Priming Strategies for Benefiting Plant Performance under Toxic Trace Metal Exposure
Abstract
1. Introduction
2. What Does Priming Really Mean? The Aspects of Priming, Acclimation, and Hormesis
3. Priming at Critical Growth Stages
4. Major Players in Chemical Priming towards Toxic Metal(Loid) Tolerance
5. Other Potent Chemical Priming Agents
6. Genes That Can Be Targeted to Induce Priming Effects for Metallic Stress
7. Hydropriming, Halopriming, Hormopriming, and Biopriming
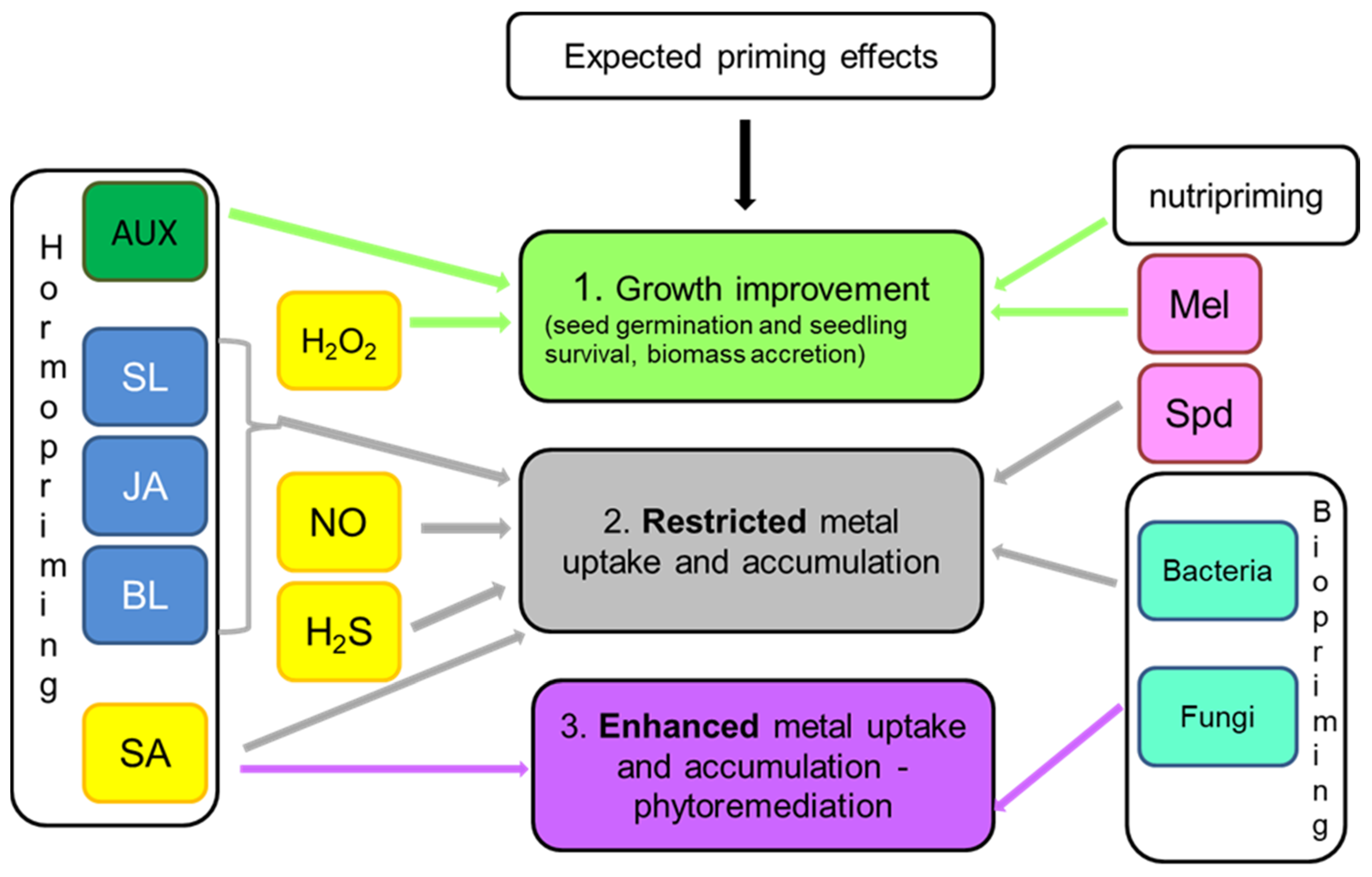
8. Effect-Oriented Priming: Growth Improvement, Metal Uptake Restriction or Metal Uptake Stimulation?
9. Prospective Priming Approaches and Concluding Remarks
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Q.; Leung, J.Y.; Geng, X.; Chen, S.; Huang, X.; Li, H.; Huang, Z.; Zhu, L.; Chen, J.; Lu, Y. Heavy metal contamination of soil and water in the vicinity of an abandoned e-waste recycling site: Implications for dissemination of heavy metals. Sci. Total. Environ. 2015, 506–507, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef]
- Hasan, K.; Cheng, Y.; Kanwar, M.K.; Chu, X.-Y.; Ahammed, G.J.; Qi, Z.-Y. Responses of plant proteins to heavy metal stress—A review. Front. Plant Sci. 2017, 8, 1492. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, M.; Tukiendorf, A. Accumulation and tolerance of lead in two contrasting ecotypes of Dianthus carthusianorum. Phytochemistry 2014, 100, 60–65. [Google Scholar] [CrossRef]
- Muszyńska, E.; Labudda, M.; Hanus-Fajerska, E. Changes in proteolytic activity and protein carbonylation in shoots of Alyssum montanum ecotypes under multi-metal stress. J. Plant Physiol. 2019, 232, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Wiszniewska, A.; Kamińska, I.; Hanus-Fajerska, E.; Sliwinska, E.; Koźmińska, A. Distinct co-tolerance responses to combined salinity and cadmium exposure in metallicolous and non-metallicolous ecotypes of Silene vulgaris. Ecotoxicol. Environ. Saf. 2020, 201, 110823. [Google Scholar] [CrossRef]
- Cabello-Conejo, M.; Prieto-Fernandez, A.; Kidd, P. Exogenous treatments with phytohormones can improve growth and nickel yield of hyperaccumulating plants. Sci. Total. Environ. 2014, 494–495, 1–8. [Google Scholar] [CrossRef]
- Rees, F.; Sterckeman, T.; Morel, J.L. Root development of non-accumulating and hyperaccumulating plants in metal-contaminated soils amended with biochar. Chemosphere 2016, 142, 48–55. [Google Scholar] [CrossRef]
- Moya, J.L.; Ros, R.; Picazo, I. Heavy metal-hormone interactions in rice plants: Effects on growth, net photosynthesis, and carbohydrate distribution. J. Plant Growth Regul. 1995, 14, 61–67. [Google Scholar] [CrossRef]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-Metal-Induced Reactive Oxygen Species: Phytotoxicity and Physicochemical Changes in Plants; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–44. [Google Scholar]
- Sruthi, P.; Shackira, A.M.; Puthur, J.T. Heavy metal detoxification mechanisms in halophytes: An overview. Wetl. Ecol. Manag. 2017, 25, 129–148. [Google Scholar] [CrossRef]
- Balmer, A.; Pastor, V.; Gamir, J.; Flors, V.; Mauch-Mani, B. The ‘prime-ome’: Towards a holistic approach to priming. Trends Plant Sci. 2015, 20, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Vincent, C.; Rowland, D.; Schaffer, B.; Bassil, E.; Racette, K.; Zurweller, B. Primed acclimation: A physiological process offers a strategy for more resilient and irrigation-efficient crop production. Plant Sci. 2020, 295, 110240. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, C.; Xenofontos, R.; Chatzimichail, G.; Christou, A.; Kashfi, K.; Fotopoulos, V. Exploring the potential of nitric oxide and hydrogen sulfide (NOSH)-releasing synthetic compounds as novel priming agents against drought stress in Medicago sativa plants. Biomolecules 2020, 10, 120. [Google Scholar] [CrossRef]
- Leuendorf, J.E.; Frank, M.; Schmülling, T. Acclimation, priming and memory in the response of Arabidopsis thaliana seedlings to cold stress. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical priming of plants against multiple abiotic stresses: Mission possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef]
- Filippou, P.; Tanou, G.; Molassiotis, A.; Fotopoulos, V. Plant Acclimation to Environmental Stress Using Priming Agents; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–27. [Google Scholar]
- Méndez, A.A.; Pena, L.B.; Benavides, M.P.; Gallego, S.M. Priming with NO controls redox state and prevents cadmium-induced general up-regulation of methionine sulfoxide reductase gene family in Arabidopsis. Biochimie 2016, 131, 128–136. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Muszyńska, E.; Kołton, A.; Kamińska, I.; Hanus-Fajerska, E. In vitro acclimation to prolonged metallic stress is associated with modulation of antioxidant responses in a woody shrub Daphne jasminea. Plant Cell. Tissue Organ Cult. 2019, 139, 339–357. [Google Scholar] [CrossRef]
- Hilker, M.; Schwachtje, J.; Baier, M.; Balazadeh, S.; Bäurle, I.; Geiselhardt, S.; Dirk, K.H.; Reinhard, K.; Bernd, M.-R.; Matthias, C.R.; et al. Priming and memory of stress responses in organisms lacking a nervous system. Biol. Rev. 2016, 91, 1118–1133. [Google Scholar] [CrossRef]
- Antoniou, C.; Savvides, A.; Christou, A.; Fotopoulos, V. Unravelling chemical priming machinery in plants: The role of reactive oxygen–nitrogen–sulfur species in abiotic stress tolerance enhancement. Curr. Opin. Plant Biol. 2016, 33, 101–107. [Google Scholar] [CrossRef]
- González-Bosch, C. Priming plant resistance by activation of redox-sensitive genes. Free. Radic. Biol. Med. 2018, 122, 171–180. [Google Scholar] [CrossRef]
- Rowland, D.L.; Faircloth, W.H.; Payton, P.; Tissue, D.T.; Ferrell, J.A.; Sorensen, R.B.; Butts, C.L. Primed acclimation of cultivated peanut (Arachis hypogaea L.) through the use of deficit irrigation timed to crop developmental periods. Agric. Water Manag. 2012, 113, 85–95. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Cabot, C.; Martos, S.; Gallego, B.; Barceló, J. Do toxic ions induce hormesis in plants? Plant Sci. 2013, 212, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Wiszniewska, A.; Hanus-Fajerska, E.; Muszyńska, E.; Smoleń, S. Comparative assessment of response to cadmium in heavy metal-tolerant shrubs cultured in vitro. Water Air Soil Pollut. 2017, 228, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wiszniewska, A.; Muszyńska, E.; Hanus-Fajerska, E.; Dziurka, K.; Dziurka, M. Evaluation of the protective role of exogenous growth regulators against Ni toxicity in woody shrub Daphne jasminea. Planta 2018, 248, 1365–1381. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Blain, R.B. Hormesis and plant biology. Environ. Pollut. 2009, 157, 42–48. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. The hormetic dose-response model is more common than the threshold model in toxicology. Toxicol. Sci. 2003, 71, 246–250. [Google Scholar] [CrossRef]
- Sivritepe, N.; Sivritepe, H.; Eris, A. The effects of NaCl priming on salt tolerance in melon seedlings grown under saline conditions. Sci. Hortic. 2003, 97, 229–237. [Google Scholar] [CrossRef]
