Native Amazonian Canga Grasses Show Distinct Nitrogen Growth Responses in Iron Mining Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Propagation
2.2. Plant Growth Conditions
2.2.1. Substrate Analysis and Preparation
2.2.2. Environmental Conditions
2.3. Plant Measurements
2.3.1. Tillering Rate, Biomass, and Nutrient Partitioning
2.3.2. Gas Exchanges
2.3.3. Pigment Contents
2.3.4. Stomatal Frequency and Size
2.4. Data Analysis
3. Results
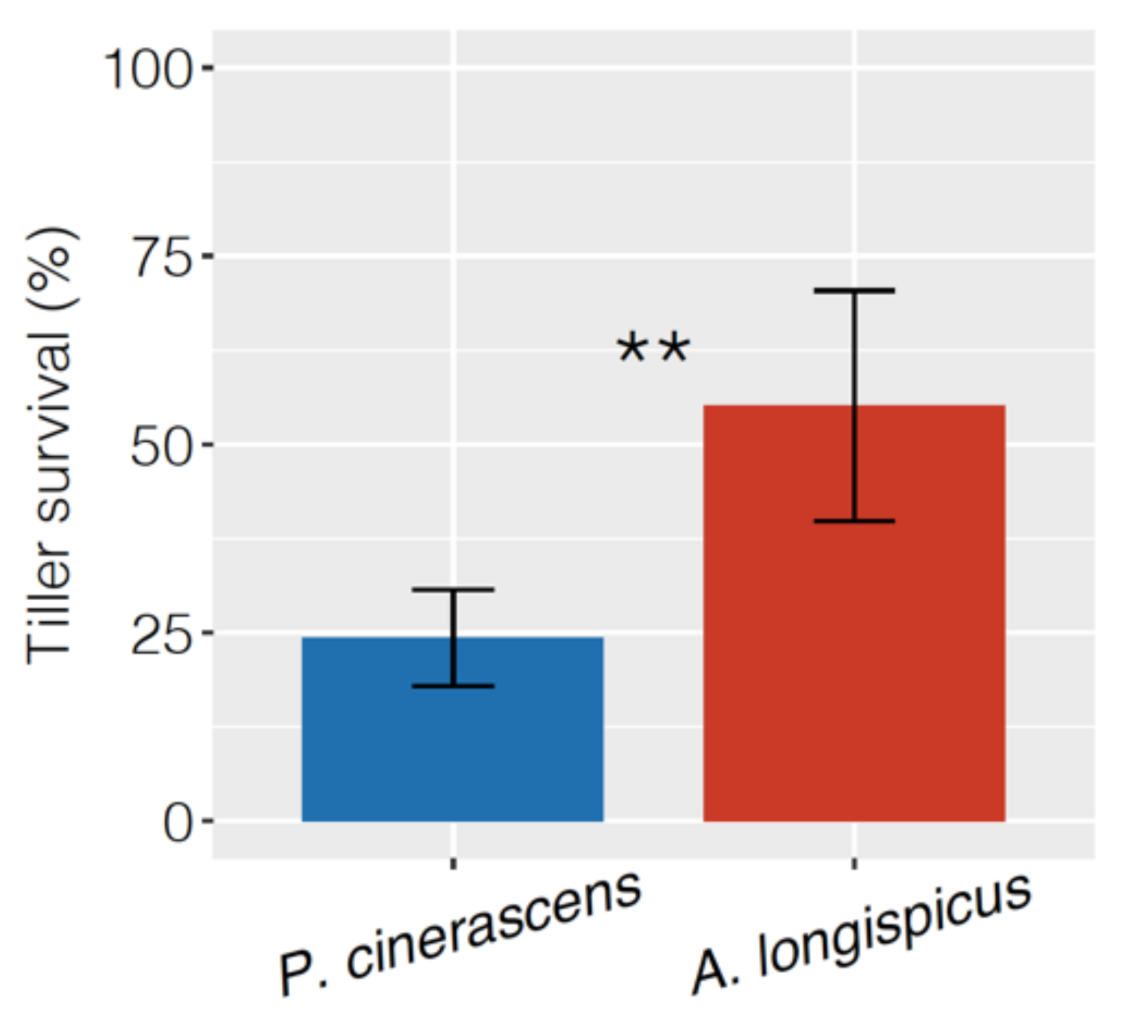
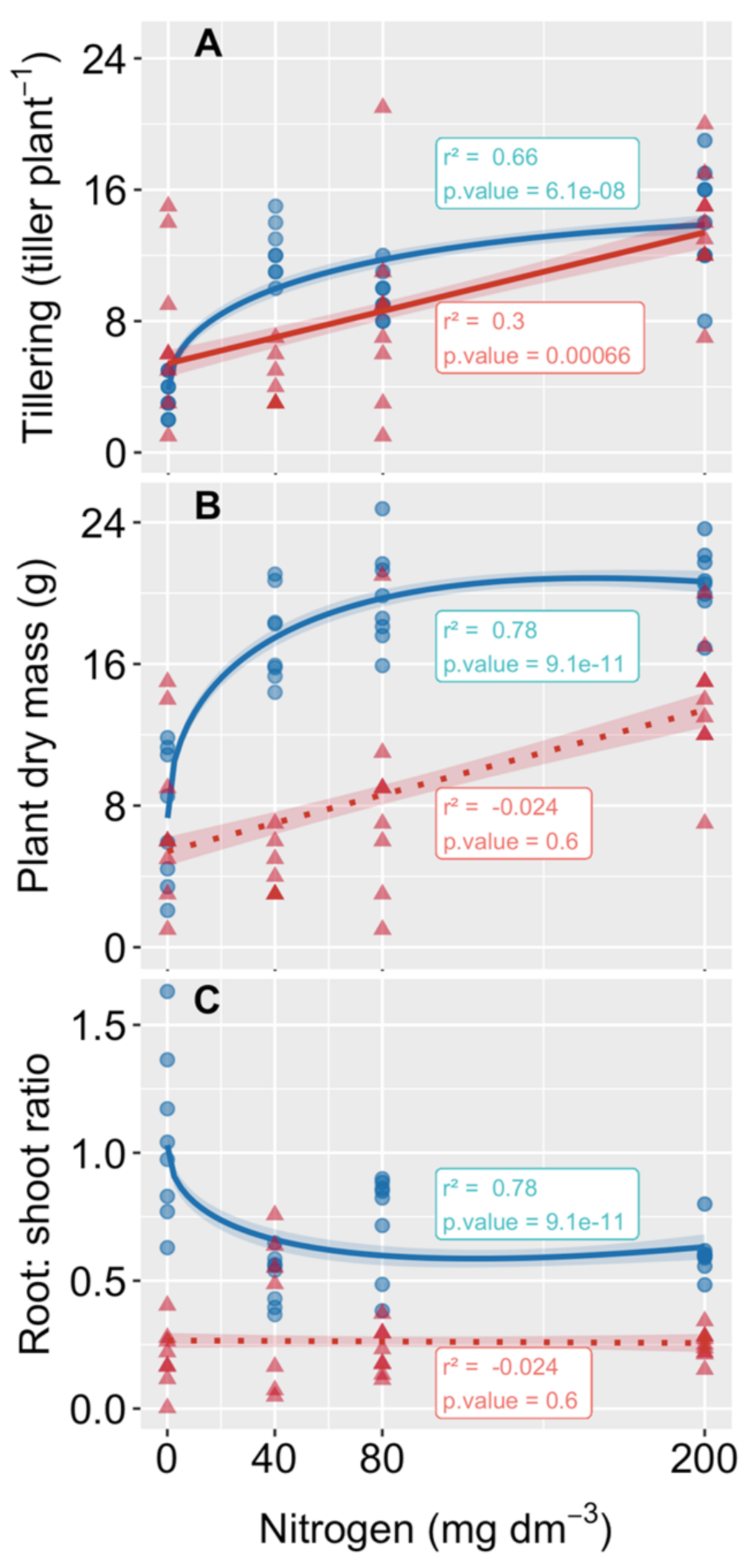
3.1. Plant Propagation and Growth
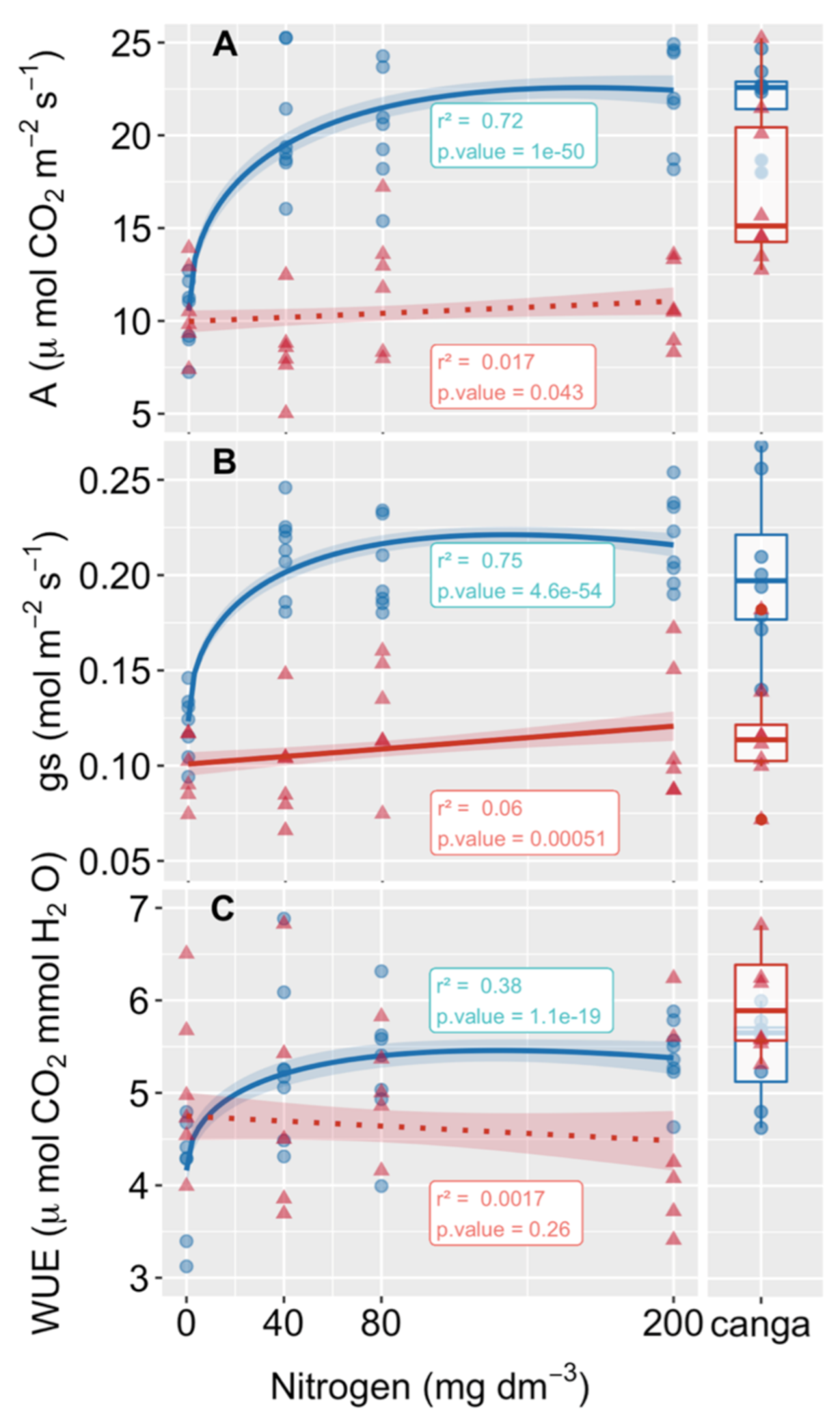
3.2. Gas Exchanges and Pigment Contents
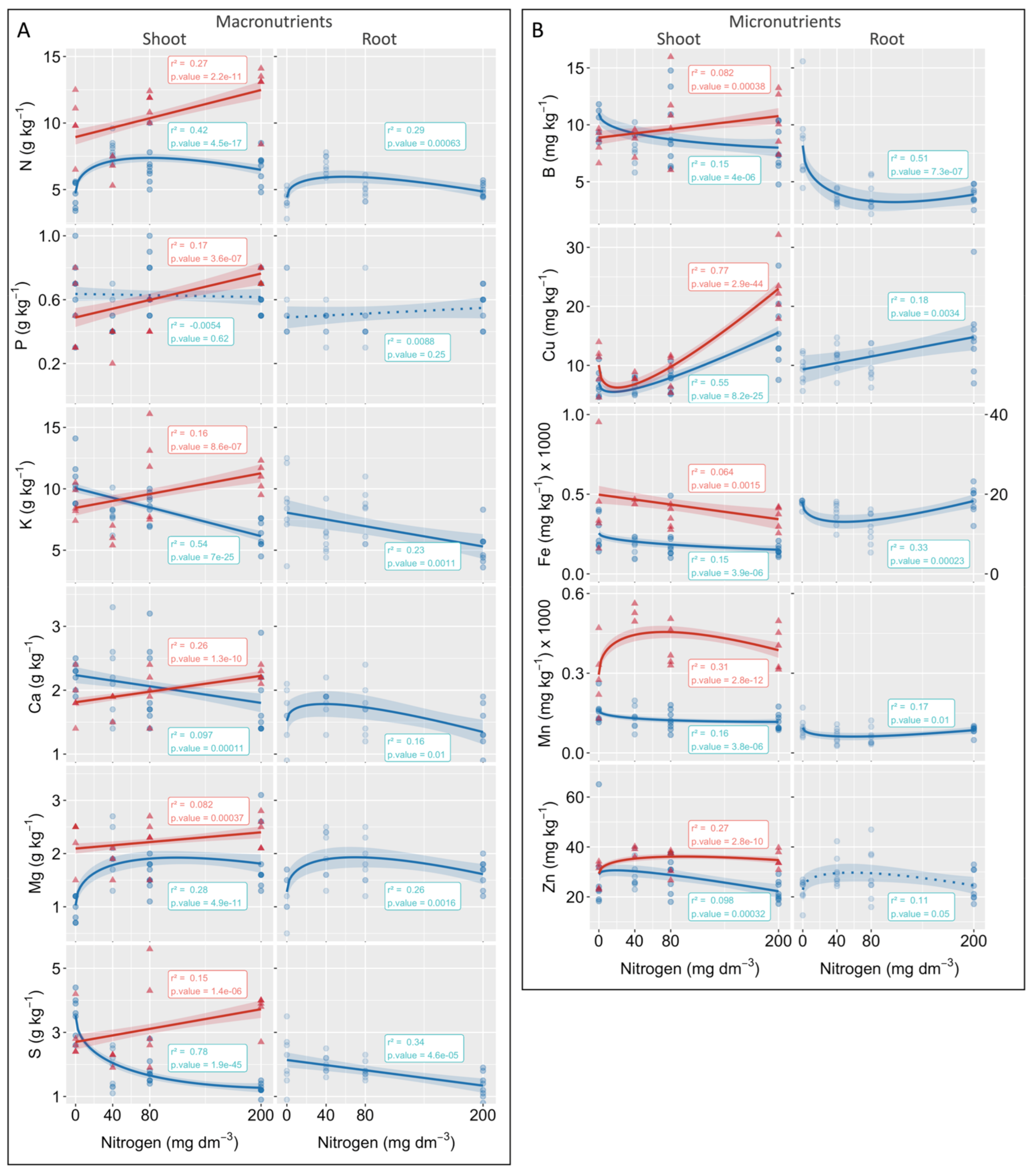
3.3. Root and Shoot Nutrient Contents
3.4. Stomatal Frequency and Size
4. Discussion
4.1. The Tillering Rates of Both Species Increased with N Addition, but Only P. cinerascens Plants Increased in Biomass
4.2. Biomass Gain Was a Consequence of Enhancing Carbon Assimilation Mediated by Leaf Pigment and Nutrient Absorption
4.3. Higher WUE Benefits Plants Growing in Water-Limiting Conditions, Such as Those Found in Areas Requiring Rehabilitation
4.4. The Iron Exclusion Behavior Reveals an Important Tolerance Mechanism Which Would Be Helpful in the Rehabilitation of Iron Mining Areas
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fields-Johnson, C.W.; Zipper, C.E.; Burger, J.A.; Evans, D.M. Forest Restoration on Steep Slopes after Coal Surface Mining in Appalachian USA: Soil Grading and Seeding Effects. For. Ecol. Manag. 2012, 270, 126–134. [Google Scholar] [CrossRef]
