Current Condition of Pannonic Salt Steppes at Their Distribution Limit: What Do Indicator Species Reveal about Habitat Quality?
Abstract
1. Introduction
- (i)
- to analyze the ecological range limits of indicator plants of Pannonic salt steppes on their border of distribution,
- (ii)
- to compare the current condition, threatening factors, and conservation measures of their target habitat with reference sites in their central distribution.
2. Materials and Methods
2.1. Study Area
2.2. Study Design
2.2.1. Selection of Study Objects
2.2.2. Vegetation Sampling
2.2.3. Soil Sampling
2.2.4. Data Processing
2.2.5. Data Analysis
3. Results and Discussion
3.1. Ecological Range Limits of Indicator Species of Pannonic Salt Steppes—Vegetation Composition and Land Use Approach
3.1.1. Artemisia santonicum Subsp. Patens (ART)
3.1.2. Camphorosma Annua (CAM)
3.1.3. Tripolium pannonicum Subsp. pannonicum (TRI)
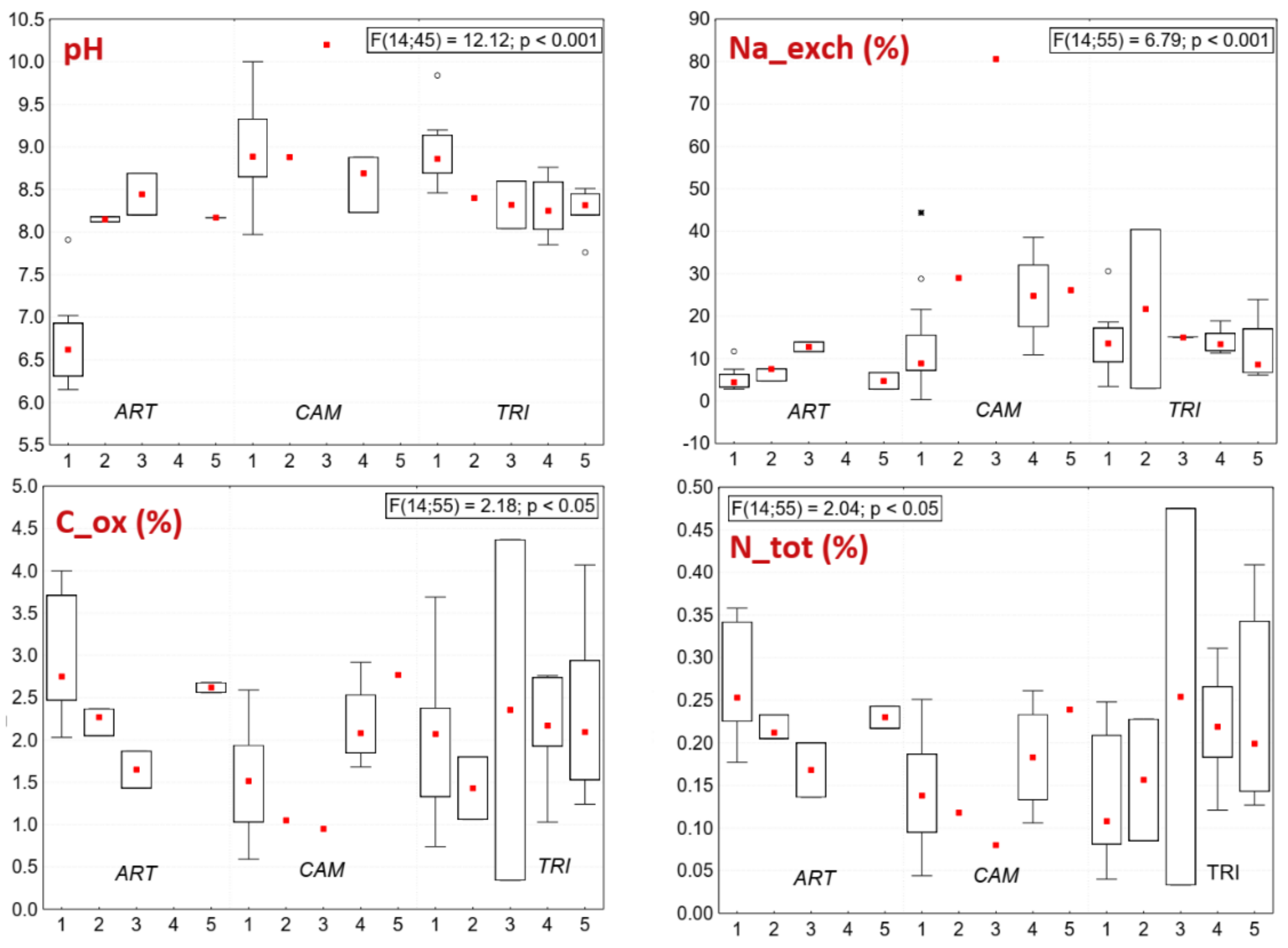
3.2. Soil Chemistry of Indicator Species of Pannonic Salt Steppes (ART, CAM, TRI)
3.3. Current Condition of Indicator Species of Pannonic Salt Steppes
3.4. Conservation Perspectives of Pannonic Salt Steppes on Their Border Area
4. Conclusions and Practical Outcomes
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Büchi, L.; Vuilleumier, S. Coexistence of specialist and generalist species is shaped by dispersal and environmental factors. Am. Nat. 2014, 183, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Hájek, M.; Horsáková, V.; Hájková, P.; Coufal, R.; Dítě, D.; Němec, T.; Horsák, M. Habitat extremity and conservation management stabilise endangered calcareous fens in a changing world. Sci. Total Environ. 2020, 719, 134693. [Google Scholar] [CrossRef] [PubMed]
- Commission of the European Communities. Habitats of the European Community. CORINE Biotopes Manual; Commission of the European Communities: Luxembourg, Luxembourg, 1991; pp. 18–26. [Google Scholar]
- Szabolcs, I. Salt-Affected Soils in Europe; Martinus Nijhoff: The Hague, The Netherlands, 1974; pp. 21–28. [Google Scholar]
- Loidi, J. The Vegetation of the Iberian Peninsula; Springer International Publishing: Cham, Switzerland, 2017; Chapter 1; Volume 1, pp. 3–28. [Google Scholar]
- Brandes, D. Flora und Vegetation Salzbeeinflußter Habitate im Binnenland: Eine Einführung; Braunschweig Institute for Plant Biology: Braunschweig, Germany, 1999. [Google Scholar]
- Molnár, Z.; Borhidi, A. Hungarian alkali vegetation: Origins, landscape history, syntaxonomy, conservation. Phytocoenologia 2003, 33, 377–408. [Google Scholar] [CrossRef]
