Phytostimulatory Influence of Comamonas testosteroni and Silver Nanoparticles on Linum usitatissimum L. under Salinity Stress
Abstract
1. Introduction
2. Results
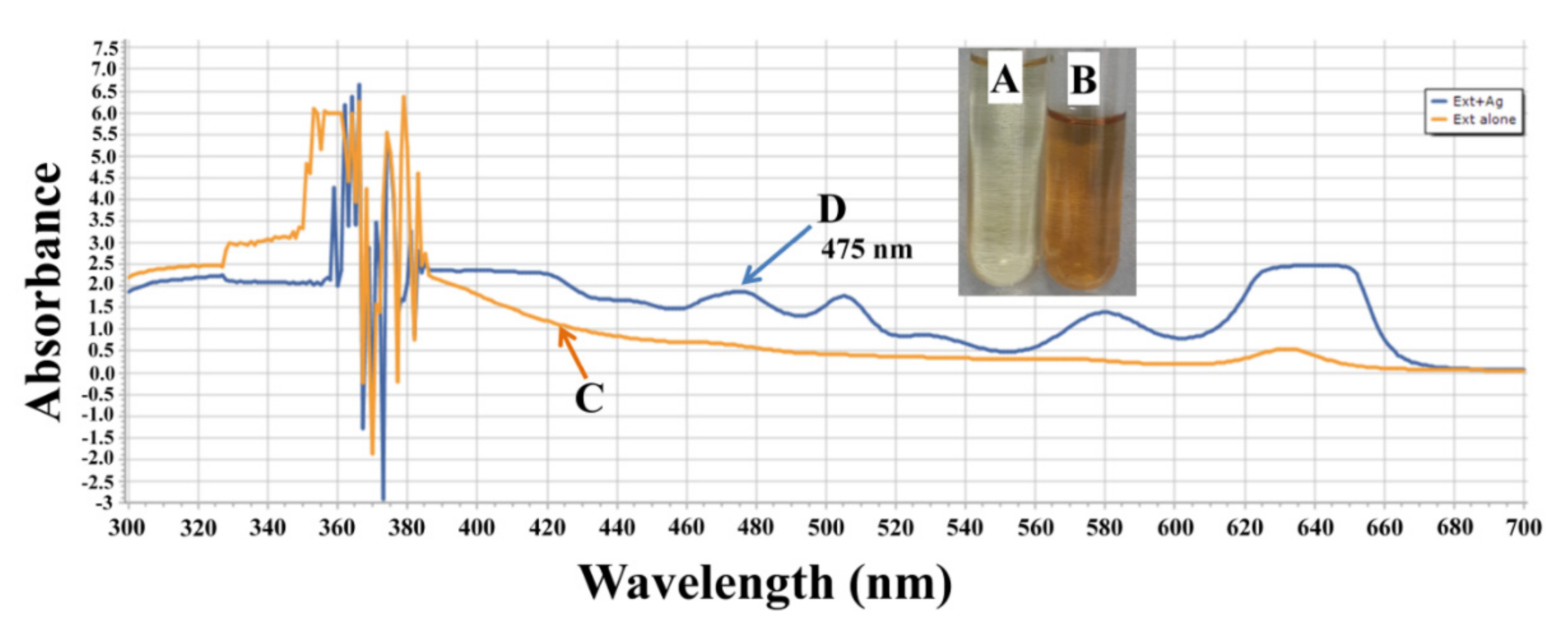
2.1. Characterization of AgNPs
2.2. Photosynthetic Pigments
2.3. Soluble Sugars, Proteins, and Proline Contents
2.4. MDA and H2O2 Contents
2.5. Determination of Nonenzymatic Antioxidants
2.6. Assay of Antioxidant Enzyme Activities
3. Discussion
4. Materials and Methods
4.1. Bacterial Culture Preparation
4.2. Preparation of AgNPs Using Rosmarinus Officinalis
4.3. Description of Bio Fabricated AgNPs
4.4. Effect of AgNPs and C. testosteroni on Plant Growth
4.5. Quantification of Photosynthetic Pigments
4.6. Determination of Non-Antioxidant Enzymes
4.6.1. Determination of Soluble Sugars, Soluble Protein, and Proline
4.6.2. Determination of Hydrogen Peroxide (H2O2) and Malondialdehyde (MDA)
4.6.3. Determination of Total Phenolic (TPC), Ascorbic Acid (AsA), and Glutathione (GSH)
4.7. Determination of Antioxidant Enzymes
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Afroz, S.; Mohammad, F.; Hayat, S.; Siddiqui, M.H. Exogenous Application of Gibberellic Acid Counteracts the Ill Effect of Sodium Chloride in Mustard. Turk. J. Biol. 2005, 29, 233–236. [Google Scholar]
- Habib, S.H.; Kausar, H.; Saud, H.M. Plant Growth-Promoting Rhizobacteria Enhance Salinity Stress Tolerance in Okra through ROS-Scavenging Enzymes. BioMed Res. Int. 2016, 2016, 2016. [Google Scholar] [CrossRef]
- Khan, M.N.; Siddiqui, M.H.; Mohammad, F.; Naeem, M.; Khan, M.M.A. Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defense system and osmoprotectant accumulation. Acta Physiol. Plant. 2010, 32, 121–132. [Google Scholar] [CrossRef]
- Allaby, R.G.; Peterson, G.W.; Merriwether, D.A.; Fu, Y.B. Evidence of the domestication history of flax (Linum usitatissimum L.) from the genetic diversity of the sad2 locus. Theor. Appl. Genet. 2005, 112, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A.; Kazem, G.G. Physiological responses of soybean (Glycine max L.) to zinc application under salinity stress. Aust. J. Crop Sci. 2011, 5, 1441–1447. [Google Scholar]
- Abdel Latef, A.A.H.; Abu Alhmad, M.F.; Abdelfattah, K.E. The Possible Roles of Priming with ZnO Nanoparticles in Mitigation of Salinity Stress in Lupine (Lupinus termis) Plants. J. Plant Growth Regul. 2017, 36, 60–70. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Singh, A.K.; Singh, S.P.; Ansari, M.I. Nanoparticles and Plant Interaction with Respect to Stress Response. In Nanomaterials and Environmental Biotechnology; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Maroufpour, N.; Mousavi, M.; Abbasi, M.; Ghorbanpour, M. Biogenic nanoparticles as novel sustainable approach for plant protection. In Biogenic Nano-Particles and Their Use in Agro-Ecosystems; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Gupta, S.D.; Agarwal, A.; Pradhan, S. Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: An insight from antioxidative enzyme activities and gene expression patterns. Ecotox. Environ. Safe. 2018, 161, 624–633. [Google Scholar] [CrossRef]
- Dewez, D.; Goltsev, V.; Kalaji, H.M.; Oukarroum, A. Inhibitory effects of silver nanoparticles on photosystem II performance in Lemna gibba probed by chlorophyll fluorescence. Curr. Plant Biol. 2018, 16, 15–21. [Google Scholar] [CrossRef]
- Anjum, N.A.; Gill, S.S.; Duarte, A.C.; Pereira, E.; Ahmad, I. Silver nanoparticles in soil-plant systems. J. Nanoparticle Res. 2013, 15, 1–26. [Google Scholar] [CrossRef]
- Dewez, C.; Domingo, G.; Onelli, E.; De Mattia, F.; Bruni, I.; Marsoni, M.; Bracale, M. Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings. J. Plant Physiol. 2014, 171, 1142–1148. [Google Scholar] [CrossRef]
- Rossi, L.; Zhang, W.; Lombardini, L.; Ma, X. The impact of cerium oxide nanoparticles on the salt stress responses of Brassica napus L. Environ. Pollut. 2016, 219, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, Z.M. Influence of silver nanoparticles on the salt resistance of tomato (Solanum lycopersicum) during germination. Int. J. Agric. Biol. 2016, 18, 449–457. [Google Scholar] [CrossRef]
- Sharma, G.K.; Mahajan, S.; Matura, R.; Subramaniam, S.; Mohapatra, J.K.; Pattnaik, B. Quantitative single dilution liquid phase blocking ELISA for sero-monitoring of foot-and-mouth disease in India. Biologicals 2015, 43, 158–164. [Google Scholar] [CrossRef]
- Zea, L.; Salama, H.M.H. Effects of silver nanoparticles in some crop plants, Common bean (Phaseolus vulgaris L.) and corn. Int. Res. J. Biotech. 2012, 3, 190–197. Available online: http://www.interesjournals.org/IRJOB/Pdf/2012/December/Salama.pdf (accessed on 5 August 2020).
