An Overview on the Conservative Management of Endometriosis from a Naturopathic Perspective: Phytochemicals and Medicinal Plants
Abstract
1. Introduction
2. Materials and Methods
3. Pathogenic Pathways of Endometriosis
3.1. Inflammatory Pathways in Endometriosis
3.2. Angiogenesis in Endometriosis
3.3. Apoptosis and Endometriosis
4. Results
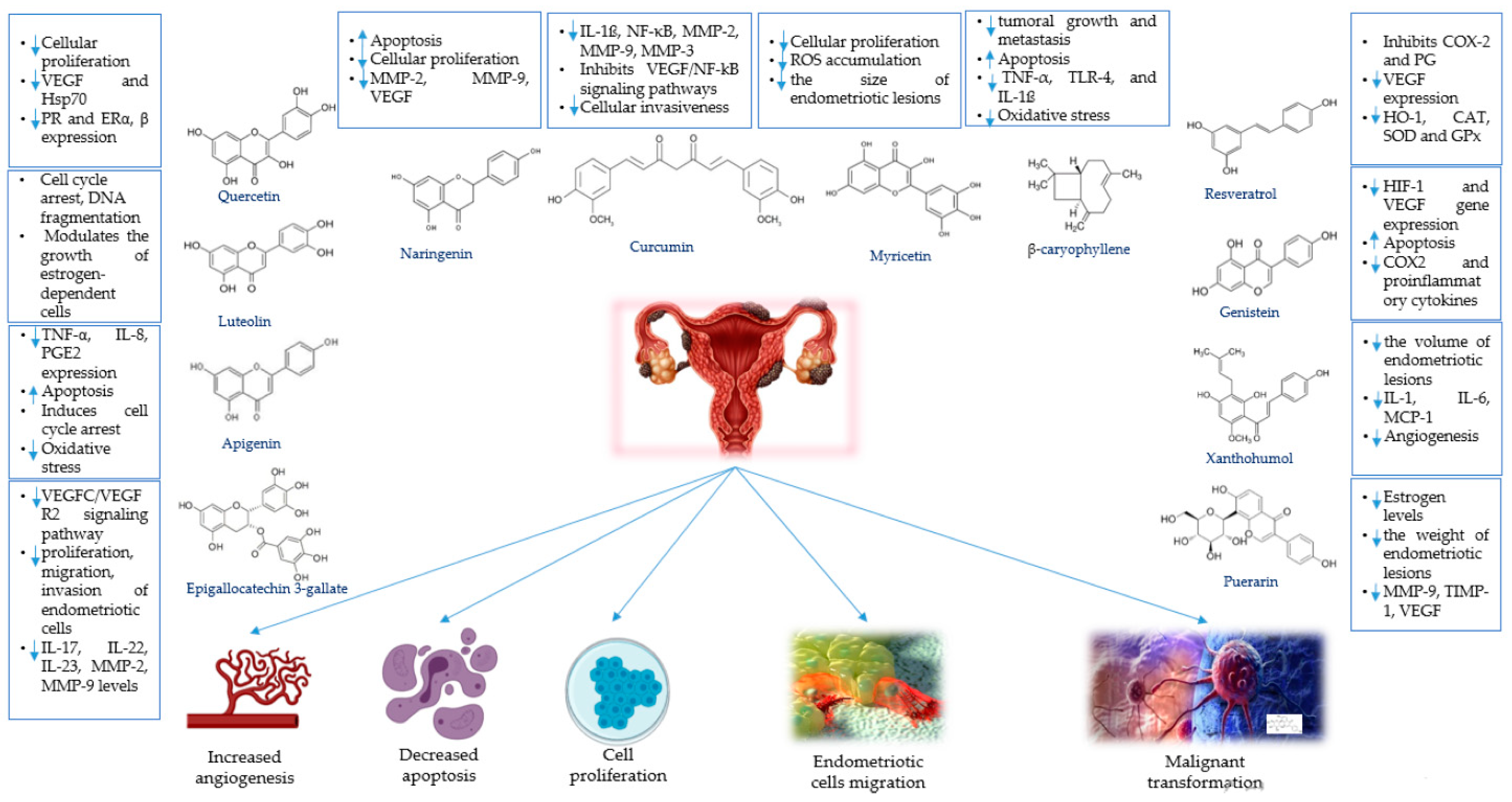
4.1. Phytochemicals for the Treatment of Endometriosis
4.1.1. Apigenin
4.1.2. β-Caryophyllene
4.1.3. Curcumin
4.1.4. Epigallocatechin-3-gallate
4.1.5. Genistein
4.1.6. Luteolin and Chrysin
4.1.7. Myricetin
4.1.8. Naringenin
4.1.9. Puerarin
4.1.10. Quercetin
4.1.11. Resveratrol
4.1.12. Xanthohumol
4.2. Medicinal Plants for the Treatment of Endometriosis
4.2.1. Angelica sinensis (Danggui)
4.2.2. Achillea biebersteinii (Yarrow)
4.2.3. Artemisia princeps
4.2.4. Allium sativum
4.2.5. Astragalus membranaceus
4.2.6. Curcuma longa
4.2.7. Prunella vulgaris
4.2.8. Sparganium stoloniferum
4.2.9. Salvia miltiorrhiza (Danshen)
4.2.10. Paeonia lactiflora (Chishao)
4.2.11. Viburnum opulus
4.2.12. Cyperus rotundus
4.2.13. Euterpe oleracea
4.2.14. Other Natural Products for Endometriosis Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| CAT | Catalase |
| COX-2 | Cyclooxygenase-2 |
| DNA | Deoxyribonucleic acid |
| E2 | Estradiol |
| ECM | Extracellular matrix |
| EGCG | Epigallocatechin-3-gallate |
| EGFR | Epithelial growth factor receptor |
| ER | Estrogen receptor |
| GnRH | Gonadotropin-releasing hormone |
| GSH | Glutathione |
| GSH-Px | Glutathione peroxidase |
| HIF | Hypoxia-inducible factor |
| HSP70 | Heat shock protein 70 |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| IFN | Interferon |
| IL | Interleukin |
| iNOS | Nitric oxide synthase |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MDA | Methylenedioxyamphetamine |
| MeSH | Medical Subject Headings |
| MIF | Macrophage migration inhibitory factor |
| miRNA | Micro-ribonucleic acid |
| MMP | Matrix metalloproteinase |
| NF-kB | Nuclear factor κB |
| NO | Nitric oxide |
| NSAID | Nonsteroidal anti-inflammatory drugs |
| P38 | Protein 38 |
| PG | Prostaglandin |
| PGE2 | Prostaglandin E2 |
| PK-1 | Prokinetitsina-1 |
| PONase | Paraoxonase |
| PR | Progesterone receptor |
| ROS | Reactive oxygen species |
| RTK | Receptor tyrosine kinase |
| SOD | Superoxide dismutase |
| TCM | Traditional Chinese Medicine |
| TGF-β | Transforming growth factor beta |
| TIMP | Tissue inhibitors of metalloproteinase |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor-alpha |
| TSP-1 | Thrombospondin-1 |
| VEGF | Vascular endothelial growth factor |
| VEGFC | Vascular endothelial growth factor C |
| VEGFR | Vascular endothelial growth factor receptor |
References
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr. Obstet. Gynecol. Rep. 2017, 6, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Monsanto, S.P.; Miller, C.; Singh, S.S.; Thomas, R.; Tayade, C. Pathophysiology and Immune Dysfunction in Endometriosis. Biomed. Res. Int. 2015, 2015, 795976. [Google Scholar] [CrossRef] [PubMed]
- Anastasiu, C.-V.; Moga, M.; Neculau, A.; Bălan, A.; Scarneciu, I.; Dragomir, R.; Dull, A.-M.; Chicea, L. Biomarkers for the Noninvasive Diagnosis of Endometriosis: State of the Art and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 1750. [Google Scholar] [CrossRef]
- Bina, F.; Soleymani, S.; Toliat, T.; Hajimahmoodi, M.; Tabarrai, M.; Abdollahi, M.; Rahimi, R. Plant-derived medicines for treatment of endometriosis: A comprehensive review of molecular mechanisms. Pharmacol. Res. 2019, 139, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Moga, M.A.; Bălan, A.; Dimienescu, O.G.; Burtea, V.; Dragomir, R.M.; Anastasiu, C.V. Circulating miRNAs as Biomarkers for Endometriosis and Endometriosis-Related Ovarian Cancer-An Overview. J. Clin. Med. 2019, 8, 735. [Google Scholar] [CrossRef]
- Andres, M.P.; Arcoverde, F.V.L.; Souza, C.C.C.; Fernandes, L.F.C.; Abrão, M.S.; Kho, R.M. Extrapelvic Endometriosis: A Systematic Review. J. Minim. Invasive Gynecol. 2020, 27, 373–389. [Google Scholar] [CrossRef]
- Yovich, J.L.; Rowlands, P.K.; Lingham, S.; Sillender, M.; Srinivasan, S. Pathogenesis of endometriosis: Look no further than John Sampson. Reprod. BioMed. Online 2020, 40, 7–11. [Google Scholar] [CrossRef]
- Sasson, I.E.; Taylor, H.S. Stem cells and the pathogenesis of endometriosis. Ann. N. Y. Acad. Sci. 2008, 1127, 106–115. [Google Scholar] [CrossRef]
- Harirchian, P.; Gashaw, I.; Lipskind, S.T.; Braundmeier, A.G.; Hastings, J.M.; Olson, M.R.; Fazleabas, A.T. Lesion kinetics in a non-human primate model of endometriosis. Hum. Hum. Reprod. 2012, 27, 2341–2351. [Google Scholar] [CrossRef]
- Hapangama, D. Theories on the Pathogenesis of Endometriosis. Int. J. Reprod. Med. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Dehoux, J.-P.; Defrere, S.; Squifflet, J.; Donnez, O.; Polet, R.; Mestdagt, M.; Foidart, J.-M.; Van Langendonckt, A.; Donnez, J. Is the baboon model appropriate for endometriosis studies? Fertil. Steril. 2011, 96, 728–733.e723. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D.C. Pathogenesis of endometriosis: The genetic/epigenetic theory. Fertil. Steril. 2019, 111, 327–340. [Google Scholar] [CrossRef]
- Laganà, A.S.; Salmeri, F.M.; Vitale, S.G.; Triolo, O.; Götte, M. Stem Cell Trafficking During Endometriosis: May Epigenetics Play a Pivotal Role? Reprod. Sci. 2018, 25, 978–979. [Google Scholar] [CrossRef]
- Ghai, V.; Jan, H.; Shakir, F.; Haines, P.; Kent, A. Diagnostic delay for superficial and deep endometriosis in the United Kingdom. J. Obstet. Gynaecol. 2020, 40, 83–89. [Google Scholar] [CrossRef]
- Becker, C.; Gattrell, W.; Gude, K.; Singh, S. Reevaluating response and failure of medical treatment of endometriosis: A systematic review. J. Fertil. Steril. 2017, 108, 125–136. [Google Scholar] [CrossRef]
- Bedaiwy, M.A.; Alfaraj, S.; Yong, P.; Casper, R. New developments in the medical treatment of endometriosis. Fertil. Steril. 2017, 107, 555–565. [Google Scholar] [CrossRef]
- Dunselman, G.A.J.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gong, P.; Chen, Y.; Nwachukwu, J.C.; Srinivasan, S.; Ko, C.; Bagchi, M.K.; Taylor, R.N.; Korach, K.S.; Nettles, K.W.; et al. Dual suppression of estrogenic and inflammatory activities for targeting of endometriosis. Sci. Transl. Med. 2015, 7, 271ra279. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.H.; Uy-Kroh, M.J. New Developments in Surgery for Endometriosis and Pelvic Pain. Clin. Obstet. Gynecol. 2017, 60, 245–251. [Google Scholar] [CrossRef]