- Garve, E.; Garve, V. Halophyten an Kalihalden in Deutschland und Frankreich (Elsass). Tuexenia 2000, 20, 375–417. [Google Scholar]
- Tóth, T.; Blaskó, L. Secondary Salinization Due to Irrigation. In The Soil as a Strategic Resource; Rodriguez, A.R., Mendoza, C.C.J., Salguero, M.L.T., Eds.; Geoforma Ediciones: Logrono, Spain, 1998; pp. 229–253. [Google Scholar]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Piernik, A. Inland halophilous vegetation as indicator of soil salinity. Basic Appl. Ecol. 2003, 4, 525–536. [Google Scholar] [CrossRef]
- Koull, N.; Chehma, A. Soil characteristics and plant distribution in saline wetlands of Oued Righ, northeastern Algeria. J. Arid. Land 2016, 8, 948–959. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.S.; Kim, J.W. Relationship between halophyte distribution and soil environmental factors in the west coast of South Korea. J. Ecol. Environ. 2018, 42, 2. [Google Scholar] [CrossRef][Green Version]
- Eliáš, P.J.; Sopotlieva, D.; Dítě, D.; Hájková, P.; Apostolova, I.; Senko, D.; Melečková, Z.; Hájek, M. Vegetation diversity of salt-rich grasslands in the south-east Europe. App. Veg. Sci. 2013, 16, 521–537. [Google Scholar] [CrossRef]
- Janssen, J.A.M.; Rodwell, J.S.; Garcia Criado, M.; Gubbay, S.; Haynes, T.; Nieto, A.; Sanders, N.; Landucci, F.; Loidi, J.; Ssymank, A.; et al. European Red List of Habitats. Part 2. Terrestrial and Freshwater Habitats; Publications Office of the European Union: Luxembourg, Luxembourg, 2016; pp. 33–34. [Google Scholar]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.P.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; García, R.G.; et al. Vegetation of Europe: Hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl. Veg. Sci. 2016, 19 (Suppl. 1), 3–264. [Google Scholar] [CrossRef]
- Török, P.; Kapocsi, I.; Deák, B. Conservation and Management of Alkali Grassland Biodiversity in Central-Europe. In Grasslands: Types, Biodiversity and Impacts; Zhang, W.J., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2011; pp. 109–118. [Google Scholar]
- Molnár, Z.; Bölöni, J.; Horváth, F. Threatening factors encountered: Actual endangerment of the Hungarian (semi-)natural habitats. Acta Bot. Hung. 2008, 50 (Suppl. 1), 199–217. [Google Scholar] [CrossRef]
- Bölöni, J.; Molnár, Z.; Horváth, F.; Illyés, E. Naturalness-based habitat quality of the Hungarian (semi-) natural habitats. Acta Bot. Hung. 2008, 50 (Suppl. 1), 149–159. [Google Scholar] [CrossRef]
- Janjatović, V.; Knežević, A.; Anðelić, M.; Merkulov, L. Ekomorphological characteristics of Camphorosma annua Pall. (Chenopodiaceae). Zbornik radova PMF Ser. Biol. 1992, 22, 31–38. [Google Scholar]