- Barriuso, J.; Ramos Solano, B.; Lucas, J.A.; Lobo, A.P.; García-Villaraco, A.; Gutiérrez Mañero, F.J. Ecology, Genetic Diversity and Screening Strategies of Plant Growth Promoting Rhizobacteria (PGPR). J. Plant Nutr. 2008, 4, 1–17. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Pseudomonas siderophore: A mechanism explaining disease-suppressive soils. Curr. Microbiol. 1980, 4, 317–320. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microb. 2002, 68, 3795–3801. [Google Scholar] [CrossRef]
- Lesueur, D.; Deaker, R.; Herrmann, L.; Bräu, L.; Jansa, J. The production and potential of biofertilizers to improve the crop. In Bioformulations: For Sustainable Agriculture; Springer: New Delhi, India, 2016. [Google Scholar] [CrossRef]
- Bae, H.S.; Lee, J.M.; Kim, Y.B.; Lee, S.T. Biodegradation of the mixtures of 4-chlorophenol and phenol by Comamonas testosteroni CPW301. Biodegradation 1996, 7, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Su, J.; Wang, L.; Yao, R.; Wang, D.; Deng, Y.; Rensing, C. Selenite reduction by the obligate aerobic bacterium Comamonas testosteroni S44 isolated from a metal-contaminated soil. BMC Microbiol. 2014, 14, 14. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Reddy, M.S.; Rodriguez-Kabana, R.; Kenney, D.S.; Kokalis-Burelle, N.; Martinez-Ochoa, N.; Vavrina, C.S. Application for rhizobacteria in transplant production and yield enhancement. Acta Hortic. 2004, 631, 217–229. [Google Scholar] [CrossRef]
- Zhu, D.; Xie, C.; Huang, Y.; Sun, J.; Zhang, W. Description of Comamonas serinivorans sp. nov., isolated from wheat straw compost. Int. J. Syst. Evol. Microbiol. 2014, 64, 4141–4146. [Google Scholar] [CrossRef] [PubMed]
- Ghramh, H.A.; Ibrahim, E.H.; Kilnay, M.; Ahmad, Z.; Alhag, S.K.; Khan, K.A.; Asiri, F.M. Silver Nanoparticle Production by Ruta graveolens and Testing Its Safety, Bioactivity, Immune Modulation, Anticancer, and Insecticidal Potentials. Bioinorg. Chem. Appl. 2020, 1–11. [Google Scholar] [CrossRef]
- Franca, J.R.; De Luca, M.P.; Ribeiro, T.G.; Castilho, R.O.; Moreira, A.N.; Santos, V.R.; Faraco, A.A.G. Propolis—Based chitosan varnish: Drug delivery, controlled release and antimicrobial activity against oral pathogen bacteria. BMC Complement Altern. Med. 2014, 14, 478. [Google Scholar] [CrossRef] [PubMed]
- Geissler, N.; Hussin, S.; Koyro, H.W. Interactive effects of NaCl salinity and elevated atmospheric CO2 concentration on growth, photosynthesis, water relations, and chemical composition of the potential cash crop halophyte Aster tripolium L. Environ. Exp. Bot. 2009, 65, 220–231. [Google Scholar] [CrossRef]
- Naumann, J.C.; Anderson, J.E.; Young, D.R. Linking physiological responses, chlorophyll fluorescence and hyperspectral imagery to detect salinity stress using the physiological reflectance index in the coastal shrub, Myrica cerifera. Remote Sens. Environ. 2008, 112, 3865–3875. [Google Scholar] [CrossRef]
- Keutgen, A.J.; Pawelzik, E. Modifications of strawberry fruit antioxidant pools and fruit quality under NaCl stress. J. Agric. Food Chem. 2007, 55, 4066–4072. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585. [Google Scholar] [CrossRef] [PubMed]
- Azimi, R.; Borzelabad, M.J.; Feizi, H.; Azimi, A. Interaction of SiO2 nanoparticles with seed prechilling on germination early seedling growth of tall wheatgrass (Agropyron elongatum L.). Pol. J. Chem. Technol. 2014, 16, 25–29. [Google Scholar] [CrossRef]
- Shakeel Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Gurunathan, S.; Chung, I.M. Physiological, metabolic, and transcriptional effects of biologically-synthesized silver nanoparticles in turnip (Brassica rapa ssp. rapa L.). Protoplasma. 2015, 252, 1031–1046. [Google Scholar] [CrossRef] [PubMed]
- Narendhran, S.; Rajiv, P.; Sivaraj, R. Influence of zinc oxide nanoparticles on the growth of Sesamum indicum L. In zinc-deficient soil. Int. J. Pharm. Pharm. Sci. 2016, 8, 365–371. [Google Scholar]
- Mohsenzadeh, S.; Moosavian, S.S. Zinc Sulphate, and Nano-Zinc Oxide Effects on Some Physiological Parameters of Rosmarinus officinalis. Am. J. Plant Sci. 2017, 8, 2635–2649. [Google Scholar] [CrossRef]