- Flower, A.; Liu, J.P.; Chen, S.; Lewith, G.; Little, P. Chinese herbal medicine for endometriosis. Cochrane Database Syst Rev. 2009, Cd006568. [Google Scholar] [CrossRef]
- Ashrafizaveh, A.; Sabouri Fard, H.; Azmoudeh, E. Application of Medicinal Plants, Acupuncture, Massage Therapy and Transcutaneous Electric Nerve Stimulation in Treatment of Endometriosis: Review Study. Iran. J. Obstet. Gynecol. Infertil. 2019, 22, 90–100. [Google Scholar]
- Zheng, W.; Wu, J.; Gu, J.; Weng, H.; Wang, J.; Wang, T.; Liang, X.; Cao, L. Modular Characteristics and Mechanism of Action of Herbs for Endometriosis Treatment in Chinese Medicine: A Data Mining and Network Pharmacology–Based Identification. Front. Pharmacol. 2020, 11, 147. [Google Scholar] [CrossRef]
- Su, S.Y.; Muo, C.H.; Sung, F.C.; Morisky, D.E. Reduction of surgery rate in endometriosis patients who take Chinese medicine: A population-based retrospective cohort study. Complement. Ther. Med. 2014, 22, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Cao, L.; Xu, Z.; Ma, Y.; Liang, X. Anti-Angiogenic Alternative and Complementary Medicines for the Treatment of Endometriosis: A Review of Potential Molecular Mechanisms. Evid. Complement. Altern. Med. 2018, 2018, 4128984. [Google Scholar] [CrossRef] [PubMed]
- Lousse, J.C.; Van Langendonckt, A.; Defrere, S.; Ramos, R.G.; Colette, S.; Donnez, J. Peritoneal endometriosis is an inflammatory disease. Front. Biosci. 2012, 4, 23–40. [Google Scholar] [CrossRef]
- Oral, E.; Olive, D.L.; Arici, A. The peritoneal environment in endometriosis. Hum. Reprod. Update 1996, 2, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Gazvani, R.; Templeton, A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction 2002, 123, 217–226. [Google Scholar] [CrossRef]
- Wu, M.-H.; Shoji, Y.; Chuang, P.-C.; Tsai, S.-J. Endometriosis: Disease pathophysiology and the role of prostaglandins. Expert Rev. Mol. Med. 2007, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-H.; Lu, C.-W.; Chuang, P.-C.; Tsai, S.-J. Prostaglandin E2: The master of endometriosis? Exp. Biol. Med. 2010, 235, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Narumiya, S.; Sugimoto, Y.; Ushikubi, F. Prostanoid receptors: Structures, properties, and functions. Physiol. Rev. 1999, 79, 1193–1226. [Google Scholar] [CrossRef]
- Sugino, N.; Karube-Harada, A.; Taketani, T.; Sakata, A.; Nakamura, Y. Withdrawal of ovarian steroids stimulates prostaglandin F2alpha production through nuclear factor-kappaB activation via oxygen radicals in human endometrial stromal cells: Potential relevance to menstruation. J. Reprod. Dev. 2004, 50, 215–225. [Google Scholar] [CrossRef]
- Banu, S.K.; Lee, J.; Speights, V.O., Jr.; Starzinski-Powitz, A.; Arosh, J.A. Cyclooxygenase-2 Regulates Survival, Migration, and Invasion of Human Endometriotic Cells through Multiple Mechanisms. Endocrinology 2008, 149, 1180–1189. [Google Scholar] [CrossRef]
- González-Ramos, R.; Van Langendonckt, A.; Defrère, S.; Lousse, J.C.; Colette, S.; Devoto, L.; Donnez, J. Involvement of the nuclear factor-κB pathway in the pathogenesis of endometriosis. Fertil. Steril. 2010, 94, 1985–1994. [Google Scholar] [CrossRef]
- González-Ramos, R.; Van Langendonckt, A.; Defrère, S.; Lousse, J.C.; Mettlen, M.; Guillet, A.; Donnez, J. Agents Blocking the Nuclear Factor-κB Pathway Are Effective Inhibitors of Endometriosis in an in vivo Experimental Model. Gynecol. Obstet. Investig. 2008, 65, 174–186. [Google Scholar] [CrossRef]
- Dull, A.-M.; Moga, M.A.; Dimienescu, O.G.; Sechel, G.; Burtea, V.; Anastasiu, C.V. Therapeutic Approaches of Resveratrol on Endometriosis via Anti-Inflammatory and Anti-Angiogenic Pathways. Molecules 2019, 24, 667. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-H.; Hsiao, K.-Y.; Tsai, S.-J. Endometriosis and possible inflammation markers. Gynecol. Minim. Invasive Ther. 2015, 4, 61–67. [Google Scholar] [CrossRef]
- Brinckerhoff, C.E.; Matrisian, L.M. Matrix metalloproteinases: A tail of a frog that became a prince. Nat. Rev. Mol. Cell Biol. 2002, 3, 207–214. [Google Scholar] [CrossRef]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Mol. Cell Res. 2010, 1803, 55–71. [Google Scholar] [CrossRef]
- Osteen, K.G.; Yeaman, G.R.; Bruner-Tran, K.L. Matrix metalloproteinases and endometriosis. Semin. Reprod. Med. 2003, 21, 155–164. [Google Scholar] [CrossRef]
- Paul, S.; Bhattacharya, P.; Mahapatra, P.; Swarnakar, S. Melatonin protects against endometriosis via regulation of matrix metalloproteinase-3 and an apoptotic pathway. J. Pineal Res. 2010, 49, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Paul, S.; Swarnakar, S. Curcumin as anti-endometriotic agent: Implication of MMP-3 and intrinsic apoptotic pathway. Biochem. Pharmacol. 2012, 83, 797–804. [Google Scholar] [CrossRef]
- Iurlaro, M.; Loverro, G.; Vacca, A.; Cormio, G.; Ribatti, D.; Minischetti, M.; Ria, R.; Bruno, M.; Selvaggi, L. Angiogenesis extent and expression of matrix metalloproteinase-2 and-9 correlate with upgrading and myometrial invasion in endometrial carcinoma. Eur. J. Clin. Investig. 1999, 29, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Lang, J.H.; Leng, J.H.; Liu, D.Y. Increased levels of prostaglandin E2 and bcl-2 in peritoneal fluid and serum of patients with endometriosis. Zhonghua Fu Chan Ke Za Zhi 2005, 40, 598–600. [Google Scholar]
- Huang, H.F.; Hong, L.H.; Tan, Y.; Sheng, J.Z. Matrix metalloproteinase 2 is associated with changes in steroid hormones in the sera and peritoneal fluid of patients with endometriosis. Fertil. Steril. 2004, 81, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Agarwal, A.; Krajcir, N.; Alvarez, J.G. Role of oxidative stress in endometriosis. Reprod. BioMed. Online. 2006, 13, 126–134. [Google Scholar] [CrossRef]
- Ruder, E.H.; Hartman, T.J.; Blumberg, J.; Goldman, M.B. Oxidative stress and antioxidants: Exposure and impact on female fertility. Hum. Reprod. Update 2008, 14, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Aon, M.A.; Cortassa, S.; O’Rourke, B. Redox-optimized ROS balance: A unifying hypothesis. Biochim. Biophys. Acta. 2010, 1797, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef]
- Van Langendonckt, A.; Casanas-Roux, F.; Donnez, J. Oxidative stress and peritoneal endometriosis. Fertil. Steril. 2002, 77, 861–870. [Google Scholar] [CrossRef]
- Guo, S.W. Nuclear Factor-κB (NF-κB): An Unsuspected Major Culprit in the Pathogenesis of Endometriosis That Is Still at Large? Gynecol. Obstet. Investig. 2007, 63, 71–97. [Google Scholar] [CrossRef]
