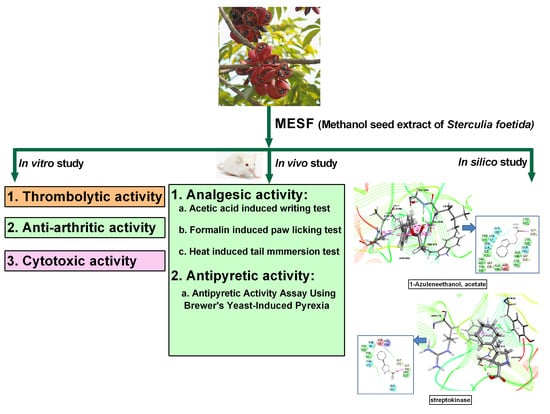
Chemical Profiling, Pharmacological Insights and In Silico Studies of Methanol Seed Extract of Sterculia foetida
Abstract
1. Introduction
2. Results
2.1. Qualitative Phytochemical Screening
2.2. GC-MS Analysis
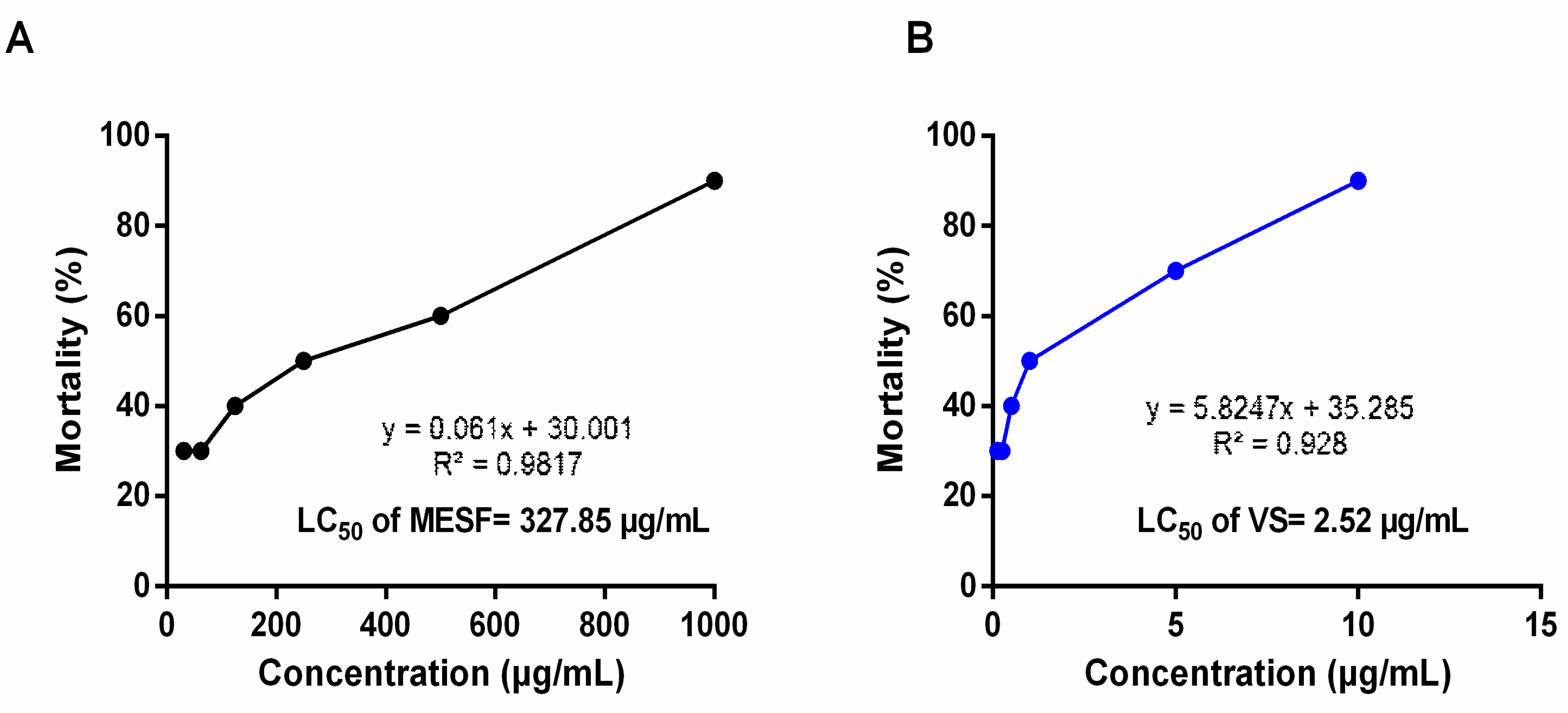
2.3. Brine Shrimp Lethality Bioassay
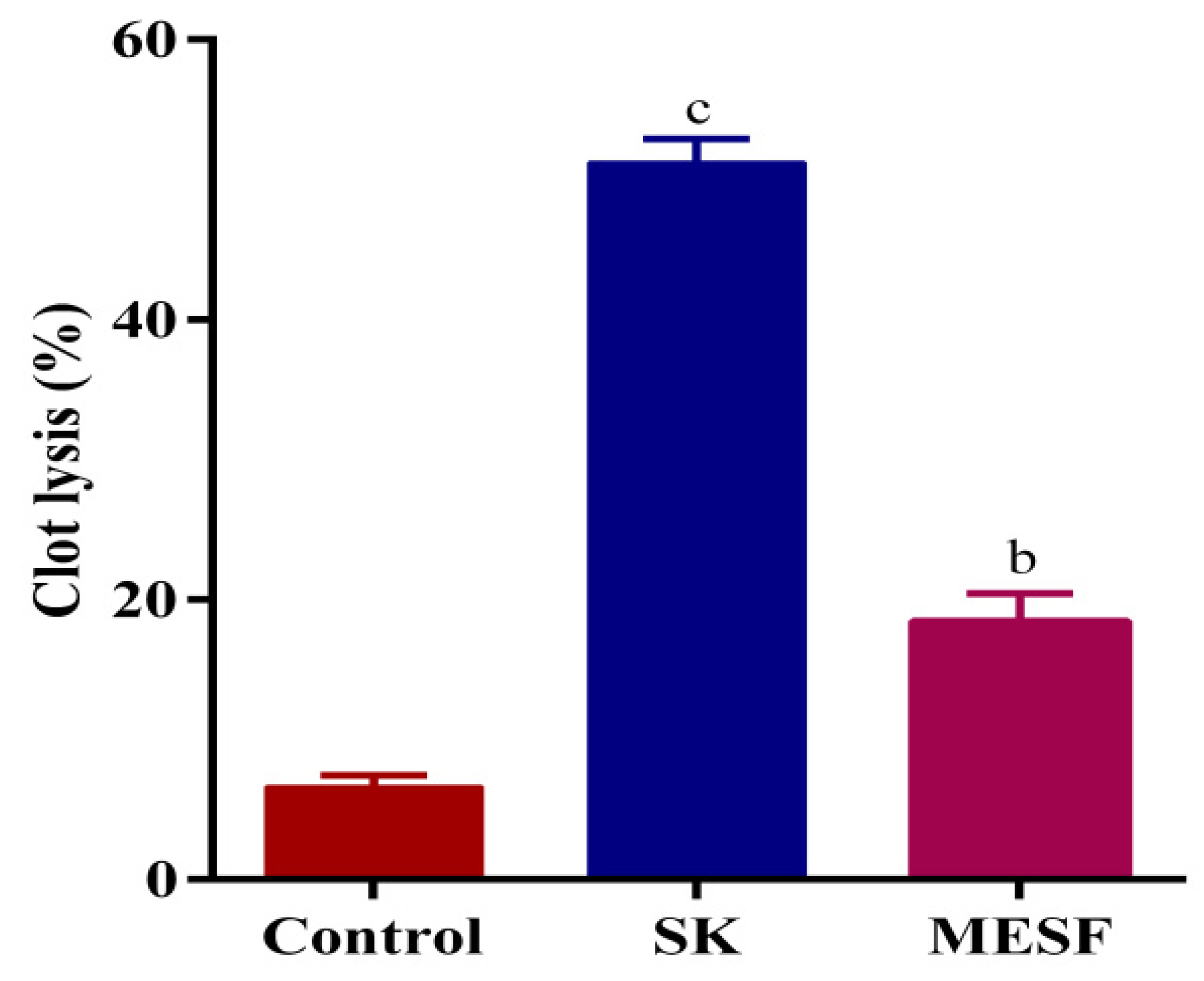
2.4. Thrombolytic Activity
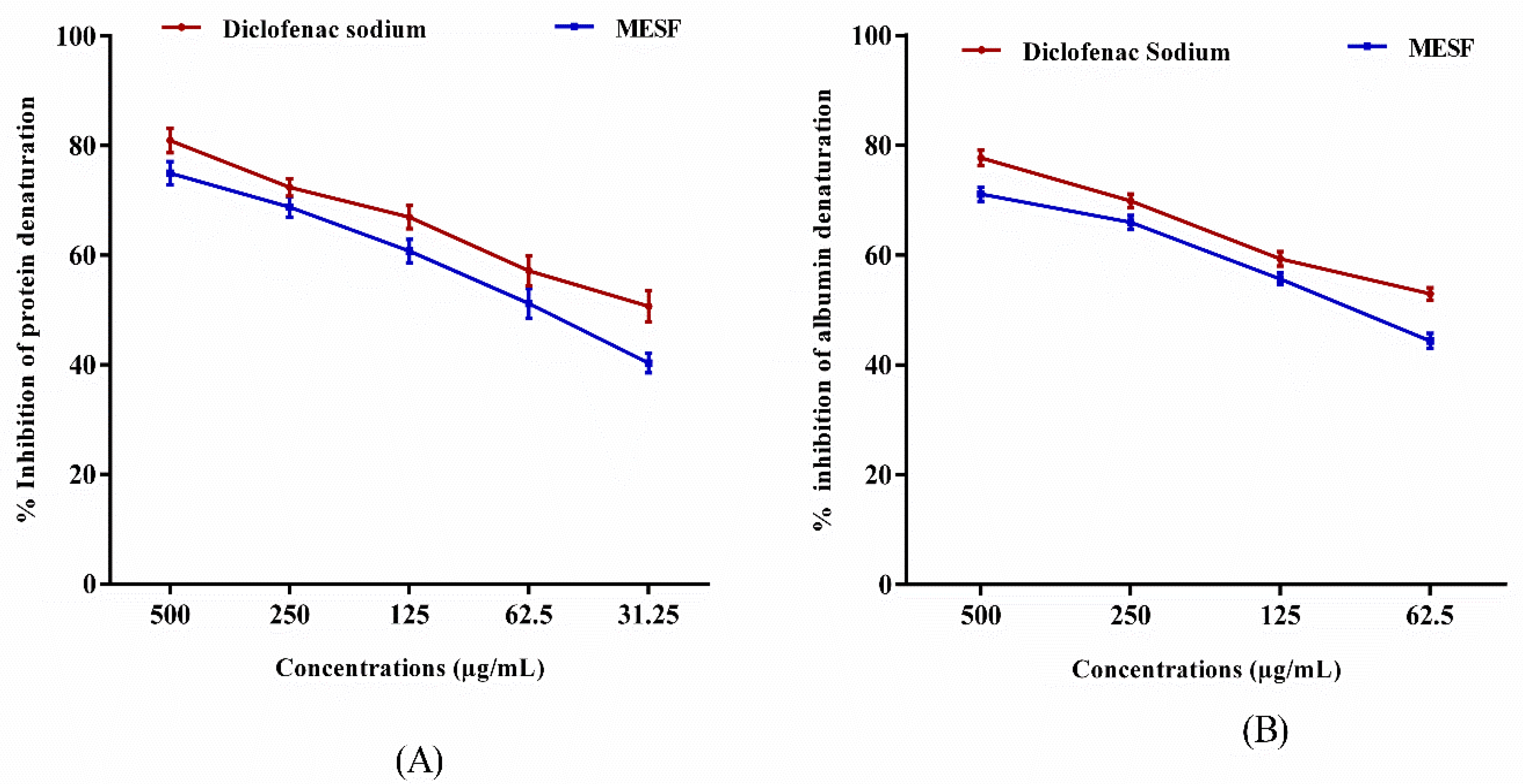
2.5. Evaluation of In Vitro Anti-Inflammatory Activity
2.5.1. Inhibition of Protein Denaturation
2.5.2. Egg Albumin Denaturation Method
2.6. Acute Toxicity Test
2.7. Analgesic Activity
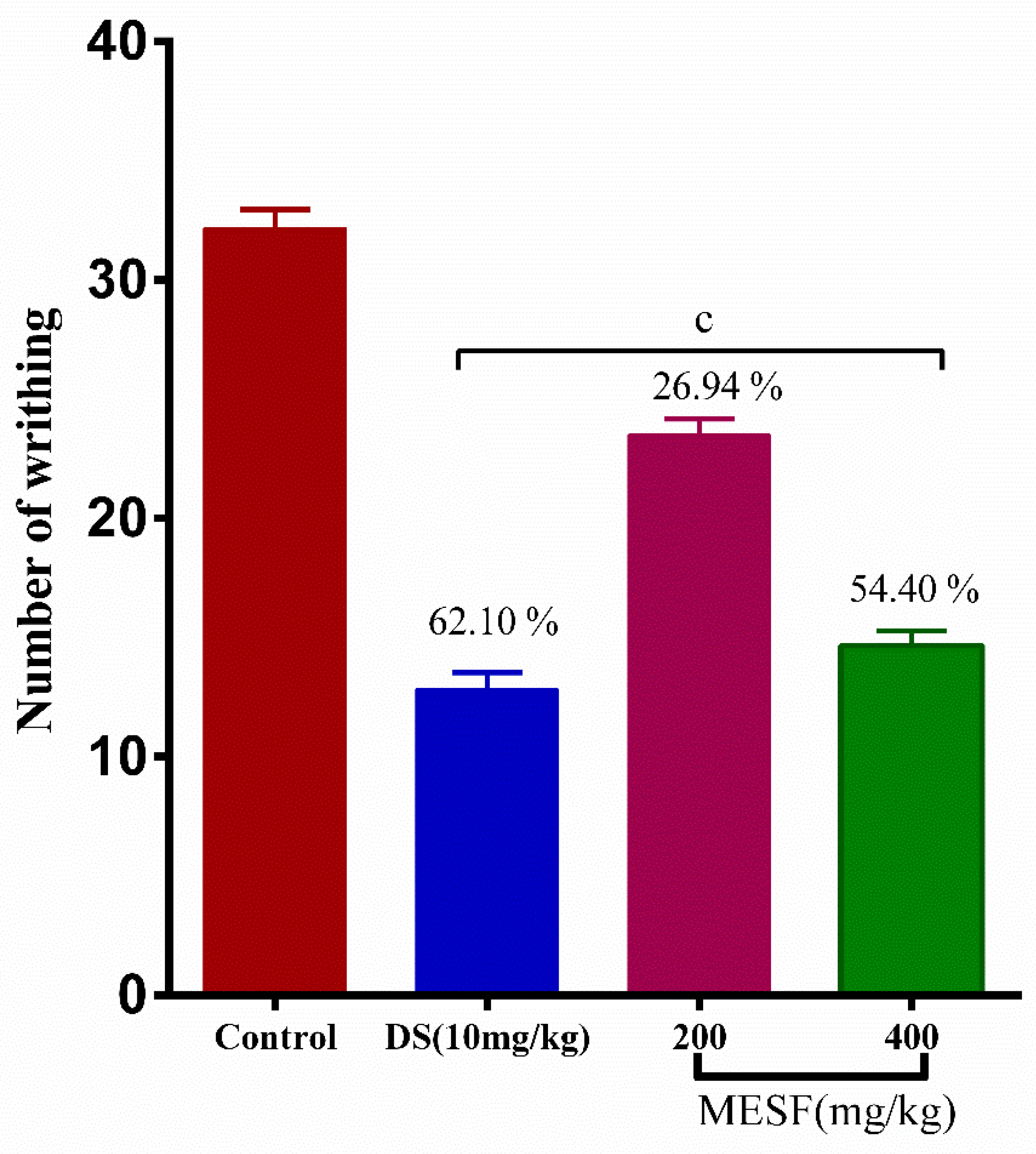
2.7.1. Acetic Acid-Induced Writhing Inhibition Test
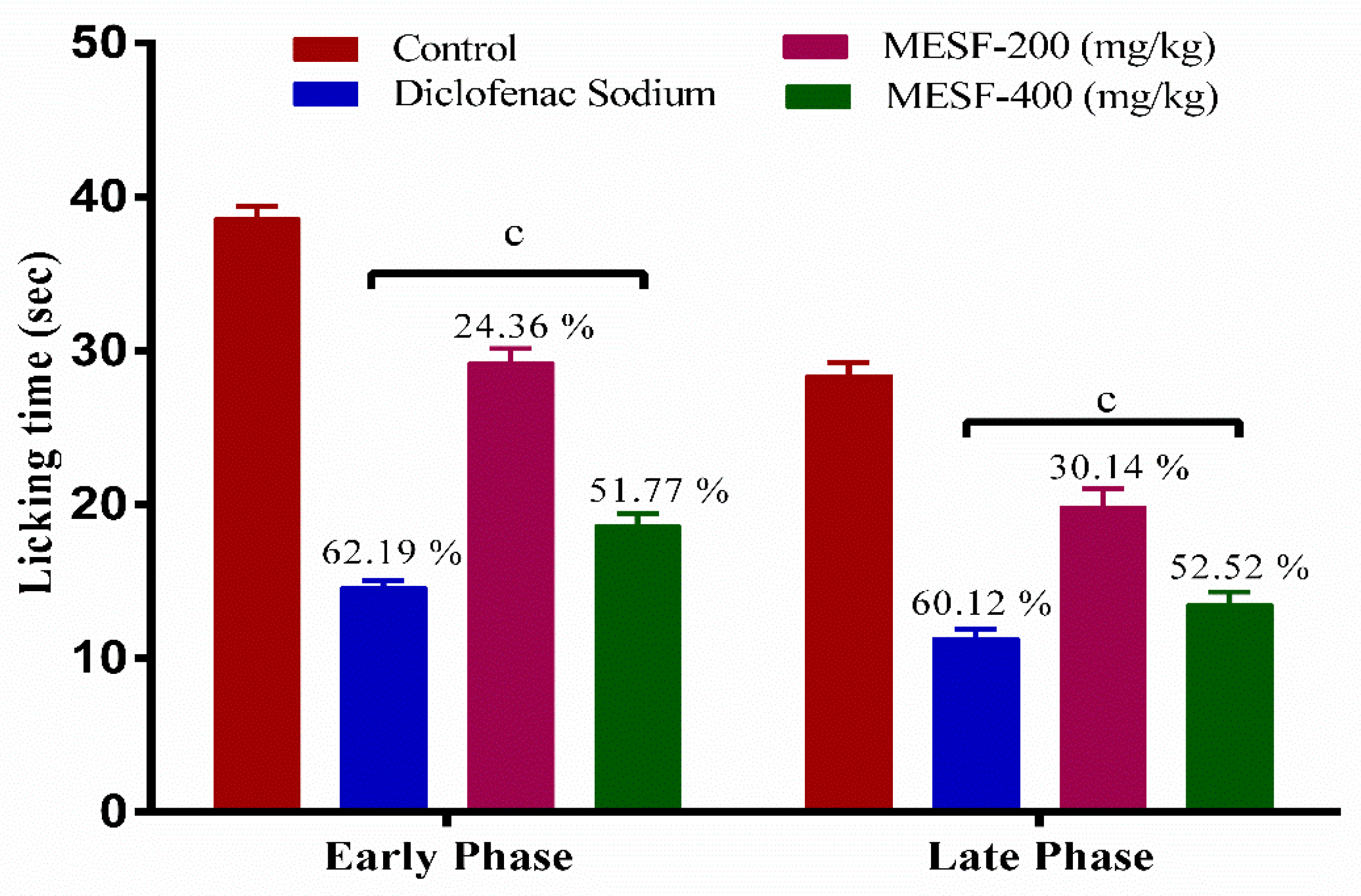
2.7.2. Formalin-Induced Paw-Licking Test
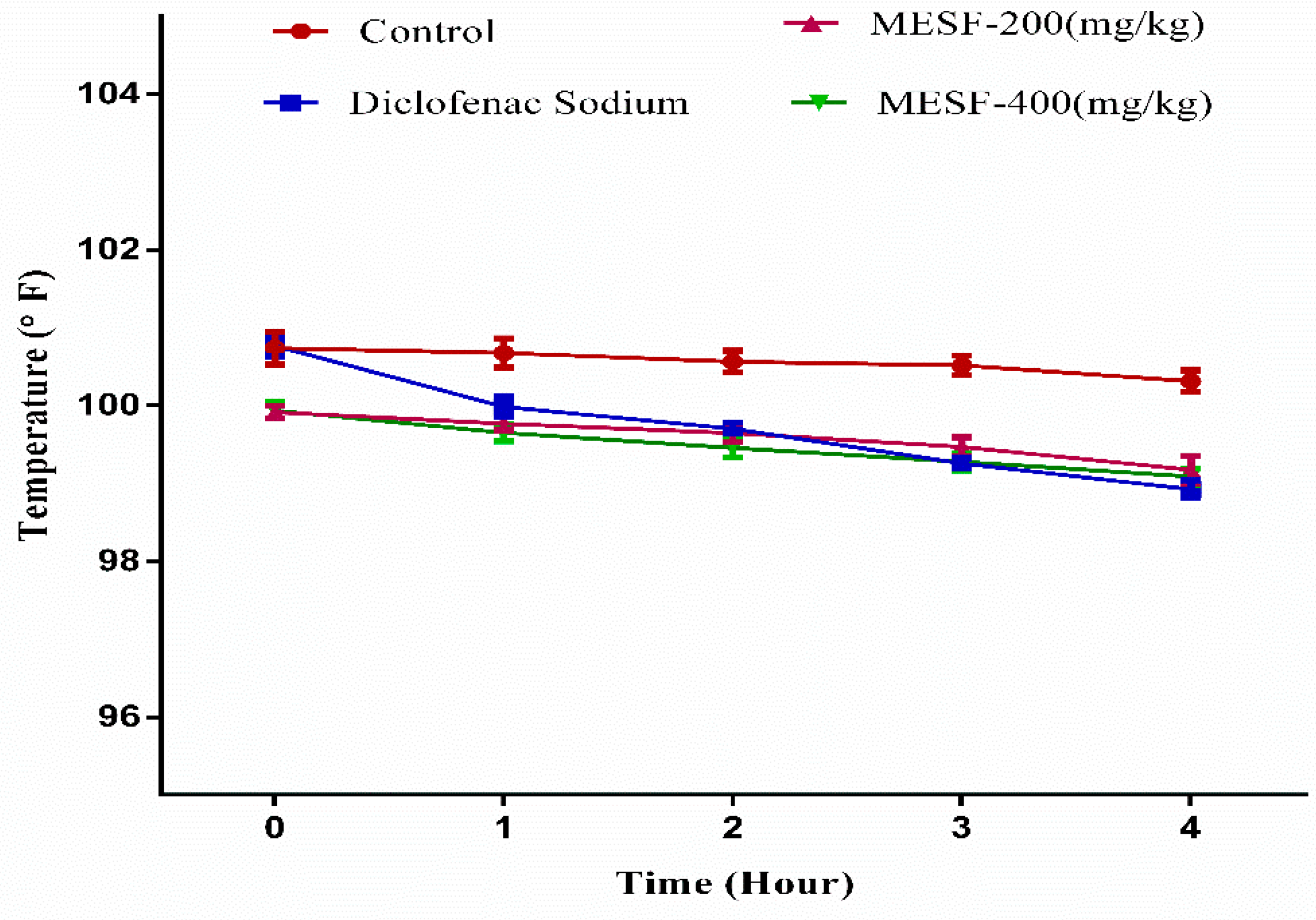
2.7.3. Tail Immersion Test
2.8. Antipyretic Test by Brewer’s Yeast-Induced Pyrexia
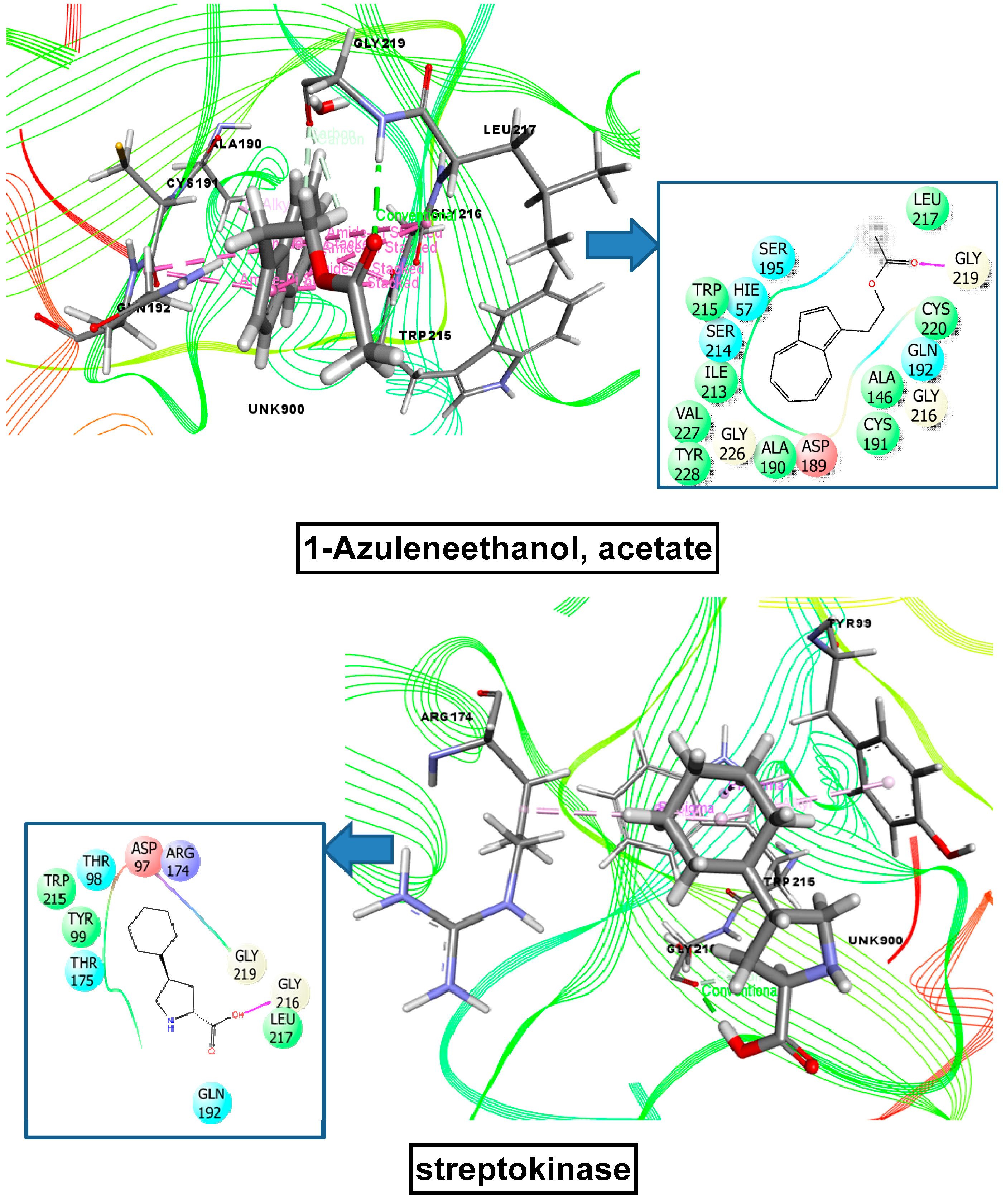
2.9. Molecular Docking Related to Cytotoxic Activity
2.10. Molecular Docking Related to Thrombolytic Activity
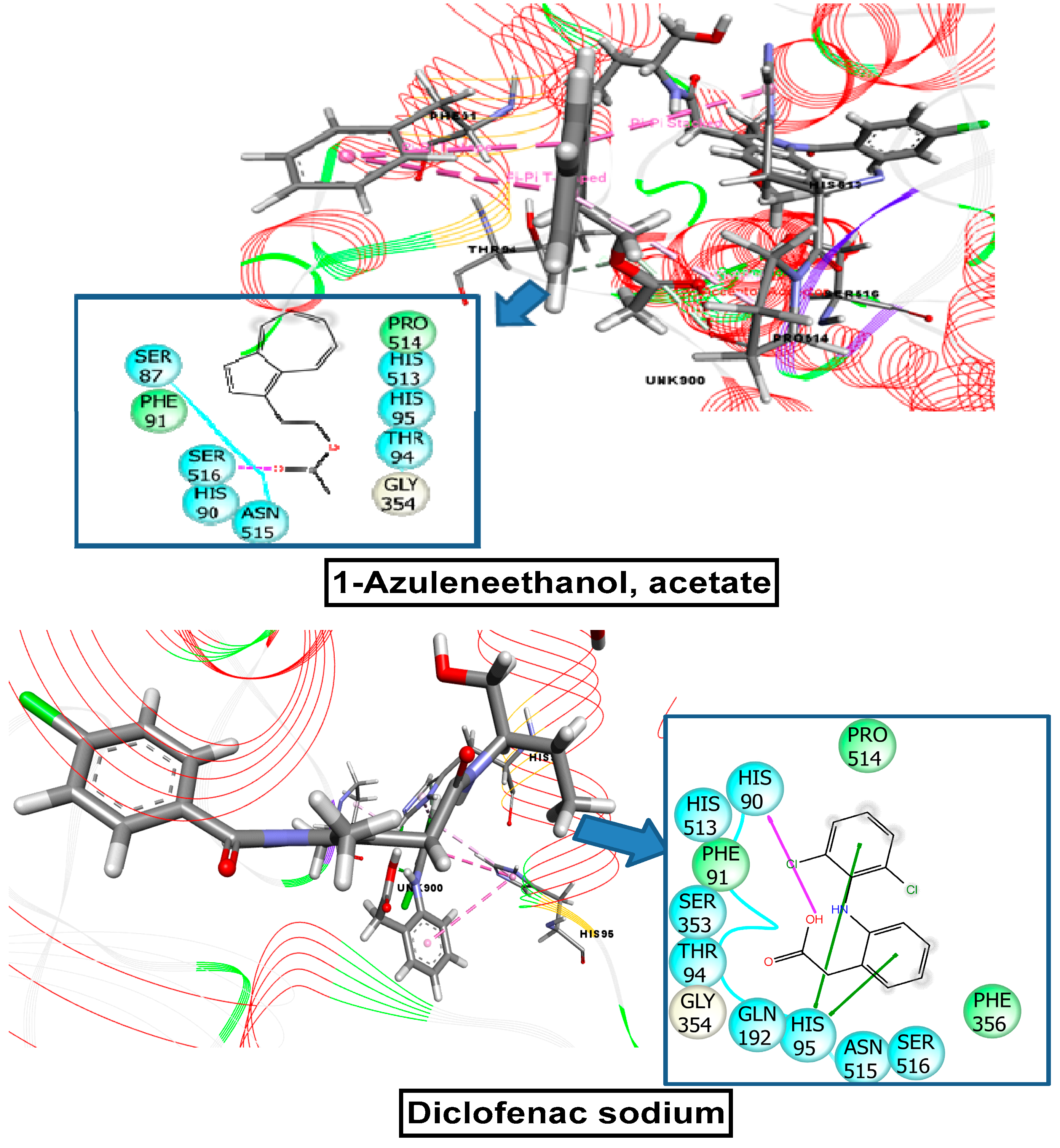
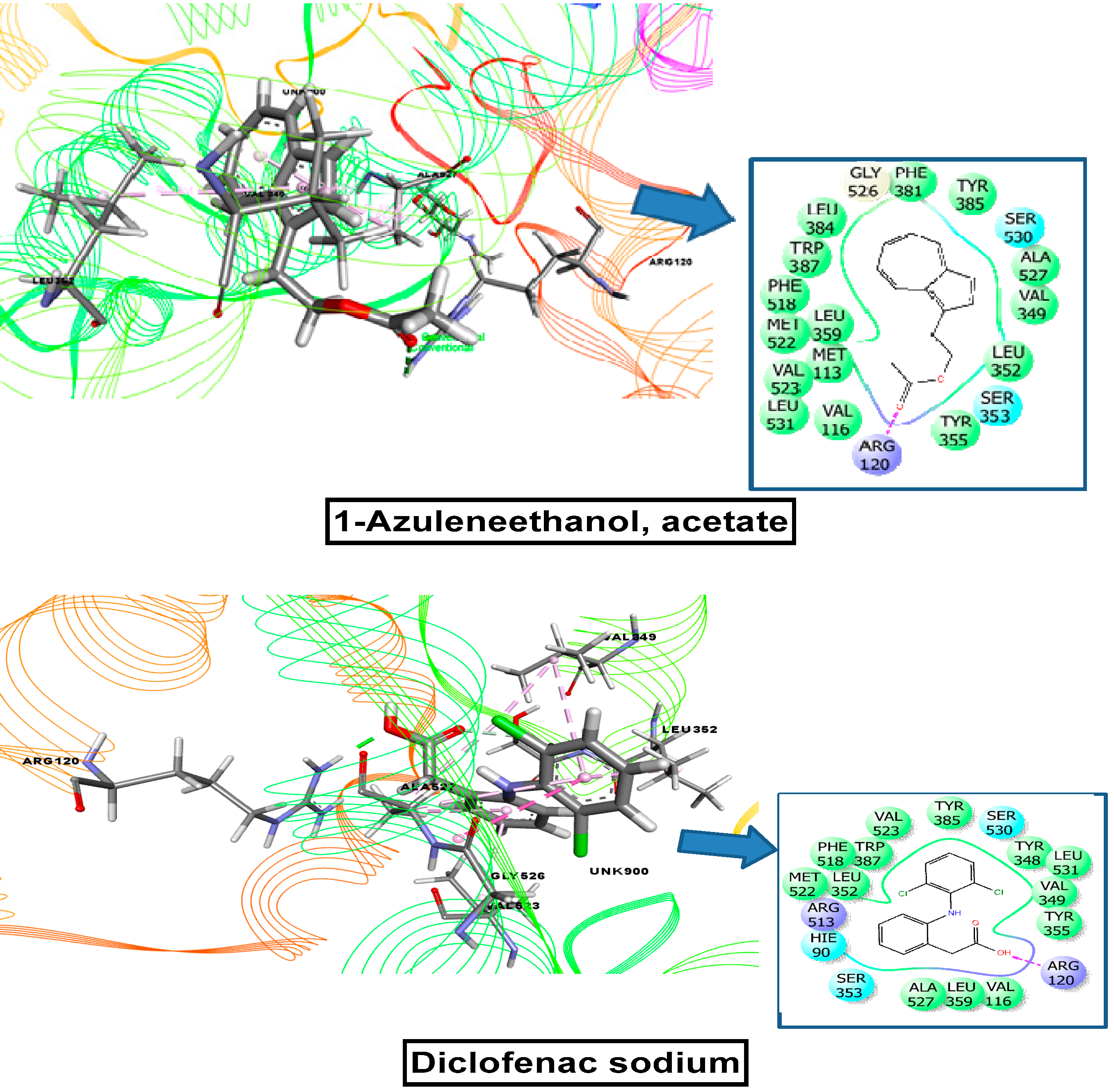
2.11. Molecular Docking Related to Anti-Inflammatory, Analgesic, and Antipyretic Activity
2.12. ADME/T and Toxicological Properties Prediction
2.13. PASS Prediction Study
3. Discussion
4. Materials and Methods
4.1. Plant Collection and Extraction Process
4.2. Chemicals
4.3. Experimental Animals and Ethical Statement
4.4. Qualitative Phytochemicals Analysis
4.5. GC-MS (Gas Chromatography-Mass Spectrometry) Analysis
4.6. Brine Shrimp Lethality Bioassay
4.7. Thrombolytic Activity
4.8. Evaluation of In Vitro Anti-Inflammatory Activity
4.8.1. Inhibition of Protein Denaturation
4.8.2. Egg Albumin Denaturation Method
4.9. Acute Toxicity Test
4.10. Analgesic Activity
4.10.1. Acetic Acid-Induced Writhing Inhibition Test
4.10.2. Formalin-Induced Paw-Licking Test
4.10.3. Tail Immersion Test
4.11. Antipyretic Activity Assay Using Brewer’s Yeast-Induced Pyrexia
4.12. Compounds Selection for the Computational Analysis
4.13. Molecular Docking Analysis
4.14. ADME Analysis and Toxicological Property Prediction
4.15. PASS Prediction
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef]
- Mukul, S.; Uddin, M.; Mr, T. Medicinal plant diversity and local healthcare among the people living in and around a conservation area of Northern Bangladesh. Int. J. For. Usufructs Manag. 2007, 8, 50–63. [Google Scholar]
- Haque, M.; Chowdhury, A.B.M.; Shahjahan, M.; Harun, G.D. Traditional healing practices in rural Bangladesh: A qualitative investigation. BMC Complement. Altern. Med. 2018, 18, 62. [Google Scholar] [CrossRef]
- Rahman, M.O.; Hassan, M.A.; Mia, M.M.; Huq, A.M. A synoptical account of the Sterculiaceae in Bangladesh. Bangladesh J. Plant Taxon. 2012, 19, 63–78. [Google Scholar] [CrossRef]
- Uddin, I.; Rubina, N.S.; Mubeen, M.A.; Kiran, N.; Vijay, P.; Butool, A. Phytochemical Screening and in-vitro Anti-Inflammatory Activity of Methanolic Extract of Sterculia foetida L. IOSR J. Pharm. Biol. Sci. 2016, 11, 28–34. [Google Scholar]