- He, Y.; Du, Z.; Lv, H.; Jia, Q.; Tang, Z.; Zheng, X.; Zhao, F. Green synthesis of silver nanoparticles by Chrysanthemum morifolium Ramat. Extract and their application in clinical ultrasound gel. Int. J. Nanomed. 2013, 8, 1809–1815. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of the phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.D.V.B.; Seldin, L.; de Araujo, F.F.; Mariano, R.D.L.R. Plant Growth Promoting Rhizobacteria: Fundamentals and Applications. In Plant Growth and Health Promoting Bacteria; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Razzaghi Komaresofla, B.; Alikhani, H.A.; Etesami, H.; Khoshkholgh-Sima, N.A. Improved growth and salinity tolerance of the halophyte Salicornia sp. by co-inoculation with endophytic and rhizosphere bacteria. Appl. Soil Ecol. 2019, 138, 160–170. [Google Scholar] [CrossRef]
- Zameer, M.; Zahid, H.; Tabassum, B.; Ali, Q.; Nasir, I.A.; Saleem, M.; Butt, S.J. PGPR Potentially Improve Growth of Tomato Plants in Salt-Stressed Environment. Turk. J. Agric. Food Sci. Technol. 2016, 4, 455. [Google Scholar] [CrossRef][Green Version]
- Abdel Latef, A.A. Changes of antioxidative enzymes in salinity tolerance among different wheat cultivars. Cereal Res. Commun. 2010, 38, 43–55. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Egamberdieva, D.; Bhardwaj, R.; Ashraf, M. Zinc application mitigates the adverse effects of NaCl stress on mustard [Brassica juncea (L.) czern & coss] through modulating compatible organic solutes, antioxidant enzymes, and flavonoid content. J. Plant Interact. 2017, 12, 429–437. [Google Scholar] [CrossRef]
- Mohamed, A.K.S.; Qayyum, M.F.; Abdel-Hadi, A.M.; Rehman, R.A.; Ali, S.; Rizwan, M. Interactive effect of salinity and silver nanoparticles on photosynthetic and biochemical parameters of wheat. Arch. Agron. Soil Sci. 2017, 63, 1736–1747. [Google Scholar] [CrossRef]
- Akanbi-Gada, M.A.; Ogunkunle, C.O.; Vishwakarma, V.; Viswanathan, K.; Fatoba, P.O. Phytotoxicity of Nano-zinc oxide to tomato plant (Solanum lycopersicum L.): Zn uptake, stress enzymes response and influence on nonenzymatic antioxidants in fruits. Environ. Technol. Innov. 2019, 14, 100325. [Google Scholar] [CrossRef]
- Dodd, I.C.; Pérez-Alfocea, F. Microbial amelioration of crop salinity stress. J. Exp. Bot. 2012, 63, 3415–3428. [Google Scholar]
- Upadhyay, S.K.; Singh, J.S.; Saxena, A.K.; Singh, D.P. Impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions. Plant Biol. 2012, 14, 605–611. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Priyadarshini, S.; Gopinath, V.; Meera Priyadharsshini, N.; MubarakAli, D.; Velusamy, P. Synthesis of anisotropic silver nanoparticles using novel strain, Bacillus flexus, and its biomedical application. Colloid Surf. B 2013, 102, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Burman, U.; Saini, M.; Kumar, P. Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Environ. Chem. 2013, 95, 605–612. [Google Scholar] [CrossRef]
- Subramanian, P.; Mageswari, A.; Kim, K.; Lee, Y.; Sa, T. Psychrotolerant endophytic pseudomonas sp. strains OB155 and OS261 induced chilling resistance in tomato plants (Solanum lycopersicum Mill.) by activation of their antioxidant capacity. Mol. Plant Microbe. Interact. 2015, 28, 1073–1081. [Google Scholar] [CrossRef]
- Soliman, A.S.; El-feky, S.A.; Darwish, E. Alleviation of salt stress on Moringa peregrina using the foliar application of Nano fertilizers. J. Hortic. Sci. For. 2015, 7, 36–47. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef]
- Rashad, Y.; Aseel, D.; Hammad, S.; Elkelish, A. Rhizophagus irregularis, and Rhizoctonia solani differentially elicit systemic transcriptional expression of polyphenol biosynthetic pathways genes in sunflower. Biomolecules 2020, 10, 379. [Google Scholar] [CrossRef]
- Akbari, M.; Mahna, N.; Ramesh, K.; Bandehagh, A.; Mazzuca, S. Ion homeostasis, osmoregulation, and physiological changes in the roots and leaves of pistachio rootstocks in response to salinity. Protoplasma 2018, 255, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.; Qari, S.H.; Abu-Elsaoud, A.; El-Esawi, M.; Alhaithloul, H.; Elkelish, A. Rapid green synthesis of silver nanoparticles from blue gum augment growth and performance of maize, fenugreek, and onion by modulating plants cellular antioxidant machinery and genes expression. Acta Physiol. Plant. 2020, 42, 148. [Google Scholar] [CrossRef]
- Ochieng, L.A. Agro-Morphological Characterization of Sweet Potato Genotypes Grown in Different Ecological Zones in Kenya. J. Hort. Plant Res. 2019, 5, 1–12. [Google Scholar] [CrossRef]
- Abbas, T.; Pervez, M.A.; Ayyub, C.M.; Ahmad, R. Assessment of morphological, antioxidant, biochemical, and ionic responses of salt-tolerant and salt-sensitive okra (Abelmoschus esculentus) under the saline regime. Pak. J. Life Soc. Sci. 2013, 11, 147–153. [Google Scholar]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Ashraf, M. Mitigation of sodium chloride toxicity in Solanum lycopersicum l. By supplementation of jasmonic acid and nitric oxide. J. Plant Interact. 2018, 13, 64–72. [Google Scholar] [CrossRef]