- Hassini, I.; Martinez-Ballesta, M.C.; Boughanmi, N.; Moreno, D.A.; Carvajal, M. Improvement of broccoli sprouts (Brassica oleracea L. var. italica) growth and quality by KCl seed priming and methyl jasmonate under salinity stress. Sci. Hortic. 2017, 226, 141–151. [Google Scholar] [CrossRef]
- Zhao, W.; Dong, H.; Zhou, Z.; Wang, Y.; Hu, W. Potassium (K) application alleviates the negative effect of drought on cotton fiber strength by sustaining higher sucrose content and carbohydrates conversion rate. Plant Physiol. Biochem. 2020, 157, 105–113. [Google Scholar] [CrossRef]
- Van Nguyen, D.; Nguyen, H.M.; Le, N.T.; Nguyen, K.H.; Nguyen, H.T.; Le, H.M.; Nguyen, A.T.; Dinh, N.T.T.; Hoang, S.A.; Van Ha, C. Copper nanoparticle application enhances plant growth and grain yield in maize under drought stress conditions. J. Plant Growth Regul. 2021, 1–12. [Google Scholar] [CrossRef]
- Nawaz, F.; Naeem, M.; Akram, A.; Ashraf, M.Y.; Ahmad, K.S.; Zulfiqar, B.; Sardar, H.; Shabbir, R.N.; Majeed, S.; Shehzad, M.A.; et al. Seed priming with KNO3 mediates biochemical processes to inhibit lead toxicity in maize (Zea mays L.). J. Sci. Food Agric. 2017, 97, 4780–4789. [Google Scholar] [CrossRef]
- Imran, M.; Boelt, B.; Mühling, K.-H. Zinc seed priming improves salt resistance in maize. J. Agron. Crop. Sci. 2018, 204, 390–399. [Google Scholar] [CrossRef]
- Basit, A.; Hussain, S.; Abid, M.; Zafar-Ul-Hye, M.; Ahmed, N. Zinc and potassium priming of maize (Zea mays L.) seeds for salt-affected soils. J. Plant Nutr. 2021, 44, 130–141. [Google Scholar] [CrossRef]
- Khan, I.; Raza, M.A.; Awan, S.A.; Shah, G.A.; Rizwan, M.; Ali, B.; Tariq, R.; Hassan, M.J.; Alyemeni, M.N.; Brestic, M.; et al. Amelioration of salt induced toxicity in pearl millet by seed priming with silver nanoparticles (AgNPs): The oxidative damage, antioxidant enzymes and ions uptake are major determinants of salt tolerant capacity. Plant Physiol. Biochem. 2020, 156, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Rojek, J.; Kozieradzka-Kiszkurno, M.; Kapusta, M.; Aksmann, A.; Jacewicz, D.; Drzezdzon, J.; Tesmar, A.; Zamojc, K.; Wyrzykowski, D.; Chmurzynski, L. The effect of vanadium(IV) complexes on development of Arabidopsis thaliana subjected to H2O2-induced stress. Funct. Plant Biol. 2019, 46, 942. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Banerjee, A.; Roychoudhury, A. Seed priming with calcium compounds abrogate fluoride-induced oxidative stress by upregulating defence pathways in an indica rice variety. Protoplasma 2019, 257, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Winter, T.R.; Borkowski, L.; Zeier, J.; Rostás, M. Heavy metal stress can prime for herbivore-induced plant volatile emission. Plant Cell Environ. 2012, 35, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Woźniak, A.; Mai, V.; Rucińska-Sobkowiak, R.; Jeandet, P. The role of heavy metals in plant response to biotic stress. Molecules 2018, 23, 2320. [Google Scholar] [CrossRef]
- Carreiras, J.; Perez-Romero, J.A.; Mateos-Naranjo, E.; Redondo, S.; Matos, A.R.; Cacador, I.; Duarte, B. The effect of heavy metal contamination pre-conditioning in the heat stress tolerance of native and invasive Mediterranean halophytes. Ecol. Indic. 2020, 111, 106045. [Google Scholar] [CrossRef]
- Chen, K.; Arora, R. Priming memory invokes seed stress-tolerance. Environ. Exp. Bot. 2013, 94, 33–45. [Google Scholar] [CrossRef]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Forti, C.; Shankar, A.; Singh, A.; Balestrazzi, A.; Prasad, V.; Macovei, A. Hydropriming and biopriming improve Medicago truncatula seed germination and upregulate DNA repair and antioxidant genes. Genes 2020, 11, 242. [Google Scholar] [CrossRef]
- Babajani, A.; Iranbakhsh, A.; Ardebili, Z.O.; Eslami, B. Seed priming with non-thermal plasma modified plant reactions to selenium or zinc oxide nanoparticles: Cold plasma as a novel emerging tool for plant science. Plasma Chem. Plasma Process. 2018, 39, 21–34. [Google Scholar] [CrossRef]
- Waqas, M.; Korres, N.E.; Khan, M.D.; Nizami, A.-S.; Deeba, F.; Ali, I.; Hussain, H. Advances in the concept and methods of seed priming. In Priming and Pretreatment of Seeds and Seedlings; Springer: Singapore, 2019; pp. 11–41. [Google Scholar]
- Bhardwaj, J.; Anand, A.; Nagarajan, S. Biochemical and biophysical changes associated with magnetopriming in germinating cucumber seeds. Plant Physiol. Biochem. 2012, 57, 67–73. [Google Scholar] [CrossRef]
- Noutoshi, Y.; Okazaki, M.; Kida, T.; Nishina, Y.; Morishita, Y.; Ogawa, T.; Suzuki, H.; Shibata, D.; Jikumaru, Y.; Hanada, A.; et al. Novel plant immune-priming compounds identified via high-throughput chemical screening target salicylic acid glucosyltransferases in Arabidopsis. Plant Cell 2012, 24, 3795–3804. [Google Scholar] [CrossRef] [PubMed]
- Demecsová, L.; Zelinová, V.; Liptáková, Ľ.; Valentovičová, K.; Tamás, L. Indole-3-butyric acid priming reduced cadmium toxicity in barley root tip via NO generation and enhanced glutathione peroxidase activity. Planta 2020, 252, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone priming: Regulator for heavy metal stress in plants. J. Plant Growth Regul. 2019, 38, 739–752. [Google Scholar] [CrossRef]
- Kobylińska, A.; Reiter, R.J.; Posmyk, M.M. Melatonin protects cultured tobacco cells against lead-induced cell death via inhibition of cytochrome c translocation. Front. Plant Sci. 2017, 8, 1560. [Google Scholar] [CrossRef]
- Viviane, C.; Sharella, S.; Kees, H.; Hervé, D.d.B.; Filip, C.; Inge, H.; Jos, M.R.; Víctor, J.C. Priming of plant growth promotion by volatiles of root-associated Microbacterium spp. Appl. Environ. Microbiol. 2018, 84, 1865–1883. [Google Scholar]
- Moghanloo, M.; Iranbakhsh, A.; Ebadi, M.; Satari, T.N.; Ardebili, Z.O. Seed priming with cold plasma and supplementation of culture medium with silicon nanoparticle modified growth, physiology, and anatomy in Astragalus fridae as an endangered species. Acta Physiol. Plant. 2019, 41, 54. [Google Scholar] [CrossRef]
- Nowak, J.; Shulaev, V. Priming for transplant stress resistance in in vitro propagation. Vitr. Cell. Dev. Biol. Anim. 2003, 39, 107–124. [Google Scholar] [CrossRef]
- Da Silva, J.A.T.; Singh, N.; Tanaka, M. Priming biotic factors for optimal protocorm-like body and callus induction in hybrid Cymbidium (Orchidaceae), and assessment of cytogenetic stability in regenerated plantlets. Plant Cell Tissue Organ Cult. (PCTOC) 2006, 84, 135–144. [Google Scholar] [CrossRef]
- Ray, A.; Bhattacharya, S. An improved micropropagation of Eclipta alba by in vitro priming with chlorocholine chloride. Plant Cell, Tissue Organ Cult. 2007, 92, 315–319. [Google Scholar] [CrossRef]
- Rezaei, F.; Kartal, M.; Erdem, S.A. Effect of priming on thymoquinone content and in vitro plant regeneration with tissue culture of black cumin (Nigella sativa L.) seeds. J. Chem. Metrol. 2018, 12, 98. [Google Scholar] [CrossRef]
- Paulert, R.; Ebbinghaus, D.; Urlass, C.; Moerschbacher, B.M. Priming of the oxidative burst in rice and wheat cell cultures by ulvan, a polysaccharide from green macroalgae, and enhanced resistance against powdery mildew in wheat and barley plants. Plant Pathol. 2010, 59, 634–642. [Google Scholar] [CrossRef]
- Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Madala, N.E.; Dubery, I.A. Priming agents of plant defense stimulate the accumulation of mono- and di-acylated quinic acids in cultured tobacco cells. Physiol. Mol. Plant Pathol. 2014, 88, 61–66. [Google Scholar] [CrossRef]