- Gastauer, M.; Caldeira, C.F.; Ramos, S.J.; Silva, D.F.; Siqueira, J.O. Active Rehabilitation of Amazonian Sand Mines Converges Soils, Plant Communities and Environmental Status to Their Predisturbance Levels. Land Degrad. Dev. 2020, 31, 607–618. [Google Scholar] [CrossRef]
- SER (Society for Ecological Restoration International Science & Policy Working Group). The SER International Primer on Ecological Restoration; SER (www.ser.org) & Tucson; Society for Ecological Restoration International: Tucson, AZ, USA, 2004; p. 16. [Google Scholar]
- Guedes, R.S.; Ramos, S.J.; Gastauer, M.; Fernandes, A.R.; Caldeira, C.F.; do Amarante, C.B.; Siqueira, J.O. Phosphorus Lability Increases with the Rehabilitation Advance of Iron Mine Land in the Eastern Amazon. Environ. Monit. Assess. 2020, 192, 390. [Google Scholar] [CrossRef] [PubMed]
- Zipper, C.E.; Burger, J.A.; McGrath, J.M.; Rodrigue, J.A.; Holtzman, G.I. Forest Restoration Potentials of Coal-Mined Lands in the Eastern United States. J. Environ. Qual. 2011, 40, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.J.; Gigante Carvalheiro, L.; Gastauer, M.; Almeida-Neto, M.; Giannini, T.C. Pollinator Restoration in Brazilian Ecosystems Relies on a Small but Phylogenetically-Diverse Set of Plant Families. Sci. Rep. 2019, 9, 17383. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, M.B.; Brancalion, P.H.S.; Rodrigues, R.R.; Loyola, R.; Cianciaruso, M.V. Functional Traits and Ecosystem Services in Ecological Restoration. Restor. Ecol. 2020, rec.13279. [Google Scholar] [CrossRef]
- Gastauer, M.; Caldeira, C.F.; Ramos, S.J.; Trevelin, L.C.; Jaffé, R.; Oliveira, G.; Vera, M.P.O.; Pires, E.; Santiago, F.L.; Carneiro, M.A.C.; et al. Integrating Environmental Variables by Multivariate Ordination Enables the Reliable Estimation of Mineland Rehabilitation Status. J. Environ. Manag. 2020, 256, 109894. [Google Scholar] [CrossRef]