- Srivastav, M.; Kishor, A.; Dahuja, A.; Sharma, R.R. Effect of paclobutrazol and salinity onion leakage, proline content, and activities of antioxidant enzymes in mango (Mangifera indica L.). Sci. Hortic. 2010, 125, 785–788. [Google Scholar] [CrossRef]
- Lei, Z.; Mingyu, S.; Xiao, W.; Chao, L.; Chunxiang, Q.; Liang, C.; Fashui, H. Antioxidant stress is promoted by Nano-anatase in spinach chloroplasts under UV-B radiation. Biol. Trace Elem. Res. 2008, 121, 69–79. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alayafi, A.A. Overexpression of rice Rab7 gene improves drought and heat tolerance and increases grain yield in rice (Oryza sativa L.). Genes 2019, 10, 56. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth-promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 23–131. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Yasmeen, T.; Arif, M.S.; Ali, S.; Ali, B.; Hameed, S.; Zhou, W. Plant growth-promoting bacteria confer salt tolerance in Vigna radiata by upregulating antioxidant defense and biological soil fertility. Plant Growth Regul. 2016, 80, 23–36. [Google Scholar] [CrossRef]
- Pérez Rodríguez, N.; Engström, E.; Rodushkin, I.; Nason, P.; Alakangas, L.; öhlander, B. Copper and iron isotope fractionation in mine tailings at the Laver and Kristineberg mines, northern Sweden. Appl. Geochem. 2013, 32, 204–215. [Google Scholar] [CrossRef]
- Gururani, M.A.; Upadhyaya, C.P.; Baskar, V.; Venkatesh, J.; Nookaraju, A.; Park, S.W. Plant Growth-Promoting Rhizobacteria Enhance Abiotic Stress Tolerance in Solanum tuberosum through Inducing Changes in the Expression of ROS-Scavenging Enzymes and Improved Photosynthetic Performance. J. Plant Growth Regul. 2013, 32, 245–258. [Google Scholar] [CrossRef]
- Kohler, J.; Knapp, B.A.; Waldhuber, S.; Caravaca, F.; Roldán, A.; Insam, H. Effects of elevated CO2, water stress, and inoculation with Glomus intraradices or Pseudomonas mendocina on dry lettuce matter and rhizosphere microbial and functional diversity under growth chamber conditions. J. Soil Sediment. 2010, 10, 1585–1597. [Google Scholar] [CrossRef]
- Dolatabadian, A.; Jouneghani, R.S. Impact of exogenous ascorbic acid on antioxidant activity and some physiological traits of common bean subjected to salinity stress. Not. Bot. Horti. Agrobot. Cluj. Napoca. 2009, 37, 165–172. [Google Scholar] [CrossRef]
- Esitken, A.; Karlidag, H.; Ercisli, S.; Turan, M.; Sahin, F. The effect of spraying a growth-promoting bacterium on the yield, growth, and nutrient element composition of leaves of apricot (Prunus armeniaca L. cv. Hacihaliloglu). Aust. J. Agric. Res. 2003, 54, 377–380. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Irigoyen, J.J.; Einerich, D.W.; Sánchez-Díaz, M. Water stress-induced changes in proline concentrations and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plantarum. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Ann. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implication of Hydrogen Peroxide–Ascorbate System on Membrane Permeability of Water Stressed Vigna Seedlings. New Phytol. 1985, 99, 355–360. [Google Scholar] [CrossRef]
- Wu, F.; Dong, J.; Cai, Y.; Chen, F.; Zhang, G. Differences in Mn uptake and subcellular distribution in different barley genotypes as a response to Cd toxicity. Sci. Total Environ. 2007, 385, 228–234. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Van Montagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Ann. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Method Enzymol. 1985, 113, 548–555. [Google Scholar] [CrossRef]
- Chen, L.S.; Cheng, L. Both xanthophyll cycle-dependent thermal dissipation and the antioxidant system are upregulated in grape (Vitis labrusca L. cv. Concord) leaves in response to N limitation. J. Exp. Bot. 2003, 54, 2165–2175. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wei, J.; Wei, X.; Tang, K.; Liang, Y.; Shu, K.; Wang, B. Effect of sound wave stress on antioxidant enzyme activities and lipid peroxidation of Dendrobium candidum. Colloid Surf. B 2008, 63, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in Vitro. Method Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Zhou, W.; Leul, M. Uniconazole-induced tolerance of rape plants to heat stress in relation to changes in hormonal levels, enzyme activities, and lipid peroxidation. Plant Growth Regul. 1999, 27, 99–104. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Statistical Analysis System; SAS Release 9.1 for Windows; SAS Institute Inc.: Cary, NC, USA, 2003.