- Jafri, A.; Bano, S.; Rais, J.; Khan, F.; Shivnath, N.; Sharma, A.K.; Arshad, M. Phytochemical screening of Sterculia foetida seed extract for anti-oxidant, anti-microbial activity, and detection of apoptosis through reactive oxygen species (ROS) generation, mitochondrial membrane potential (MMP) decrease, and nuclear fragmentation in human osteosarcoma cells. J. Histotechnol. 2019, 42, 68–79. [Google Scholar]
- Kavitha, M.; Vadivu, R.; Radha, R. A Review on Sterculia foetida Linn. Res. J. Pharm. Phytochem. 2015, 7, 239. [Google Scholar]
- Singh, S.P.; Vidyasagar, G.M. In-vitro antidermatophytic activity of low polar petroleum ether and inter polar methanolic seed extracts of Sterculia foetida L. Int. J. Pharma. Bio. Sci. 2014, 5, 872–879. [Google Scholar]
- Sharma, H.; Garg, M. A review of traditional use, phytoconstituents and biological activities of Himalayan yew, Taxus wallichiana. J. Integr. Med. 2015, 13, 80–90. [Google Scholar] [CrossRef]
- Amuthavalli, A.; Ramesh, T. Phytochemical, Fluorescence and GC-MS Analysis of Methanolic Extract of Sterculia foetida L. Seeds. Int. J. Environ. Agric. Biotech. 2021, 6, 046-053. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.; McLaughlin, J. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Ozçelik, B.; Kartal, M.; Erdogan Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharma. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Gordanian, B.; Behbahani, M.; Carapetian, J.; Fazilati, M. In vitro evaluation of cytotoxic activity of flower, leaf, stem and root extracts of five Artemisia species. Res. Pharma. Sci. 2014, 9, 91–96. [Google Scholar]
- Nobori, T.; Miura, K.; Wu, D.J.; Lois, A.; Takabayashi, K.; Carson, D.A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature 1994, 368, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Ratnasooriya, W.; Fernando, T.; Madubashini, P. In vitro thrombolytic activity of Sri Lankan black tea, Camellia sinensis (L.) O. Kuntze. J. Natn. Sci. Found. Sri. Lanka 2009, 36, 179–181. [Google Scholar] [CrossRef]
- Joshipura, K.; Ascherio, A.; Manson, J.; Stampfer, M.; Rimm, E.; Speizer, F.; Hennekens, C.; Spiegelman, D.; Willett, W. Fruit and Vegetable Intake in Relation to Risk of Ischemic Stroke. JAMA 1999, 282, 1233–1239. [Google Scholar] [CrossRef]
- Liu, S.; Manson, J.E.; Lee, I.M.; Cole, S.R.; Hennekens, C.H.; Willett, W.C.; Buring, J.E. Fruit and vegetable intake and risk of cardiovascular disease: The Women’s Health Study. Am. J. Clin. Nutr. 2000, 72, 922–928. [Google Scholar] [CrossRef]
- Prasad, S.B.; Yashwant, A.V. In vitro anti-inflammatory activity of Raupya (Silver) Bhasma. J. Chem. Pharma. Res. 2013, 5, 194–197. [Google Scholar]
- Khan, R. Study of analgesic and anti-inflammatory activity from plant extracts of Lactuca scariola and Artemisia absinthium. J. Islamic. Acad. Sci. 1992, 5, 111–114. [Google Scholar]