- IPBES. Report of the Plenary of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on the Work of Its Seventh Session; Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Paris, France, 2019; p. 47. [Google Scholar]
- Skirycz, A.; Castilho, A.; Chaparro, C.; Carvalho, N.; Tzotzos, G.; Siqueira, J.O. Canga Biodiversity, a Matter of Mining. Front. Plant Sci. 2014, 5, 653. [Google Scholar] [CrossRef]
- Gastauer, M.; Silva, J.R.; Caldeira Junior, C.F.; Ramos, S.J.; Souza Filho, P.W.M.; Furtini Neto, A.E.; Siqueira, J.O. Mine Land Rehabilitation: Modern Ecological Approaches for More Sustainable Mining. J. Clean. Prod. 2018, 172, 1409–1422. [Google Scholar] [CrossRef]
- Giannini, T.C.; Giulietti, A.M.; Harley, R.M.; Viana, P.L.; Jaffe, R.; Alves, R.; Pinto, C.E.; Mota, N.F.O.; Caldeira, C.F.; Imperatriz-Fonseca, V.L.; et al. Selecting Plant Species for Practical Restoration of Degraded Lands Using a Multiple-Trait Approach. Austral Ecol. 2017, 42, 510–521. [Google Scholar] [CrossRef]
- Ramos, S.J.; Gastauer, M.; Mitre, S.K.; Caldeira, C.F.; Silva, J.R.; Furtini Neto, A.E.; Oliveira, G.; Souza Filho, P.W.M.; Siqueira, J.O. Plant Growth and Nutrient Use Efficiency of Two Native Fabaceae Species for Mineland Revegetation in the Eastern Amazon. J. For. Res. 2019. [Google Scholar] [CrossRef]
- Ramos, S.J.; Caldeira, C.F.; Gastauer, M.; Costa, D.L.P.; Furtini Neto, A.E.; de Souza, F.B.M.; Souza-Filho, P.W.M.; Siqueira, J.O. Native Leguminous Plants for Mineland Revegetation in the Eastern Amazon: Seed Characteristics and Germination. New For. 2019, 50, 859–872. [Google Scholar] [CrossRef]