| Salinity Levels/Treatments | Chlorophyll-a (mg/g) | Chlorophyll-b (mg/g) | Total Chlorophyll (mg/g) | Carotenoids (mg/g) | Anthocyanin (mg/g) | |
|---|---|---|---|---|---|---|
| 0.0 mM NaCl | Control | 1.27 ± 0.089 | 0.97 ± 0.082 | 2.06 ± 0.008 | 0.63 ± 0.033 | 0.31 ± 0.024 |
| AgNPs | 1.32 ± 0.065 | 1.02 ± 0.036 | 2.34 ± 0.003 | 0.68 ± 0.045 | 0.35 ± 0.169 | |
| C.t | 1.29 ± 0.031 | 0.99 ± 0.024 | 2.28 ± 0.006 | 0.65 ± 0.061 | 0.33 ± 0.143 | |
| AgNPs + C. t | 1.38 ± 0.022 | 1.05 ± 0.017 | 2.43 ± 0.001 | 0.73 ± 0.183 | 0.39 ± 0.008 | |
| NaCl treatment Mean | 1.32 ± 0.052 A | 1.01 ± 0.04 A | 2.28 ± 0.005 A | 0.67 ± 0.08 D | 0.35 ± 0.087 C | |
| 25 mM NaCl | Control | 1.19 ± 0.034 | 0.91 ± 0.046 | 2.10 ± 0.012 | 0.69 ± 0.026 | 0.39 ± 0.014 |
| AgNPs | 1.23 ± 0.068 | 0.97 ± 0.023 | 2.20 ± 0.067 | 0.74 ± 0.003 | 0.47 ± 0.157 | |
| C.t. | 1.21 ± 0.047 | 0.93 ± 0.054 | 2.14 ± 0.005 | 0.71 ± 0.117 | 0.43 ± 0.082 | |
| AgNPs + C. t | 1.25 ± 0.021 | 0.99 ± 0.091 | 2.24 ± 0.002 | 0.78 ± 0.093 | 0.51 ± 0.163 | |
| NaCl treatment Mean | 1.23 ± 0.043 B | 0.95 ± 0.038 A | 2.17 ± 0.022 A | 0.73 ± 0.06 C | 0.45 ± 0.104 B | |
| 50 mM NaCl | Control | 1.12 ± 0.040 | 0.82 ± 0.076 | 1.94 ± 0.008 | 0.74 ± 0.085 | 0.42 ± 0.079 |
| AgNPs | 1.16 ± 0.079 | 0.89 ± 0.090 | 2.05 ± 0.036 | 0.80 ± 0.131 | 0.46 ± 0.181 | |
| C.t. | 1.14 ± 0.022 | 0.84 ± 0.054 | 1.98 ± 0.004 | 0.76 ± 0.060 | 0.44 ± 0.003 | |
| AgNPs + C. t | 1.18 ± 0.011 | 0.93 ± 0.027 | 2.11 ± 0.073 | 0.83 ± 0.007 | 0.49 ± 0.001 | |
| NaCl treatment Mean | 1.15 ± 0.038 B | 0.87 ± 0.050 B | 2.02 ± 0.03 B | 0.78 ± 0.07 B | 0.45 ± 0.07 B | |
| 100 mM NaCl | Control | 0.98 ± 0.045 | 0.63 ± 0.033 | 1.61 ± 0.021 | 0.82 ± 0.012 | 0.45 ± 0.086 |
| AgNPs | 1.06 ± 0.037 | 0.69 ± 0.025 | 1.75 ± 0.009 | 0.85 ± 0.078 | 0.51 ± 0.191 | |
| C.t. | 1.02 ± 0.081 | 0.67 ± 0.091 | 1.69 ± 0.016 | 0.83 ± 0.164 | 0.48 ± 0.054 | |
| AgNPs + C. t | 1.10 ± 0.034 | 0.72 ± 0.086 | 1.82 ± 0.042 | 0.87 ± 0.051 | 0.53 ± 0.008 | |
| NaCl treatment Mean | 1.04 ± 0.05 C | 0.68 ± 0.06 C | 1.72 ± 0.022 C | 0.84 ± 0.08 A | 0.49 ± 0.085 A | |
| (Ag/Ct) treatment Mean | ||||||
| Control | 1.14 ± 0.052 c | 0.83 ± 0.060 d | 1.93 ± 0.012 d | 0.72 ± 0.041 d | 0.39 ± 0.050 d | |
| AgNPs | 1.19 ± 0.062 b | 0.89 ± 0.044 b | 2.08 ± 0.028 b | 0.77 ± 0.064 b | 0.45 ± 0.174 b | |
| C. t | 1.17 ± 0.045 b | 0.86 ± 0.056 c | 2.02 ± 0.008 c | 0.74 ± 0.101 c | 0.42 ± 0.071 c | |
| AgNPs + C. t | 1.23 ± 0.022 a | 0.92 ± 0.055 a | 2.15 ± 0.029 a | 0.81 ± 0.084 a | 0.48 ± 0.045 a | |
| An honestly significant difference (HSD) at p < 0.05 probability level using Tukey′s test for: | ||||||
| NaCl treatments | 0.075 | 0.052 | 0.126 | 0.059 | 0.035 | |
| (Ag/Ct) treatment | 0.033 | 0.023 | 0.056 | 0.025 | 0.015 | |
| NaCl × Ag/C. t Interaction | ns | ns | ns | ns | ns | |
| Salinity Levels/Treatments | Soluble Sugar mg/g | Soluble Proteins mg/g | Proline mg/g | Hydrogen Peroxide µg/L | Lipid Peroxidation µg/L | |