- González-Ramos, R.; Donnez, J.; Defrère, S.; Leclercq, I.; Squifflet, J.; Lousse, J.-C.; Van Langendonckt, A. Nuclear factor-kappa B is constitutively activated in peritoneal endometriosis. Mol. Hum. Reprod. 2007, 13, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Lousse, J.C.; Van Langendonckt, A.; González-Ramos, R.; Defrère, S.; Renkin, E.; Donnez, J. Increased activation of nuclear factor-kappa B (NF-kappaB) in isolated peritoneal macrophages of patients with endometriosis. Fertil. Steril. 2008, 90, 217–220. [Google Scholar] [CrossRef]
- Dalton, T.P.; Shertzer, H.G.; Puga, A. Regulation of gene expression by reactive oxygen. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 67–101. [Google Scholar] [CrossRef] [PubMed]
- Hey-Cunningham, A.J.; Peters, K.M.; Zevallos, H.B.; Berbic, M.; Markham, R.; Fraser, I.S. Angiogenesis, lymphangiogenesis and neurogenesis in endometriosis. Front. Biosci. 2013, 5, 1033–1056. [Google Scholar] [CrossRef]
- Gargett, C.E.; Masuda, H. Adult stem cells in the endometrium. Mol. Hum. Reprod. 2010, 16, 818–834. [Google Scholar] [CrossRef] [PubMed]
- Esser, S.; Lampugnani, M.G.; Corada, M.; Dejana, E.; Risau, W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J. Cell Sci. 1998, 111 Pt 13, 1853–1865. [Google Scholar]
- Kevil, C.G.; Payne, D.K.; Mire, E.; Alexander, J.S. Vascular permeability factor/vascular endothelial cell growth factor-mediated permeability occurs through disorganization of endothelial junctional proteins. J. Biol. Chem. 1998, 273, 15099–15103. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am. J. Physiol. Cell Physiol. 2001, 280, C1358–C1366. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.R.; Claffey, K.P.; Benes, J.E.; Perruzzi, C.A.; Sergiou, A.P.; Detmar, M. Angiogenesis promoted by vascular endothelial growth factor: Regulation through alpha1beta1 and alpha2beta1 integrins. Proc. Natl. Acad. Sci. USA 1997, 94, 13612–13617. [Google Scholar] [CrossRef]
- Pardo, O.E.; Arcaro, A.; Salerno, G.; Raguz, S.; Downward, J.; Seckl, M.J. Fibroblast growth factor-2 induces translational regulation of Bcl-XL and Bcl-2 via a MEK-dependent pathway. Correlation with resistance to etoposide-induced apoptosis. J. Biol. Chem. 2015, 290, 15390. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.F.; Lee, Y.L.; Chan, R.W.; Cheong, A.W.; Ng, E.H.; Ho, P.C.; Yeung, W.S. Up-regulation of endocrine gland-derived vascular endothelial growth factor but not vascular endothelial growth factor in human ectopic endometriotic tissue. Fertil. Steril. 2010, 93, 1052–1060. [Google Scholar] [CrossRef][Green Version]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Reed, J.C. Mechanisms of apoptosis. Am. J. Pathol. 2000, 157, 1415–1430. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Abrams, J.M.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; Dawson, T.M.; Dawson, V.L.; El-Deiry, W.S.; Fulda, S.; et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012, 19, 107–120. [Google Scholar] [CrossRef]
- Shi, Y. Mechanical aspects of apoptosome assembly. Curr. Opin. Cell Biol. 2006, 18, 677–684. [Google Scholar] [CrossRef]
- Harada, T.; Taniguchi, F.; Izawa, M.; Ohama, Y.; Takenaka, Y.; Tagashira, Y.; Ikeda, A.; Watanabe, A.; Iwabe, T.; Terakawa, N. Apoptosis and endometriosis. Front. Biosci. 2007, 12, 3140–3151. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Luo, X.; Toloubeydokhti, T.; Chegini, N. The expression profile of micro-RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol. Hum. Reprod. 2007, 13, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-Y.; Gu, L.; Chen, J.; Guo, X.-R.; Shi, Y.-L. Downregulation of miR-183 inhibits apoptosis and enhances the invasive potential of endometrial stromal cells in endometriosis. Int. J. Mol. Med. 2014, 33, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Xu, L.; Wang, P. MiR-191 inhibits TNF-α induced apoptosis of ovarian endometriosis and endometrioid carcinoma cells by targeting DAPK1. Int. J. Clin. Exp. Pathol. 2015, 8, 4933–4942. [Google Scholar]
- Corte, L.D.; Noventa, M.; Ciebiera, M.; Magliarditi, M.; Sleiman, Z.; Karaman, E.; Catena, U.; Salvaggio, C.; Falzone, G.; Garzon, S. Phytotherapy in endometriosis: An up-to-date review %J Journal of Complementary and Integrative Medicine. J. Complement. Integr. Med. 2019, 20190084. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Suou, K.; Iwabe, T.; Harada, T. Apigenin inhibits tnf-induced cell proliferation in endometriotic stromal cells. Fertil. Steril. 2009, 92, S11. [Google Scholar] [CrossRef]
- Suou, K.; Taniguchi, F.; Tagashira, Y.; Kiyama, T.; Terakawa, N.; Harada, T. Apigenin inhibits tumor necrosis factor α–induced cell proliferation and prostaglandin E2 synthesis by inactivating NFκB in endometriotic stromal cells. Fertil. Steril. 2011, 95, 1518–1521. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Bazer, F.W.; Song, G. Apigenin induces ROS-dependent apoptosis and ER stress in human endometriosis cells. J. Cell Physiol. 2018, 233, 3055–3065. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Austin, J.; Jinhong, R.; Johnson, M.E.; Lantvit, D.D.; Burdette, J.E. The Flavonoid Apigenin Is a Progesterone Receptor Modulator with In Vivo Activity in the Uterus. Horm. Cancer 2018, 9, 265–277. [Google Scholar] [CrossRef]
- Sharma, C.; Al Kaabi, J.M.; Nurulain, S.; Goyal, S.; Amjad Kamal, M.; Ojha, S. Polypharmacological Properties and Therapeutic Potential of β-Caryophyllene: A Dietary Phytocannabinoid of Pharmaceutical Promise. Curr. Pharm. Design. 2016, 22, 3237–3264. [Google Scholar] [CrossRef]
- Kim, C.; Cho, S.K.; Kim, K.-D.; Nam, D.; Chung, W.-S.; Jang, H.-J.; Lee, S.-G.; Shim, B.S.; Sethi, G.; Ahn, K.S. β-Caryophyllene oxide potentiates TNFα-induced apoptosis and inhibits invasion through down-modulation of NF-κB-regulated gene products. Apoptosis 2014, 19, 708–718. [Google Scholar] [CrossRef]
- Yang, M.; Lv, Y.; Tian, X.; Lou, J.; An, R.; Zhang, Q.; Li, M.; Xu, L.; Dong, Z. Neuroprotective effect of β-caryophyllene on cerebral ischemia-reperfusion injury via regulation of necroptotic neuronal death and inflammation: In vivo and in vitro. Front. Neurosci. 2017, 11, 583. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.A.; Taha, M.O.; Zihlif, M.A.; Disi, A.M. β-Caryophyllene causes regression of endometrial implants in a rat model of endometriosis without affecting fertility. Eur. J. Pharmacol. 2013, 702, 12–19. [Google Scholar] [CrossRef]