- Gell, P.; Benacerraf, B. Studies on hypersensitivity. II. Delayed hypersensitivity to denatured proteins in guinea pigs. Immunology 1959, 2, 64–70. [Google Scholar]
- Uddin Chy, M.N.; Adnan, M.; Chowdhury, M.R.; Pagano, E.; Kamal, A.T.M.M.; Oh, K.K.; Cho, D.H.; Capasso, R. Central and peripheral pain intervention by Ophiorrhiza rugosa leaves: Potential underlying mechanisms and insight into the role of pain modulators. J. Ethnopharmacol. 2021, 276, 114182. [Google Scholar] [CrossRef]
- Insel, P.A. Analgesic-Antipyretic and Antiinflammatory Agents and Drugs Employed in the Treatment of Gout. Pharmacol. Basic Ther. 1996, 617–657. [Google Scholar]
- Omar, F.; Tareq, A.M.; Alqahtani, A.M.; Dhama, K.; Sayeed, M.A.; Emran, T.B.; Simal-Gandara, J. Plant-based Indole Alkaloids: A Comprehensive Overview from a Pharmacological Perspective. Molecules 2021, 26, 2297. [Google Scholar] [CrossRef]
- Calixto, J.; Beirith, A.; Ferreira, J.; Santos, A.; Cechinel Filho, V.; Yunes, R. Naturally occurring antinociceptive substances from plants. Phytother. Res. 2000, 14, 401–418. [Google Scholar] [CrossRef]
- Rakib, A.; Ahmed, S.; Islam, M.A.; Uddin, M.M.N.; Paul, A.; Chy, M.N.U.; Emran, T.B.; Seidel, V. Pharmacological studies on the antinociceptive, anxiolytic and antidepressant activity of Tinospora crispa. Phytother. Res. 2020, 34, 2978–2984. [Google Scholar] [CrossRef] [PubMed]
- Ikeda-Matsuo, Y.; Ueno, A.; Naraba, H.; Oh-ishi, S. Involvement of vanilloid receptor TRPV1 and prostanoids in the acid-induced writhing responses of mice. Life Sci. 2001, 69, 2911–2919. [Google Scholar] [CrossRef]
- Bley, K.; Hunter, J.; Eglen, R.; Smith, J.A.M. The role of IP prostanoid receptors in inflammatory pain. Trends Pharm. Sci. 1998, 19, 141–147. [Google Scholar] [CrossRef]
- Silva, K.; Gritsenko, K.; Wahezi, S. Animal Models of Nociception and Pain; Springer: Cham, Switzerland, 2017; pp. 23–24. [Google Scholar]
- Parada, C.A.; Tambeli, C.H.; Cunha, F.Q.; Ferreira, S.H. The major role of peripheral release of histamine and 5-hydroxytryptamine in formalin-induced nociception. Neuroscience 2001, 102, 937–944. [Google Scholar] [CrossRef]
- Uddin, M.Z.; Rana, M.S.; Hossain, S.; Dutta, E.; Ferdous, S.; Dutta, M.; Emran, T.B. In vivo neuroprotective, antinociceptive, anti-inflammatory potential in Swiss albino mice and in vitro antioxidant and clot lysis activities of fractionated Holigarna longifolia Roxb. bark extract. J. Complement. Integr. Med. 2019, 17, 1–10. [Google Scholar] [CrossRef]
- Takahashi, R.N.; de Lima, T.C.; Morato, G.S. Pharmacological actions of tannic acid; II. Evaluation of CNS activity in animals. Planta Med. 1986, 31, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Starec, M.; Waitzová, D.; Elis, J. Evaluation of the analgesic effect of RG-tannin using the “hot plate”and“tailflick”method in mice. Ceskoslovenská Farm. 1988, 37, 319–321. [Google Scholar]
- Bone, K.; Mills, S. Principles and Practice of Phytotherapy: Modern Herbal Medicine; Elsevier: New York, NY, USA, 2012; pp. 1–1051. [Google Scholar]