|---|---|---|---|---|---|---|
| 0.0 mM NaCl | Control | 121.56 ± 1.394 | 24.87 ± 1.851 | 10.23 ± 0.174 | 2.58 ± 0.045 | 20.87 ± 0.021 |
| AgNPs | 127.22 ± 1.271 | 27.39 ± 1.664 | 15.19 ± 0.153 | 1.61 ± 0.005 | 19.33 ± 0.065 | |
| C. t | 124.61 ± 1.382 | 25.01 ± 1.041 | 12.68 ± 0.12 | 1.81 ± 0.079 | 20.01 ± 0.028 | |
| AgNPs + Ct | 131.80 ± 1.190 | 30.44 ± 1.009 | 17.45 ± 0.18 | 1.98 ± 0.012 | 19.82 ± 0.061 | |
| NaCl treatment Mean | 126.3 ± 1.31 B | 26.93 ± 1.40 D | 13.89 ± 0.16 D | 2.0 ± 0.035 D | 19.93 ± 0.044 D | |
| 25 mM NaCl | Control | 128.64 ± 1.006 | 28.03 ± 1.061 | 13.97 ± 0.120 | 4.04 ± 0.004 | 23.88 ± 0.011 |
| AgNPs | 134.21 ± 1.037 | 31.68 ± 1.043 | 17.99 ± 0.104 | 3.72 ± 0.091 | 21.91 ± 0.083 | |
| C. t | 131.99 ± 1.982 | 28.99 ± 1.927 | 14.05 ± 0.145 | 3.96 ± 0.067 | 22.06 ± 0.003 | |
| AgNPs + Ct | 137.01 ± 1.003 | 35.05 ± 1.082 | 20.74 ± 0.191 | 3.89 ± 0.043 | 22.01 ± 0.092 | |
| NaCl treatment Mean | 131.61 ± 1.257 AB | 30.94 ± 1.28 C | 16.69 ± 0.14 C | 3.90 ± 0.051 C | 22.47 ± 0.047 C | |
| 50 mM NaCl | Control | 131.85 ± 1.481 | 31.40 ± 1.049 | 19.62 ± 0.157 | 5.35 ± 0.0028 | 29.65 ± 0.006 |
| AgNPs | 136.42 ± 1.031 | 34.26 ± 1.003 | 23.08 ± 0.140 | 4.92 ± 0.001 | 27.34 ± 0.073 | |
| C. t | 133.97 ± 1.156 | 32.41 ± 1.017 | 22.49 ± 0.128 | 5.12 ± 0.035 | 28.51 ± 0.039 | |
| AgNPs + Ct | 137.39 ± 1.294 | 38.98 ± 1.050 | 26.73 ± 0.123 | 5.03 ± 0.009 | 28.93 ± 0.020 | |
| NaCl treatment Mean | 134.91 ± 1.241 A | 34.26 ± 1.03 B | 22.98 ± 0.137 B | 4.65 ± 0.012 B | 28.61 ± 0.035 B | |
| 100 mM NaCl | Control | 137.79 ± 1.003 | 35.29 ± 1.001 | 22.89 ± 0.182 | 7.19 ± 0.015 | 35.40 ± 0.031 |
| AgNPs | 141.23 ± 1.932 | 39.58 ± 1.054 | 26.65 ± 0.115 | 6.80 ± 0.070 | 34.21 ± 0.002 | |
| C. t | 139.95 ± 1.096 | 36.99 ± 1.082 | 23.04 ± 0.171 | 6.93 ± 0.041 | 34.76 ± 0.007 | |
| AgNPs + C. t | 143.11 ± 1.800 | 42.17 ± 1.038 | 29.95 ± 0.185 | 6.90 ± 0.082 | 35.02 ± 0.059 | |
| NaCl treatment Mean | 140.52 ± 1.458 A | 38.51 ± 1.04 A | 25.63 ± 0.163 AA | 6.96 ± 0.052 A | 34.85 ± 0.025 A | |
| (Ag/ Ct) treatment Mean | ||||||
| Control | 129.96 ± 1.221 c | 29.9 ± 1.24 d | 16.68 ± 0.16 d | 4.79 ± 0.02 a | 27.45 ± 0.02 a | |
| AgNPs | 134.77 ± 1.32 ab | 33.23 ± 1.19 b | 20.73 ± 0.13 b | 4.26 ± 0.04 c | 25.7 ± 0.06 b | |
| C.t | 132.63 ± 1.40 bc | 30.85 ± 1.27 c | 18.07 ± 0.14 c | 4.46 ± 0.06 bc | 26.36 ± 0.02 ab | |
| AgNPs + C. t | 137.33 ± 1.32 a | 36.66 ± 1.04 a | 23.72 ± 0.17 a | 4.45 ± 0.04 b | 26.45 ± 0.06 a | |
| An honestly significant difference (HSD) at p < 0.05 probability level using Tukey′s test for: | ||||||
| NaCl treatments | 9.71 | 2.79 | 1.99 | 0.482 | 0.502 | |
| (Ag/Ct) treatment Mean | 4.14 | 1.21 | 0.853 | 0.188 | 0.015 | |
| NaCl × Ag/C. t Interaction | ns | ns | 2.4; 3.6* | 0.53; 2.42 | ns | |
| Salinity/AgNPs + Ct Treatments | TPC mg/g | AsA mg/g | GSH nM/g | |
|---|---|---|---|---|
| 0.0 mM NaCl | Control | 5.24 ± 0.115 | 7.66 ± 0.005 | 360.34 ± 0.176 |