- Gangopadhyay, M.; Gantait, S.; Palchoudhury, S.; Ali, N.; Mondal, C.; Pal, A.K. UVC-priming mediated modulation of forskolin biosynthesis key genes against Macrophomina root rot of Coleus forskohlii - a tissue culture based sustainable approach. Phytochem. Lett. 2016, 17, 36–44. [Google Scholar] [CrossRef]
- Ding, M.; Wang, X.; Li, Y. Acquired tolerance to cadmium following long-term acclimation to CdCl2 in rice suspension cultures. Plant Cell Tissue Organ Cult. 2016, 124, 47–55. [Google Scholar] [CrossRef]
- Chun, H.J.; Baek, D.; Cho, H.M.; Jung, H.S.; Jeong, M.S.; Jung, W.-H.; Choi, C.W.; Lee, S.H.; Jin, B.J.; Park, M.S.; et al. Metabolic adjustment of Arabidopsis root suspension cells during adaptation to salt stress and mitotic stress memory. Plant Cell Physiol. 2018, 60, 612–625. [Google Scholar] [CrossRef]
- Khairy, A.I.H.; Oh, M.J.; Lee, S.M.; Kim, D.S.; Roh, K.S. Nitric oxide overcomes Cd and Cu toxicity in in vitro-grown tobacco plants through increasing contents and activities of rubisco and rubisco activase. Biochim. Open 2016, 2, 41–51. [Google Scholar] [CrossRef]
- Petrov, V.D.; Van Breusegem, F. Hydrogen peroxide—a central hub for information flow in plant cells. AoB Plants 2012, 2012, pls014. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, Y.; He, L.-F. The central role of hydrogen sulfide in plant responses to toxic metal stress. Ecotoxicol. Environ. Saf. 2018, 157, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; González-Gordo, S.; Cañas, A.; Palma, J.M. Nitric oxide and hydrogen sulfide in plants: Which comes first? J. Exp. Bot. 2019, 70, 4391–4404. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Calderón-Urrea, A.; Yu, J.; Liao, W.; Xie, J.; Lv, J.; Feng, Z.; Tang, Z. The role of hydrogen sulfide in plant alleviates heavy metal stress. Plant Soil 2020, 449, 1–10. [Google Scholar] [CrossRef]
- Wang, H.; Ji, F.; Zhang, Y.; Hou, J.; Liu, W.; Huang, J.; Liang, W. Interactions between hydrogen sulphide and nitric oxide regulate two soybean citrate transporters during the alleviation of aluminum toxicity. Plant Cell Environ. 2019, 42, 2340–2356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, M.; Zhou, H.; Zhao, D.; Gotor, C.; Romero, L.C.; Shen, J.; Ge, Z.; Zhang, Z.; Shen, W.; et al. Hydrogen sulfide (H2S), a signaling molecule in plant stress responses. J. Integr. Plant Biol 2020, 63, 146–160. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.-M.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef]
- Cuypers, A.; Hendrix, S.; Amaral dos Reis, R.; De Smet, S.; Deckers, J.; Gielen, H.; Jozefczak, M.; Loix, C.; Vercampt, H.; Vangronsveld, J.; et al. Hydrogen peroxide, signaling in disguise during metal phytotoxicity. Front. Plant Sci. 2016, 7, 470. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Khan, N.A.; Masood, A.; Per, T.S.; Asgher, M. Hydrogen peroxide alleviates nickel-inhibited photosynthetic responses through increase in use-efficiency of nitrogen and sulfur, and glutathione production in mustard. Front. Plant Sci. 2016, 7, 44. [Google Scholar] [CrossRef]
- Nazir, F.; Hussain, A.; Fariduddin, Q. Hydrogen peroxide modulate photosynthesis and antioxidant systems in tomato (Solanum lycopersicum L.) plants under copper stress. Chemosphere 2019, 230, 544–558. [Google Scholar] [CrossRef]
- Xu, F.J.; Jin, C.W.; Liu, W.J.; Zhang, Y.S.; Lin, X.Y. Pretreatment with H2O2 alleviates aluminum-induced oxidative stress in wheat seedlings. J. Integr. Plant Biol. 2010, 53, 44–53. [Google Scholar] [CrossRef]
- Asgher, M.; Ahmed, S.; Sehar, Z.; Gautam, H.; Gandhi, S.G.; Khan, N.A. Hydrogen peroxide modulates activity and expression of antioxidant enzymes and protects photosynthetic activity from arsenic damage in rice (Oryza sativa L.). J. Hazard. Mater. 2021, 401, 123365. [Google Scholar] [CrossRef]
- Bai, X.-J.; Liu, L.-J.; Zhang, C.-H.; Ge, Y.; Cheng, W.-D. Effect of H2O2 Pretreatment on Cd tolerance of different rice cultivars. Rice Sci. 2011, 18, 29–35. [Google Scholar] [CrossRef]
- Yıldız, M.; Terzi, H.; Bingül, N.; Yıldız, M. Protective role of hydrogen peroxide pretreatment on defense systems and BnMP1 gene expression in Cr(VI)-stressed canola seedlings. Ecotoxicology 2013, 22, 1303–1312. [Google Scholar] [CrossRef]
- Kabała, K.; Zboińska, M.; Głowiak, D.; Reda, M.; Jakubowska, D.; Janicka, M. Interaction between the signaling molecules hydrogen sulfide and hydrogen peroxide and their role in vacuolar H+-ATPase regulation in cadmium-stressed cucumber roots. Physiol. Plant. 2019, 166, 688–704. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Alharby, H.F.; Razafindrabe, B.H.N.; Fujita, M. Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress in Brassica napus L.: An intrinsic study on antioxidant defense and glyoxalase systems. Front. Plant Sci. 2017, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.-F.; Gong, M.; Liu, Y.; Hu, J.-L.; Deng, M.-H. Effect of hydrogen peroxide on growth and activity of some enzymes involved in proline metabolism of sweet corn seedlings under copper stress. Sci. Hortic. 2013, 164, 366–371. [Google Scholar] [CrossRef]
- Kopyra, M.; Gwóźdź, E.A. Nitric oxide stimulates seed germination and counteracts the inhibitory effect of heavy metals and salinity on root growth of Lupinus luteus. Plant Physiol. Biochem. 2003, 41, 1011–1017. [Google Scholar] [CrossRef]
- Yang, L.; Ji, J.; Harris-Shultz, K.R.; Wang, H.; Wang, H.; Abd-Allah, E.F.; Luo, Y.; Hu, X. The dynamic changes of the plasma membrane proteins and the protective roles of nitric oxide in rice subjected to heavy metal cadmium stress. Front. Plant Sci. 2016, 7, 190. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, R.; Pan, Y.; Ma, M.; Pan, J.; Zhao, Y.; Cheng, Q.; Wu, M.; Wang, M.; Zhang, L. Nitric oxide contributes to minerals absorption, proton pumps and hormone equilibrium under cadmium excess in Trifolium repens L. plants. Ecotoxicol. Environ. Saf. 2015, 119, 35–46. [Google Scholar] [CrossRef]
- Seabra, A.B.; Oliveira, H.C. How nitric oxide donors can protect plants in a changing environment: What we know so far and perspectives. AIMS Mol. Sci. 2016, 3, 692–718. [Google Scholar] [CrossRef]
- Fang, P.; Sun, T.; Wang, Y.; Ding, Y.; Pandey, A.K.; Zhu, C.; Xu, P. Plant gasotransmitters: Light molecules interplaying with heavy metals. In Reviews in Environmental Science and Bio-Technology; Springer Science and Business Media B.V.: New York, NY, USA, 2021; pp. 1–23. [Google Scholar]
- Gill, S.S.; Hasanuzzaman, M.; Nahar, K.; Macovei, A.; Tuteja, N. Importance of nitric oxide in cadmium stress tolerance in crop plants. Plant Physiol. Biochem. 2013, 63, 254–261. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, H.P.; Batish, D.R.; Mahajan, P.; Kohli, R.K.; Rishi, V. Exogenous nitric oxide (NO) interferes with lead (Pb)-induced toxicity by detoxifying reactive oxygen species in hydroponically grown wheat (Triticum aestivum) roots. PLoS ONE 2015, 10, e0138713. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Mostofa, M.G.; Ahmad, M.Z.; Imtiaz, M.; Mehmood, S.; Adeel, M.; Dai, Z.; Li, Z.; Aziz, O.; Zhang, Y.; et al. Nitric oxide induces rice tolerance to excessive nickel by regulating nickel uptake, reactive oxygen species detoxification and defense-related gene expression. Chemosphere 2018, 191, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol. Plant. 2019, 168, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Fujita, M. Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 2013, 22, 584–596. [Google Scholar] [CrossRef]