- Giulietti, A.M.; Giannini, T.C.; Mota, N.F.O.; Watanabe, M.T.C.; Viana, P.L.; Pastore, M.; Silva, U.C.S.; Siqueira, M.F.; Pirani, J.R.; Lima, H.C.; et al. Edaphic Endemism in the Amazon: Vascular Plants of the Canga of Carajás, Brazil. Bot. Rev. 2019, 85, 357–383. [Google Scholar] [CrossRef]
- Viana, P.L.; Mota, N.F.; Gil, A.D.S.B.; Salino, A.; Zappi, D.C.; Harley, R.M.; Ilkiu-Borges, A.L.; Secco, R.D.S.; Almeida, T.E.; Watanabe, M.T.C.; et al. Flora Das Cangas Da Serra Dos Carajás, Pará, Brasil: História, Área de Estudos e Metodologia. Rodriguésia Inst. Pesqui. Jard. Botânico Rio Janeirosia 2016, 67, 1107–1124. [Google Scholar] [CrossRef]
- Mota, N.F.D.O.; Watanabe, M.T.C.; Zappi, D.C.; Hiura, A.L.; Pallos, J.; Viveros, R.S.; Giulietti, A.M.; Viana, P.L. Amazon Canga: The Unique Vegetation of Carajás Revealed by the List of Seed Plants. Rodriguésia 2018, 69, 1435–1488. [Google Scholar] [CrossRef]
- Franks, P.J.; Farquhar, G.D. The Mechanical Diversity of Stomata and Its Significance in Gas-Exchange Control. Plant Physiol. 2007, 143, 78–87. [Google Scholar] [CrossRef]
- Galatis, B.; Apostolakos, P. The Role of the Cytoskeleton in the Morphogenesis and Function of Stomatal Complexes: Tansley Review. New Phytol. 2004, 161, 613–639. [Google Scholar] [CrossRef]
- Viana, P.L.; Rocha, A.E.S.D.; Silva, C.; Afonso, E.A.L.; Oliveira, R.P.; Oliveira, R.C. Flora das cangas da Serra dos Carajás, Pará, Brasil: Poaceae. Rodriguésia Inst. Pesqui. Jard. Botânico Rio Janeirosia 2018, 69, 1311–1368. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, Y.; Lu, H.; Yuan, J.; Zhu, Y.; Liang, Y. Grass Hedge Effects on Controlling Soil Loss from Concentrated Flow: A Case Study in the Red Soil Region of China. Soil Tillage Res. 2015, 148, 97–105. [Google Scholar] [CrossRef]
