Metal and Metalloid Toxicity in Plants: An Overview on Molecular Aspects
Abstract
1. Introduction
2. Plant Evolution for Hyperaccumulation and Tolerance
3. Metal and Metalloid Toxicity in Plants
3.1. The Effects and Responses of Zinc (Zn) in Plants
3.2. Induction of ROS by Copper (Cu) and the Response in Plants
3.3. Aluminium Response Mechanisms: Exclusion and Tolerance in Plants
3.4. Toxic Effects of Lead (Pb) in Plants and Their Response
3.5. Effects, Response, and Cadmium (Cd) Tolerance in Plants
3.6. Arsenic (As) Uptake, Response, and Detoxification by Plants
4. Transcriptomes and Metabolic and Functional Characterization of Metal-Toxicity-Related Genes
5. Biotechnological and Ecological Applications of Accumulator and Hyperaccumulator Plants
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kopittke, P.M.; Wang, P.; Lombi, E.; Donner, E. Synchrotron-based X-Ray Approaches for Examining Toxic Trace Metal(loid)s in Soil-Plant Systems. J. Environ. Qual. 2017, 46, 1175–1189. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Singh, V.P. The Relative Impact of Toxic Heavy Metals (THMs)(Arsenic (As), Cadmium (Cd), Chromium (Cr)(VI), Mercury (Hg), and Lead (Pb)) On the Total Environment: An Overview. Environ. Monit. Assess. 2019, 191, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Narendrula-Kotha, R.; Theriault, G.; Mehes-Smith, M.; Kalubi, K.; Nkongolo, K. Metal Toxicity and Resistance in Plants and Microorganisms in Terrestrial Ecosystems. Residue Rev. 2019, 249, 1–27. [Google Scholar] [CrossRef]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.-Y.; Li, J.; Wang, P.-Y.; Qin, F.; et al. Plant Abiotic Stress Response and Nutrient Use Efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef] [PubMed]
- Chmielowska-Bąk, J.; Deckert, J. Plant Recovery after Metal Stress—A Review. Plants 2021, 10, 450. [Google Scholar] [CrossRef]
- Di Marzio, A.; Lambertucci, S.; Fernandez, A.G.; Martínez-López, E. From Mexico to the Beagle Channel: A Review of Metal and Metalloid Pollution Studies on Wildlife Species in Latin America. Environ. Res. 2019, 176, 108462. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Hernández, J.C.; Vázquez-Delgado, O.R.; Castillo-Morales, M.; Varela-Caselis, J.L.; Santamaría-Juárez, J.D.; Olivares-Xometl, O.; Morales, J.A.; Pérez-Osorio, G. Phytoremediation of Mine Tailings by Brassica Juncea Inoculated with Plant Growth-Promoting Bacteria. Microbiol. Res. 2019, 228, 126308. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.E.; Cruz-Ortega, R.; Meza-Figueroa, D.; Romero, F.M.; Sanchez-Escalante, J.J.; Maier, R.M.; Neilson, J.W.; Alcaraz, L.D.; Freaner, F.E.M. Plants from the Abandoned Nacozari Mine Tailings: Evaluation of Their Phytostabilization Potential. PeerJ 2017, 5, e3280. [Google Scholar] [CrossRef]
- Fryzova, R.; Pohanka, M.; Martinkova, P.; Cihlarova, H.; Brtnicky, M.; Hladky, J.; Kynicky, J. Oxidative Stress and Heavy Metals in Plants. Residue Rev. 2017, 245, 129–156. [Google Scholar] [CrossRef]
- Von Uexküll, H.R.; Mutert, E. Global Extent, Development and Economic Impact of Acid Soils. Plant Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Clemens, S.; Ma, J.F. Toxic Heavy Metal and Metalloid Accumulation in Crop Plants and Foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef]
- Qiao, X.; Li, Q.; Yin, H.; Qi, K.; Li, L.; Wang, R.; Zhang, S.; Paterson, A.H. Gene Duplication and Evolution in Recurring Polyploidization–Diploidization Cycles in Plants. Genome Biol. 2019, 20, 1–23. [Google Scholar] [CrossRef]
- Alloway, B.J. Sources of Heavy Metals and Metalloids in Soils. In Environmental Pollution; Metzler, J.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 11–50. [Google Scholar]
- Yadav, V.; Arif, N.; Kováč, J.; Singh, V.P.; Tripathi, D.K.; Chauhan, D.K.; Vaculík, M. Structural Modifications of Plant Organs and Tissues by Metals and Metalloids in the Environment: A Review. Plant Physiol. Biochem. 2021, 159, 100–112. [Google Scholar] [CrossRef]
- Armendariz, A.L.; Talano, M.A.; Travaglia, C.; Reinoso, H.; Oller, A.L.W.; Agostini, E. Arsenic Toxicity in Soy-Bean Seedlings and Their Attenuation Mechanisms. Plant Physiol. Biochem. 2016, 98, 119–127. [Google Scholar] [CrossRef]
- Pereira, F.J.; de Castro, E.M.; Pires, M.F.; Oliveira, C.; de Pasqual, M. Anatomical and Physiological Modifications in Water Hyacinth under Cadmium Contamination. J. Appl. Bot. Food Qual. 2017, 90, 10–17. [Google Scholar]
- Naila, A.; Meerdink, G.; Jayasena, V.; Sulaiman, A.Z.; Ajit, A.B.; Berta, G. A Review on Global Metal Accumu-Lators—Mechanism, Enhancement, Commercial Application, and Research Trend. Environ. Sci. Pollut. Res. 2019, 26, 26449–26471. [Google Scholar] [CrossRef] [PubMed]
- Poschenrieder, C.; Busoms, S.; Barceló, J. How Plants Handle Trivalent (+3) Elements. Int. J. Mol. Sci. 2019, 20, 3984. [Google Scholar] [CrossRef]
- Xia, J.; Yamaji, N.; Kasai, T.; Ma, J.F. Plasma Membrane-Localized Transporter for Aluminum in Rice. Proc. Natl. Acad. Sci. USA 2010, 107, 18381–18385. [Google Scholar] [CrossRef]
- Mosa, K.A.; Saadoun, I.; Kumar, K.; Helmy, M.; Dhankher, O.P. Potential Biotechnological Strategies for the Cleanup of Heavy Metals and Metalloids. Front. Plant Sci. 2016, 7, 303. [Google Scholar] [CrossRef]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally Sustainable Way for Reclamation of Heavy Metal Polluted Soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef]
- Chandra, R.; Saxena, G.; Kumar, V. Phytoremediation of Environmental Pollutants: An Eco-Sustainable Green Technology to Environmental Management. Adv. Biodegrad. Bioremediation Ind. Waste 2015, 1–29. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.; Wenzel, W.W.; Rinklebe, J. Trace Elements in the Soil-Plant Interface: Phytoavailability, Translocation, and Phytoremediation–A Review. Earth Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Oh, S.; Park, Y.-K. Overview of Biochar Production from Preservative-Treated Wood with Detailed Analysis of Biochar Characteristics, Heavy Metals Behaviors, and Their Ecotoxicity. J. Hazard. Mater. 2020, 384, 121356. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Bednik, M.; Chohura, P. Assessing the Influence of Compost and Biochar Amend-ments on the Mobility and Uptake of Heavy Metals by Green Leafy Vegetables. Int. J. Environ. Res. Public Health 2020, 17, 7861. [Google Scholar] [CrossRef]
- Khan, A.Z.; Ding, X.; Khan, S.; Ayaz, T.; Fidel, R.; Khan, M.A. Biochar Efficacy for Reducing Heavy Metals up-Take by Cilantro (Coriandrum sativum) and Spinach (Spinaccia oleracea) to Minimize Human Health Risk. Chemosphere 2020, 244, 125543. [Google Scholar] [CrossRef]
- Melbinger, A.; Vergassola, M. The Impact of Environmental Fluctuations on Evolutionary Fitness Functions. Sci. Rep. 2015, 5, 15211. [Google Scholar] [CrossRef]
- Manara, A.; Fasani, E.; Furini, A.; DalCorso, G. Evolution of the Metal Hyperaccumulation and Hypertolerance Traits. Plant, Cell Environ. 2020, 43, 2969–2986. [Google Scholar] [CrossRef]
- Johnson, M.T.J.; Prashad, C.M.; Lavoignat, M.; Saini, H.S. Contrasting the Effects of Natural Selection, Genetic Drift and Gene Flow on Urban Evolution in White Clover (Trifolium repens). Proc. R. Soc. B Boil. Sci. 2018, 285, 20181019. [Google Scholar] [CrossRef]