- Morteza-Semnani, K.; Mahmoudi, M.; Heidar, M.R. Analgesic activity of the methanol extract and total alkaloids of Glaucium paucilobum. Methods Find. Exp. Clin. Pharm. 2006, 28, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Emran, T.B.; Dash, R.; Uddin, M.M.N.; Rahman, M.A. Sedative, anxiolytic, antinociceptive, anti-inflammatory and antipyretic effects of a chloroform extract from the leaves of Urena sinuata (Borss) L. in rodents. J. Appl. Life Sci. Int. 2018, 16, 1–19. [Google Scholar] [CrossRef]
- Jinsmaa, Y.; Fujita, Y.; Shiotani, K.; Miyazaki, A.; Li, T.; Tsuda, Y.; Okada, Y.; Ambo, A.; Sasaki, Y.; Bryant, S.D.; et al. Differentiation of opioid receptor preference by [Dmt1]endomorphin-2-mediated antinociception in the mouse. Eur. J. Pharm. 2005, 509, 37–42. [Google Scholar] [CrossRef]
- Jinsmaa, Y.; Okada, Y.; Tsuda, Y.; Shiotani, K.; Sasaki, Y.; Ambo, A.; Bryant, S.D.; Lazarus, L.H. Novel 2′,6′-Dimethyl-Tyrosine-Containing Pyrazinone Opioid Mimetic μ-Agonists with Potent Antinociceptive Activity in Mice. J. Pharm. Exp. Ther. 2004, 309, 432–438. [Google Scholar] [CrossRef]
- Uddin, M.M.N.; Ahmed, S.; Kabir, M.S.H.; Rahman, M.S.; Sultan, R.A.; Emran, T.B. In vivo analgesic, anti-inflammatory potential in Swiss albino mice and in vitro thrombolytic activity of hydroalcoholic fruits extract from Daemonorops robusta Warb. J. Appl. Pharma. Sci. 2017, 7, 104–113. [Google Scholar] [CrossRef]
- Goda, Y.; Kiuchi, F.; Shibuya, M.; Sankawa, U. Inhibitors of prostaglandin biosynthesis from Dalbergia odorifera. Chem. Pharma. Bull. 1992, 40, 2452–2457. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rakib, A.; Ahmed, S.; Islam, M.A.; Haye, A.; Uddin, S.N.; Uddin, M.M.N.; Hossain, M.K.; Paul, A.; Emran, T.B. Antipyretic and hepatoprotective potential of Tinospora crispa and investigation of possible lead compounds through in silico approaches. Food Sci. Nutr. 2020, 8, 547–556. [Google Scholar] [CrossRef]
- Khan, S.; Nazir, M.; Raiz, N.; Saleem, M.; Zengin, G.; Fazal, G.; Saleem, H.; Mukhtar, M.; Tousif, M.I.; Tareen, R.B.; et al. Phytochemical profiling, in vitro biological properties and in silico studies on Caragana ambigua stocks (Fabaceae): A comprehensive approach. Ind. Crop. Prod. 2019, 131, 117–124. [Google Scholar] [CrossRef]
- Harbone, J.B. Phytochemical methods, a guide to modern techniques of plants analysis. Comp. Biochem. Flavonoids 1973, 221–222. [Google Scholar] [CrossRef]
- Al Mahmud, Z.; Qais, N.; Bachar, S.C.; Hasan, C.M.; Emran, T.B.; Uddin, M.M.N. Phytochemical investigations and antioxidant potential of leaf of Leea macrophylla (Roxb.). BMC Res. Notes 2017, 10, 245. [Google Scholar] [CrossRef]
- Rahman, J.; Tareq, A.M.; Hossain, M.M.; Sakib, S.A.; Islam, M.N.; Uddin, A.B.M.N.; Hoque, M.; Nasrin, M.S.; Ali, M.H.; Caiazzo, E.; et al. Biological evaluation, DFT calculations and molecular docking studies on the antidepressant and cytotoxicity activities of Cycas pectinata Buch.-Ham. Compounds. Pharmaceuticals 2020, 13, 232. [Google Scholar] [CrossRef]