- Ojha, S.; Javed, H.; Azimullah, S.; Haque, M.E. β-Caryophyllene, a phytocannabinoid attenuates oxidative stress, neuroinflammation, glial activation, and salvages dopaminergic neurons in a rat model of Parkinson disease. Mol. Cell Biochem. 2016, 418, 59–70. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, H.; Yu, Z.; Peng, H.-Y.; Zhang, C.-J. Curcumin inhibits endometriosis endometrial cells by reducing estradiol production. Iranian J. Reprod. Med. 2013, 11, 415–422. [Google Scholar]
- Cao, W.-G.; Morin, M.; Metz, C.; Maheux, R.; Akoum, A. Stimulation of Macrophage Migration Inhibitory Factor Expression in Endometrial Stromal Cells by Interleukin 1, beta Involving the Nuclear Transcription Factor NFκB1. Biol. Reprod. 2005, 73, 565–570. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, S.; Yang, Z.; Fan, Y.; Guan, B.; Jia, J.; Gao, Y.; Wang, K.; Wu, K.; Wang, X.; Zheng, P. Curcumin enhances temsirolimus-induced apoptosis in human renal carcinoma cells through upregulation of YAP/p53. Oncol. Lett. 2016, 12, 4999–5006. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Al-Salam, S.; Al Suleimani, Y.; Al Kalbani, J.; Al Bahlani, S.; Ashique, M.; Manoj, P.; Al Dhahli, B.; Al Abri, N.; Naser, H.T.; et al. Curcumin Ameliorates Kidney Function and Oxidative Stress in Experimental Chronic Kidney Disease. Basic Clin. Pharmacol. Toxicol. 2018, 122, 65–73. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, H.; Hu, Y.-Y.; Wang, H.; Zhang, C.-J. Inhibitory effect of curcumin on angiogenesis in ectopic endometrium of rats with experimental endometriosis. Int. J. Mol. Med. 2011, 27, 87–94. [Google Scholar] [CrossRef]
- Lee, A.Y.-L.; Fan, C.-C.; Chen, Y.-A.; Cheng, C.-W.; Sung, Y.-J.; Hsu, C.-P.; Kao, T.-Y. Curcumin Inhibits Invasiveness and Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma Through Reducing Matrix Metalloproteinase 2, 9 and Modulating p53-E-Cadherin Pathway. Integr. Cancer Ther. 2015, 14, 484–490. [Google Scholar] [CrossRef]
- Chen, Q.; Gao, Q.; Chen, K.; Wang, Y.; Chen, L.; Li, X. Curcumin suppresses migration and invasion of human endometrial carcinoma cells. Oncol. Lett. 2015, 10, 1297–1302. [Google Scholar] [CrossRef]
- Ahn, W.S.; Huh, S.W.; Bae, S.M.; Lee, I.P.; Lee, J.M.; Namkoong, S.E.; Kim, C.K.; Sin, J.I. A major constituent of green tea, EGCG, inhibits the growth of a human cervical cancer cell line, CaSki cells, through apoptosis, G(1) arrest, and regulation of gene expression. DNA Cell Biol. 2003, 22, 217–224. [Google Scholar] [CrossRef]
- Xu, H.; Becker, C.M.; Lui, W.T.; Chu, C.Y.; Davis, T.N.; Kung, A.L.; Birsner, A.E.; D’Amato, R.J.; Wai Man, G.C.; Wang, C.C. Green tea epigallocatechin-3-gallate inhibits angiogenesis and suppresses vascular endothelial growth factor C/vascular endothelial growth factor receptor 2 expression and signaling in experimental endometriosis in vivo. Fertil. Steril. 2011, 96, 1021–1028.e1021. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Darcha, C. Antifibrotic properties of epigallocatechin-3-gallate in endometriosis. Hum. Reprod. 2014, 29, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.-H.; Wang, C.-C. Prodrug of Green Tea Epigallocatechin-3-Gallate (Pro-EGCG) for Use in the Treatment of Endometriosis. U.S. Patent No. 9,713,603, 25 July 2017. [Google Scholar]
- Uchiyama, Y.; Suzuki, T.; Mochizuki, K.; Goda, T. Dietary Supplementation with (−)-Epigallocatechin-3-gallate Reduces Inflammatory Response in Adipose Tissue of Non-obese Type 2 Diabetic Goto-Kakizaki (GK) Rats. J. Agric. Food Chem. 2013, 61, 11410–11417. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Mei, L.; Wang, H.; Fang, F. Epigallocatechin-3-gallate (EGCG) inhibits imiquimod-induced psoriasis-like inflammation of BALB/c mice. BMC Complement. Altern. Med. 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Han, Z.; Li, X.; Xie, H.-H.; Zhu, S.-S. Mechanism of EGCG promoting apoptosis of MCF-7 cell line in human breast cancer. Oncol. letters. 2017, 14, 3623–3627. [Google Scholar] [CrossRef]
- Chen, S.J.; Yao, X.D.; Peng, B.; Xu, Y.F.; Wang, G.C.; Huang, J.; Liu, M.; Zheng, J.H. Epigallocatechin-3-gallate inhibits migration and invasion of human renal carcinoma cells by downregulating matrix metalloproteinase-2 and matrix metalloproteinase-9. Experimental Ther. Med. 2016, 11, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, E.; Oktem, M.; Esinler, I.; Toru, S.A.; Zeyneloglu, H.B. Genistein causes regression of endometriotic implants in the rat model. Fertil. Steril. 2007, 88, 1129–1134. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, J.; Mi, M.; Chen, W.; Pan, Q.; Wei, M.J. Anti-angiogenic genistein inhibits VEGF-induced endothelial cell activation by decreasing PTK activity and MAPK activation. Med. Oncol. 2012, 29, 349–357. [Google Scholar] [CrossRef]
- Wei, D.; Yang, L.; Lv, B.; Chen, L. Genistein suppresses retinoblastoma cell viability and growth and induces apoptosis by upregulating miR-145 and inhibiting its target ABCE1. Mol. Vis. 2017, 23, 385–394. [Google Scholar]
- Surico, D.; Ercoli, A.; Farruggio, S.; Raina, G.; Filippini, D.; Mary, D.; Minisini, R.; Surico, N.; Pirisi, M.; Grossini, E. Modulation of Oxidative Stress by 17 β-Estradiol and Genistein in Human Hepatic Cell Lines In Vitro. Cell Phys. Biochem. 2017, 42, 1051–1062. [Google Scholar] [CrossRef]
- Geng, Y.; Zhu, S.; Cheng, P.; Lu, Z.-M.; Xu, H.-Y.; Shi, J.-S.; Xu, Z.-H. Bioassay-guided fractionation of ethyl acetate extract from Armillaria mellea attenuates inflammatory response in lipopolysaccharide (LPS) stimulated BV-2 microglia. Phytomedicine 2017, 26, 55–61. [Google Scholar] [CrossRef]
- Cotroneo, M.S.; Lamartiniere, C.A. Pharmacologic, but Not Dietary, Genistein Supports Endometriosis in a Rat Model. Toxic Sci. 2001, 61, 68–75. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M. Distribution and Biological Activities of the Flavonoid Luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; You, S.; Song, G. Ameliorative effects of luteolin against endometriosis progression in vitro and in vivo. J. Nutr. Biochem. 2019, 67, 161–172. [Google Scholar] [CrossRef]
- Nordeen, S.K.; Bona, B.J.; Jones, D.N.; Lambert, J.R.; Jackson, T.A. Endocrine disrupting activities of the flavonoid nutraceuticals luteolin and quercetin. Homones Cancer. 2013, 4, 293–300. [Google Scholar] [CrossRef]
- Moga, M.A.; Dimienescu, O.G.; Arvatescu, C.A.; Mironescu, A.; Dracea, L.; Ples, L. The Role of Natural Polyphenols in the Prevention and Treatment of Cervical Cancer-An Overview. Molecules 2016, 21, 1055. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Bazer, F.W.; Lim, W.; Song, G. Chrysin leads to cell death in endometriosis by regulation of endoplasmic reticulum stress and cytosolic calcium level. J. Cell Physiol. 2019, 234, 2480–2490. [Google Scholar] [CrossRef]