- Klerks, P.L.; Xie, L.; Levinton, J.S. Quantitative Genetics Approaches to Study Evolutionary Processes in Eco-Toxicology; A Perspective from Research on the Evolution of Resistance. Ecotoxicology 2011, 20, 513–523. [Google Scholar] [CrossRef]
- Dupont, C.L.; Butcher, A.; Valas, R.E.; Bourne, P.E.; Caetano-Anollés, G. History of Biological Metal Utilization Inferred through Phylogenomic Analysis of Protein Structures. Proc. Natl. Acad. Sci. USA 2010, 107, 10567–10572. [Google Scholar] [CrossRef]
- Capdevila, M.; Atrian, S. Metallothionein Protein Evolution: A Miniassay. JBIC J. Biol. Inorg. Chem. 2011, 16, 977–989. [Google Scholar] [CrossRef]
- Reeves, R.D. Metal-Accumulating Plants. In Phytoremediation of Toxic Metals: Using Plants to Clean Up the Environment; Ensley, B.D., Ed.; John Wiley & Son: London, UK, 2000; pp. 193–229. [Google Scholar]
- Reeves, R.D.; Baker, A.J.M.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; Van Der Ent, A. A Global Database for Plants That Hyperaccumulate Metal and Metalloid Trace Elements. New Phytol. 2018, 218, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Reeves, R.D.; Baker, A.J. Facultative Hyperaccumulation of Heavy Metals and Metalloids. Plant Sci. 2014, 217–218, 8–17. [Google Scholar] [CrossRef]
- Hanikenne, M.; Nouet, C. Metal Hyperaccumulation and Hypertolerance: A Model for Plant Evolutionary Genomics. Curr. Opin. Plant Biol. 2011, 14, 252–259. [Google Scholar] [CrossRef]
- Kabouw, P.; Van Dam, N.M.; Van Der Putten, W.H.; Biere, A. How Genetic Modification of Roots Affects Rhizosphere Processes and Plant Performance. J. Exp. Bot. 2011, 63, 3475–3483. [Google Scholar] [CrossRef] [PubMed]
- Seregin, I.V.; Kozhevnikova, A.D. Low-Molecular-Weight Ligands in Plants: Role in Metal Homeostasis and Hyperaccumulation. Photosynth. Res. 2020, 1–46. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular Mechanisms of Metal Hyperaccumulation in Plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef] [PubMed]
- Yogeeswaran, K.; Frary, A.; York, T.L.; Amenta, A.; Lesser, A.H.; Nasrallah, J.B.; Tanksley, S.D.; Nasrallah, M.E. Comparative Genome Analyses of Arabidopsis spp.: Inferring Chromosomal Rearrangement Events in the Evolutionary History of A. thaliana. Genome Res. 2005, 15, 505–515. [Google Scholar] [CrossRef]
- Clauss, M.J.; Koch, M.A. Poorly Known Relatives of Arabidopsis thaliana. Trends Plant Sci. 2006, 11, 449–459. [Google Scholar] [CrossRef]
- van de Mortel, J.E.; Villanueva, L.A.; Schat, H.; Kwekkeboom, J.; Coughlan, S.; Moerland, P.D.; van Themaat, E.V.L.; Koornneef, M.; Aarts, M.G.M. Large Expression Differences in Genes for Iron and Zinc Homeostasis, Stress Response and Lignin Biosynthesis Distinguish Roots of Arabidopsis Thaliana and the Related Metal Hyper-Accumulator Thlaspi Caerulescens. Plant Physiol. 2006, 142, 1127–1147. [Google Scholar] [CrossRef]
- Guimarães, M.D.A.; Gustin, J.L.; Salt, D.E. Reciprocal Grafting Separates the Roles of the Root and Shoot in Zinc Hyperaccumulation in Thlaspi caerulescens. New Phytol. 2009, 184, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Nouet, C.; Charlier, J.-B.; Carnol, M.; Bosman, B.; Farnir, F.; Motte, P.; Hanikenne, M. Functional Analysis of the Three HMA4 Copies of the Metal Hyperaccumulator Arabidopsis halleri. J. Exp. Bot. 2015, 66, 5783–5795. [Google Scholar] [CrossRef]
- Hanikenne, M.; Talke, I.N.; Haydon, M.J.; Lanz, C.; Nolte, A.; Motte, P.; Kroymann, J.; Weigel, D.; Krämer, U. Evolution of Metal Hyperaccumulation Required Cis-Regulatory Changes and Triplication of HMA4. Nat. Cell Biol. 2008, 453, 391–395. [Google Scholar] [CrossRef]
- Pauwels, M.; Saumitou-Laprade, P.; Holl, A.C.; Petit, D.; Bonnin, I. Multiple Origin of Metallicolous Pop-Ulations of the Pseudometallophyte Arabidopsis Halleri (Brassicaceae) in Central Europe: The cpDNA Testimony. Mol. Ecol. 2005, 14, 4403–4414. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy Metals Toxicity in Plants: An Overview on the Role of Glutathione and Phytochelatins in Heavy Metal Stress Tolerance of Plants. South Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy Metal Hyperaccumulating Plants: How and Why Do They Do It? and What Makes Them So Interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil Amendments for Immobilization of Potentially Toxic Elements in Contaminated Soils: A Critical Review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Fu, S.-F.; Chen, P.-Y.; Nguyen, Q.T.T.; Huang, L.-Y.; Zeng, G.-R.; Huang, T.-L.; Lin, C.-Y.; Huang, H.-J. Tran-Scriptome Profiling of Genes and Pathways Associated with Arsenic Toxicity and Tolerance in Arabidopsis. BMC Plant Biol. 2014, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.-F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive Oxygen Species and Heavy Metal Stress in Plants: Impact on the Cell Wall and Secondary Metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Huang, X.; Zhou, Y.; Quan, Q.; Li, Y.; Zhu, X. Response of Photosynthesis to Different Concentrations of Heavy Metals in Davidia involucrata. PLoS ONE 2020, 15, e0228563. [Google Scholar] [CrossRef]
- Jalmi, S.K.; Bhagat, P.K.; Verma, D.; Noryang, S.; Tayyeba, S.; Singh, K.; Sharma, D.; Sinha, A.K. Traversing the Links between Heavy Metal Stress and Plant Signaling. Front. Plant Sci. 2018, 9, 12. [Google Scholar] [CrossRef]
- Mehes-Smith, M.; Nkongolo, K.; Cholewa, E. Coping Mechanisms of Plants to Metal Contaminated Soil. Environ. Change Sustain. 2013, 54, 53–90. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy Metal Tolerance in Plants: Role of Transcriptomics, Proteomics, Metabolomics, and Ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef]
- Rizvi, A.; Zaidi, A.; Ameen, F.; Ahmed, B.; Alkahtani, M.D.F.; Khan, M.S. Heavy Metal Induced Stress on Wheat: Phytotoxicity and Microbiological Management. RSC Adv. 2020, 10, 38379–38403. [Google Scholar] [CrossRef]
- Wilkens, S. Structure and Mechanism of ABC Transporters. F1000Prime Rep. 2015, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Park, J.; Choi, H.; Burla, B.; Kretzschmar, T.; Lee, Y.; Martinoia, E. Plant ABC Transporters. Arab. Book 2011, 9, e0153. [Google Scholar] [CrossRef]
- Hwang, J.-U.; Song, W.-Y.; Hong, D.; Ko, D.; Yamaoka, Y.; Jang, S.; Yim, S.; Lee, E.; Khare, D.; Kim, K.; et al. Plant ABC Transporters Enable Many Unique Aspects of a Terrestrial Plant’s Lifestyle. Mol. Plant 2016, 9, 338–355. [Google Scholar] [CrossRef]
- Frelet-Barrand, A.; Kolukisaoglu, H.; Üner; Plaza, S.; Rüffer, M.; Azevedo, L.; Hörtensteiner, S.; Marinova, K.; Weder, B.; Schulz, B.; Klein, M. Comparative Mutant Analysis of Arabidopsis ABCC-Type ABC Transporters: AtMRP2 Contributes to Detoxification, Vacuolar Organic Anion Transport and Chlorophyll Degradation. Plant Cell Physiol. 2008, 49, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Cózatl, D.G.; Jobe, T.O.; Hauser, F.; Schroeder, J.I. Long-Distance Transport, Vacuolar Sequestration, Tolerance, and Transcriptional Responses Induced by Cadmium and Arsenic. Curr. Opin. Plant Biol. 2011, 14, 554–562. [Google Scholar] [CrossRef]
- Briat, J.-F. Arsenic Tolerance in Plants: “Pas de deux” Between Phytochelatin Synthesis and ABCC Vacuolar Transporters. Proc. Natl. Acad. Sci. USA 2010, 107, 20853–20854. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of Heavy Metals—Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Ali, W.; Isayenkov, S.V.; Zhao, F.-J.; Maathuis, F.J.M. Arsenite Transport in Plants. Cell. Mol. Life Sci. 2009, 66, 2329–2339. [Google Scholar] [CrossRef]