- Prasad, S.; Kashyap, R.; Deopujari, J.; Purohit, H.; Taori, G.; Daginawala, H. Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thromb. J. 2006, 4, 14. [Google Scholar] [CrossRef]
- Emran, T.B.; Rahman, M.A.; Uddin, M.M.N.; Rahman, M.M.; Uddin, M.Z.; Dash, R.; Layzu, C. Effects of organic extracts and their different fractions of five Bangladeshi plants on in vitro thrombolysis. BMC Complement. Altern. Med. 2015, 15, 128. [Google Scholar] [CrossRef]
- Barua, N.; Ibn Aziz, M.A.; Tareq, A.M.; Sayeed, M.A.; Alam, N.; Alam, N.U.; Uddin, M.A.; Lyzu, C.; Emran, T.B. In vivo and in vitro evaluation of pharmacological activities of Adenia trilobata (Roxb.). Biochem. Biophys. Rep. 2020, 23, 100772. [Google Scholar] [CrossRef]
- Mizushima, Y.; Kobayashi, M. Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J. Pharm. Pharm. 1968, 20, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Jahan, I.; Tona, M.R.; Sharmin, S.; Sayeed, M.A.; Tania, F.Z.; Paul, A.; Chy, M.; Uddin, N.; Rakib, A.; Emran, T.B. GC-MS phytochemical profiling, pharmacological properties, and in silico studies of Chukrasia velutina leaves: A novel source for bioactive agents. Molecules 2020, 25, 3536. [Google Scholar] [CrossRef]
- Bristy, T.A.; Barua, N.; Tareq, A.M.; Sakib, S.A.; Etu, S.T.; Chowdhury, K.H.; Jyoti, M.A.; Aziz, M.; Ibn, A.; Reza, A. Deciphering the pharmacological properties of methanol extract of Psychotria calocarpa leaves by in vivo, in vitro and in silico approaches. Pharmaceuticals 2020, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Yesmin, S.; Paul, A.; Naz, T.; Rahman, A.B.M.A.; Akhter, S.F.; Wahed, M.I.I.; Emran, T.B.; Siddiqui, S.A. Membrane stabilization as a mechanism of the anti-inflammatory activity of ethanolic root extract of Choi (Piper chaba). Clin. Phytosci. 2020, 6, 59. [Google Scholar] [CrossRef]
- Taur, D.D.; Waghmare, M.; Bandal, R.; Patil, R. Antinociceptive activity of Ricinus communis L. leaves. Asian Pac. J. Trop. Biomed. 2011, 1, 139–141. [Google Scholar] [CrossRef]
- Ahmed, S.; Rakib, A.; Islam, M.; Khanam, H.; Faiz, F.; Paul, A.; Chy, M.N.; Bhuiya, N.M.M.; Uddin, M.M.N.; Ullah, S.M.; et al. In vivo and in vitro pharmacological activities of Tacca integrifolia rhizome and investigation of possible lead compounds against breast cancer through in silico approaches. Clin. Phytosci. 2019, 5, 1–13. [Google Scholar] [CrossRef]
- Sakib, S.A.; Tareq, A.M.; Islam, A.; Rakib, A.; Islam, M.N.; Uddin, M.A.; Rahman, M.M.; Emran, T.B.; Seidel, V. Anti-Inflammatory, Thrombolytic and Hair-Growth Promoting Activity of the n-Hexane Fraction of the Methanol Extract of Leea indica Leaves. Plants 2021, 10, 1081. [Google Scholar] [CrossRef]
- Adnan, M.; Nazim Uddin Chy, M.; Mostafa Kamal, A.T.M.; Barlow, J.W.; Faruque, M.O.; Yang, X.; Uddin, S.B. Evaluation of anti-nociceptive and anti-inflammatory activities of the methanol extract of Holigarna caustica (Dennst.) Oken leaves. J. Ethnopharmacol. 2019, 236, 401–411. [Google Scholar] [CrossRef]