- Yao, Z.; Li, C.; Gu, Y.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Bao, X.; Zhang, S.; Sun, S.; et al. Dietary myricetin intake is inversely associated with the prevalence of type 2 diabetes mellitus in a Chinese population. Nutr. Res. 2019, 68, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Song, G.; Lim, W. Myricetin inhibits endometriosis growth through cyclin E1 down-regulation in vitro and in vivo. J. Nutr. Biochem. 2020, 78, 108328. [Google Scholar] [CrossRef] [PubMed]
- Kanno, S.; Tomizawa, A.; Ohtake, T.; Koiwai, K.; Ujibe, M.; Ishikawa, M. Naringenin-induced apoptosis via activation of NF-kappaB and necrosis involving the loss of ATP in human promyeloleukemia HL-60 cells. Toxicol. Lett. 2006, 166, 131–139. [Google Scholar] [CrossRef]
- Ratnam, D.V.; Ankola, D.D.; Bhardwaj, V.; Sahana, D.K.; Kumar, M.N.V.R. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J. Control. Release 2006, 113, 189–207. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Bazer, F.W.; Song, G. Naringenin induces mitochondria-mediated apoptosis and endoplasmic reticulum stress by regulating MAPK and AKT signal transduction pathways in endometriosis cells. Mol. Hum. Reprod. 2017, 23, 842–854. [Google Scholar] [CrossRef]
- Kapoor, R.; Sirohi, V.K.; Gupta, K.; Dwivedi, A. Naringenin ameliorates progression of endometriosis by modulating Nrf2/Keap1/HO1 axis and inducing apoptosis in rats. J. Nutr. Biochem. 2019, 70, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, C.; Shi, S.; Han, J.; Wang, J.; Hu, J.; Liu, Y.; Cai, Z.; Yu, C. Endometriotic Implants Regress in Rat Models Treated With Puerarin by Decreasing Estradiol Level. Reprod. Sci. 2011, 18, 886–891. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.; Han, J.; Zai, D.; Ji, M.; Cheng, W.; Xu, L.; Yang, L.; He, M.; Ni, J. Puerarin suppresses invasion and vascularization of endometriosis tissue stimulated by 17β-estradiol. PLoS ONE 2011, 6, e25011. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Chen, L.; Yang, S.; Han, J.; Zhai, D.; Ni, J.; Yu, C.; Cai, Z. Puerarin Suppresses Proliferation of Endometriotic Stromal Cells Partly via the MAPK Signaling Pathway Induced by 17ß-estradiol-BSA. PLoS ONE 2012, 7, e45529. [Google Scholar] [CrossRef] [PubMed]
- Murahari, M.; Singh, V.; Chaubey, P.; Suvarna, V. A Critical Review on Anticancer Mechanisms of Natural Flavonoid Puerarin. Anti-Cancer Agents Med. Chem. 2020, 20, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, H.M.; Lehtinen, O.; Suomela, J.P.; Viitanen, M.; Kallio, H. Flavonol glycosides of sea buckthorn (Hippophaë rhamnoides ssp. sinensis) and lingonberry (Vaccinium vitis-idaea) are bioavailable in humans and monoglucuronidated for excretion. J. Agric. Food Chem. 2010, 58, 620–627. [Google Scholar] [CrossRef]
- Scambia, G.; Ranelletti, F.O.; Panici, P.B.; Piantelli, M.; Bonanno, G.; De Vincenzo, R.; Ferrandina, G.; Maggiano, N.; Capelli, A.; Mancuso, S. Inhibitory effect of quercetin on primary ovarian and endometrial cancers and synergistic activity with cis-diamminedichloroplatinum(II). Gynecol. Oncol. 1992, 45, 13–19. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Wang, H.-J.; Yang, Q.; Qie, M.-R. Inhibition effect and mechanisms of quercetin on surgically induced endometriosis. J. Sichuan Univ. 2009, 40, 228–231, 244. [Google Scholar]
- Cao, Y.; Zhuang, M.-F.; Yang, Y.; Xie, S.-W.; Cui, J.-G.; Cao, L.; Zhang, T.-T.; Zhu, Y. Preliminary Study of Quercetin Affecting the Hypothalamic-Pituitary-Gonadal Axis on Rat Endometriosis Model. Evid. Complement. Altern. Med. 2014, 2014, 781684. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef]
- Chu, M.; Almagro, L.; Chen, B.; Burgos, L.; Pedreño, M.A. Recent trends and comprehensive appraisal for the biotechnological production of trans-resveratrol and its derivatives. Phytochem. Rev. 2018, 17, 491–508. [Google Scholar] [CrossRef]
- Nakata, R.; Takahashi, S.; Inoue, H. Recent advances in the study on resveratrol. Biol. Pharm. Bull. 2012, 35, 273–279. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Chu, H.; Li, H.; Guan, X.; Yan, H.; Zhang, X.; Cui, X.; Li, X.; Cheng, M. Resveratrol protects late endothelial progenitor cells from TNF-α-induced inflammatory damage by upregulating Krüppel-like factor-2. Mol. Med. Rep. 2018, 17, 5708–5715. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Buhrmann, C.; Mobasheri, A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J. Biol. Chem. 2011, 286, 11492–11505. [Google Scholar] [CrossRef] [PubMed]
- Trapp, V.; Parmakhtiar, B.; Papazian, V.; Willmott, L.; Fruehauf, J.P. Anti-angiogenic effects of resveratrol mediated by decreased VEGF and increased TSP1 expression in melanoma-endothelial cell co-culture. Angiogenesis 2010, 13, 305–315. [Google Scholar] [CrossRef]
- Polak, G.; Mazurek, D.; Rogala, E.; Nowicka, A.; Derewianka-Polak, M.; Kotarski, J. Increased oxidized LDL cholesterol levels in peritoneal fluid of women with advanced-stage endometriosis. Ginekol Pol. 2011, 82, 191–194. [Google Scholar] [PubMed]
- Truong, V.-L.; Jun, M.; Jeong, W.-S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors 2018, 44, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Sunkaria, A.; Singhal, N.; Sandhir, R. Resveratrol loaded solid lipid nanoparticles attenuate mitochondrial oxidative stress in vascular dementia by activating Nrf2/HO-1 pathway. Neurochem. Int. 2018, 112, 239–254. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus). Molecules 2015, 20, 754–779. [Google Scholar] [CrossRef]
- Rudzitis-Auth, J.; Körbel, C.; Scheuer, C.; Menger, M.D.; Laschke, M.W. Xanthohumol inhibits growth and vascularization of developing endometriotic lesions. Hum. Reprod. 2012, 27, 1735–1744. [Google Scholar] [CrossRef]
- Dell’Eva, R.; Ambrosini, C.; Vannini, N.; Piaggio, G.; Albini, A.; Ferrari, N. AKT/NF-κB inhibitor xanthohumol targets cell growth and angiogenesis in hematologic malignancies. Cancer. 2007, 110, 2007–2011. [Google Scholar] [CrossRef]
- Dorn, C.; Massinger, S.; Wuzik, A.; Heilmann, J.; Hellerbrand, C. Xanthohumol suppresses inflammatory response to warm ischemia–reperfusion induced liver injury. Exper Mol. Pathol. 2013, 94, 10–16. [Google Scholar] [CrossRef]