- Araujo, T.O.D.; Isaure, M.-P.; Alchoubassi, G.; Bierla, K.; Szpunar, J.; Trcera, N.; Chay, S.; Alcon, C.; Campos da Silva, L.; Curie, C.; et al. Paspalum Urvillei and Setaria Parviflora, Two Grasses Naturally Adapted to Extreme Iron-Rich Environments. Plant Physiol. Biochem. 2020, 151, 144–156. [Google Scholar] [CrossRef]
- Figueiredo, M.A.; Diniz, A.P.; Messias, M.C.T.B.; Kozovits, A.R. Propagation and Establishment of Rupestrian Grassland Grasses for Restoration of Degraded Areas by Mining. Braz. J. Bot. 2018, 41, 287–295. [Google Scholar] [CrossRef]
- Silva, J.R.; Gastauer, M.; Ramos, S.J.; Mitre, S.K.; Furtini Neto, A.E.; Siqueira, J.O.; Caldeira, C.F. Initial Growth of Fabaceae Species: Combined Effects of Topsoil and Fertilizer Application for Mineland Revegetation. Flora 2018, 246–247, 109–117. [Google Scholar] [CrossRef]
- Carvalho, J.M.; Ramos, S.J.; Furtini Neto, A.E.; Gastauer, M.; Caldeira, C.F., Jr.; Siqueira, J.O.; Silva, M.L.S. Influence of Nutrient Management on Growth and Nutrient Use Efficiency of Two Plant Species for Mineland Revegetation. Restor. Ecol. 2018, 26, 303–310. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Chapter 6—Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 135–189. ISBN 978-0-12-384905-2. [Google Scholar]