- Kale, S.S.; Darade, V.; Thakur, H.A. Analysis of fixed oil from Sterculia foetida linn. Int. J. Pharma. Sci. Res. 2011, 2, 2908. [Google Scholar]
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S.; et al. The Protein Data Bank. Acta Cryst. D Biol. Cryst. 2002, 58, 899–907. [Google Scholar] [CrossRef]
- Shiau, A.K.; Barstad, D.; Loria, P.M.; Cheng, L.; Kushner, P.J.; Agard, D.A.; Greene, G.L. The Structural Basis of Estrogen Receptor/Coactivator Recognition and the Antagonism of This Interaction by Tamoxifen. Cell 1998, 95, 927–937. [Google Scholar] [CrossRef]
- Renatus, M.; Bode, W.; Huber, R.; Stürzebecher, J.; Prasa, D.; Fischer, S.; Kohnert, U.; Stubbs, M.T. Structural mapping of the active site specificity determinants of human tissue-type plasminogen activator. Implications for the design of low molecular weight substrates and inhibitors. J. Biol. Chem. 1997, 272, 21713–21719. [Google Scholar] [CrossRef]
- Harman, C.A.; Turman, M.V.; Kozak, K.R.; Marnett, L.J.; Smith, W.L.; Garavito, R.M. Structural basis of enantioselective inhibition of cyclooxygenase-1 by S-alpha-substituted indomethacin ethanolamides. J. Biol. Chem. 2007, 282, 28096–28105. [Google Scholar] [CrossRef]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Miyashiro, J.M.; Penning, T.D.; Seibert, K.; et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef] [PubMed]
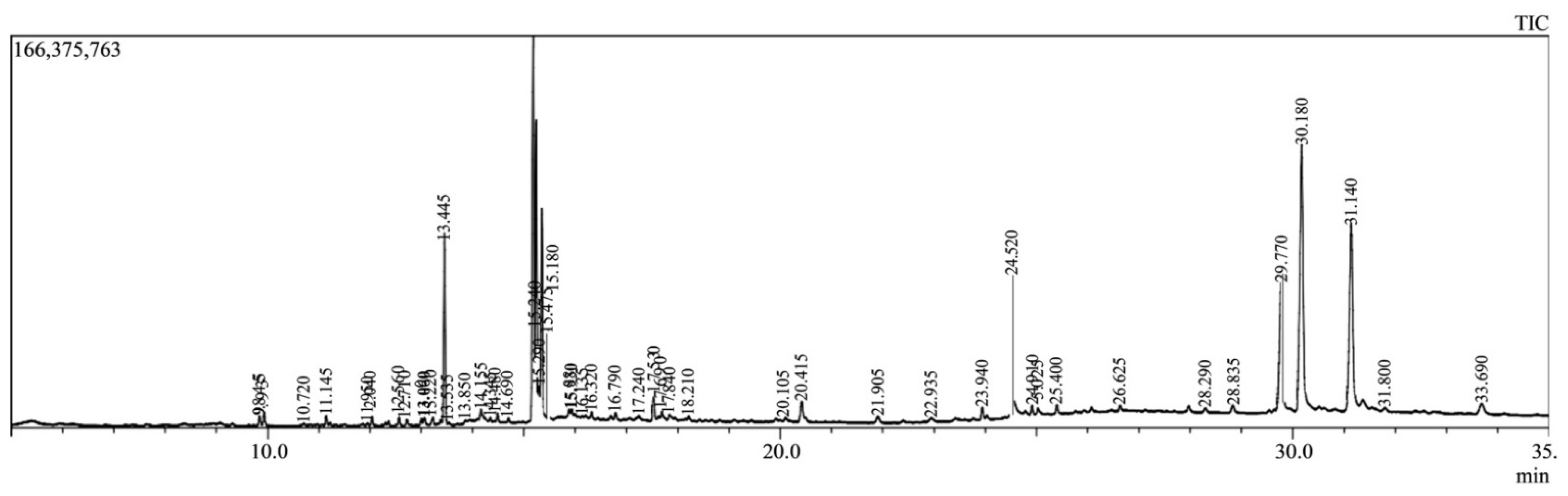

| SL. No. | Compound Name | Retention Time | Chemical Formula |
|---|---|---|---|
| 1. | Acetamiprid | 9.843, 12.039, 13.450, 14.484 and 15.882 | C10H11ClN4 |
| 2. | Halfenprox | 9.843, 12.713, 15.179, 17.529 and 17.841 | C24H23BrF2O3 |
| 3. | Alpha-BHC | 9.843, 13.450, 13.450, 14.161, 15.179, 17.836, 20.417 and 24.525 | C6H6Cl6 |
| 4. | Delta-BHC | 9.843, 11.143, 12.713 and 15.882 | C6H6Cl6 |
| 5. | Beta-BHC | 9.843 | C6H6Cl6 |
| 6. | 9-octadecenoic acid (Z), methyl ester | 9.843 | C21H38O4 |
| 7. | Tralomethrin | 9.927, 12.562, 15.178, 15.178, 16.789 and 29.780 | C22H19Br4NO3 |
| 8. | Tetradecanoic acid, methyl ester | 11.143 | C15H30O2 |
| 9. | Hexadecanoic acid, methyl ester | 11.143 | C17H34O2 |
| 10. | Gamma-BHC | 12.039, 13.450, 15.179, 20.416 and 22.935 | C6H6Cl6 |
| 11. | Terbufos | 12.039, 15.477 and 17.529 | C9H21O2PS3 |
| 12. | Benfuresate | 12.039, 13.450, 14.483, 16.321, 17.530 and 31.146 | C12H16O4S |
| 13. | Dichlofluanid | 13.450, 14.162, 17.529, 17.530 and 22.935, | C9H11Cl2FN2O2S2 |
| 14. | DEP (Trichlorfon) | 13.449, 15.179, 15.882 and 20.417 | C4H8Cl3O4P |
| 15. | Sterculic acid | 14.483 | C19H34O2 |
| 16. | 1-azuleneethanol, acetate | 14.484 | C14H14O2 |
| 17. | Captan | 15.178 | C9H8Cl3NO2S |
| 18. | Etridiazole | 15.882 | C5H5Cl3N2OS |
| 19. | Diethofencarb | 17.841 and 28.319 | C14H21NO4 |
| 20. | p, p′-DDT | 21.907 | C14H9Cl5 |
| 21. | Etobennzanid | 23.940 | |
| 22. | Cyfluthrin | 24.526 and 25.404 | C22H18Cl2FNO3 |
| 23. | Cypermethrin | 24.526 and 25.404 | C22H19Cl2NO3 |
| 24. | Pendimethalin | 26.624 | C13H19N3O4 |