| AgNPs | 5.61 ± 0.102 | 12.04 ± 0.062 | 386.98 ± 0.139 | |
| C. t | 5.38 ± 0.176 | 10.92 ± 0.017 | 379.01 ± 0.023 | |
| AgNPs + C.t | 5.57 ± 0.192 | 9.97 ± 0.043 | 380.43 ± 0.105 | |
| NaCl treatment Mean | 5.45 ± 0.156 D | 10.15 ± 0.032 D | 376.69 ± 0.11 C | |
| 25 mM NaCl | Control | 8.12 ± 0.171 | 11.84 ± 0.098 | 371.92 ± 0.199 |
| AgNPs | 9.03 ± 0.173 | 14.73 ± 0.002 | 390.06 ± 0.132 | |
| C. t | 8.72 ± 0.105 | 12.06 ± 0.083 | 386.55 ± 0.101 | |
| AgNPs + C. t | 8.50 ± 0.183 | 12.87 ± 0.063 | 388.72 ± 0.102 | |
| NaCl treatment Mean | 8.59 ± 0.158 C | 12.88 ± 0.062 C | 384.31 ± 0.133 BC | |
| 50 mM NaCl | Control | 11.01 ± 0.197 | 15.25 ± 0.065 | 386.59 ± 0.011 |
| AgNPs | 11.37 ± 0.131 | 17.44 ± 0.0188 | 406.31 ± 0.162 | |
| C. t | 11.25 ± 0.122 | 16.91 ± 0.024 | 399.89 ± 0.190 | |
| AgNPs + C. t | 11.32 ± 0.134 | 17.11 ± 0.029 | 401.57 ± 0.122 | |
| NaCl treatment Mean | 11.24 ± 0.146 B | 16.68 ± 0.034 B | 398.59 ± 0.121 AB | |
| 100 mM NaCl | Control | 13.06 ± 0.165 | 19.06 ± 0.088 | 409.36 ± 0.145 |
| AgNPs | 13.28 ± 0.193 | 20.82 ± 0.005 | 416.80 ± 0.057 | |
| C.t | 13.09 ± 0.126 | 19.75 ± 0.005 | 410.58 ± 0.168 | |
| AgNPs + C. t | 13.15 ± 0.104 | 20.09 ± 0.061 | 412.11 ± 0.102 | |
| NaCl treatment Mean | 13.145 ± 0.147 A | 19.93 ± 0.04 A | 412.21 ± 0.12 A | |
| (Ag/C. t) treatment Mean | ||||
| Control | 9.36 ± 0.172 b | 13.45 ± 0.064 c | 382.05 ± 0.133 b | |
| AgNPs | 9.82 ± 0.15 a | 16.26 ± 0.022 a | 400.04 ± 0.122 a | |
| C.t | 9.61 ± 0.132 ab | 14.91 ± 0.032 b | 394.01 ± 0.12 a | |
| AgNPs + C. t | 9.64 ± 0.153 a | 15.01 ± 0.049 b | 395.71 ± 0.11 a | |
| An honestly significant difference (HSD) at p < 0.05 probability level using Tukey′s test for: | ||||
| NaCl treatments | 0.93 | 1.42 | 28.39 | |
| (Ag/C. t) treatment Mean | 0.36 | 0.56 | 11.97 | |
| NaCl × Ag/C. t Interaction | ns | 1.60; 2.51* | ns | |
| Salinity /AgNPs + C. t Treatments | SOD U/mg Protein | CAT U/mg Protein | POD U/mg Protein | APX U/mg Protein | G.R U/mg Protein | |
|---|---|---|---|---|---|---|
| 0.0 mM NaCl | Control | 103.42 ± 0.199 | 205.64 ± 0.191 | 101.26 ± 0.153 | 96.97 ± 0.124 | 120.53 ± 0.112 |
| AgNPs | 129.50 ± 0.112 | 220.87 ± 0.113 | 134.56 ± 0.189 | 118.26 ± 0.157 | 145.37 ± 0.117 | |
| C. t | 116.39 ± 0.117 | 217.02 ± 0.081 | 117.92 ± 0.127 | 105.67 ± 0.129 | 132.65 ± 0.106 | |
| AgNPs + C.t | 121.05 ± 0.143 | 209.63 ± 0.175 | 126.55 ± 0.130 | 112.89 ± 0.108 | 129.80 ± 0.187 | |
| NaCl treatment Mean | 117.59 ± 0.13 C | 213.29 ± 0.14 B | 120.073 ± 0.150 C | 108.45 ± 0.13 C | 132.088 ± 0.131 C | |
| 25 mM NaCl | Control | 119.10 ± 0.156 | 210.75 ± 0.118 | 113.68 ± 0.107 | 104.69 ± 0.113 | 126.43 ± 0.144 |
| AgNPs | 132.89 ± 0.103 | 232.69 ± 0.137 | 153.54 ± 0.114 | 130.62 ± 0.194 | 157.26 ± 0.156 | |
| C. t | 127.75 ± 0.107 | 224.55 ± 0.122 | 129.72 ± 0.172 | 124.17 ± 0.198 | 140.05 ± 0.107 | |
| AgNPs + C. t | 129.64 ± 0.119 | 217.22 ± 0.119 | 134.89 ± 0.183 | 118.45 ± 0.176 | 146.33 ± 0.159 | |