- Guengerich, F.P. Thematic Minireview Series: Metals in Biology 2012. J. Biol. Chem. 2012, 287, 13508–13509. [Google Scholar] [CrossRef]
- Gustin, J.L.; Zanis, M.J.E.; Salt, D. Structure and Evolution of the Plant Cation Diffusion Facilitator Family of Ion Transporters. BMC Evol. Biol. 2011, 11, 76. [Google Scholar] [CrossRef]
- Song, W.-Y.; Park, J.; Mendoza-Cózatl, D.G.; Suter-Grotemeyer, M.; Shim, D.; Hörtensteiner, S.; Geisler, M.; Weder, B.; Rea, P.A.; Rentsch, D. Arsenic Tolerance in Arabidopsis Is Mediated by Two ABCC-Type Phyto-Chelatin Transporters. Proc. Natl. Acad. Sci. USA 2010, 107, 21187–21192. [Google Scholar] [CrossRef]
- Palmgren, M.G.; Clemens, S.; Williams, L.E.; Krämer, U.; Borg, S.; Schjørring, J.K.; Sanders, D. Zinc Biofortifi-Cation of Cereals: Problems and Solutions. Trends Plant Sci. 2008, 13, 464–473. [Google Scholar] [CrossRef]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling Cadmium Toxicity and Tolerance in Plants: Insight into Regulatory Mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Jakubowska, D.; Janicka-Russak, M.; Kabała, K.; Migocka, M.; Reda, M. Modification of Plasma Membrane NADPH Oxidase Activity in Cucumber Seedling Roots in Response to Cadmium Stress. Plant Sci. 2015, 234, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhaes, J.V. Plant Adaptation to Acid Soils: The Molecular Basis for Crop Aluminum Resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Huang, C.-F.; Yamaji, N.; Ma, J.F. Knockout of a Bacterial-Type ATP-Binding Cassette Transporter Gene, AtSTAR1, Results in Increased Aluminum Sensitivity in Arabidopsis. Plant Physiol. 2010, 153, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Reyna-Llorens, I.; Corrales, I.; Poschenrieder, C.; Barcelo, J.; Cruz-Ortega, R. Both Aluminum and ABA Induce the Expression of an ABC-like Transporter Gene (FeALS3) in the Al-tolerant Species Fagopyrum esculentum. Environ. Exp. Bot. 2015, 111, 74–82. [Google Scholar] [CrossRef]
- Mattiello, L.; Begcy, K.; Da Silva, F.R.; Jorge, R.A.; Menossi, M. Transcriptome Analysis Highlights Changes in the Leaves of Maize Plants Cultivated in Acidic Soil Containing Toxic Levels of Al3+. Mol. Biol. Rep. 2014, 41, 8107–8116. [Google Scholar] [CrossRef]
- Thounaojam, T.C.; Panda, P.; Mazumdar, P.; Kumar, D.; Sharma, G.; Sahoo, L.; Sanjib, P. Excess Copper Induced Oxidative Stress and Response of Antioxidants in Rice. Plant Physiol. Biochem. 2012, 53, 33–39. [Google Scholar] [CrossRef]
- Mei, L.; Daud, M.K.; Ullah, N.; Ali, S.; Khan, M.; Malik, Z.; Zhu, S.J. Pretreatment with Salicylic Acid and Ascorbic Acid Significantly Mitigate Oxidative Stress Induced by Copper in Cotton Genotypes. Environ. Sci. Pollut. Res. 2015, 22, 9922–9931. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.L.; de Almeida, A.-A.F.; de Souza, J.S.; Mangabeira, P.A.O.; de Jesus, R.M.; Pirovani, C.P.; Ahnert, D.; Baligar, V.C.; Loguercio, L.L. Altered Physiology, Cell Structure, and Gene Expression of Theobroma Cacao Seed-Lings Subjected to Cu Toxicity. Environ. Sci. Pollut. Res. 2014, 21, 1217–1230. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Seraj, Z.I.; Fujita, M. Exogenous Sodium Nitroprusside and Glutathione Alleviate Copper Tox-Icity by Reducing Copper Uptake and Oxidative Damage in Rice (Oryza sativa L.) Seedlings. Protoplasma 2014, 251, 1373–1386. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Bali, S.; Khanna, K.; Arora, S.; Sharma, A.; Bhardwaj, R. Current Scenario of Pb Toxicity in Plants: Unraveling Plethora of Physiological Responses. Residue Rev. 2019, 249, 153–197. [Google Scholar] [CrossRef]
- Kaur, G.; Kaur, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K.; Rishi, V. Biochemical Adaptations in Zea mays Roots to Short-Term Pb2+ Exposure: ROS Generation and Metabolism. Bull. Environ. Contam. Toxicol. 2015, 95, 246–253. [Google Scholar] [CrossRef]
- Usman, K.; Abu-Dieyeh, M.H.; Zouari, N.; Al-Ghouti, M.A. Lead (Pb) Bioaccumulation and Antioxidative Responses in Tetraena Qataranse. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Reis, G.S.M.; de Almeida, A.-A.F.; de Almeida, N.M.; de Castro, A.V.; Mangabeira, P.A.O.; Pirovani, C.P. Molecular, Biochemical and Ultrastructural Changes Induced by Pb Toxicity in Seedlings of Theobroma cacao L. PLoS ONE 2015, 10, e0129696. [Google Scholar] [CrossRef] [PubMed]
- Castillo-González, J.; Ojeda-Barrios, D.; Hernández-Rodríguez, A.; González-Franco, A.C.; Ro-bles-Hernández, L.; López-Ochoa, G.R. Zinc Metalloenzymes in Plants. Interciencia 2018, 43, 242–248. [Google Scholar]
- Reis, S.; Pavia, I.; Carvalho, A.; Moutinho-Pereira, J.; Correia, C.; Lima-Brito, J. Seed Priming with Iron and Zinc in Bread Wheat: Effects in Germination, Mitosis and Grain Yield. Protoplasma 2018, 255, 1179–1194. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, A.; Namdjoyan, S.; Soorki, A.A. Effects of Exogenous Melatonin and Glutathione on Zinc Toxicity in Safflower (Carthamus tinctorius L.) Seedlings. Ecotoxicol. Environ. Saf. 2020, 201, 110853. [Google Scholar] [CrossRef] [PubMed]
- Ricachenevsky, F.K.; Menguer, P.K.; Sperotto, R.A.; Fett, J.P. Got to Hide Your Zn Away: Molecular Control of Zn Accumulation and Biotechnological Applications. Plant Sci. 2015, 236, 1–17. [Google Scholar] [CrossRef]
- Pochodylo, A.L.; Aristilde, L. Molecular Dynamics of Stability and Structures in Phytochelatin Complexes with Zn, Cu, Fe, Mg, and Ca: Implications for Metal Detoxification. Environ. Chem. Lett. 2017, 15, 495–500. [Google Scholar] [CrossRef]
- Grennan, A.K. Metallothioneins, a Diverse Protein Family. Plant Physiol. 2011, 155, 1750–1751. [Google Scholar] [CrossRef]
- Pineau, C.; Loubet, S.; Lefoulon, C.; Chalies, C.; Fizames, C.; Lacombe, B.; Ferrand, M.; Loudet, O.; Berthomieu, P.; Richard, O. Natural Variation at the FRD3 MATE Transporter Locus Reveals Cross-Talk between Fe Homeostasis and Zn Tolerance in Arabidopsis Thaliana. PLoS Genet. 2012, 8, e1003120. [Google Scholar] [CrossRef] [PubMed]
- Delhaize, E.; Ma, J.F.; Ryan, P.R. Transcriptional Regulation of Aluminium Tolerance Genes. Trends Plant Sci. 2012, 17, 341–348. [Google Scholar] [CrossRef]
- Seigneurin-Berny, D.; Gravot, A.; Auroy, P.; Mazard, C.; Kraut, A.; Finazzi, G.; Grunwald, D.; Rappaport, F.; Vavasseur, A.; Joyard, J.; et al. HMA1, a New Cu-ATPase of the Chloro plast Envelope, Is Essential for Growth under Adverse Light Conditions. J. Biol. Chem. 2006, 281, 2882–2892. [Google Scholar] [CrossRef]
- Schaaf, G.; Ludewig, U.; Erenoglu, B.E.; Mori, S.; Kitahara, T.; von Wirén, N. ZmYS1 Functions as a Proton-Coupled Symporter for Phytosiderophore-and Nicotianamine-Chelated Metals. J. Biol. Chem. 2004, 279, 9091–9096. [Google Scholar] [CrossRef]
- Liu, X.; Chen, J.; Wang, G.-H.; Wang, W.-H.; Shen, Z.-J.; Luo, M.-R.; Gao, G.-F.; Simon, M.; Ghoto, K.; Zheng, H.-L. Hydrogen Sulfide Alleviates Zinc Toxicity by Reducing Zinc Uptake and Regulating Genes Expression of Antioxidative Enzymes and Metallothioneins in Roots of the Cadmium/Zinc Hyperaccumulator Solanum nigrum L. Plant Soil 2016, 400, 177–192. [Google Scholar] [CrossRef]