- Kováčik, J.; Babula, P.; Hedbavny, J.; Švec, P. Manganese-induced oxidative stress in two ontogenetic stages of chamomile and amelioration by nitric oxide. Plant Sci. 2014, 215–216, 1–10. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, W.; Xu, L.; Kong, J.; Liu, S.; He, Z. Nitric oxide can induce tolerance to oxidative stress of peanut seedlings under cadmium toxicity. Plant Growth Regul. 2016, 79, 19–28. [Google Scholar] [CrossRef]
- Rey, P.; Tarrago, L. Physiological roles of plant methionine sulfoxide reductases in redox homeostasis and signaling. Antioxidants 2018, 7, 114. [Google Scholar] [CrossRef]
- Xiong, J.; An, L.; Lu, H.; Zhu, C. Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 2009, 230, 755–765. [Google Scholar] [CrossRef]
- Lisjak, M.; Teklic, T.; Wilson, I.D.; Whiteman, M.; Hancock, J.T. Hydrogen sulfide: Environmental factor or signalling molecule? Plant Cell Environ. 2013, 36, 1607–1616. [Google Scholar] [CrossRef]
- Shan, C.; Dai, H.; Sun, Y. Hydrogen sulfide protects wheat seedlings against copper stress by regulating the ascorbate and glutathione metabolism in leaves. Aust. J. Crop Sci. 2012, 6, 248–254. [Google Scholar]
- Valivand, M.; Amooaghaie, R.; Ahadi, A. Seed priming with H2S and Ca2+ trigger signal memory that induces cross-adaptation against nickel stress in zucchini seedlings. Plant Physiol. Biochem. 2019, 143, 286–298. [Google Scholar] [CrossRef]
- Zanganeh, R.; Jamei, R.; Rahmani, F. Role of salicylic acid and hydrogen sulfide in promoting lead stress tolerance and regulating free amino acid composition in Zea mays L. Acta Physiol. Plant. 2019, 41, 94. [Google Scholar] [CrossRef]
- Ali, S.; Farooq, M.A.; Hussain, S.; Yasmeen, T.; Abbasi, G.; Zhang, G. Alleviation of chromium toxicity by hydrogen sulfide in barley. Environ. Toxicol. Chem. 2013, 32, 2234–2239. [Google Scholar] [CrossRef]
- Fu, M.-M.; Dawood, M.; Wang, N.-H.; Wu, F. Exogenous hydrogen sulfide reduces cadmium uptake and alleviates cadmium toxicity in barley. Plant Growth Regul. 2019, 89, 227–237. [Google Scholar] [CrossRef]
- Kharbech, O.; Ben Massoud, M.; Sakouhi, L.; Djebali, W.; Mur, L.A.J.; Chaoui, A. Exogenous application of hydrogen sulfide reduces chromium toxicity in maize seedlings by suppressing NADPH oxidase activities and methylglyoxal accumulation. Plant Physiol. Biochem. 2020, 154, 646–656. [Google Scholar] [CrossRef]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.M.; Brown, E.M.; Grace, J.P.; Salem, A.K.; Irish, E.E.; Bowden, N.B. Improved growth of pea, lettuce, and radish plants using the slow release of hydrogen sulfide from GYY-4137. PLoS ONE 2018, 13, e0208732. [Google Scholar] [CrossRef]
- Calderone, V.; Martelli, A.; Testai, L.; Citi, V.; Breschi, M.C. Using hydrogen sulfide to design and develop drugs. Expert Opin. Drug Discov. 2015, 11, 163–175. [Google Scholar] [CrossRef]
- Popova, L.P.; Maslenkova, L.T.; Ivanova, A.; Stoinova, Z.G. Role of salicylic acid in alleviating heavy metal stress. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer: New York, NY, USA, 2011; pp. 447–466. [Google Scholar]
- Gharbi, E.; Martínez, J.P.; Benahmed, H.; Dailly, H.; Quinet, M.; Lutts, S. The salicylic acid analog 2,6-dichloroisonicotinic acid has specific impact on the response of the halophyte plant species Solanum chilense to salinity. Plant Growth Regul. 2017, 82, 517–525. [Google Scholar] [CrossRef]
- Khan, F.; Hussain, S.; Tanveer, M.; Khan, S.; Hussain, H.A.; Iqbal, B.; Geng, M. Coordinated effects of lead toxicity and nutrient deprivation on growth, oxidative status, and elemental composition of primed and non-primed rice seedlings. Environ. Sci. Pollut. Res. 2018, 25, 21185–21194. [Google Scholar] [CrossRef]
- Khan, F.; Hussain, S.; Khan, S.; Geng, M. Seed priming improved antioxidant defense system and alleviated Ni-induced adversities in rice seedlings under N, P, or K deprivation. Front. Plant Sci. 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudi, K.; Arvin, M.J.; Ashraf, M. Mitigation of arsenic toxicity in wheat by the exogenously applied salicylic acid, 24-epi-brassinolide and silicon. J. Soil Sci. Plant Nutr. 2019, 20, 577–588. [Google Scholar] [CrossRef]
- Safari, F.; Akramian, M.; Salehi-Arjmand, H.; Khadivi, A. Physiological and molecular mechanisms underlying salicylic acid-mitigated mercury toxicity in lemon balm (Melissa officinalis L.). Ecotoxicol. Environ. Saf. 2019, 183, 109542. [Google Scholar] [CrossRef] [PubMed]
- Agami, R.A.; Mohamed, G.F. Exogenous treatment with indole-3-acetic acid and salicylic acid alleviates cadmium toxicity in wheat seedlings. Ecotoxicol. Environ. Saf. 2013, 94, 164–171. [Google Scholar] [CrossRef]
- Karalija, E.; Selović, A.; Dahija, S.; Demir, A.; Samardžić, J.; Vrobel, O.; Parić, A. Use of seed priming to improve Cd accumulation and tolerance in Silene sendtneri, novel Cd hyper-accumulator. Ecotoxicol. Environ. Saf. 2021, 210, 111882. [Google Scholar] [CrossRef]
- Belkadhi, A.; De Haro, A.; Obregon, S.; Chaïbi, W.; Djebali, W. Positive effects of salicylic acid pretreatment on the composition of flax plastidal membrane lipids under cadmium stress. Environ. Sci. Pollut. Res. 2014, 22, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Belkhadi, A.; Hediji, H.; Abbes, Z.; Nouairi, I.; Barhoumi, Z.; Zarrouk, M.; Chaibi, W.; Djebali, W. Effect of exogenous salicylic acid pre-treatment on cadmium toxicity and leaf lipid content in Linum usitatissimum L. Ecotoxicol Environ. Saf. 2010, 73, 1004–1011. [Google Scholar] [CrossRef]
- Tao, S.; Sun, L.; Ma, C.; Li, L.; Li, G.; Hao, L. Reducing basal salicylic acid enhances Arabidopsis tolerance to lead or cadmium. Plant Soil 2013, 372, 309–318. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Koźmińska, A.; Hanus-Fajerska, E.; Dziurka, M.; Dziurka, K. Insight into mechanisms of multiple stresses tolerance in a halophyte Aster tripolium subjected to salinity and heavy metal stress. Ecotoxicol. Environ. Saf. 2019, 180, 12–22. [Google Scholar] [CrossRef]
- Takahashi, T.; Kakehi, J.-I. Polyamines: Ubiquitous polycations with unique roles in growth and stress responses. Ann. Bot. 2009, 105, 1–6. [Google Scholar] [CrossRef]
- Bae, D.-H.; Lane, D.J.; Jansson, P.J.; Richardson, D.R. The old and new biochemistry of polyamines. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2053–2068. [Google Scholar] [CrossRef]
- Paul, S.; Banerjee, A.; Roychoudhury, A. Role of polyamines in mediating antioxidant defense and epigenetic regulation in plants exposed to heavy metal toxicity. In Plants under Metal and Metalloid Stress; Springer: Singapore, 2018; pp. 229–247. [Google Scholar]
- Hasanuzzaman, M.; Alhaithloul, H.A.S.; Parvin, K.; Bhuyan, M.H.M.B.; Tanveer, M.; Mohsin, S.M.; Nahar, K.; Soliman, M.H.; Mahmud, J.A.; Fujita, M. Polyamine action under metal/metalloid stress: Regulation of biosynthesis, metabolism, and molecular Interactions. Int. J. Mol. Sci. 2019, 20, 3215. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Tong, W. Regulation and diversity of polyamine biosynthesis in plants. In Polyamines: A Universal Molecular Nexus for Growth, Survival, and Specialized Metabolism; Springer: Tokyo, Japan, 2014; pp. 27–44. [Google Scholar]