- Jin, M.; Zhao, K.; Huang, Q.; Xu, C.; Shang, P. Isolation, structure and bioactivities of the polysaccharides from Angelica sinensis (Oliv.) Diels: A review. Carbohydr. Polym. 2012, 89, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.-W.; Lin, B.-F. Bioactivities of major constituents isolated from Angelica sinensis (Danggui). Chinese Med. 2011, 6, 29. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Meng, F.Y.; Liang, S.X.; Deng, R.; Li, C.K.; Pong, N.H.; Lau, C.P.; Cheng, S.W.; Ye, J.Y.; et al. Polysaccharides from the root of Angelica sinensis promotes hematopoiesis and thrombopoiesis through the PI3K/AKT pathway. BMC Complement. Alternat Med. 2010, 10, 79. [Google Scholar] [CrossRef]
- Xiong, Q.-X.; Ruan, X.-Y.; Deng, A.-P.; Liu, J.; Zhou, Q. Anti-endometriotic effect of Angelica sinensis (Oliv.) Diels extract in human endometriotic cells and rats. Trop. J. Pharm. Res. 2020, 19, 817–821. [Google Scholar] [CrossRef]
- Yeşilada, E.; Honda, G.; Sezik, E.; Tabata, M.; Fujita, T.; Tanaka, T.; Takeda, Y.; Takaishi, Y. Traditional medicine in Turkey. V. Folk medicine in the inner Taurus Mountains. J. Ethnopharmacol. 1995, 46, 133–152. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Bolego, C.; Cignarella, A.; Gaion, R.M.; Innocenti, G. Vasoprotective activity of standardized Achillea millefolium extract. Phytomedicine 2011, 18, 1031–1036. [Google Scholar] [CrossRef]
- Demirel, M.A.; Suntar, I.; Ilhan, M.; Keles, H.; Kupeli Akkol, E. Experimental endometriosis remission in rats treated with Achillea biebersteinii Afan.: Histopathological evaluation and determination of cytokine levels. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 175, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Mazandarani, M.; Osia, N.; Ghafourian, M. Antioxidant activity and ethno pharmacological survey of Achillea biebersteinii Afan. in the treatment of dysmenorrhoea in traditional medicine of Golestan province, Iran. J. Women Health Reprod. Sci. 2015, 3, 107–110. [Google Scholar] [CrossRef]
- Jaffal, S.M.; Abbas, M.A. Antinociceptive action of Achillea biebersteinii methanolic flower extract is mediated by interaction with cholinergic receptor in mouse pain models. Inflammopharmacology 2019, 27, 961–968. [Google Scholar] [CrossRef]
- Umano, K.; Hagi, Y.; Nakahara, K.; Shoji, A.; Shibamoto, T. Volatile Chemicals Identified in Extracts from Leaves of Japanese Mugwort (Artemisia princeps Pamp.). J. Agric. Food Chem. 2000, 48, 3463–3469. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Jung, S.-H.; Yang, Y.-I.; Ahn, J.-H.; Cho, J.-G.; Lee, K.-T.; Baek, N.-I.; Choi, J.-H. Artemisia leaf extract induces apoptosis in human endometriotic cells through regulation of the p38 and NFκB pathways. J. Ethnopharmacol. 2013, 145, 767–775. [Google Scholar] [CrossRef]
- Cho, J.-H.; Lee, J.-G.; Yang, Y.-I.; Kim, J.-H.; Ahn, J.-H.; Baek, N.-I.; Lee, K.-T.; Choi, J.-H. Eupatilin, a dietary flavonoid, induces G2/M cell cycle arrest in human endometrial cancer cells. Food Chem. Toxicol. 2011, 49, 1737–1744. [Google Scholar] [CrossRef]
- Han, J.-M.; Kim, M.-J.; Baek, S.-H.; An, S.; Jin, Y.-Y.; Chung, H.-G.; Baek, N.-I.; Choi, M.-S.; Lee, K.-T.; Jeong, T.-S. Antiatherosclerotic Effects of Artemisia princeps Pampanini cv. Sajabal in LDL Receptor Deficient Mice. J. Agric. Food Chem. 2009, 57, 1267–1274. [Google Scholar] [CrossRef]
- Kim, M.-J.; Han, J.-M.; Jin, Y.-Y.; Baek, N.-I.; Bang, M.-H.; Chung, H.-G.; Choi, M.-S.; Lee, K.-T.; Sok, D.-E.; Jeong, T.-S. In Vitro antioxidant and anti-inflammatory activities of Jaceosidin from Artemisia princeps Pampanini cv. Sajabal. Arch Pharm. Res. 2008, 31, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Park, J.K.; Choi, Y.-W.; Kim, Y.-H.; Lee, E.N.; Lee, J.-R.; Kim, H.-S.; Baek, S.-Y.; Kim, B.-S.; Lee, K.-S. Hexane extract of aged black garlic reduces cell proliferation and attenuates the expression of ICAM-1 and VCAM-1 in TNF-α-activated human endometrial stromal cells. Int. J. Mol. Med. 2013, 32, 67–78. [Google Scholar] [CrossRef]
- Xiao, D.; Li, M.; Herman-Antosiewicz, A.; Antosiewicz, J.; Xiao, H.; Lew, K.L.; Zeng, Y.; Marynowski, S.W.; Singh, S.V. Diallyl trisulfide inhibits angiogenic features of human umbilical vein endothelial cells by causing Akt inactivation and down-regulation of VEGF and VEGF-R2. Nutr. Cancer 2006, 55, 94–107. [Google Scholar] [CrossRef]
- Hodge, G.; Hodge, S.; Han, P. Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: Potential therapeutic use in the treatment of inflammatory bowel disease. J. Quant. Cell Sci. 2002, 48, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Padiya, R.; Chowdhury, D.; Borkar, R.; Srinivas, R.; Pal Bhadra, M.; Banerjee, S.K. Garlic Attenuates Cardiac Oxidative Stress via Activation of PI3K/AKT/Nrf2-Keap1 Pathway in Fructose-Fed Diabetic Rat. PLoS ONE 2014, 9, e94228. [Google Scholar] [CrossRef] [PubMed]
- Avci, A.; Atli, T.; Ergüder, İ.B.; Varli, M.; Devrim, E.; Demir, Ö.; Durak, I.; Turgay, M. Effects of grape consumption on plasma and erythrocyte antioxidant parameters in elderly subjects. Turkish J. Med. Sci. 2010, 40, 525–529. [Google Scholar] [CrossRef][Green Version]
- Kim, Y.-S.; Lim, E.-M.; Cho, H.-J. Effects of Astragalus membranaceus on Surgically Induced Endometriosis in Rats. J. Korean Obstet. Gynecol. 2007, 20, 43–59. [Google Scholar]
- Orkhon, B.; Kobayashi, K.; Javzan, B.; Sasaki, K. Astragalus root induces ovarian β-oxidation and suppresses estrogen-dependent uterine proliferation. Mol. Med. Rep. 2018, 18, 5198–5206. [Google Scholar] [CrossRef]
- Zhao, R.-H.; Hao, Z.-P.; Zhang, Y.; Lian, F.-M.; Sun, W.-W.; Liu, Y.; Wang, R.; Long, L.; Cheng, L.; Ding, Y.-F. Controlling the recurrence of pelvic endometriosis after a conservative operation: Comparison between Chinese herbal medicine and western medicine. Chin. J. Integr. Med. 2013, 19, 820–825. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, J.-w. Experimental study on rat model of endometriosis treated with tamoxifen and rhizoma curcumae oil. J. Sichuan Univ 2006, 37, 596–598. [Google Scholar]
- Swarnakar, S.; Paul, S. Curcumin arrests endometriosis by downregulation of matrix metalloproteinase-9 activity. Indian J. Biochem. Biophys 2009, 46, 59–65. [Google Scholar] [PubMed]
- Uchio, R.; Higashi, Y.; Kohama, Y.; Kawasaki, K.; Hirao, T.; Muroyama, K.; Murosaki, S. A hot water extract of turmeric (Curcuma longa) suppresses acute ethanol-induced liver injury in mice by inhibiting hepatic oxidative stress and inflammatory cytokine production. J. Nutr. Sci. 2017, 6, e3. [Google Scholar] [CrossRef]