- Ramakrishna, B.; Rao, S.S.R. Foliar Application of Brassinosteroids Alleviates Adverse Effects of Zinc Toxicity in Radish (Raphanus sativus L.) Plants. Protoplasma 2015, 252, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Viehweger, K. How Plants Cope with Heavy Metals. Bot. Stud. 2014, 55, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Elsevier BV: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Kumar, V.; Pandita, S.; Sidhu, G.P.S.; Sharma, A.; Khanna, K.; Kaur, P.; Bali, A.S.; Setia, R. Copper Bioavailability, Uptake, Toxicity and Tolerance in Plants: A Comprehensive Review. Chemosphere 2021, 262, 127810. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Tripathi, D.K.; Singh, S.; Sharma, S.; Dubey, N.K.; Chauhan, D.K.; Vaculík, M. Toxicity of Aluminium on Various Levels of Plant Cells and Organism: A Review. Environ. Exp. Bot. 2017, 137, 177–193. [Google Scholar] [CrossRef]
- Hänsch, R.; Mendel, R.R. Physiological Functions of Mineral Micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B., Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Yadav, S.K.; Singla-Pareek, S.L.; Reddy, M.K.; Sopory, S.K. Methylglyoxal Detoxification by Glyoxalase System: A Survival Strategy during Environmental Stresses. Physiol. Mol. Biol. Plants 2005, 11, 1. [Google Scholar]
- Shin, L.-J.; Lo, J.-C.; Yeh, K.-C. Copper Chaperone Antioxidant Protein1 Is Essential for Copper Homeostasis. Plant Physiol. 2012, 159, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.; Nath, S.; Chanu, T.T.; Sharma, G.D.; Panda, S.K. Cadmium Stress-Induced Oxidative Stress and Role of Nitric Oxide in Rice (Oryza sativa L.). Acta Physiol. Plant. 2011, 33, 1737–1747. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.H.; Hu, L.Y.; Wang, S.H.; Zhang, F.Q.; Hu, K.D. Effects of Exogenous Nitric Oxide Donor on Antioxidant Metabolism in Wheat Leaves under Aluminum Stress. Russ. J. Plant Physiol. 2008, 55, 469–474. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Basalah, M.O. Role of Nitric Oxide in Tolerance of Plants to Abiotic Stress. Protoplasma 2011, 248, 447–455. [Google Scholar] [CrossRef]
- Metwally, A.; Finkemeier, I.; Georgi, M.; Dietz, K.-J. Salicylic Acid Alleviates the Cadmium Toxicity in Barley Seedlings. Plant Physiol. 2003, 132, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Arunakumara, K.K.I.U.; Walpola, B.C.; Yoon, M.-H. Aluminum Toxicity and Tolerance Mechanism in Cereals and Legumes—A Review. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 1–9. [Google Scholar] [CrossRef]
- Pickar-Oliver, A.; Gersbach, C.A. The Next Generation of CRISPR–Cas Technologies and Applications. Nat. Rev. Mol. Cell Biol. 2019, 20, 490–507. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Riaz, M.; Liu, J.; Yu, M.; Cuncang, J. The Aluminum Tolerance and Detoxification Mechanisms in Plants; Recent Advances and Prospects. Crit. Rev. Environ. Sci. Technol. 2021, 1–37. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Y.; Fan, S.; Wang, S.; Shi, Q.; Zhang, M.; Zhang, J. Detoxification of Heavy Metals Attributed to Biological and Non–Biological Complexes in Soils around Copper Producing Areas throughout China. J. Clean. Prod. 2021, 292, 125999. [Google Scholar] [CrossRef]
- Samad, R.; Rashid, P.; Karmoker, J. Anatomical Responses of Rice (Oryza sativa, L.) to Aluminium Toxicity. J. Bangladesh Acad. Sci. 2020, 43, 123–131. [Google Scholar] [CrossRef]
- Xu, L.-M.; Liu, C.; Cui, B.-M.; Wang, N.; Zhao, Z.; Zhou, L.-N.; Huang, K.-F.; Ding, J.-Z.; Du, H.-M.; Jiang, W.; et al. Transcriptomic Responses to Aluminum (Al) Stress in Maize. J. Integr. Agric. 2018, 17, 1946–1958. [Google Scholar] [CrossRef]
- Riaz, M.; Yan, L.; Wu, X.; Hussain, S.; Aziz, O.; Jiang, C. Mechanisms of Organic Acids and Boron Induced Tolerance of Aluminum Toxicity: A Review. Ecotoxicol. Environ. Saf. 2018, 165, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.J.; Baker, A.; Muench, S.P. The Varied Functions of Aluminium-Activated Malate Transporters–Much More Than Aluminium Resistance. Biochem. Soc. Trans. 2016, 44, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, K.; Shitan, N.; Yazaki, K. The Multidrug and Toxic Compound Extrusion (MATE) Family in Plants. Plant Biotechnol. 2014, 31, 417–430. [Google Scholar] [CrossRef]
- Salazar-Chavarría, V.; Sánchez-Nieto, S.; Cruz-Ortega, R. Fagopyrum Esculentum at Early Stages Copes with Aluminum Toxicity by Increasing ABA Levels and Antioxidant System. Plant Physiol. Biochem. 2020, 152, 170–176. [Google Scholar] [CrossRef]
- Fu, Z.; Jiang, X.; Li, W.-W.; Shi, Y.; Lai, S.; Zhuang, J.; Yao, S.; Liu, Y.; Hu, J.; Gao, L.; et al. Proanthocyanidin–Aluminum Complexes Improve Aluminum Resistance and Detoxification of Camellia sinensis. J. Agric. Food Chem. 2020, 68, 7861–7869. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Hashida, K.; Otsuka, Y.; Ohara, S.; Kojima, K.; Shinohara, K. Identification of a Hydrolyzable Tannin, Oenothein B, as an Aluminum-Detoxifying Ligand in a Highly Aluminum-Resistant Tree, Eucalyptus camaldulensis. Plant Physiol. 2014, 164, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, U.; Ali, S.; Shakoor, M.B.; Farid, M.; Hussain, S.; Yasmeen, T.; Adrees, M.; Bharwana, S.A.; Abbas, F. EDTA Ameliorates Phytoextraction of Lead and Plant Growth by Reducing Morphological and Biochemical Injuries in Brassica napus L. under Lead Stress. Environ. Sci. Pollut. Res. 2014, 21, 9899–9910. [Google Scholar] [CrossRef] [PubMed]
- Herbette, S.; Taconnat, L.; Hugouvieux, V.; Piette, L.; Magniette, M.-L.; Cuine, S.; Auroy, P.; Richaud, P.; Forestier, C.; Bourguignon, J.; et al. Genome-Wide Transcriptome Profiling of the Early Cadmium Response of Arabidopsis Roots and Shoots. Biochim. 2006, 88, 1751–1765. [Google Scholar] [CrossRef] [PubMed]
- Uzu, G.; Sobanska, S.; Sarret, G.; Muñoz, M.; Dumat, C. Foliar Lead Uptake by Lettuce Exposed to Atmospheric Fallouts. Environ. Sci. Technol. 2010, 44, 1036–1042. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Morphological, Anatomical, and Ultrastructural Changes (Visualized through Scanning Electron Microscopy) Inducedin Triticum Aestivum by Pb2+ Treatment. Protoplasma 2014, 251, 1407–1416. [Google Scholar] [CrossRef]
- Madhu, P.M.; Sadagopan, R.S. Effect of Heavy Metals on Growth and Development of Cultivated Plants with Reference to Cadmium, Chromium and Lead–A Review. J. Stress Physiol. Biochem. 2020, 16, 84–102. [Google Scholar]
- Gupta, D.; Nicoloso, F.; Schetinger, M.; Rossato, L.; Pereira, L.; Castro, G.; Srivastava, S.; Tripathi, R. Antioxidant Defense Mechanism in Hydroponically Grown Zea mays Seedlings under Moderate Lead Stress. J. Hazard. Mater. 2009, 172, 479–484. [Google Scholar] [CrossRef]
- Pereira, A.S.; Cortez, P.A.; De Almeida, A.-A.F.; Prasad, M.N.V.; França, M.G.C.; Da Cunha, M.; De Jesus, R.M.; Mangabeira, P.A.O. Morphology, Ultrastructure, and Element Uptake in Calophyllum brasiliense Cambess. (Calophyllaceae, J. Agardh) Seedlings under Cadmium Exposure. Environ. Sci. Pollut. Res. 2017, 24, 15576–15588. [Google Scholar] [CrossRef]
- Grant, C.A.; Buckley, W.T.; Bailey, L.D.; Selles, F. Cadmium Accumulation in Crops. Can. J. Plant Sci. 1998, 78, 1–17. [Google Scholar] [CrossRef]
- Kushwaha, A.; Rani, R.; Kumar, S.; Gautam, A. Heavy Metal Detoxification and Tolerance Mechanisms in Plants: Implications for Phytoremediation. Environ. Rev. 2016, 24, 39–51. [Google Scholar] [CrossRef]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.; Hu, C. Cadmium in Plants: Uptake, Toxicity, and Its Interactions with Selenium Fertilizers. Metals 2019, 11, 255–277. [Google Scholar] [CrossRef]