- Taie, H.A.A.; El-Yazal, M.A.S.; Ahmed, S.M.A.; Rady, M.M. Polyamines modulate growth, antioxidant activity, and genomic DNA in heavy metal–stressed wheat plant. Environ. Sci. Pollut. Res. 2019, 26, 22338–22350. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.P.; Kanwar, M.; Bhardwaj, R.; Yu, J.-Q.; Tran, L.-S.P. Chromium stress mitigation by polyamine-brassinosteroid application involves phytohormonal and physiological strategies in Raphanus sativus L. PLoS ONE 2012, 7, e33210. [Google Scholar] [CrossRef] [PubMed]
- Agami, R.A. Pre-soaking in indole-3-acetic acid or spermidine enhances copper tolerance in wheat seedlings. South Afr. J. Bot. 2016, 104, 167–174. [Google Scholar] [CrossRef]
- Altaf, M.M.; Shahid, R.; Ren, M.; Mora-Poblete, F.; Arnao, M.B.; Naz, S.; Anwar, M.; Shahid, S.; Shakoor, A.; Sohail, H.; et al. Phytomelatonin: An overview of the importance and mediating functions of melatonin against environmental stresses. Physiol. Plant. 2020, 1–27. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Xu, H.; Li, D.; Gao, X.; Li, T.L.; Wang, R. Effect of melatonin priming on photosynthetic capacity of tomato leaves under low-temperature stress. Photosynthetica 2017, 56, 884–892. [Google Scholar] [CrossRef]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Rizwan, M.; Fahad, S.; Xu, Z.; Hu, L. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crop. Prod. 2019, 140, 111597. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Jiao, Y.; Chen, C.; Shireen, F.; Zheng, Z.; Imtiaz, M.; Bie, Z.; Huang, Y. Melatonin pretreatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J. Plant Physiol. 2018, 220, 115–127. [Google Scholar] [CrossRef]
- Xie, C.; Xiong, X.; Huang, Z.; Sun, L.; Ma, J.; Cai, S.; Yu, F.; Zhong, W.; Chen, S.; Li, X. Exogenous melatonin improves lead tolerance of bermudagrass through modulation of the antioxidant defense system. Int. J. Phytoremed. 2018, 20, 1408–1417. [Google Scholar] [CrossRef]
- Shah, A.A.; Ahmed, S.; Ali, A.; Yasin, N.A. 2-Hydroxymelatonin mitigates cadmium stress in Cucumis sativus seedlings: Modulation of antioxidant enzymes and polyamines. Chemosphere 2020, 243, 125308. [Google Scholar] [CrossRef]
- Ni, J.; Wang, Q.; Shah, F.A.; Liu, W.; Wang, D.; Huang, S.; Fu, S.; Wu, L. Exogenous melatonin confers cadmium tolerance by counterbalancing the hydrogen peroxide homeostasis in wheat seedlings. Molecules 2018, 23, 799. [Google Scholar] [CrossRef] [PubMed]
- Tousi, S.; Zoufan, P.; Ghahfarrokhie, A.R. Alleviation of cadmium-induced phytotoxicity and growth improvement by exogenous melatonin pretreatment in mallow (Malva parviflora) plants. Ecotoxicol. Environ. Saf. 2020, 206, 111403. [Google Scholar] [CrossRef]
- Abd_Allah, E.F.; Hashem, A.; Alam, P.; Ahmad, P. Silicon alleviates nickel-induced oxidative stress by regulating antioxidant defense and glyoxalase systems in mustard plants. J. Plant Growth Regul. 2019, 38, 1260–1273. [Google Scholar] [CrossRef]
- Kaya, C.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Exogenously supplied silicon (Si) improves cadmium tolerance in pepper (Capsicum annuum L.) by up-regulating the synthesis of nitric oxide and hydrogen sulfide. J. Biotechnol. 2020, 316, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Moulick, D.; Santra, S.C.; Ghosh, D. Effect of selenium induced seed priming on arsenic accumulation in rice plant and subsequent transmission in human food chain. Ecotoxicol. Environ. Saf. 2018, 152, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Ragab, G.; Saad-Allah, K. Seed priming with greenly synthesized sulfur nanoparticles enhances antioxidative defense machinery and restricts oxidative injury under manganese stress in Helianthus annuus (L.) seedlings. J. Plant Growth Regul. 2020, 1–9. [Google Scholar] [CrossRef]
- Fatemi, H.; Pour, B.E.; Rizwan, M. Foliar application of silicon nanoparticles affected the growth, vitamin C, flavonoid, and antioxidant enzyme activities of coriander (Coriandrum sativum L.) plants grown in lead (Pb)-spiked soil. Environ. Sci. Pollut. Res. 2021, 28, 1417–1425. [Google Scholar] [CrossRef]
- Sotoodehnia-Korani, S.; Iranbakhsh, A.; Ebadi, M.; Majd, A.; Ardebili, Z.O. Selenium nanoparticles induced variations in growth, morphology, anatomy, biochemistry, gene expression, and epigenetic DNA methylation in Capsicum annuum; an in vitro study. Environ. Pollut. 2020, 265, 114727. [Google Scholar] [CrossRef]
- Frost, C.J.; Mescher, M.C.; Dervinis, C.; Davis, J.M.; Carlson, J.E.; de Moraes, C.M. Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis -3-hexenyl acetate. New Phytol. 2008, 180, 722–734. [Google Scholar] [CrossRef]
- Conrath, U. Molecular aspects of defence priming. Trends Plant Sci. 2011, 16, 524–531. [Google Scholar] [CrossRef]
- Kubala, S.; Garnczarska, M.; Wojtyla, L.; Clippe, A.; Kosmala, A.; Zmienko, A.; Lutts, S.; Quinet, M. Deciphering priming-induced improvement of rapeseed (Brassica napus L.) germination through an integrated transcriptomic and proteomic approach. Plant Sci. 2015, 231, 94–113. [Google Scholar] [CrossRef]
- Mo, H.-J.; Sun, Y.-X.; Zhu, X.-L.; Wang, X.-F.; Zhang, Y.; Yang, J.; Yan, G.-J.; Ma, Z.-Y. Cotton S-adenosylmethionine decarboxylase-mediated spermine biosynthesis is required for salicylic acid- and leucine-correlated signaling in the defense response to Verticillium dahliae. Planta 2016, 243, 1023–1039. [Google Scholar] [CrossRef]
- Qi, C.; Zhang, H.; Liu, Y.; Wang, X.; Dong, D.; Yuan, X.; Li, X.; Zhang, X.; Li, X.; Zhang, N.; et al. CsSNAT positively regulates salt tolerance and growth of cucumber by promoting melatonin biosynthesis. Environ. Exp. Bot. 2020, 175, 104036. [Google Scholar] [CrossRef]
- Hu, T.; Liu, S.Q.; Amombo, E.; Fu, J.M. Stress memory induced rearrangements of HSP transcription, photosystem II photochemistry and metabolism of tall fescue (Festuca arundinacea Schreb.) in response to high-temperature stress. Front. Plant Sci. 2015, 6, 403. [Google Scholar] [CrossRef]
- De Camargos, L.F.; Fraga, O.T.; Oliveira, C.C.; Da Silva, J.C.F.; Fontes, E.P.B.; Reis, P.A.B. Development and cell death domain-containing asparagine-rich protein (DCD/NRP): An essential protein in plant development and stress responses. Theor. Exp. Plant Physiol. 2019, 31, 59–70. [Google Scholar] [CrossRef]
- Ederli, L.; Reale, L.; Madeo, L.; Ferranti, F.; Gehring, C.; Fornaciari, M.; Romano, B.; Pasqualini, S. NO release by nitric oxide donors in vitro and in planta. Plant Physiol. Biochem. 2009, 47, 42–48. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic acid biosynthesis in plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef]
- Liu, J.-H.; Nada, K.; Honda, C.; Kitashiba, H.; Wen, X.-P.; Pang, X.-M.; Moriguchi, T. Polyamine biosynthesis of apple callus under salt stress: Importance of the arginine decarboxylase pathway in stress response. J. Exp. Bot. 2006, 57, 2589–2599. [Google Scholar] [CrossRef]