- Kumar, G.; Tajpara, P.; Maru, G. Dietary Turmeric Post-Treatment Decreases DMBA-Induced Hamster Buccal Pouch Tumor Growth by Altering Cell Proliferation and Apoptosis-Related Markers. J. Environ. Pathol Toxicol. Oncol. 2012, 31, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Yuliani, S.; Mustofa; Partadiredja, G. The neuroprotective effects of an ethanolic turmeric (Curcuma longa L.) extract against trimethyltin-induced oxidative stress in rats. Nutr. Neurosci. 2019, 22, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-F.; Chyau, C.-C.; Wang, T.-S.; Li, C.-R.; Hu, T.-J. Enhanced Antioxidant and Anti-inflammatory Activities of Monascus pilosus Fermented Products by Addition of Turmeric to the Medium. J. Agric. Food Chem. 2009, 57, 11397–11405. [Google Scholar] [CrossRef]
- Psotová, J.; Kolář, M.; Soušek, J.; Švagera, Z.; Vičar, J.; Ulrichová, J. Biological activities of Prunella vulgaris extract. Phytoter. Res. 2003, 17, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.H.; Lessey, E.C.; DuSell, C.D.; McDonnell, D.P.; Fowler, L.; Palomino, W.A.; Illera, M.J.; Yu, X.; Mo, B.; Houwing, A.M.; et al. Characterization of Antiestrogenic Activity of the Chinese Herb, Prunella vulgaris, Using In Vitro and In Vivo (Mouse Xenograft) Models1. Biol. Reprod. 2009, 80, 375–383. [Google Scholar] [CrossRef]
- Yin, D.T.; Lei, M.; Xu, J.; Li, H.; Wang, Y.; Liu, Z.; Ma, R.; Yu, K.; Li, X. The Chinese herb Prunella vulgaris promotes apoptosis in human well-differentiated thyroid carcinoma cells via the B-cell lymphoma-2/Bcl-2-associated X protein/caspase-3 signaling pathway. Oncol. Lett. 2017, 14, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Koo, H.J.; Sung, Y.Y.; Kim, H.K. The protective effect of Prunella vulgaris ethanol extract against vascular inflammation in TNF-α-stimulated human aortic smooth muscle cells. BMB Rep. 2013, 46, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wei, Y.-H. A new alkaloid-aluminum glycoside isolated from Rhizoma Sparganii (Sparganium stoloniferum Buch.-Ham.). J. Med. Plants Res. 2011, 5, 3128–3131. [Google Scholar] [CrossRef]
- Sun, J.; Wang, S.; Wei, Y.-H. Reproductive toxicity of Rhizoma Sparganii (Sparganium stoloniferum Buch.-Ham.) in mice: Mechanisms of anti-angiogenesis and anti-estrogen pharmacologic activities. J. Ethnopharmacol. 2011, 137, 1498–1503. [Google Scholar] [CrossRef]
- Wu, Y.-z.; Sun, J.; Wang, Y.-b. Selective estrogen receptor modulator: A novel polysaccharide from Sparganii Rhizoma induces apoptosis in breast cancer cells. Carbohydr. Polym. 2017, 163, 199–207. [Google Scholar] [CrossRef]
- Jiang, Y.-Y.; Li, Y.-B.; Yu, J.; Chen, H.; Zhou, J.; Wang, L.; Zhang, L.; Zhao, M.-J.; Zhou, Y.-H.; Yu, L. Preliminary structure and bioactivities of polysaccharide SMWP-U&E isolated from Salvia miltiorrhiza Bunge Residue. Int. j Biol. Mol. 2020, 157, 434–443. [Google Scholar] [CrossRef]
- Wang, B.Q. Salvia miltiorrhiza chemical and pharmacological review of a medicinal plant. J. Med. Plants Res. 2010, 4, 2813–2820. [Google Scholar]
- Chen, Z.-z.; Gong, X. Tanshinone IIA contributes to the pathogenesis of endometriosis via renin angiotensin system by regulating the dorsal root ganglion axon sprouting. Life Sci. 2020, 240, 117085. [Google Scholar] [CrossRef]
- Liu, J.-J.; Lin, D.-J.; Liu, P.-Q.; Huang, M.; Li, X.-D.; Huang, R.-W. Induction of apoptosis and inhibition of cell adhesive and invasive effects by tanshinone IIA in acute promyelocytic leukemia cells in vitro. J. Biomed. Sci. 2006, 13, 813–823. [Google Scholar] [CrossRef]
- Tsai, M.-Y.; Yang, R.-C.; Wu, H.-T.; Pang, J.-H.S.; Huang, S.-T. Anti-angiogenic effect of Tanshinone IIA involves inhibition of matrix invasion and modification of MMP-2/TIMP-2 secretion in vascular endothelial cells. Cancer Lett. 2011, 310, 198–206. [Google Scholar] [CrossRef]
- Kim, S.Y.; Moon, T.C.; Chang, H.W.; Son, K.H.; Kang, S.S.; Kim, H.P. Effects of tanshinone I isolated from Salvia miltiorrhiza Bunge on arachidonic acid metabolism and in vivo inflammatory responses. Phytoter. Res. 2002, 16, 616–620. [Google Scholar] [CrossRef]
- Zhou, Z.-H.; Weng, Q.; Zhou, J.-H.; Zhou, J. Extracts of Salvia miltiorrhiza bunge on the cytokines of rat endometriosis models. Afr. J. Trad. Complement. Altern. Med. 2012, 9, 303–314. [Google Scholar] [CrossRef][Green Version]
- Tu, Q.; Wang, R.; Ding, B.; Zhong, W.; Cao, H. Protective and antioxidant effect of Danshen polysaccharides on cerebral ischemia/reperfusion injury in rats. Int. J. Biol. Macromol. 2013, 60, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Qian, S.; Fan, P.; Huo, D.; Wang, S. Effect of Salvia miltiorrhiza Hydrophilic Extract on Antioxidant Enzymes in Diabetic Patients with Chronic Heart Disease: A Randomized Controlled Trial. Phytoter. Res. 2012, 26, 60–66. [Google Scholar] [CrossRef] [PubMed]
- He, D.-Y.; Dai, S.-M. Anti-inflammatory and immunomodulatory effects of Paeonia lactiflora Pall., a traditional Chinese herbal medicine. Front. Pharmacol. 2011, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-X.; Hu, L.; Zhu, S.-H.; Han, Y.; Liu, W.-T.; Yang, Y.-J.; Li, Q.-P. Paeoniflorin attenuates postoperative pain by suppressing Matrix Metalloproteinase-9/2 in mice. Eur. J. Pain. 2018, 22, 272–281. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, F.; Wang, H.; Wang, Y.; Wu, Y.; Xu, H.; Su, C. Paeoniflorin inhibits proliferation of endometrial cancer cells via activating MAPK and NF-κB signaling pathways. Experim Ther. Med. 2017, 14, 5445–5451. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ohno, O.; Suenaga, K.; Miyamoto, K. Apoptosis-inducing activity and antiproliferative effect of Paeoniflorigenone from moutan cortex. Biosci. Biotechnol. Biochem. 2017, 81, 1106–1113. [Google Scholar] [CrossRef]
- Rop, O.; Reznicek, V.; Valsikova, M.; Jurikova, T.; Mlcek, J.; Kramarova, D. Antioxidant properties of European cranberrybush fruit (Viburnum opulus var. edule). Molecules 2010, 15, 4467–4477. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, B.; David, L.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Baldea, I.; Clichici, S.; Filip, G.A. In vitro and in vivo anti-inflammatory properties of green synthesized silver nanoparticles using Viburnum opulus L. fruits extract. Mater. Sci. Engin. 2017, 79, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Saltan, G.; Süntar, I.; Ozbilgin, S.; Ilhan, M.; Demirel, M.A.; Oz, B.E.; Keleş, H.; Akkol, E.K. Viburnum opulus L.: A remedy for the treatment of endometriosis demonstrated by rat model of surgically-induced endometriosis. J. Ethnopharmacol. 2016, 193, 450–455. [Google Scholar] [CrossRef]
- Zayachkivska, O.; Gzhegotsky, M.; Terletska, O.; Lutsyk, D.; Yaschenko, A.; Dzhura, O. Influence of Viburnum opulus proanthocyanidins on stress-induced gastrointestinal mucosal damage. J. Physiol. Pharmacol. 2006, 57, 155. [Google Scholar]