- Bayçu, G.; Gevrek-Kürüm, N.; Moustaka, J.; Csatári, I.; Rognes, S.E.; Moustakas, M. Cadmium-Zinc Accumulation and Photosystem II Responses of Noccaea Caerulescens to Cd and Zn Exposure. Environ. Sci. Pollut. Res. 2016, 24, 2840–2850. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Abbas, T.; Adrees, M.; Zia-Ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Qayyum, M.F.; Nawaz, R. Residual Effects of Biochar on Growth, Photosynthesis and Cadmium Uptake in Rice (Oryza sativa L.) under Cd Stress with Different Water Conditions. J. Environ. Manag. 2018, 206, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Mayonde, S.; Cron, G.V.; Glennon, K.L.; Byrne, M.J. Effects of Cadmium Toxicity on the Physiology and Growth of a Halophytic Plant, Tamarix usneoides (E. Mey. ex Bunge). Int. J. Phytoremediation 2021, 23, 130–138. [Google Scholar] [CrossRef]
- Murtaza, G.; Javed, W.; Hussain, A.; Qadir, M.; Aslam, M. Soil-Applied Zinc and Copper Suppress Cadmium Uptake and Improve the Performance of Cereals and Legumes. Int. J. Phytoremediation 2016, 19, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, P.; Zanella, L.; De Paolis, A.; Di Litta, D.; Cecchetti, V.; Falasca, G.; Barbieri, M.; Altamura, M.M.; Costantino, P.; Cardarelli, M. Cadmium-Inducible Expression of the ABC-Type Transporter AtABCC3 Increases Phytochelatin-Mediated Cadmium Tolerance in Arabidopsis. J. Exp. Bot. 2015, 66, 3815–3829. [Google Scholar] [CrossRef]
- Heyno, E.; Klose, C.; Krieger-Liszkay, A. Origin of Cadmium-Induced Reactive Oxygen Species Production: Mitochondrial Electron Transfer versus Plasma Membrane NADPH Oxidase. New Phytol. 2008, 179, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Janik, E.; Maksymiec, W.; Mazur, R.; Garstka, M.; Gruszecki, W.I. Structural and Functional Modifications of the Major Light-Harvesting Complex II in Cadmium- or Copper-Treated Secale cereale. Plant Cell Physiol. 2010, 51, 1330–1340. [Google Scholar] [CrossRef]
- Li, H.; Hu, T.; Amombo, E.; Fu, J. Transcriptome Profilings of Two Tall Fescue (Festuca arundinacea) Cultivars in Response to Lead (Pb) stress. BMC Genom. 2017, 18, 145. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.; Mukherjee, A. Investigating the Underlying Mechanism of Cadmium-Induced Plant Adaptive Response to Genotoxic Stress. Ecotoxicol. Environ. Saf. 2021, 209, 111817. [Google Scholar] [CrossRef] [PubMed]
- Shiyu, Q.I.N.; Hongen, L.I.U.; Zhaojun, N.I.E.; Rengel, Z.; Wei, G.A.O.; Chang, L.I.; Peng, Z. Toxicity of Cadmium and Its Competition with Mineral Nutrients for Uptake by Plants: A Review. Pedosphere 2020, 30, 168–180. [Google Scholar]
- Abbas, G.; Murtaza, B.; Niazi, N.K.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M. Natasha Arsenic Uptake, Toxicity, Detoxification, and Speciation in Plants: Physiological, Biochemical, and Molecular Aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Khan, E. Mechanism of Arsenic Toxicity and Tolerance in Plants: Role of Silicon and Signalling Molecules. In Stress Responses in Plants; Metzler, J.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 143–157. [Google Scholar]
- Li, N.; Wang, J.; Song, W.-Y. Arsenic Uptake and Translocation in Plants. Plant Cell Physiol. 2016, 57, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Mattusch, J.; Wennrich, R. Accumulation and Transformation of Inorganic and Organic Arsenic in Rice and Role of Thiol-Complexation to Restrict Their Translocation to Shoot. Sci. Rep. 2017, 7, 40522. [Google Scholar] [CrossRef] [PubMed]
- Castrillo, G.; Sánchez-Bermejo, E.; de Lorenzo, L.; Crevillén, P.; Fraile-Escanciano, A.; Mohan, T.C.; Mouriz, A.; Catarecha, P.; Sobrino-Plata, J.; Olsson, S. WRKY6 Transcription Factor Restricts Arsenate Uptake and Transposon Activation in Arabidopsis. Plant Cell. 2013, 25, 2944–2957. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.I.; Latif, H.H.; Hanafy, R.S. Influence of Nitric Oxide Application on Some Biochemical Aspects, Endogenous Hormones, Minerals and Phenolic Compounds of Vicia faba Plant Grown under Arsenic Stress. Gesunde Pflanz. 2016, 68, 99–107. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Gupta, M. Exposure of Brassica Juncea L. to Arsenic Species in Hydroponic Medium: Comparative Analysis in Accumulation and Bio-Chemical and Transcriptional Alterations. Environ. Sci. Pollut. Res. 2013, 20, 8141–8150. [Google Scholar] [CrossRef]
- Peryea, F. Heavy Metal Contamination in Deciduous Tree Fruit Orchards: Implications for Mineral Nutrient Management. Acta Hortic. 2001, 31–39. [Google Scholar] [CrossRef]
- Larsen, P.B.; Geisler, M.; Jones, C.A.; Williams, K.M.; Cancel, J.D. ALS3 Encodes a Phloem-Localized ABC Transporter-like Protein That Is Required for Aluminum Tolerance in Arabidopsis. Plant J. 2005, 41, 353–363. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, W.U.; Shah, A.A.; Yasin, N.A.; Naz, S.; Ali, A.; Tahir, A.; Batool, A.I. Synergistic Effects of Nitric Oxide and Silicon on Promoting Plant Growth, Oxidative Stress Tolerance and Reduction of Arsenic Uptake in Brassica juncea. Chemosphere 2021, 262, 128384. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Corpas, F.J.; Ahmad, P. Salicylic Acid-Induced Nitric Oxide Enhances Arsenic Toxicity Tolerance in Maize Plants by Upregulating the Ascorbate-Glutathione Cycle and Glyoxalase System. J. Hazard. Mater. 2020, 399, 123020. [Google Scholar] [CrossRef]
- Dubey, S.; Shri, M.; Misra, P.; Lakhwani, D.; Bag, S.K.; Asif, M.H.; Trivedi, P.K.; Tripathi, R.D.; Chakrabarty, D. Heavy Metals Induce Oxidative Stress and Genome-Wide Modulation in Transcriptome of Rice Root. Funct. Integr. Genom. 2014, 14, 401–417. [Google Scholar] [CrossRef]
- Shri, M.; Chakrabarty, D.; Verma, G. Mechanisms of Arsenic Hyperaccumulation by Plants. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives II; Metzler, J.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 767–785. [Google Scholar]
- Xu, X.; Zhang, S.; Cheng, Z.; Li, T.; Jia, Y.; Wang, G.; Yang, Z.; Xian, J.; Yang, Y.; Zhou, W. Transcriptome Analysis Revealed Cadmium Accumulation Mechanisms in Hyperaccumulator Siegesbeckia orientalis L. Environ. Sci. Pollut. Res. 2020, 27, 18853–18865. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, H.; Reinfelder, J.R.; Liang, X.; Sun, C.; Liu, C.; Li, F.; Yi, J. A Transcriptomic (RNA-seq) Analysis of Genes Responsive to Both Cadmium and Arsenic Stress in Rice Root. Sci. Total. Environ. 2019, 666, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-Q.; Bai, Z.-Y.; Xiao, Y.-F.; Li, Y.; Liu, Q.-L.; Zhang, L.; Pan, Y.-Z.; Jiang, B.-B.; Zhang, F. Transcriptomic Analysis of Verbena Bonariensis Roots in Response to Cadmium Stress. BMC Genom. 2019, 20, 877. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wang, Y.; Zhang, S.; Guo, Q.; Jin, Y.; Chen, J.; Gao, Y.; Ma, H. Transcriptomic and Physiological Analyses of Medicago sativa L. Roots in Response to Lead Stress. PLoS ONE 2017, 12, e0175307. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Lin, S. Transcriptomic Revelation of Phenolic Compounds Involved in Aluminum Toxicity Responses in Roots of Cunninghamia lanceolata (Lamb) Hook. Genes 2019, 10, 835. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, B.; Yuan, Y.; Xu, Q.; Chen, P. Transcriptome Profiling of Fagopyrum Tataricum Leaves in Response to Lead Stress. BMC Plant Biol. 2020, 20, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cui, J.; Cai, Y.; Yang, S.; Liu, J.; Wang, W.; Gai, J.; Hu, Z.; Li, Y. Comparative Transcriptome Analysis of Two Contrasting Soybean Varieties in Response to Aluminum Toxicity. Int. J. Mol. Sci. 2020, 21, 4316. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, M.; Sun, J.; Mao, X.; Wang, J.; Liu, H.; Zheng, H.; Li, X.; Zhao, H.; Zou, D. Heavy Metal Stress-Associated Proteins in Rice and Arabidopsis: Genome-Wide Identification, Phylogenetics, Duplication, and Expression Profiles Analysis. Front. Genet. 2020, 11, 477. [Google Scholar] [CrossRef]