| NaCl treatment Mean | 127.3 ± 0.121 C | 221.31 ± 0.124 B | 132.96 ± 0.144 AB | 119.48 ± 0.17 B | 142.52 ± 0.142 B | |
| 50 mM NaCl | Control | 125.93 ± 0.130 | 235.10 ± 0.116 | 118.51 ± 0.145 | 113.89 ± 0.151 | 132.64 ± 0.169 |
| AgNPs | 154.80 ± 0.127 | 257.29 ± 0.132 | 136.92 ± 0129 | 128.86 ± 0.126 | 167.39 ± 0.126 | |
| C. t | 141.61 ± 0.109 | 243.77 ± 0.069 | 121.87 ± 0.136 | 119.31 ± 0.023 | 151.67 ± 0.141 | |
| AgNPs + C. t | 148.78 ± 0.111 | 248.99 ± 0.185 | 126.12 ± 0.118 | 123.11 ± 0.160 | 162.90 ± 0.101 | |
| NaCl treatment Mean | 142.78 ± 120 B | 246.23 ± 0.125 A | 125.86 ± 0.132 BC | 121.29 ± 0.115 B | 153.65 ± 0.134 A | |
| 100 mM NaCl | Control | 133.30 ± 0.106 | 249.67 ± 0.168 | 126.63 ± 0.112 | 116.59 ± 0.127 | 146.51 ± 0.124 |
| AgNPs | 168.88 ± 0.159 | 263.86 ± 0.172 | 147.95 ± 0.190 | 148.94 ± 0.004 | 158.10 ± 0.116 | |
| C. t | 156.31 ± 0.197 | 251.84 ± 0.015 | 135.16 ± 0.135 | 123.66 ± 0.139 | 149.14 ± 0.110 | |
| AgNPs + C. t | 161.07 ± 0.120 | 257.62 ± 0.04 | 139.74 ± 0.080 | 135.07 ± 0.028 | 153.25 ± 0.108 | |
| NaCl treatment Mean | 154.85 ± 0.145 A | 255.75 ± 0.098 A | 137.37 ± 0.129 A | 131.07 ± 0.075 A | 151.75 ± 0.115 A | |
| (Ag/C. t) treatment Mean | ||||||
| Control | 120.44 ± 0.150 b | 225.29 ± 0.148 c | 120.44 ± 0.152 d | 108.04 ± 0.13 d | 131.53 ± 0.14 d | |
| AgNPs | 146.48 ± 0.125 b | 243.66 ± 0.139 a | 143.24 ± 0.16 a | 131.67 ± 0.12 a | 157.03 ± 0.144 a | |
| C. t | 135.5 ± 0.133 a | 234.278 ± 0.072 b | 126.17 ± 0.143 c | 118.203 ± 0.122 c | 143.38 ± 0.116 c | |
| AgNPs + C. t | 140.11 ± 0.123 a | 233.34 ± 0.130 b | 131.83 ± 0.128 b | 122.38 ± 0.118 b | 148.07 ± 0.139 b | |
| An honestly significant difference (HSD) at p < 0.05 probability level using Tukey′s test for: | ||||||
| NaCl treatments | 11.19 | 17.97 | 9.39 | 9.27 | 10.86 | |
| (Ag/Ct) treatment Mean | 4.63 | 7.45 | 4.06 | 3.94 | 4.57 | |
| NaCl × Ag/C. t Interaction | 13.13; 20.04* | ns | 11.51; 17.04 | 11.17; 16.74 | 3.07; 19.53 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalofah, A.; Kilany, M.; Migdadi, H. Phytostimulatory Influence of Comamonas testosteroni and Silver Nanoparticles on Linum usitatissimum L. under Salinity Stress. Plants 2021, 10, 790. https://doi.org/10.3390/plants10040790
Khalofah A, Kilany M, Migdadi H. Phytostimulatory Influence of Comamonas testosteroni and Silver Nanoparticles on Linum usitatissimum L. under Salinity Stress. Plants. 2021; 10(4):790. https://doi.org/10.3390/plants10040790
Chicago/Turabian StyleKhalofah, Ahlam, Mona Kilany, and Hussein Migdadi. 2021. "Phytostimulatory Influence of Comamonas testosteroni and Silver Nanoparticles on Linum usitatissimum L. under Salinity Stress" Plants 10, no. 4: 790. https://doi.org/10.3390/plants10040790
APA StyleKhalofah, A., Kilany, M., & Migdadi, H. (2021). Phytostimulatory Influence of Comamonas testosteroni and Silver Nanoparticles on Linum usitatissimum L. under Salinity Stress. Plants, 10(4), 790. https://doi.org/10.3390/plants10040790