- Gaju, O.; DeSilva, J.; Carvalho, P.; Hawkesford, M.J.; Griffiths, S.; Greenland, A.; Foulkes, M.J. Leaf Photosynthesis and Associations with Grain Yield, Biomass and Nitrogen-Use Efficiency in Landraces, Synthetic-Derived Lines and Cultivars in Wheat. Field Crops Res. 2016, 193, 1–15. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, Y.; Wang, Q.; Meng, D.; Wang, S. Effects of Nitrogen and 6-Benzylaminopurine on Rice Tiller Bud Growth and Changes in Endogenous Hormones and Nitrogen. Crop Sci. 2011, 51, 786–792. [Google Scholar] [CrossRef]
- Deng, F.; Wang, L.; Ren, W.J.; Mei, X.F.; Li, S.X. Optimized Nitrogen Managements and Polyaspartic Acid Urea Improved Dry Matter Production and Yield of Indica Hybrid Rice. Soil Tillage Res. 2015, 145, 1–9. [Google Scholar] [CrossRef]
- Aravindhakshan, S.C.; Epplin, F.M.; Taliaferro, C.M. Switchgrass, Bermudagrass, Flaccidgrass, and Lovegrass Biomass Yield Response to Nitrogen for Single and Double Harvest. Biomass Bioenergy 2011, 35, 308–319. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Kanter, D.R.; Bartolini, F.; Kugelberg, S.; Leip, A.; Oenema, O.; Uwizeye, A. Nitrogen Pollution Policy beyond the Farm. Nat. Food 2020, 1, 27–32. [Google Scholar] [CrossRef]
- Sylvester-Bradley, R.; Kindred, D.R. Analysing Nitrogen Responses of Cereals to Prioritize Routes to the Improvement of Nitrogen Use Efficiency. J. Exp. Bot. 2009, 60, 1939–1951. [Google Scholar] [CrossRef]
- Dhital, S.; Raun, W.R. Variability in Optimum Nitrogen Rates for Maize. Agron. J. 2016, 108, 2165–2173. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Teixeira, P.C., Ed.; Embrapa Solos: Brasilia, Brazil, 2017; Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1085209/manual-de-metodos-de-analise-de-solo (accessed on 18 April 2021).