- Tian, W.; He, G.; Qin, L.; Li, D.; Meng, L.; Huang, Y.; He, T. Genome-Wide Analysis of the NRAMP Gene Family in Potato (Solanum tuberosum): Identification, Expression Analysis and Response to Five Heavy Metals Stress. Ecotoxicol. Environ. Saf. 2021, 208, 111661. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, J.; Zhao, L.; Yang, S.; Song, Y. Impact of Heavy Metal Stresses on the Growth and Auxin Homeostasis of Arabidopsis Seedlings. BioMetals 2014, 28, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, Y.; Lu, C.; Peng, H.; Luo, M.; Li, G.; Shen, Y.; Ding, H.; Zhang, Z.; Pan, G.; et al. The Development Dynamics of the Maize Root Transcriptome Responsive to Heavy Metal Pb Pollution. Biochem. Biophys. Res. Commun. 2015, 458, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-L.; Huang, L.-Y.; Fu, S.-F.; Trinh, N.-N.; Huang, H.-J. Genomic Profiling of Rice Roots with Short-and Long-Term Chromium Stress. Plant Mol. Biol. 2014, 86, 157–170. [Google Scholar] [CrossRef]
- Yokosho, K.; Yamaji, N.; Ma, J.F. Global Transcriptome Analysis of Al-Induced Genes in an Al-Accumulating Species, Common Buckwheat (Fagopyrum esculentum Moench). Plant Cell Physiol. 2014, 55, 2077–2091. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, H.; Zhu, Y.; Zou, J.; Zhao, F.-J.; Huang, C.-F. Genome-Wide Transcriptomic and Phylogenetic Analyses Reveal Distinct Aluminum-Tolerance Mechanisms in the Alumi-Num-Accumulating Species Buckwheat (Fagopyrum tataricum). BMC Plant Biol. 2015, 15, 16. [Google Scholar] [CrossRef]
- Ariani, A.; Di Baccio, D.; Romeo, S.; Lombardi, L.; Andreucci, A.; Lux, A.; Horner, D.S.; Sebastiani, L. RNA Sequencing of Populus x canadensis Roots Identifies Key Molecular Mechanisms Underlying Physiological Adaption to Excess Zinc. PLoS ONE 2015, 10, e0117571. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cao, F.; Chen, F.; Sun, H.; Zhang, G.; Chen, Z.-H.; Wu, F. Genome-Wide Transcriptome and Functional Analysis of Two Contrasting Genotypes Reveals Key Genes for Cadmium Tolerance in Barley. BMC Genom. 2014, 15, 611. [Google Scholar] [CrossRef]
- Han, M.; Lu, X.; Yu, J.; Chen, X.; Wang, X.; Malik, W.A.; Wang, J.; Wang, D.; Wang, S.; Guo, L.; et al. Transcriptome Analysis Reveals Cotton (Gossypium hirsutum) Genes That Are Differentially Expressed in Cadmium Stress Tolerance. Int. J. Mol. Sci. 2019, 20, 1479. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Nesi, A.; Brito, D.S.; Inostroza-Blancheteau, C.; Fernie, A.R.; Araújo, W.L. The Complex Role of Mitochondrial Metabolism in Plant Aluminum Resistance. Trends Plant Sci. 2014, 19, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kobayashi, Y.; Devi, S.R.; Rikiishi, S.; Matsumoto, H. Aluminum Toxicity Is Associated with Mitochondrial Dysfunction and the Production of Reactive Oxygen Species in Plant Cells. Plant Physiol. 2002, 128, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xing, D. Mechanistic Study of Mitochondria-Dependent Programmed Cell Death Induced by Aluminium Phytotoxicity Using Fluorescence Techniques. J. Exp. Bot. 2010, 62, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chen, Y.; Yin, S.; Zhang, M.; Wang, W. Over-expression of TaEXPB23, a Wheat Expansin Gene, Improves Oxidative Stress Tolerance in Transgenic Tobacco Plants. J. Plant Physiol. 2015, 173, 62–71. [Google Scholar] [CrossRef]
- Sun, L.; Liang, C.; Chen, Z.; Liu, P.; Tian, J.; Liu, G.; Liao, H. Superior Aluminium (Al) Tolerance of Stylosanthes Is Achieved Mainly by Malate Synthesis through an Al-Enhanced Malic Enzyme, Sg ME 1. New Phytol. 2014, 202, 209–219. [Google Scholar] [CrossRef]
- Singh, H.; Bhat, J.A.; Singh, V.P.; Corpas, F.J.; Yadav, S.R. Auxin Metabolic Network Regulates the Plant Response to Metalloids Stress. J. Hazard. Mater. 2021, 405, 124250. [Google Scholar] [CrossRef]
- Sharma, A.; Sidhu, G.P.S.; Araniti, F.; Bali, A.S.; Shahzad, B.; Tripathi, D.K.; Brestic, M.; Skalicky, M.; Landi, M. The Role of Salicylic Acid in Plants Exposed to Heavy Metals. Molecules 2020, 25, 540. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone Priming: Regulator for Heavy Metal Stress in Plants. J. Plant Growth Regul. 2019, 38, 739–752. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, H.; Ruan, W.; Deng, M.; Wang, F.; Peng, J.; Luo, J.; Chen, Z.; Yi, K. Abnormal Inflorescence Meristem1 Functions in Salicylic Acid Biosynthesis to Maintain Proper Reactive Oxygen Species Levels for Root Meristem Activity in Rice. Plant Cell 2017, 29, 560–574. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.F.; Zhou, G.; Na, X.F.; Yang, L.; Bin Nan, W.; Liu, X.; Zhang, Y.Q.; Li, J.L.; Bi, Y.R. Cadmium Interferes with Maintenance of Auxin Homeostasis in Arabidopsis Seedlings. J. Plant Physiol. 2013, 170, 965–975. [Google Scholar] [CrossRef]
- Husain, T.; Fatima, A.; Suhel, M.; Singh, S.; Sharma, A.; Prasad, S.M.; Singh, V.P. A Brief Appraisal of Ethylene Signaling under Abiotic Stress in Plants. Plant Signal. Behav. 2020, 15, 1782051. [Google Scholar] [CrossRef]
- Noman, A.; Aqeel, M. miRNA-Based Heavy Metal Homeostasis and Plant Growth. Environ. Sci. Pollut. Res. 2017, 24, 10068–10082. [Google Scholar] [CrossRef] [PubMed]
- Millar, A.A. The Function of miRNAs in Plants. Plants 2020, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Pegler, J.L.; Oultram, J.M.J.; Nguyen, D.Q.; Grof, C.P.L.; Eamens, A.L. MicroRNA-Mediated Responses to Cadmium Stress in Arabidopsis thaliana. Plants 2021, 10, 10–130. [Google Scholar] [CrossRef] [PubMed]
- Koźmińska, A.; Wiszniewska, A.; Hanus-Fajerska, E.; Muszyńska, E. Recent Strategies of Increasing Metal Tolerance and Phytoremediation Potential Using Genetic Transformation of Plants. Plant Biotechnol. Rep. 2018, 12, 1–14. [Google Scholar] [CrossRef]
- Fan, W.; Liu, C.; Cao, B.; Qin, M.; Long, D.; Xiang, Z.; Zhao, A. Genome-Wide Identification and Characterization of Four Gene Families Putatively Involved in Cadmium Uptake, Translocation and Sequestration in Mulberry. Front. Plant Sci. 2018, 9, 879. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Bortesi, L.; Baysal, C.; Twyman, R.M.; Fischer, R.; Capell, T.; Schillberg, S.; Christou, P. Characteristics of Genome Editing Mutations in Cereal Crops. Trends Plant Sci. 2017, 22, 38–52. [Google Scholar] [CrossRef]