- Lee, K.-W.; Cha, J.-Y.; Kim, K.-H.; Kim, Y.-G.; Lee, B.-H.; Lee, S.-H. Overexpression of alfalfa mitochondrial HSP23 in prokaryotic and eukaryotic model systems confers enhanced tolerance to salinity and arsenic stress. Biotechnol. Lett. 2011, 34, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Santhanagopalan, I.; Basha, E.; Ballard, K.N.; Bopp, N.E.; Vierling, E. Model Chaperones: Small heat shock proteins from plants. In Heat Shock Proteins; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; Volume 8, pp. 119–153. [Google Scholar]
- Lee, S.-H.; Lee, K.-W.; Lee, D.-G.; Son, D.; Park, S.J.; Kim, K.-Y.; Park, H.S.; Cha, J.-Y. Identification and functional characterization of Siberian wild rye (Elymus sibiricus L.) small heat shock protein 16.9 gene (EsHsp16.9) conferring diverse stress tolerance in prokaryotic cells. Biotechnol. Lett. 2015, 37, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-W.; Choi, G.J.; Kim, K.-Y.; Ji, H.J.; Park, H.S.; Kim, Y.-G.; Lee, B.H.; Lee, S.-H. Transgenic expression of MsHsp23 confers enhanced tolerance to abiotic stresses in tall fescue. Asian Australas. J. Anim. Sci. 2012, 25, 818–823. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, J.; Ahn, Y.-J. Heterologous expression of a carrot small heat shock protein increased Escherichia coli viability under lead and arsenic stresses. HortScience 2013, 48, 1323–1326. [Google Scholar] [CrossRef]
- Zhao, L.; Peng, B.; Hernandez-Viezcas, J.A.; Rico, C.; Sun, Y.; Peralta-Videa, J.R.; Tang, X.; Niu, G.; Jin, L.; Varela-Ramirez, A.; et al. Stress response and tolerance of Zea mays to CeO2 nanoparticles: Cross talk among H2O2, heat shock protein, and lipid peroxidation. ACS Nano 2012, 6, 9615–9622. [Google Scholar] [CrossRef] [PubMed]
- Balestrazzi, A.; Confalonieri, M.; Macovei, A.; Donà, M.; Carbonera, D. Genotoxic stress and DNA repair in plants: Emerging functions and tools for improving crop productivity. Plant Cell Rep. 2011, 30, 287–295. [Google Scholar] [CrossRef]
- Koźmińska, A.; Wiszniewska, A.; Hanus-Fajerska, E.; Muszyńska, E. Recent strategies of increasing metal tolerance and phytoremediation potential using genetic transformation of plants. Plant Biotechnol. Rep. 2018, 12, 1–14. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Ding, Y.; Wang, X.; Xu, J. Overexpression of PtPCS enhances cadmium tolerance and cadmium accumulation in tobacco. Plant Cell, Tissue Organ Cult. 2015, 121, 389–396. [Google Scholar] [CrossRef]
- Bertini, L.; Proietti, S.; Focaracci, F.; Sabatini, B.; Caruso, C. Epigenetic control of defense genes following MeJA-induced priming in rice (O. sativa). J. Plant Physiol. 2018, 228, 166–177. [Google Scholar]
- Souza, L.A.; Monteiro, C.C.; Carvalho, R.F.; Gratão, P.L.; Azevedo, R.A. Dealing with abiotic stresses: An integrative view of how phytohormones control abiotic stress-induced oxidative stress. Theor. Exp. Plant Physiol. 2017, 29, 109–127. [Google Scholar] [CrossRef]
- Singh, S.; Prasad, S.M. IAA alleviates Cd toxicity on growth, photosynthesis and oxidative damages in eggplant seedlings. Plant Growth Regul. 2015, 77, 87–98. [Google Scholar] [CrossRef]
- Bashri, G.; Prasad, S.M. Exogenous IAA differentially affects growth, oxidative stress and antioxidants system in Cd stressed Trigonella foenum-graecum L. seedlings: Toxicity alleviation by up-regulation of ascorbate-glutathione cycle. Ecotoxicol. Environ. Saf. 2016, 132, 329–338. [Google Scholar] [CrossRef]
- Li, S.-W.; Zeng, X.-Y.; Leng, Y.; Feng, L.; Kang, X.-H. Indole-3-butyric acid mediates antioxidative defense systems to promote adventitious rooting in mung bean seedlings under cadmium and drought stresses. Ecotoxicol. Environ. Saf. 2018, 161, 332–341. [Google Scholar] [CrossRef]
- Khan, M.Y.; Prakash, V.; Yadav, V.; Chauhan, D.K.; Prasad, S.M.; Ramawat, N.; Singh, V.P.; Tripathi, D.K.; Sharma, S. Regulation of cadmium toxicity in roots of tomato by indole acetic acid with special emphasis on reactive oxygen species production and their scavenging. Plant Physiol. Biochem. 2019, 142, 193–201. [Google Scholar] [CrossRef]
- Zhang, C.; He, Q.; Wang, M.; Gao, X.; Chen, J.; Shen, C. Exogenous indole acetic acid alleviates Cd toxicity in tea (Camellia sinensis). Ecotoxicol. Environ. Saf. 2020, 190, 110090. [Google Scholar] [CrossRef]
- Ochoa, L.; Medina-Velo, I.A.; Barrios, A.C.; Bonilla-Bird, N.J.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Modulation of CuO nanoparticles toxicity to green pea (Pisum sativum Fabaceae) by the phytohormone indole-3-acetic acid. Sci. Total. Environ. 2017, 598, 513–524. [Google Scholar] [CrossRef]
- Massoud, M.B.; Sakouhi, L.; Karmous, I.; Zhu, Y.; El Ferjani, E.; Sheehan, D.; Chaoui, A. Protective role of exogenous phytohormones on redox status in pea seedlings under copper stress. J. Plant Physiol. 2018, 221, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yu, W.; Zhang, J.; Rengel, Z.; Xu, J.; Han, Q.; Chen, L.; Li, K.; Yu, Y.; Chen, Q. Auxin enhances aluminum-induced citrate exudation through upregulation of GmMATE and activation of the plasma membrane H+-ATPase in soybean roots. Ann. Bot. 2016, 118, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Nazir, F.; Fariduddin, Q.; Hussain, A.; Alam Khan, T. Brassinosteroid and hydrogen peroxide improve photosynthetic machinery, stomatal movement, root morphology and cell viability and reduce Cu-triggered oxidative burst in tomato. Ecotoxicol. Environ. Saf. 2021, 207, 111081. [Google Scholar] [CrossRef]
- Shah, A.A.; Ahmed, S.; Abbas, M.; Yasin, N.A. Seed priming with 3-epibrassinolide alleviates cadmium stress in Cucumis sativus through modulation of antioxidative system and gene expression. Sci. Hortic. 2020, 265, 109203. [Google Scholar] [CrossRef]
- Galhaut, L.; De Lespinay, A.; Walker, D.J.; Bernal, M.P.; Correal, E.; Lutts, S. Seed priming of Trifolium repens L. improved germination and early seedling growth on heavy metal-contaminated soil. Water, Air, Soil Pollut. 2014, 225, 1905. [Google Scholar] [CrossRef]
- Sneideris, L.C.; Gavassi, M.A.; Campos, M.L.; D’Amico-Damião, V.; Carvalho, R.F. Effects of hormonal priming on seed germination of pigeon pea under cadmium stress. An. Acad. Bras. Cienc. 2015, 87, 1847–1852. [Google Scholar] [CrossRef]
- Wu, X.; He, J.; Ding, H.; Zhu, Z.; Chen, J.; Xu, S.; Zha, D. Modulation of zinc-induced oxidative damage in Solanum melongena by 6-benzylaminopurine involves ascorbate–glutathione cycle metabolism. Environ. Exp. Bot. 2015, 116, 1–11. [Google Scholar] [CrossRef]
- Agnihotri, A.; Seth, C.S. Does jasmonic acid regulate photosynthesis, clastogenecity, and phytochelatins in Brassica juncea L. in response to Pb-subcellular distribution? Chemosphere 2020, 243, 125361. [Google Scholar] [CrossRef]
- Tai, Z.; Yin, X.; Fang, Z.; Shi, G.; Lou, L.; Cai, Q. Exogenous GR24 alleviates cadmium toxicity by reducing cadmium uptake in switchgrass (Panicum virgatum) seedlings. Int. J. Environ. Res. Public Health 2017, 14, 852. [Google Scholar] [CrossRef]
- Jisha, K.C.; Puthur, J.T. Halopriming of seeds imparts tolerance to NaCl and PEG induced stress in Vigna radiata (L.) Wilczek varieties. Physiol. Mol. Biol. Plants 2014, 20, 303–312. [Google Scholar] [CrossRef]
- Kumar, M.; Pant, B.; Mondal, S.; Bose, B. Hydro and halo priming: Influenced germination responses in wheat Var-HUW-468 under heavy metal stress. Acta Physiol. Plant. 2016, 38, 1–7. [Google Scholar] [CrossRef]