- Nagulendran, K.; Velavan, S.; Mahesh, R.; Begum, V.H. In vitro antioxidant activity and total polyphenolic content of Cyperus rotundus rhizomes. J. Chem. 2007, 4, 440–449. [Google Scholar]
- Ahn, J.-H.; Lee, T.-W.; Kim, K.-H.; Byun, H.; Ryu, B.; Lee, K.-T.; Jang, D.S.; Choi, J.-H. 6-Acetoxy Cyperene, a Patchoulane-type Sesquiterpene Isolated from Cyperus rotundus Rhizomes Induces Caspase-dependent Apoptosis in Human Ovarian Cancer Cells. Phytother Res. 2015, 29, 1330–1338. [Google Scholar] [CrossRef]
- Sabbe, S.; Verbeke, W.; Deliza, R.; Matta, V.M.; Van Damme, P. Consumer Liking of Fruit Juices with Different Açaí (Euterpe oleracea Mart.) Concentrations. Food Sci. 2009, 74, S171–S176. [Google Scholar] [CrossRef]
- Machado, D.E.; Rodrigues-Baptista, K.C.; Alessandra-Perini, J.; Soares de Moura, R.; Santos, T.A.d.; Pereira, K.G.; Marinho da Silva, Y.; Souza, P.J.C.; Nasciutti, L.E.; Perini, J.A. Euterpe oleracea Extract (Açaí) Is a Promising Novel Pharmacological Therapeutic Treatment for Experimental Endometriosis. PLoS ONE 2016, 11, e0166059. [Google Scholar] [CrossRef]
- Del Pozo-Insfran, D.; Percival, S.S.; Talcott, S.T. Açai (Euterpe oleracea Mart.) Polyphenolics in Their Glycoside and Aglycone Forms Induce Apoptosis of HL-60 Leukemia Cells. J. Agric. Food Chem. 2006, 54, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Kang, J.; Li, Z.; Schauss, A.G.; Badger, T.M.; Nagarajan, S.; Wu, T.; Wu, X. The açaí flavonoid velutin is a potent anti-inflammatory agent: Blockade of LPS-mediated TNF-α and IL-6 production through inhibiting NF-κB activation and MAPK pathway. J. Nutr. Biochem. 2012, 23, 1184–1191. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Wang, C.; Qu, S.; Zhu, Y.; Yang, Z.; Wang, L. Açaí (Euterpe oleracea Mart.) attenuates alcohol-induced liver injury in rats by alleviating oxidative stress and inflammatory response. Exp. Ther. Med. 2018, 15, 166–172. [Google Scholar] [CrossRef]
- Iravani, S.; Zolfaghari, B. Pharmaceutical and nutraceutical effects of Pinus pinaster bark extract. Res. Pharm. Sci. 2011, 6, 1–11. [Google Scholar]
- Maia Junior, H.; Haddad, C.; Casoy, J. Combining oral contraceptives with a natural nuclear factor-kappa B inhibitor for the treatment of endometriosis-related pain. Int. J. Women’s Health 2013, 6, 35–39. [Google Scholar] [CrossRef][Green Version]
- Kohama, T.; Herai, K.; Inoue, M. Effect of French maritime pine bark extract on endometriosis as compared with leuprorelin acetate. J. Reprod. Med. Chicago 2007, 52, 703. [Google Scholar]
- Yang, I.-H.; Shin, J.-A.; Cho, S.-D. Pycnogenol Induces Nuclear Translocation of Apoptosis-inducing Factor and Caspase-independent Apoptosis in MC-3 Human Mucoepidermoid Carcinoma Cell Line. J. Cancer Prev. 2014, 19, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cong, H.; Zhao, S.; Zhang, Q.; Gu, X. Jing Tong Yu Shu, a traditional Chinese medicine, suppresses IL-1β and IL-6 gene expressions in macrophages, and alleviates endometriosis. Trop. J. Pharm. Res. 2017, 16, 2953–2958. [Google Scholar] [CrossRef][Green Version]
- Ilhan, M.; Ali, Z.; Khan, I.A.; Taştan, H.; Küpeli Akkol, E. Bioactivity-guided isolation of flavonoids from Urtica dioica L. and their effect on endometriosis rat model. J. Ethnopharmacol. 2019, 243, 112100. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Kikuzaki, H.; Hisamoto, M.; Nakatani, N. Antioxidant properties of gingerol related compounds from ginger. Biofactors 2004, 21, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiang, Y.; Yang, X.; Wei, Q.; Wang, H. 6-Shogaol reduces progression of experimental endometriosis in vivo and in vitro via regulation of VGEF and inhibition of COX-2 and PGE2-mediated inflammatory responses. Korean J. Physiol. Pharmacol. 2018, 22, 627–636. [Google Scholar] [CrossRef] [PubMed]
| Medicinal Plant | Experimental Model | Biological Effects | Molecular Mechanisms | Reference | |
|---|---|---|---|---|---|
| Angelica sinensis |  | Rats with surgically induced endometriosis (human endometriotic cells) |
|
| [140] |
| Achillea biebersteinii |  | Rats with surgically induced endometriosis Mouse pain models |
|
| [143,144,145] |
| Artemisia princeps |  | Human endometriotic cells |
|
| [148,149,150,151] |
| Allium sativum |  | Human endometriotic cells |
|
| [151,153] |
| Astragalus membranaceus |  | Rats with surgically induced endometriosis |
|
| [156] |
| Curcuma longa |  | Rat with surgically induced endometriosis |
|
| [159,160,161,162,163] |
| Prunella vulgaris |  | Xenograft mice |
|
| [166] |
| Rhizoma sparganii |  | Pregnant rodents |
|
| [170] |
| Salvia miltiorrhiza |  | Rats with surgically induced endometriosis |
|
| [174,178] |
| Paeonia lactiflora |  | Tumoral endometrial cells |
|
| [183] |
| Viburnum opulus |  | Rats with surgically induced endometriosis |
|
| [187,188] |
| Cyperus rotundus |  | Human endometrial cells |
|
| [22] |
| Euterpe oleracea |  | Rats with surgically induced endometriosis |
|
| [193] |
| Pinus pinaster |  | Human clinical trial |
|
| [198,199] |
| Urtica dioica |  | Rats with surgically induced endometriosis |
|
| [201] |
| Zinguber officinale |  | Rats with surgically induced endometriosis |
|
| [203] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balan, A.; Moga, M.A.; Dima, L.; Dinu, C.G.; Martinescu, C.C.; Panait, D.E.; Irimie, C.A.; Anastasiu, C.V. An Overview on the Conservative Management of Endometriosis from a Naturopathic Perspective: Phytochemicals and Medicinal Plants. Plants 2021, 10, 587. https://doi.org/10.3390/plants10030587
Balan A, Moga MA, Dima L, Dinu CG, Martinescu CC, Panait DE, Irimie CA, Anastasiu CV. An Overview on the Conservative Management of Endometriosis from a Naturopathic Perspective: Phytochemicals and Medicinal Plants. Plants. 2021; 10(3):587. https://doi.org/10.3390/plants10030587
Chicago/Turabian StyleBalan, Andreea, Marius Alexandru Moga, Lorena Dima, Catalina Georgeta Dinu, Carmen Constantina Martinescu, Diana Elena Panait, Claudia Alexandrina Irimie, and Costin Vlad Anastasiu. 2021. "An Overview on the Conservative Management of Endometriosis from a Naturopathic Perspective: Phytochemicals and Medicinal Plants" Plants 10, no. 3: 587. https://doi.org/10.3390/plants10030587
APA StyleBalan, A., Moga, M. A., Dima, L., Dinu, C. G., Martinescu, C. C., Panait, D. E., Irimie, C. A., & Anastasiu, C. V. (2021). An Overview on the Conservative Management of Endometriosis from a Naturopathic Perspective: Phytochemicals and Medicinal Plants. Plants, 10(3), 587. https://doi.org/10.3390/plants10030587