- Songmei, L.; Jie, J.; Yang, L.; Jun, M.; Shouling, X.; Yuanyuan, T.; Youfa, L.; Qingyao, S.; Jianzhong, H. Characterization and Evaluation of OsLCT1 and OsNramp5 Mutants Generated Through CRISPR/Cas9-Mediated Mutagenesis for Breeding Low Cd Rice. Rice Sci. 2019, 26, 88–97. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Zhou, Y.; Gong, T.; Wang, J.; Ge, Y. Enhanced Phytoremediation of Mixed Heavy Metal (Mercury)–Organic Pollutants (Trichloroethylene) with Transgenic Alfalfa Co-expressing Glutathione S-Transferase and Human P450 2E1. J. Hazard. Mater. 2013, 260, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Viktorovtá, J.; Novakova, M.; Trbolova, L.; Vrchotova, B.; Lovecka, P.; Mackova, M.; Macek, T. Characterization of Transgenic Tobacco Plants Containing Bacterial bphc Gene and Study of Their Phytoremediation Ability. Int. J. Phytoremediation 2014, 16, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.K.A.; Ahmad, A.; Umar, S.; Zia, M.H.; Iqbal, M.; Owens, G. Genotypic Variation in Phytoremediation Potential of Indian Mustard Exposed to Nickel Stress: A Hydroponic Study. Int. J. Phytoremediation 2015, 17, 135–144. [Google Scholar] [CrossRef]
- Chang, S.; Wei, F.; Yang, Y.; Wang, A.; Jin, Z.; Li, J.; He, Y.; Shu, H. Engineering Tobacco to Remove Mercury from Polluted Soil. Appl. Biochem. Biotechnol. 2015, 175, 3813–3827. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Shimo, H.; Bashir, K.; Senoura, T.; Sugimoto, K.; Ono, K.; Suzui, N.; Kawachi, N.; Ishii, S.; et al. From Laboratory to Field: OsNRAMP5-Knockdown Rice Is a Promising Candidate for Cd Phytoremediation in Paddy Fields. PLoS ONE 2014, 9, e98816. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Xu, J.; Li, Q.; Li, S.; Wang, P.; Xiang, F. Cloning and Characterization of a Phragmites australis Phytochelatin Synthase (PaPCS) and Achieving Cd Tolerance in Tall Fescue. PLoS ONE 2014, 9, e103771. [Google Scholar] [CrossRef]
- Liu, D.; An, Z.; Mao, Z.; Ma, L.; Lu, Z. Enhanced Heavy Metal Tolerance and Accumulation by Transgenic Sugar Beets Expressing Streptococcus thermophilus StGCS-GS in the presence of Cd, Zn and Cu Alone or in Combination. PLoS ONE 2015, 10, e0128824. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, W.L.; You, J.F.; Di Bian, M.; Qin, X.M.; Yu, H.; Liu, Q.; Ryan, P.R.; Yang, Z.M. Transgenicarabidopsis Thalianaplants Expressing aβ-1,3-Glucanase from Sweet Sorghum (Sorghum bicolor L.) Show Reduced Callose Deposition and Increased Tolerance to Aluminium Toxicity. Plant, Cell Environ. 2014, 38, 1178–1188. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, N.; Chen, P.; Gao, M.; Liu, J.; Wang, Y.; Zhao, T.; Li, Y.; Gai, J. Overexpression of a Soybean Ariadne-Like Ubiquitin Ligase Gene GmARI1 Enhances Aluminum Tolerance in Arabidopsis. PLoS ONE 2014, 9, e111120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, K.; Zhang, Y.; Dang, Y.; Wei, H.; Wang, Q. Removal of Cr(VI) from Aqueous Solutions Using Buckwheat (Fagopyrum esculentum Moench) Hull through Adsorption-Reduction: Affecting Factors, Isotherm, and Mechanisms. CLEAN Soil Air Water 2014, 42, 1549–1557. [Google Scholar] [CrossRef]
- Andreazza, R.; Bortolon, L.; Pieniz, S.; Barcelos, A.A.; Quadro, M.S.; Camargo, F.A.O. Phytoremediation of Vineyard Copper Contaminated Soil and Copper Mining Waste by a High Potential Bioenergy Crop (Helianthus annus L.). J. Plant Nutr. 2014, 38, 1580–1594. [Google Scholar] [CrossRef]
- López-Bucio, J.; Hernández-Madrigal, F.; Cervantes, C.; Ortiz-Castro, R.; Carreón-Abud, Y.; Martínez-Trujillo, M. Phosphate Relieves Chromium Toxicity in Arabidopsis Thaliana Plants by Interfering with Chromate Uptake. BioMetals 2014, 27, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Habiba, U.; Ali, S.; Farid, M.; Shakoor, M.B.; Rizwan, M.; Ibrahim, M.; Abbasi, G.H.; Hayat, T.; Ali, B. EDTA Enhanced Plant Growth, Antioxidant Defense System, and Phytoextraction of Copper by Brassica napus L. Environ. Sci. Pollut. Res. 2015, 22, 1534–1544. [Google Scholar] [CrossRef]
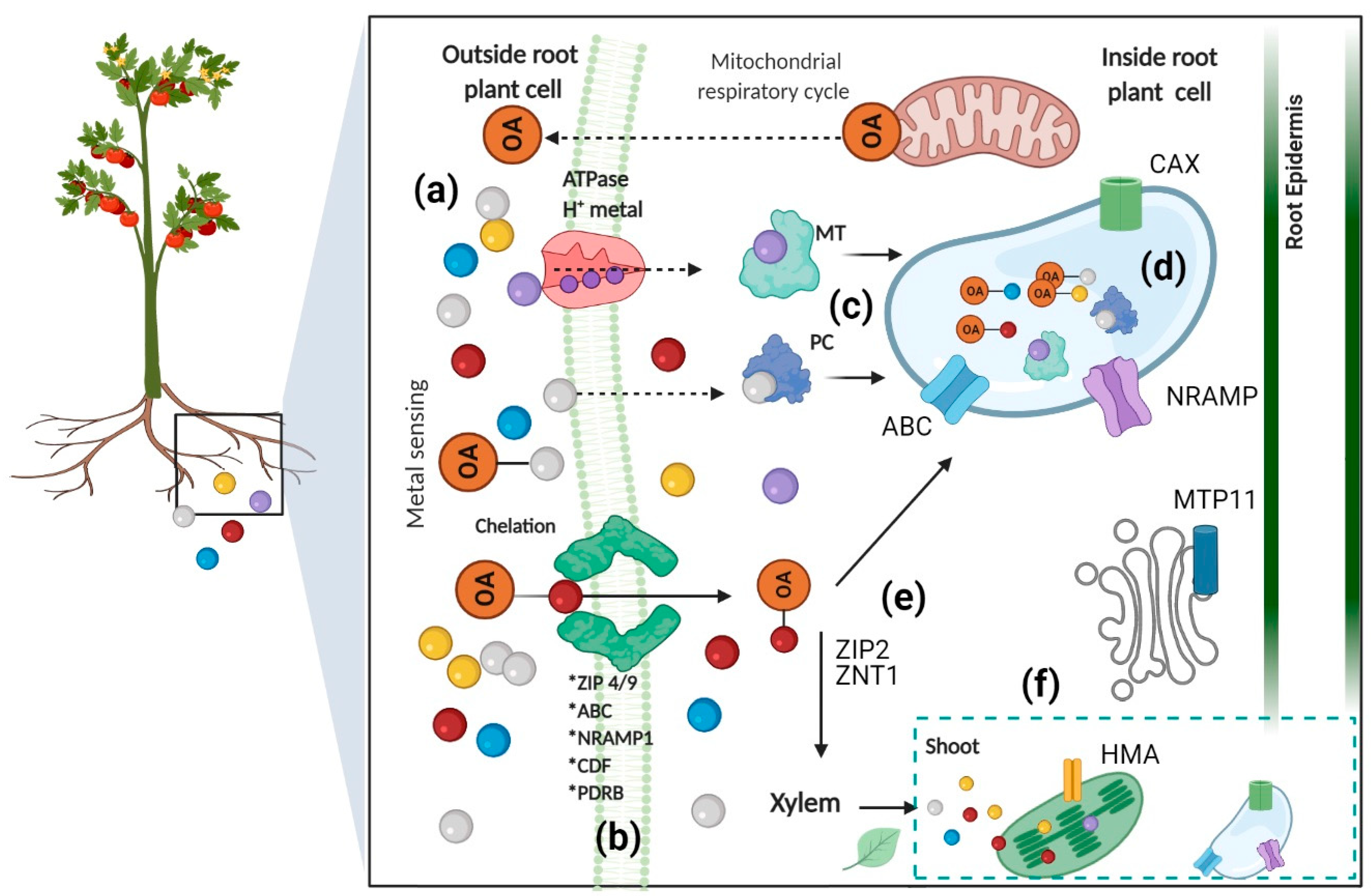
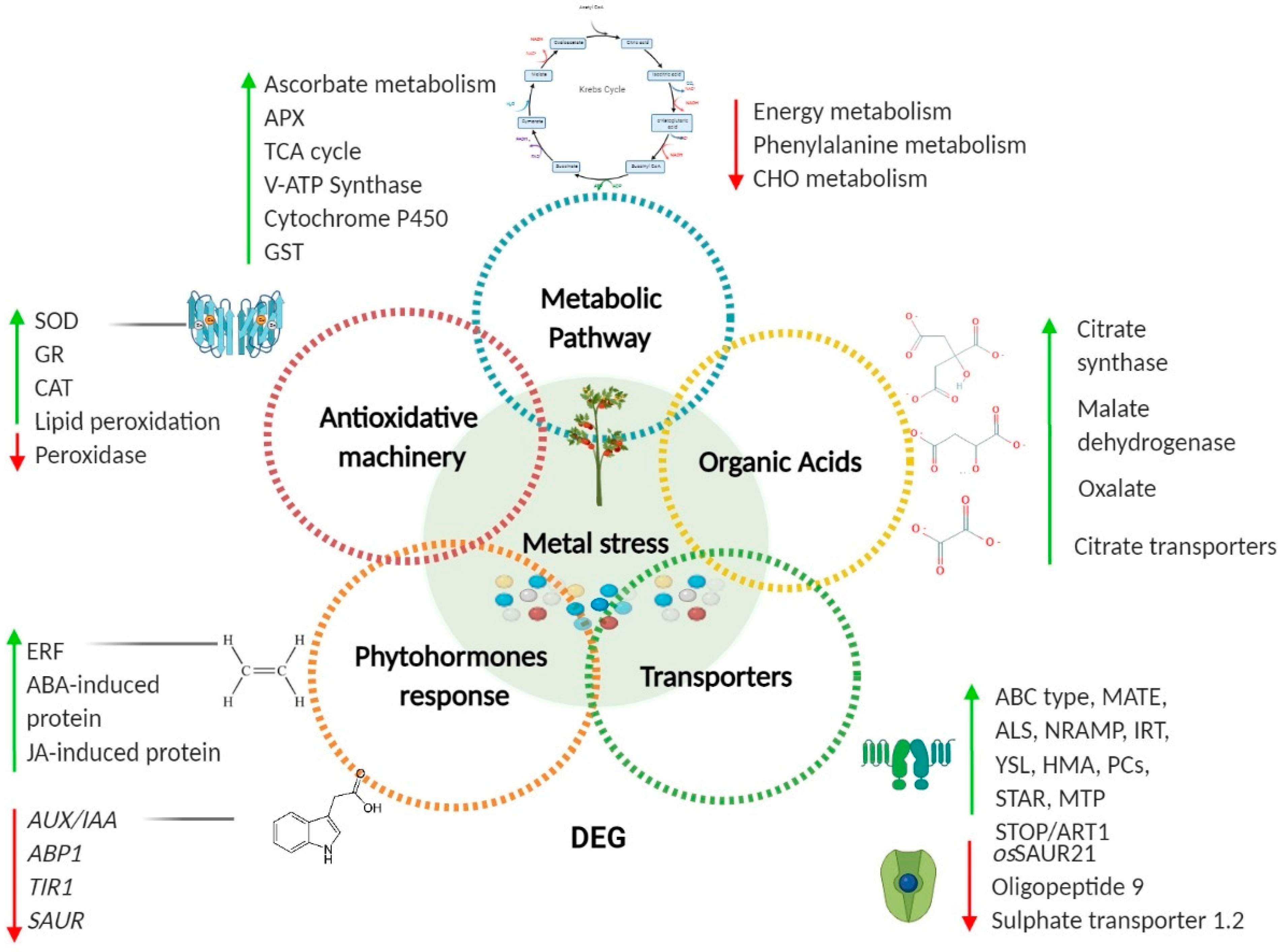
| Metal | Plant Model | Entrance | Chelation/Translocation | Accumulation | Effects | References |
|---|---|---|---|---|---|---|
| As | Oryza sativa Arabidopsis thaliana | Aquaporins [(As(III)] Roots P transporters [As(V)] | AtNIP5;1, AtNIP6;1, AtNIP7, OsNIP2;1, OsNIP3;2, AtABCCJ AtABCC2 | Roots Shoots, Leaves, not always available in edible parts. PCS | ROS increase Lipid peroxidation | [61,62] [63,64] |
| Zn | Arabidopsis thaliana Zea mays | Zn transporters Roots ZRT/IRT YS YSL | PCS+GSH MTs Citrate, malate At-PCR2 root to shoot translocation AtMPT1 translocation to vacuoles AtHMA2-AtHMA4 Fe-phytosiderophores | Tonoplast, vacuoles from dividing cells and roots (YS) Leaves to seeds during senescence (YSL) | Photosynthesis and growth inhibition Chlorosis ROS increase | [65,66,67,68] |
| Cd | Arabidopsis thaliana | NI * | AtABCC1 and AtABCC2 GSH Cd(II) transport AtABCC3 | NI | Growth inhibition, nutrition imbalance, photosynthesis supression, chlorosis, ROS increase | [69,70] |
| Al | Arabidopsis thaliana | Root system Cell wall Plasma membrane Symplasm Apoplasm | ALMT (malate), MATE (citrate), STAR1-STAR2. ALS3-ALS1 FeALS3 AvABCG1 Oxalate | Cell wall Leaf Roots Tonoplast Vacuoles | Growth inhibition ROS increase Lipid peroxidation | [71,72,73,74] |
| Cu | Theobroma cacao Arabidopsis thaliana Oryza sativa | NI * | GSH. PCs, MTs, phytochelatins, YSL, COP, Cu transporters, ERF | Roots Leaves Stem | ROS increase, lipid peroxidation, ionic leakage, protein and nucleic acids damage, changes in chloroplasts, thylakoids. Plasmolysis, chlorosis, rolling of leaves | [47,75,76,77,78] |
| Pb | Lactuca sativa Triticum aestivum Tetraena qataranse | H+/ATPase systems Apoplasm | Thiol compounds PCs, MTs | Inactivation in vacuoles | Stimulation of ROS Disrupts the electron transport in the energy transformation efficiency of photosystem II | [79,80,81,82] |
| Plant Model | Metal/Metalloid | Differentially Expressed Upregulated Genes | Reference |
|---|---|---|---|
| Platanus acerifolia | Pb | Photosynthesis, gibberellins, glutathione, antioxidant enzymes chelating compounds Metal transporters Detoxification mechanisms | [160] |
| Zea mays | Pb | ZmNAS2, ZmNAS4, ZmNAS9, transcription factors, cell wall synthesis, metal redox, ethylene response factors | [161] |
| Oryza sativa | Cd, As, Pb, Cr | Secondary metabolites, flavonoid biosynthesis, lipid metabolism, AA metabolism, CHO metabolism, xenobiotic biodegradation, ascorbate and alderate metabolism, membrane transport, multidrug resistance proteins, iron coupled transporters, major facilitator superfamily, ABC transporters, GST, MAPK signaling pathway proteins. | [149] |
| Oryza sativa | Cr(VI) | Binding activity, metabolic and cellular process proteins, catalytic activity, ethylene related, cytokinin, MAPK pathway, CDKs, ubiquitin proteasome. | [162] |
| Zea mays | Al | TCA cycle, auxin related genes, gibberellic (GA) and jasmonic acid (JA) pathway, brassinosterioids pathway | [74] |
| Oryza sativa (Fe deficient) | Cd | Heavy metal transporters, auxin, NO and gibberellic acid pathway proteins, YSL genes | [149] |
| Fagopyrum esculentum Moench cv Jianxi | Al | STOP/ART1, FeSTAR1, FeALS3, FeALS1, FeMate, FeMate2 | [163] |
| Fagopyrum tataricum | Al | Citrate transporters, ART1/STOP, ALS1, STAR1, STAR2/ALS3, antioxidant activity, xenobiotics biodegradation, lipid metabolism, FtFRDL1, FtFRDL2 | [164] |
| Populus x canadensis | Zn | Redox process, transport and cellular Fe ion homeostasis, ZIP2, NRAMP1, metal chelation, detoxification or glutathione conjugated molecules, metal uptake | [165] |