- Kettler, T.A.; Doran, J.W.; Gilbert, T.L. Simplified Method for Soil Particle-Size Determination to Accompany Soil-Quality Analyses. Soil Sci. Soc. Am. J. 2001, 65, 849. [Google Scholar] [CrossRef]
- CFSEMG. Recomendação Para o Uso de Corretivos e Fertilizantes Em Minas Gerais: 5. Aproximação. 1999. Available online: https://edisciplinas.usp.br/pluginfile.php/5330754/mod_resource/content/1/5%C2%AA%20-%20Aproxima%C3%A7%C3%A3o%20-%20Recomenda%C3%A7%C3%A3o%20para%20o%20uso%20de%20corretivos%20e%20fertilizantes%20em%20Minas%20Gerais.pdf (accessed on 18 April 2021).
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Muir, J.P.; Sanderson, M.A.; Ocumpaugh, W.R.; Jones, R.M.; Reed, R.L. Biomass Production of ‘Alamo’ Switchgrass in Response to Nitrogen, Phosphorus, and Row Spacing. Agron. J. 2001, 93, 896–901. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Ren, T.; Hussain, S.; Guo, C.; Wang, S.; Cong, R.; Li, X. Effects of Nitrogen and Tiller Type on Grain Yield and Physiological Responses in Rice. AoB PLANTS 2017, 9. [Google Scholar] [CrossRef]
- Sarath, G.; Baird, L.M.; Mitchell, R.B. Senescence, Dormancy and Tillering in Perennial C4 Grasses. Plant Sci. 2014, 217–218, 140–151. [Google Scholar] [CrossRef]
- Bochet, E.; Poesen, J.; Rubio, J.L. Runoff and Soil Loss under Individual Plants of a Semi-Arid Mediterranean Shrubland: Influence of Plant Morphology and Rainfall Intensity. Earth Surf. Process. Landf. 2006, 31, 536–549. [Google Scholar] [CrossRef]
- Duan, L.; Huang, M.; Zhang, L. Differences in Hydrological Responses for Different Vegetation Types on a Steep Slope on the Loess Plateau, China. J. Hydrol. 2016, 537, 356–366. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Hu, J.-M.; Wang, T.-W.; Cai, C.-F.; Li, Z.-X.; Zhang, Y. Effects of Vegetation Cover and Road-Concentrated Flow on Hillslope Erosion in Rainfall and Scouring Simulation Tests in the Three Gorges Reservoir Area, China. CATENA 2016, 136, 108–117. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Reed, R.L. Switchgrass Growth and Development: Water, Nitrogen, and Plant Density Effects. J. Range Manag. 2000, 53, 221. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Schmer, M.; Owens, V.; Keyser, P.; Elbersen, W. Crop Management of Switchgrass. In Switchgrass; Monti, A., Ed.; Green Energy and Technology; Springer: London, UK, 2012; pp. 87–112. ISBN 9781447129028. [Google Scholar]
- Taylaran, R.D.; Adachi, S.; Ookawa, T.; Usuda, H.; Hirasawa, T. Hydraulic Conductance as Well as Nitrogen Accumulation Plays a Role in the Higher Rate of Leaf Photosynthesis of the Most Productive Variety of Rice in Japan. J. Exp. Bot. 2011, 62, 4067–4077. [Google Scholar] [CrossRef]
- Ghannoum, O.; Evans, J.R.; Chow, W.S.; Andrews, T.J.; Conroy, J.P.; von Caemmerer, S. Faster Rubisco Is the Key to Superior Nitrogen-Use Efficiency in NADP-Malic Enzyme Relative to NAD-Malic Enzyme C 4 Grasses. Plant Physiol. 2005, 137, 638–650. [Google Scholar] [CrossRef]
- Bassi, D.; Menossi, M.; Mattiello, L. Nitrogen Supply Influences Photosynthesis Establishment along the Sugarcane Leaf. Sci. Rep. 2018, 8, 2327. [Google Scholar] [CrossRef]
- Hansch, R.; Mendel, R.R. Physiological Functions of Mineral Micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Chapter 2—Ion Uptake Mechanisms of Individual Cells and Roots: Short-distance Transport. In Marschner’s Mineral Nutrition of Higher Plants (Third Edition); Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 7–47. ISBN 978-0-12-384905-2. [Google Scholar]
- Caldeira, C.F.; Jeanguenin, L.; Chaumont, F.; Tardieu, F. Circadian Rhythms of Hydraulic Conductance and Growth Are Enhanced by Drought and Improve Plant Performance. Nat. Commun. 2014, 5, 5365. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.A.; Rabei, S.H.; Nada, R.M.; Abogadallah, G.M. Water Use Efficiency in the Drought-Stressed Sorghum and Maize in Relation to Expression of Aquaporin Genes. Biol. Plant. 2017, 61, 127–137. [Google Scholar] [CrossRef]