- Román-Ponce, B.; Reza-Vázquez, D.M.; Gutiérrez-Paredes, S.; Haro-Cruz, M.D.J.D.; Maldonado-Hernández, J.; Bahena-Osorio, Y.; Santos, P.E.-D.L.; Wang, E.T.; Vásquez-Murrieta, M.S. Plant growth-promoting traits in rhizobacteria of heavy metal-resistant plants and their effects on Brassica nigra seed germination. Pedosphere 2017, 27, 511–526. [Google Scholar] [CrossRef]
- Desoky, E.-S.M.; Merwad, A.-R.M.; Semida, W.M.; Ibrahim, S.A.; El-Saadony, M.T.; Rady, M.M. Heavy metals-resistant bacteria (HM-RB): Potential bioremediators of heavy metals-stressed Spinacia oleracea plant. Ecotoxicol. Environ. Saf. 2020, 198, 110685. [Google Scholar] [CrossRef]
- Benidire, L.; Pereira, S.I.A.; Castro, P.M.L.; Boularbah, A. Assessment of plant growth promoting bacterial populations in the rhizosphere of metallophytes from the Kettara mine, Marrakech. Environ. Sci. Pollut. Res. 2016, 23, 21751–21765. [Google Scholar] [CrossRef]
- Dabral, S.; Yashaswee; Varma, A.; Choudhary, D.K.; Bahuguna, R.N.; Nath, M. Biopriming with Piriformospora indica ameliorates cadmium stress in rice by lowering oxidative stress and cell death in root cells. Ecotoxicol. Environ. Saf. 2019, 186, 109741. [Google Scholar] [CrossRef]
- Hou, L.; Yu, J.; Zhao, L.; He, X. Dark septate endophytes improve the growth and the tolerance of Medicago sativa and Ammopiptanthus mongolicus under cadmium stress. Front. Microbiol. 2020, 10, 3061. [Google Scholar] [CrossRef]
- Qadir, M.; Hussain, A.; Hamayun, M.; Shah, M.; Iqbal, A.; Husna; Murad, W. Phytohormones producing rhizobacterium alleviates chromium toxicity in Helianthus annuus L. by reducing chromate uptake and strengthening antioxidant system. Chemosphere 2020, 258, 127386. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, L.; Ma, Z.; Wang, J. Bacillus amyloliquefaciens SAY09 increases cadmium resistance in plants by activation of auxin-mediated signaling pathways. Genes 2017, 8, 173. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Huang, Y.; Zhang, Y.; Wang, X.; Hu, Z. Cadmium-resistant rhizobacterium Bacillus cereus M4 promotes the growth and reduces cadmium accumulation in rice (Oryza sativa L.). Environ. Toxicol. Pharmacol. 2019, 72, 103265. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Javed, M.T.; Tanwir, K.; Akram, M.S.; Tazeen, S.K.; Saleem, M.H.; Masood, S.; Mujtaba, S.; Chaudhary, H.J. Plant growth-promoting Bacillus sp. strain SDA-4 confers Cd tolerance by physio-biochemical improvements, better nutrient acquisition and diminished Cd uptake in Spinacia oleracea L. Physiol. Mol. Biol. Plants 2020, 26, 2417–2433. [Google Scholar] [CrossRef] [PubMed]
- Da, K.; Nowak, J.; Flinn, B. Potato cytosine methylation and gene expression changes induced by a beneficial bacterial endophyte, Burkholderia phytofirmans strain PsJN. Plant. Physiol. Biochem. 2012, 50, 24–34. [Google Scholar] [CrossRef]
- Versluys, M.; Tarkowski, Ł.P.; Ende, W.V.D. Fructans as DAMPs or MAMPs: Evolutionary prospects, cross-tolerance, and multistress resistance potential. Front. Plant Sci. 2017, 7, 1264. [Google Scholar] [CrossRef]
- Fodorpataki, L.; Molnar, K.; Tompa, B.; Plugaru, S.R.C. Priming with vitamin U enhances cold tolerance of lettuce (Lactuca sativa L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 592–598. [Google Scholar] [CrossRef]
- Kataria, S.; Baghel, L.; Jain, M.; Guruprasad, K. Magnetopriming regulates antioxidant defense system in soybean against salt stress. Biocatal. Agric. Biotechnol. 2019, 18, 101090. [Google Scholar] [CrossRef]
- Sobhy, S.; Allah, K.; Kassem, E.; Hafez, E.; Sewelam, N. Seed priming in natural weed extracts represents a promising practice for alleviating lead stress toxicity. Egypt. J. Exp. Boil. 2019, 15, 453. [Google Scholar] [CrossRef]
| Priming Agent | Plant Species | Culture Type | Priming Benefits | Reference |
|---|---|---|---|---|
| 6.3 µM chlorocholine chloride | Eclipta alba | Shoot culture | ↑ root number and length ↑biomass of regenerated plantlets ↑ chlorophyll content ↑survival after acclimatization to ex vitro conditions | [56] |
| 1 mg·mL−1 ulvan (sulphated polysaccharide from green macroalga Ulva fasciata) | Triticum aestivum Oryza sativa Hordeum vulgare | Suspension culture | prevention from oxidative burst caused by chitosan ↓symptoms of infection with pathogen Blumeria graminis | [58] |
| Imprimatins | Arabidopsis thaliana | Suspension culture | suppressed growth of bacterial pathogens ↑level of endogenous SA ↑expression of PR genes ↓reduced SA inactivation by glucosylation | [48] |
| Pathogen-derived compounds (1 mM isonitroacetophenon, 300 µM acibenzolar-S-methyl, 200 nM flagellin, 100 µg·mL−1 chitosan, 100 µg·mL−1 lipopolysaccharide) | Nicotiana tabacum | Suspension culture | ↑synthesis of mono- and di-acetylated chlorogenic acids | [59] |
| Ultraviolet C (UVC) | Coleus forskohlii | Callus culture | callus organogenesis ↑resistance to Macrophomina root rot disease (M. phaseolina pathogen) ↑forskolin synthesis via upregulation of forskolin biosynthesis genes | [60] |
| 0.05 mM SNP (nitric oxide donor) | Nicotiana tabacum | Shoot culture | ↑tolerance to Cd and Cu, manifested by higher biomass, chlorophyll content and rubisco activity | [63] |
| 200 nM melatonin | Nicotiana tabacum | Suspension culture | ↑cell survival in the presence of Pb | [51] |
| Volatile compounds released by Microbacterium strain EC8 | Arabidopsis thaliana Lactuca sativa Solanum lycopersicum | In vitro-grown seedlings | ↑growth promotion-shoot and root biomass, lateral root density | [52] |
| Cold plasma 5–80 mg·L−1SiO2 nanoparticles (nSi) | Astragalus fridae | Seeds germinating in vitro | ↑root system development ↑ shoot biomass ↑chlorophyll and carotenoid contents ↑ root NR activity, shoot CAT activity modified anatomy and tissue differentiation patterns interactive effect of cold plasma and nSi | [53] |
| Metallic/Metalloid Stress | Priming Details | Species | Priming Effects in Comparison with Non-Primed Plants | References | |||||
|---|---|---|---|---|---|---|---|---|---|
| Dose (mM) | Duration | Plant Part | ROS Production/ Oxidation Activity | Antioxidant Response | Photosynthetic Performance/Biomass Accretion/Growth | Other | |||
| Hydrogen peroxide H2O2 | |||||||||
| As, (20–50 µM) | 0.05 H2O2 | Oryza sativa | ↑ SOD, APX, GR, GSH | ↑ | ↑ expression of genes encoding photosystem proteins and antioxidant enzymes ↑ proline | [75] | |||
| Cd (10–150 µM CdCl2) | 5 H2O2 | 24 h | roots | Cucumis sativus | ↔ | ↑ endogenous H2S ↓ ATP hydrolysis and proton transport ↑expression of genes encoding V-ATPase subunits | [78] | ||
| Cu, (10–100 mg kg−1 soil) | 0.1–0.5 H2O2 | 4 h | roots | Solanum lycopersicum | ↓O2·−, MDA | ↑ CAT, POD, SOD | ↑ | ↑ proline | [73] |
| Cd (0.5–1.0 mM CdCl2) | 0.05 H2O2 | 24 h | roots of seedlings | Brassica napus | ↓ H2O2, O2·−, ↓LOX | ↑ AsA, GSH, APX, DHAR, GR, GST, CAT, GPX ↓DHA | ↑ glyoxalase I and II activity | [79] | |
| Ni (200 mg kg−1 soil NiCl2) | 0.05 H2O2 | 15 days | seedlings | Brassica juncea | ↓TBARS | ↑APX, GR, GSH ↓GSSG | ↑rubisco, PSII activity, leaf area | ↑N and S assimilation (enzyme activity) | [72] |
| Cu (50 µM CuCl2) | 0.3 H2O2 | 6–8 h | seedlings | Zea mays | ↑ | ↑gene expression and activities of enzymes involved in proline synthesis (GDH, P5CS, arginase, OAT) ↑ proline ↓ activity of proline degrading enzyme ProDH | [80] | ||