| Hordeum vulgare (Cd sensitive and Cd tolerant varieties) | Cd | CHO metabolism, catalase, ABA induced protein, JA induced protein 1, defensin, chitinase, dehydrin, ABC transporters, β- ketoacyl CoA synthase, acyl CoA synthase, cytochrome p450, V-ATP synthase, expansin, β-xylosidase | [166] |
| Medicago sativa | Pb | GO enriched pathways: binding, transport, membranes, signaling and energy metabolism. DEGs for POD, SOD, GST, flavonoids and isoflavonoids, chalcone synthase, ABC transporters, IRT, CDFs, WRKY, ERFs and bZIP | [151] |
| Festuca arundinacea | Pb | GO enrchiched in the metabolisms for energy production, terpenoids, poliketides and carbohydrates. Zeatin biosynthesis was increased as well as limonene and pinene degradation, | [135] |
| Oryza sativa L. indica | Cd, As | Redox control, stress response, transcriptional regulation, transmembrane transport, signal transduction, macromolecule and sulfur compound metabolism | [152] |
| Cunninghamia lanceolata (Lamb.) Hook | Al | Cell wall and lipid metabolism, signaling pathways and secondary metabolism, flavonoids and phenylpropanoids. Transcription factors such as bHLH, C2H2, ERF, bZIP, GRAS and MYB | [155] |
| Verbena bonariensis | Cd | Lignin synthesis, chalcone synthase (CHS), anthocyanidin synthase (ANS) | [153] |
| Siegesbeckia orientalis | Cd | NRAMP5 isoform X1, HMA genes, ABC transporter 1, Pleotropic Resitance 1 and 8, GSH, CAT, Peroxidase, L-ascorbate peroxidase, phenylpropanoid biosynthesis | [154] |
| Gossypium hirsutum | Cd | Oxidation-reduction process, metal ion binding. DEGs for metal transporter genes ABC, CDF, HMA, annexin genes and heat shock proteins. GhHMAD5 aids in Cd tolerance | [167] |
| Fagopyrum tataricum | Pb | Metal Transporter Protein C2 (MTPC2), phytochelatin synthetase-like family protein (PCSL), a Vacuolar cation/proton exchanger 1a (VCE1a), NRAMP3, and phytochelatin synthetase (PCS),d | [156] |
| Glycine max (2 varieties: M90-24 and Pella) | Al | 11 genes enriched in the GO for cellulose production: cellulose synthases, which indicates an important role for cellulose in soybean Al tolerance | [137,157] |
| Plant Model | Toxic Metal | Strategy Employed | Main Results | References |
|---|---|---|---|---|
| Medicago sativa | Trichloroethylene (TCE) contaminants | Genetic transformation | Transgenic alfalfa plants coexpressing GST and human P4502El (CYP2El) resulted in an increased resistance and accumulation of heavy metals. | [186] |
| Nicotiana tabacum | PBC Delor 103 | Genetic transformation | bphC, a bacterial gene from the degradation pathway of polychlorinated biphenyls (PBS). The bphC codes for an enzyme 23-dihidroxybipbenyl-1,2-dioxygenase. Production of higher biomass. | [187] |
| Brassica juncea | Ni | Genotype screening in hydroponically grown plants | 10 genotypes screened in hydroponic culture with varying concentrations of nickel chloride (0–50 µM). One genotype, Pusajai Kisan was the most tolerant accumulating up to 1.7 µg Ni g-1 dry weight in aerial parts | [188] |
| Nicotiana tabacum | Hg | Genetic transformation | Two transgenic lines were transformed with merT, merP, merBJ, and merB2 from Hg resistant Pseudomonas K-62. PpK polyphosphate kinase from Enterobacter aerogenes. PCSI, phytochelatin syntbase from N. tabacum. Both transgenic lines were more resistant to organic Hg against the WT. In the Ntp-36 roots, Hg content was up to 251 µg/g. | [189] |
| Oryza sativa | Cd | Knockdown OsNRAMP5 by RNAi | Cd phytoremediation was done producing OsNRA.MP5 RNAi plants using the natural high Cd accumulating cultivar Anjana Dhan (A5i). The biomass of A5i was unchanged by suppression of OsNRA.MP5 expression. These plants accumulated twice as Cd in their shoots as the WT plant. | [190] |
| Festuca arundinacea | Cd | Genetic transformation | PaPCSJ phytochelating synthase 1; PaGCS alpha- glutamyl cysteine synthetase gene. Single and double transformants showed increase tolerance and accumulation of Cd and PC than WT plants. PaGCS appears to have greater influence that PaPCS over synthesis and Cd tolerance/accumulation. | [191] |
| Beta vulgaris | Cd, Zn, Cu, or combinated | Genetic transformation | y-glutamylcysteine synthetase-glutathione synthetase (StGCS-GS) from Streptococcus thermophilus. This GCS has limited feedback inhibition and was overexpressed in sugar beet. Transgenic sugar beets accumulated more Cd, Zn, and Cu ions in shoots than WT and higher GSH and PC levels under different metal stresses. When multiple metals were present at the same time transgenic sugar beets resisted 2 or 3 metal concentrations at the same time. | [192] |
| Arabidopsis thaliana | Al | Genetic transformation | SbGlul, which encodes a β-1,3-glucanase enzyme, was expressed in A. thaliana. Greater Al tolerance than WT plants was observed due to an increase in a P-1,3-glucanase with decrease in Al accumulation and a decrease in callose deposition. | [193] |
| Arabidospis thaliana | Al | Genetic transformation | GmARI gene codes for soybean Ariadne-like ubiquitin ligase gene. Overexpressed in A. thaliana enhanced Al tolerance. | [194] |
| Fagopyrum. esculentum | Cr(VI) | Removal of Cr(VI) from aqueous solutions | pH had significant effect on Cr(VI) removal; optimal pH = 2.0. Removal was analyzed using batch experiments. Cr(VI) was partially reduced to Cr(III). The proposed mechanisms for Cr(VI) removal using buckwheat hull were found by electrostatic attraction, chemical reduction and complex interaction. | [195] |
| Helianthus annus | Cu | Cu-phytoaccumulation | Plants grown in the two vineyard soils showed an increase in height. The bioaccumulation factors (BFC) decreased in plants grown in the three different types of soils, suggesting that they were able to accumulate Cu within them. | [196] |
| Arabidopsis thaliana | Cr(VI) | Analysis of two transgenic lines | At.PTJ and AtPT2 genes in A. thaliana transgenic lines When exposed to 140 µM potassium chromate, an increase in levels of Pi and sulfate was observed and a supplementation to the seedlings with Cr(VI) toxicity completely and partially restored the root growth, respectively. | [197] |
| Brassica napus | Cu | Analysis of EDTA effect of phytoextraction | Cu alone significantly decreased plant growth biomass, photosynthetic pigments, and gas exchange characteristics. Cu stress also reduced the activities of antioxidants such as SOD. POD, APX, and CAT. The application of EDTA significantly alleviated Cu-induced toxic effects. | [198] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angulo-Bejarano, P.I.; Puente-Rivera, J.; Cruz-Ortega, R. Metal and Metalloid Toxicity in Plants: An Overview on Molecular Aspects. Plants 2021, 10, 635. https://doi.org/10.3390/plants10040635
Angulo-Bejarano PI, Puente-Rivera J, Cruz-Ortega R. Metal and Metalloid Toxicity in Plants: An Overview on Molecular Aspects. Plants. 2021; 10(4):635. https://doi.org/10.3390/plants10040635
Chicago/Turabian StyleAngulo-Bejarano, Paola I., Jonathan Puente-Rivera, and Rocío Cruz-Ortega. 2021. "Metal and Metalloid Toxicity in Plants: An Overview on Molecular Aspects" Plants 10, no. 4: 635. https://doi.org/10.3390/plants10040635
APA StyleAngulo-Bejarano, P. I., Puente-Rivera, J., & Cruz-Ortega, R. (2021). Metal and Metalloid Toxicity in Plants: An Overview on Molecular Aspects. Plants, 10(4), 635. https://doi.org/10.3390/plants10040635