- Hui, D.; Yu, C.-L.; Deng, Q.; Saini, P.; Collins, K.; Koff, J. Weak Effects of Biochar and Nitrogen Fertilization on Switchgrass Photosynthesis, Biomass, and Soil Respiration. Agriculture 2018, 8, 143. [Google Scholar] [CrossRef]
- Gastauer, M.; Sarmento, P.S.D.M.; Santos, V.C.A.; Caldeira, C.F.; Ramos, S.J.; Teodoro, G.S.; Siqueira, J.O. Vegetative Functional Traits Guide Plant Species Selection for Initial Mineland Rehabilitation. Ecol. Eng. 2020, 148, 105763. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M.R. Stomatal Size, Speed, and Responsiveness Impact on Photosynthesis and Water Use Efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef]
- Tambussi, E.A.; Bort, J.; Araus, J.L. Water Use Efficiency in C3 Cereals under Mediterranean Conditions: A Review of Physiological Aspects. Ann. Appl. Biol. 2007, 150, 307–321. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Zhao, P.; Xu, P.; Yu, G.; Zhang, L.; Xiong, Y.; Xiang, C. AtEDT1/HDG11 Regulates Stomatal Density and Water-use Efficiency via ERECTA and E2Fa. New Phytol. 2019, 223, 1478–1488. [Google Scholar] [CrossRef]
- Masle, J.; Gilmore, S.R.; Farquhar, G.D. The ERECTA Gene Regulates Plant Transpiration Efficiency in Arabidopsis. Nature 2005, 436, 866–870. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The Role of Stomata in Sensing and Driving Environmental Change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Chen, G.; Dai, F.; Wang, Y.; Hills, A.; Ruan, Y.-L.; Zhang, G.; Franks, P.J.; Nevo, E.; Blatt, M.R. Molecular Evolution of Grass Stomata. Trends Plant Sci. 2017, 22, 124–139. [Google Scholar] [CrossRef]
- Maurel, C.; Nacry, P. Root Architecture and Hydraulics Converge for Acclimation to Changing Water Availability. Nat. Plants 2020, 6, 744–749. [Google Scholar] [CrossRef]
- Jung, J.K.H.; McCouch, S. Getting to the Roots of It: Genetic and Hormonal Control of Root Architecture. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef]
- Kaldenhoff, R.; Grote, K.; Zhu, J.; Zimmermann, U. Significance of Plasmalemma Aquaporins for Water-transport in Arabidopsis Thaliana. Plant J. 1998, 14, 121–128. [Google Scholar] [CrossRef]
- Briat, J.-F.; Ravet, K.; Arnaud, N.; Duc, C.; Boucherez, J.; Touraine, B.; Cellier, F.; Gaymard, F. New Insights into Ferritin Synthesis and Function Highlight a Link between Iron Homeostasis and Oxidative Stress in Plants. Ann. Bot. 2010, 105, 811–822. [Google Scholar] [CrossRef]
- Siqueira-Silva, A.I.; Rios, C.O.; Pereira, E.G. Iron Toxicity Resistance Strategies in Tropical Grasses: The Role of Apoplastic Radicular Barriers. J. Environ. Sci. 2019, 78, 257–266. [Google Scholar] [CrossRef]
- Araujo, T.O.D.; de Freitas-Silva, L.; Santana, B.V.N.; Kuki, K.N.; Pereira, E.G.; Azevedo, A.A.; da Silva, L.C. Tolerance to Iron Accumulation and Its Effects on Mineral Composition and Growth of Two Grass Species. Environ. Sci. Pollut. Res. 2014, 21, 2777–2784. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caldeira, C.F.; Lima, M.O.; Ramos, S.J.; Gastauer, M. Native Amazonian Canga Grasses Show Distinct Nitrogen Growth Responses in Iron Mining Substrates. Plants 2021, 10, 849. https://doi.org/10.3390/plants10050849
Caldeira CF, Lima MO, Ramos SJ, Gastauer M. Native Amazonian Canga Grasses Show Distinct Nitrogen Growth Responses in Iron Mining Substrates. Plants. 2021; 10(5):849. https://doi.org/10.3390/plants10050849
Chicago/Turabian StyleCaldeira, Cecilio F., Madson O. Lima, Silvio J. Ramos, and Markus Gastauer. 2021. "Native Amazonian Canga Grasses Show Distinct Nitrogen Growth Responses in Iron Mining Substrates" Plants 10, no. 5: 849. https://doi.org/10.3390/plants10050849
APA StyleCaldeira, C. F., Lima, M. O., Ramos, S. J., & Gastauer, M. (2021). Native Amazonian Canga Grasses Show Distinct Nitrogen Growth Responses in Iron Mining Substrates. Plants, 10(5), 849. https://doi.org/10.3390/plants10050849