| 25. | CNP | 28.932 | C93H157N27O28S3 |
| 26. | Kresoxim-methyl | 29.780 and 30.179 | C18H19NO4 |
| 27. | Tetraconazole | 29.780 and 30.179 | C13H11Cl2F4N3O |
| 28. | Pyributicarb | 29.780 and 30.179 | C18H22N2O2S |
| 29. | Permethrin | 25.404, 26.624, 32.125 and 33.460 | C21H20Cl2O3 |
| Treatment | Dose (mg/kg) | Response Times (s) (% MPE) | ||||
|---|---|---|---|---|---|---|
| Pretreatment | 30 min | 60 min | 90 min | 120 min | ||
| Control | 3.24 ± 0.26 | 3.95 ± 0.12 | 3.62 ± 0.12 | 3.25 ± 0.23 | 2.89 ± 0.14 | |
| Diclofenacsodium | 10 | 3.72 ± 0.11 | 6.67 ± 0.32 c (18.10) | 8.66 ± 0.25 c (30.32) | 7.33 ± 0.21 c (22.20) | 6.88 ± 0.26 c (19.37) |
| MESF | 200 | 3.57 ± 0.24 | 5.02 ± 0.16 b (8.81) | 5.44 ± 0.08 c (11.40) | 5.89 ± 0.16 c (14.14) | 5.42 ± 0.14 c (11.27) |
| 400 | 3.30 ± 0.08 | 5.82 ± 0.18 c (15.14) | 6.83 ± 0.12 c (21.14) | 6.42 ± 0.11 c (18.70) | 6.0 9± 0.12 c (16.72) | |
| Compounds Name | CID Number | Docking Score (kcal/mol) | |||
|---|---|---|---|---|---|
| Cytotoxic (3ERT) | Thrombolytic (1A5H) | COX1 (2OYE) | COX2 (6COX) | ||
| 1-azuleneethanol, acetate | 588184 | −7.215 | −5.836 | −5.000 | −7.602 |
| 7,10-octadecadienoic acid, methyl ester | 549028 | −2.482 | −0.945 | 0.420 | −1.617 |
| 9-octadecenoic acid (Z), methyl ester | 5354176 | - | - | - | - |
| Heptadecanoic acid, 14-methyl, methyl ester | 17219 | - | 1.04 | 2.359 | - |
| Hexadecanoic acid, methyl ester | 8181 | −0.166 | −0.761 | 0.864 | −0.943 |
| Sterculic acid | 12921 | −2.759 | −1.612 | 0.525 | −3.946 |
| Tetradecanoic acid, methyl ester | 31284 | −0.055 | 0.686 | 0.527 | −1.018 |
| Standard drug (Vincristine sulfate, Streptokinase, Diclofenac sodium) | −7.059 | −4.533 | −4.590 | −7.260 | |
| Compounds | MW | HBA | HBD | LogP | AMR | nRB | TPSA | Lipinski’s Violations |
|---|---|---|---|---|---|---|---|---|
| Rules | <500 | <5 | ≤10 | ≤5 | 40–130 | ≤10 | ≤140 | ≤1 |
| 1-azuleneethanol, acetate | 214.26 g/mol | 2 | 0 | 2.95 | 63.74 | 4 | 26.30 Å2 | 0 |
| 7, 10-octadecadienoic acid, methyl ester | 294.47 g/mol | 2 | 0 | 5.68 | 93.78 | 15 | 26.30 Å2 | 1 |
| 9-octadecenoic acid (Z), methyl ester | 354.52 g/mol | 4 | 0 | 5.74 | 105.16 | 18 | 52.60 Å2 | 1 |
| Heptadecanoic acid, 14-methyl, methyl ester | 298.50 g/mol | 2 | 0 | 6.21 | 94.73 | 16 | 26.30 Å2 | 1 |
| Hexadecanoic acid, methyl ester | 270.45 g/mol | 2 | 0 | 5.54 | 85.12 | 15 | 26.30 Å2 | 1 |
| Sterculic acid | 294.47 g/mol | 2 | 1 | 5.42 | 92.63 | 15 | 37.30 Å2 | 1 |
| Tetradecanoic acid, methyl ester | 242.40 g/mol | 2 | 0 | 4.81 | 75.50 | 13 | 26.30 Å2 | 0 |
| Compounds | Ames Toxicity | Carcinogens | Acute Oral Toxicity | Rat Acute Toxicity |
|---|---|---|---|---|
| 1-azuleneethanol, acetate | AT | NC | III | 1.8435 |
| 7,10-octadecadienoic acid, methyl ester | NAT | C | III | 1.7357 |
| 9-octadecenoic acid (Z), methyl ester | NAT | NC | III | 1.6684 |
| Heptadecanoic acid, 14-methyl, methyl ester | NAT | C | III | 1.5877 |
| Hexadecanoic acid, methyl ester | NAT | C | III | 1.4915 |