| Cr(VI) (50 µM) | 0.2 H2O2 | 24 h | seedlings | Brassica napus | ↓MDA | ↑NPT, PT, APX, POD | ↑ | ↑ Cr translocation to shoots ↑ expression of metallothionein gene | [77] |
| Cd (50 µM) | 0.1 H2O2 | 3-leafed seedlings | Oryza sativa | ↔ MDA | ↑GSH, NPT, PCs, GST | ↑ | ↓ Cd translocation to shoots | [76] | |
| Al (30 µM) | 0.6 H2O2 | 2 h | root tips | Triticum aestivum | ↓ H2O2, O2·- | ↑ SOD, CAT, POD, APX, MDHAR, GPX, GR, GSH, AsA | ↓ root elongation | [74] | |
| Nitric oxide NO | |||||||||
| Cd (0.1 mM CdCl2) | 0.1 SNP | 28 days | seedlings | Triticum aestivum | ↓ H2O2, MDA | ↑ SOD, CAT, POD | ↑ | ↓ proline ↓ Cd uptake and accumulation ↑endogenous H2S ↑ Zn2+, Fe2+, Ca2+, K+ content | [89] |
| Cd/Cu (0.2 mM each) | 0.05 SNP | 35 days | in vitro grown plants | Nicotiana tabacum | ↑rubisco and rubisco activase content and activity, depending on the metal | [63] | |||
| Cd (10 µM CdCl2) | 0.03 SNAP | 2 h | 3-leafed seedlings | Oryza sativa | ↓ H2O2 | ↑GSH, APX, SOD, GR | ↑ | affected abundance of plasma membrane proteins (transporters, ATPases, kinases, phosphatases, phospholipases, enzymes, antiporters, structural proteins, aquaporins, signal, and hormone-related proteins) ↑phosphatidic acid | [82] |
| Cd (100–200 µM CdCl2) | 0.25 SNP | 14 days | seedlings | Arachis hypogaea | ↓O2·−, MDA | ↑ SOD, CAT, POD, AsA | ↑ | ↓ Cd translocation to shoots, ↑ enhanced Cd binding in cell walls ↑ proline | [92] |
| Mn (1000 µM MnCl2) | 0.1–1 SNP | 7 days | seeds | Matricaria chamomilla | ↓ ROS | ↑ APX ↓ CAT | ↑ | ↓ Mn content in roots and shoots | [91] |
| Al (100 µM AlCl3) | 0.01–0.05SNP | 12 h | seedlings | Glycine max | cooperates with H2S in induction of citrate transporter expression ↑ endogenous H2S ↑ activities of H2S biosynthesis enzymes (cysteine desulfhydrases, CAS) ↓activity of H2S-degrading enzyme (OAS-TL) | [68] | |||
| Pb (50 µM Pb(NO3)2) | 0.1 SNP | 2–8 h | germinating seeds | Triticum aestivum | ↓MDA, conjugated dienes, O2·−, ·HO | ↑ APX, GPX, GR, SOD | ↑ radicle and plumule length | ↑intracellular nitrite content | [87] |
| Cd (100 µM) | 0.05 SNP | Trifolium repens | ↓ H2O2, MDA | ↑ ROS scavengers (enzymatic and non-enzymatic) | ↑ | ↓ inhibition of H+-ATPase proton pumps ↑ jasmonic acid, proline ↓ salicylic acid, ethylene ↑ Mg2+, Cu2+, Ca2+, Fe2+ | [83] | ||
| Ni | SNP | Oryza sativa | ↓ H2O2, MDA | ↑AsA, POD, CAT | ↑ | ↑soluble proteins↑ proline↑ transcript levels of CAT, POD, APX, GR, SOD genes | [88] | ||
| As (0.25–0.5 mM NaHAsO4) | 0.25 SNP | 72 h | seedlings | Triticum aestivum | ↓ H2O2, MDA | ↑AsA, GSH, GSH/GSSG, MDHAR, DHAR, GR, GPX, CAT ↓GSSG | ↑ proline ↑ glyoxalase I and II activity | [90] | |
| Cd (100 µM CdCl2) | 0.1 SNP | 3 days | plants | Arabidopsis thaliana | ↓protein oxidation ↓ROS/peroxides | ↑GPX, APX, CAT | ↓expression of methionine sulfoxide reductase family genes | [18] | |
| Hydrogen sulfide H2S | |||||||||
| Cd (0.1 mM CdCl2) | 0.2 NaHS | 28 days | seedlings | Triticum aestivum | ↓ H2O2, MDA, | ↑ SOD, CAT, POD | ↑ | ↓ proline ↓ Cd content ↑endogenous NO ↑ Zn2+, Fe2+, Ca2+, K+ contents | [89] |
| Al (100 µM AlCl3) | 0.01–0.1 NaHS | 12 h | seedlings | Glycine max | ↑ root growth | ↓ Al content in root tips ↑ citrate secretion upregulation of plasma membrane H+-ATPase (proton pump) act downstream of NO activity in Al-tolerance | [68] | ||
| Cd (5–50 µM CdCl2) | 0.2 NaHS | 48 h | seedlings | Hordeum vulgare | ↓ MDA,H2O2, O2·- | ↑SOD, POD, APX ↑GSH, AsA (at moderate Cd level) ↓CAT | ↑ | ↓ Cd content | [100] |
| Cd (10–150 µM CdCl2) | 0.1 NaHS | 24 h | roots | Cucumis sativus | ↑ | ↑ endogenous H2S ↔ endogenous H2O2 ↑ ATP hydrolysis and proton transport ↑expression of genes encoding V-ATPase subunits ↑activity and transcripts of plasma membrane NADPH oxidase | [78] | ||
| Pb (100–400 µM Pb(NO3)2) | 0.1–0.2 NaHS | 15 d | seedlings | Brassica napus | ↓ MDA, H2O2, O2·−, -OH | ↑SOD, POD, APX, CAT, GR, AsA, GSH, GSSG | ↑biomass | ↓ Na+ uptake ↑micro- and macroelement uptake ↑ total soluble proteins | [99] |
| Pb (2.5 mM Pb(NO3)2) | 0.2–2 NaHS | 12 h | seeds | Zea mays | ↑ GSH | ↑ | ↓ Pb content ↓ amino acids: Asp, Glu, Asn, Ser, Hist, Gly, Threo, Ala, Cyst ↑amino acids: Tyr, Tryp ↑NR activity ↑protein content | [98] | |
| Cr (200 µM K2Cr2O7) | 0.5 | 9 d | seeds | Zea mays | ↓ H2O2 | ↔GPOX ↓GST, SOD, GSNOR | ↑ radicle length | ↓protein carbonylation and thiol oxidation ↑endogenous NO ↓NADPH oxidase activity ↑S-nitrosoglutathione | [101] |
| Salicylic acid (SA) | |||||||||
| Cd (0.25–0.5 mM Cd(NO3)2) | 0.5 SA | 24 h | seeds | Silenesendtneri | ↑POD | ↑germination and seedling development (root length, shoot biomass) | ↑ Cd content in shoots ↓ Cd content in roots altered profile of secondary metabolites: phenolic compounds and organic acids | [112] | |
| Pb (1 mM PbCl2) | 100 mg·L−1 SA | 24 h | seeds | Oryza sativa | ↓ H2O2, O2·−, −OH, MDA | ↑ SOD, POD, CAT ↑GSH | ↑ shoot growth | ↓Pb content in shoots ↔ Pb content in roots ↔ macronutrient uptake | [107] |
| Ni (0.25 mM NiSO4 | 100 mg·L−1 SA | 24 h | seeds | Oryza sativa | ↓ H2O2, O2·−, -OH, MDA ↓XOD, MAO | ↑ CAT, GR, SOD, GPX, POD ↑GSH, AsA, Ve | ↑ shoot growth | ↑uptake of macronutrients ↓Ni content in shoots ↔ Ni content in roots | [108] |
| Pb (2.5 mM Pb(NO3)2) | 0.5 SA | 12 h | seeds | Zea mays | ↑GSH | ↑ | ↓ Pb content ↓ amino acids: Asp, Glu, Asn, Ser, Hist, Gly, Threo, Ala, Ile ↑NR activity ↑protein content | [98] | |
| Cd (500–1000 µM CdCl2) | 0.5 SA | 12 h | seeds | Triticum aestivum | ↑ SOD, POD, CAT | ↑ biomass | ↓ Cd content in the leaves and roots ↑proline altered leaf anatomy | [111] | |
| Cd (50–100 µM CdCl2) | 0.25–1 SA | 8 h | seeds | Linum usitatissimum | ↓ membrane stability | changed profile of membrane lipids, preserved composition of plastidial lipids | [113] | ||
| Cd (50–100 µM CdCl2) | 0.25–1.0 SA | 8 h | seeds | Linum usitatissimum | ↓MDA | ↑ biomass, chlorophylls | ↓ Cd content ↓ carotenoids ↑total lipid content, altered fatty acid composition altered nutrient distribution between roots and shoots | [114] | |
| Hg (50 µM HgCl2) | 0.05 SA | 2 w-old transplants | Melissa offcinalis | ↓MDA | ↑DPPH, FRAP | ↑ | ↑proline ↑phenolic compounds ↑expression of chlorophyll synthase and PAL | [110] | |
| As (50–100 µM Na3AsO4) | 0.5–1.0 SA | 12 h | seeds | Triticum aestivum | ↓ MDA, H2O2 | ↑ SOD, POD ↔ CAT, APX | ↑ photosynthetic rate, chlorophylls | ↓ As content, also in grains ↑soluble sugars ↑soluble proteins ↑proline | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiszniewska, A. Priming Strategies for Benefiting Plant Performance under Toxic Trace Metal Exposure. Plants 2021, 10, 623. https://doi.org/10.3390/plants10040623
Wiszniewska A. Priming Strategies for Benefiting Plant Performance under Toxic Trace Metal Exposure. Plants. 2021; 10(4):623. https://doi.org/10.3390/plants10040623
Chicago/Turabian StyleWiszniewska, Alina. 2021. "Priming Strategies for Benefiting Plant Performance under Toxic Trace Metal Exposure" Plants 10, no. 4: 623. https://doi.org/10.3390/plants10040623
APA StyleWiszniewska, A. (2021). Priming Strategies for Benefiting Plant Performance under Toxic Trace Metal Exposure. Plants, 10(4), 623. https://doi.org/10.3390/plants10040623






