemp (Cannabis sativa L.) Phenotypes" Plants 10, no. 5: 966. https://doi.org/10.3390/plants10050966
APA StyleEržen, M., Košir, I. J., Ocvirk, M., Kreft, S., & Čerenak, A. (2021). Metabolomic Analysis of Cannabinoid and Essential Oil Profiles in Different Hemp (Cannabis sativa L.) Phenotypes. Plants, 10(5), 966. https://doi.org/10.3390/plants10050966