| Sterculic acid | NAT | C | IV | 1.5128 |
| Tetradecanoic acid, methyl ester | NAT | C | III | 1.4915 |
| Compounds | Biological Activities Predicted by Pass Online | Pa | Pi |
|---|---|---|---|
| 1-azuleneethanol, acetate | Anti-inflammatory Anti-hypoxic Antipyretic Antithrombotic Antinociceptive Anti-seborrheic | 0.517 0.692 0.366 0.413 0.414 0.812 | 0.052 0.008 0.030 0.048 0.099 0.017 |
| 7, 10-octadecadienoic acid, methyl ester | Anti-inflammatory Anti-eczematic Anti-secretoric Antithrombotic Antinociceptive Antipyretic | 0.728 0.953 0.781 0.706 0.593 0.255 | 0.013 0.002 0.005 0.008 0.008 0.068 |
| 9-octadecenoic acid (Z), methyl ester | Anti-inflammatory Hypolipemic Antipruritic Antithrombotic Antinociceptive Antipyretic | 0.768 0.767 0.694 0.754 0.449 0.270 | 0.009 0.009 0.008 0.005 0.072 0.059 |
| Heptadecanoic acid, 14-methyl, methyl ester | Anti-inflammatory Anti-secretoric Antipruritic Antithrombotic Antinociceptive Acetylesterase inhibitor | 0.444 0.736 0.558 0.629 0.487 0.790 | 0.075 0.007 0.023 0.013 0.044 0.005 |
| Hexadecanoic acid, methyl ester | Anti-inflammatory Anti-hypoxic Antipruritic Antithrombotic Antinociceptive Antiparasitic | 0.758 0.620 0.566 0.587 0.538 0.429 | 0.002 0.016 0.021 0.017 0.019 0.025 |
| Sterculic acid | Anti-inflammatory Antipyretic Anti-secretoric Antithrombotic Antinociceptive Anti-eczematic | 0.558 0.349 0.785 0.576 0.436 0.866 | 0.004 0.033 0.005 0.018 0.081 0.008 |
| Tetradecanoic acid, methyl ester | Anti-inflammatory Anti-eczematic Anti-seborrheic Antithrombotic Antinociceptive Antipyretic | 0.728 0.854 0.777 0.706 0.593 0.323 | 0.013 0.009 0.002 0.008 0.008 0.038 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, N.; Banu, N.; Aziz, M.A.I.; Barua, N.; Ruman, U.; Jahan, I.; Chy, F.J.; Denath, S.; Paul, A.; Chy, M.N.U.; et al. Chemical Profiling, Pharmacological Insights and In Silico Studies of Methanol Seed Extract of Sterculia foetida. Plants 2021, 10, 1135. https://doi.org/10.3390/plants10061135
Alam N, Banu N, Aziz MAI, Barua N, Ruman U, Jahan I, Chy FJ, Denath S, Paul A, Chy MNU, et al. Chemical Profiling, Pharmacological Insights and In Silico Studies of Methanol Seed Extract of Sterculia foetida. Plants. 2021; 10(6):1135. https://doi.org/10.3390/plants10061135
Chicago/Turabian StyleAlam, Najmul, Naureen Banu, Md. Arfin Ibn Aziz, Niloy Barua, Umme Ruman, Israt Jahan, Farhana Jahan Chy, Susmita Denath, Arkajyoti Paul, Md. Nazim Uddin Chy, and et al. 2021. "Chemical Profiling, Pharmacological Insights and In Silico Studies of Methanol Seed Extract of Sterculia foetida" Plants 10, no. 6: 1135. https://doi.org/10.3390/plants10061135
APA StyleAlam, N., Banu, N., Aziz, M. A. I., Barua, N., Ruman, U., Jahan, I., Chy, F. J., Denath, S., Paul, A., Chy, M. N. U., Sayeed, M. A., Emran, T. B., & Simal-Gandara, J. (2021). Chemical Profiling, Pharmacological Insights and In Silico Studies of Methanol Seed Extract of Sterculia foetida. Plants, 10(6), 1135. https://doi.org/10.3390/plants10061135