- Dajić Stevanović, Z.; Kresović, M.; Pećinar, I.; Aćić, S.; Obratov-Petković, D. Distribution of the halophytic grass Puccinellia limosa (Schur.) Holomb. on salt affected soils in Serbia in relation to its main adaptive responses to salinity. Ekológia 2010, 29, 258–268. [Google Scholar] [CrossRef]
- Mucina, L. Puccinellio-Salicornietea. In Die Pflanzengesellschaften Osterreichs. Teil 1. Anthropogene Vegetation; Mucina, L., Grabherr, G., Ellmauer, T., Eds.; Fischer: Stuttgart, Germany; New York, NY, USA, 1993; pp. 522–549. [Google Scholar]
- Šumberová, K.; Novák, J.; Sádlo, J. Slaniskové Trávníky (Festuco-Puccinellietea). In Vegetace České Republiky 1. Travinná a Keříčková Vegetace; Chytrý, M., Ed.; Academia: Prague, Czech Republic, 2007; pp. 150–164. [Google Scholar]
- Sanda, V.; Öllerer, K.; Burescu, P. Fitocenozele din România. Sintaxonomie, Structură, Dinamică și Evoluţie; Editura Ars Docendi: Bucharest, Romania, 2008; pp. 152–155. [Google Scholar]
- Borhidi, A.; Kevey, B.; Lendvai, G. Plant Communities of Hungary; Akadémiai Kiadó: Budapest, Hungary, 2012; pp. 200–225. [Google Scholar]
- Fekete, G.; Molnár, Z.; Magyari, E.; Somodi, I.; Varga, Z. A new framework for understanding Pannonian vegetation patterns: Regularities, deviations and uniqueness. Community Ecol. 2014, 15, 12–26. [Google Scholar] [CrossRef]
- Dítě, D.; Melečková, Z.; Eliáš, P.J. Festuco-Puccinellietea. In Rastlinné Spoločenstvá Slovenska. 5. Travinno-Bylinná Vegetácia; Hegedüšová Vantarová, K., Škodová, I., Eds.; Veda: Bratislava, Slovakia, 2014; pp. 483–510. [Google Scholar]
- Sádovský, M.; Eliáš, P.J.; Dítě, D. Historické a súčasné rozšírenie slaniskových spoločenstiev na juhozápadnom Slovensku. Bull. Slov. Bot. Spoločn. 2004, 10, 127–129. [Google Scholar]
- Šefferová Stanová, V.; Janák, M.; Ripka, J. Management of Natura 2000 Habitats. * Pannonic Salt Steppes and Salt Marshes 1530; European Commission: Brussels, Belgium, 2008; pp. 8–10. [Google Scholar]
- Valkó, O.; Tóthmérész, B.; Kelemen, A.; Simon, E.; Miglécz, T.; Lukács, B.A.; Török, P. Environmental factors driving vegetation and seed bank diversity in alkali grasslands. Agric. Ecosyst. Environ. 2014, 182, 80–87. [Google Scholar] [CrossRef]
- Szujkó-Lacza, J.; Kováts, D. The Flora of the Kiskunság National Park; Magyar Természettudományi Múzeum: Budapest, Hungary, 1993; pp. 43–66. [Google Scholar]
- Boros, E.; Ecsedi, Z.; Oláh, J. Ecology and Management of Soda Pans in the Carpathian Basin; Hortobágy Environmental Association: Balmazújváros, Hungary, 2013; pp. 23–33. [Google Scholar]
- Bihari, Z.; Babolcsai, G.; Bartholy, J.; Ferenczi, Z.; Gerhátné Kerényi, J.; Haszpra, L.; Homokiné Ujváry, K.; Kovács, T.; Lakatos, M.; Németh, Á.; et al. Climate. In National Atlas of Hungary. Natural Conditions; Kocsis, K., Gercsák, G., Horváth, G., Keresztesi, Z., Nemerkényi, Z., Eds.; Geographical Research Institute, Research Centre for Astronomy and Earth Sciences, Hungarian Academy of Sciences: Budapest, Hungary, 2018; pp. 58–69. [Google Scholar]
- Alexander, C.; Deák, B.; Heilmeier, H. Micro-topography driven vegetation patterns in open mosaic landscapes. Ecol. Indic. 2016, 60, 906–920. [Google Scholar] [CrossRef]
- Niklfeld, H.; Schratt-Ehrendorfer, L. Rote Liste gefährdeter Farn- und Blütenpflanzen (Pteridophyta und Spermatophyta) Österreichs. 2. Fassung. In Rote Listen gefährdeter Pflanzen Österreichs, 2nd ed.; Niklfeld, H., Ed.; Grüne Reihe des Bundesministerium für Umwelt, Jugend und Familie: Vienna, Austria, 1999; pp. 33–151. [Google Scholar]
- Eliáš, P.J.; Dítě, D.; Kliment, J.; Hrivnák, R.; Feráková, V. Red list of ferns and flowering plants of Slovakia, 5th edition (October 2014). Biologia 2015, 70, 218–228. [Google Scholar] [CrossRef]
- Bölöni, J.; Molnár, Z.; Kun, A. (Eds.) Magyarország Élőhelyei. Vegetációtípusok Leírása és Határozója: ÁNÉR 2011; MTA Ökológiai és Botanikai Kutatóintézete: Vácrátót, Hungary, 2011; p. 441. [Google Scholar]
- Dítě, D.; Eliáš, P.J.; Dítě, Z.; Píš, V.; Šuvada, R. Vegetation classification and ecology of Pannonian salt lake beds. Phytocoenologia 2017, 47, 329–344. [Google Scholar] [CrossRef]
- Nikolić, T. Red Data Book On-Line. Flora Croatica Database; Faculty of Science, University of Zagreb: Zagreb: Croatia, 2018. [Google Scholar]
- Kaplan, Z.; Danihelka, J.; Šumberová, K.; Chrtek, J., Jr.; Rotreklová, O.; Ekrt, L.; Štěpánková, J.; Taraška, V.; Trávníček, B.; Prančl, J.; et al. Distributions of vascular plants in the Czech Republic. Part 5. Preslia 2017, 89, 333–439. [Google Scholar] [CrossRef]
- Grulich, V. Red List of vascular plants of the Czech Republic: 3rd edition. Preslia 2012, 84, 631–645. [Google Scholar]
- Chytrý, M.; Tichý, L.; Hennekens, S.M.; Knollová, I.; Janssen, J.A.M.; Rodwell, J.S.; Peterka, T.; Marcenò, C.; Landucci, F.; Danihelka, J.; et al. EUNIS Habitat Classification: Expert system, characteristic species combinations and distribution maps of European habitats. Appl. Veg. Sci. 2020, 23, 648–675. [Google Scholar] [CrossRef]
- Šerá, B. Road vegetation in Central Europe—An example from the Czech Republic. Biologia 2008, 63, 1081–1084. [Google Scholar] [CrossRef]
- Dítě, D.; Dítětová, Z. Halophytes spreading along roadsides of northern Slovakia. Thaiszia J. Bot. 2016, 26, 165–172. [Google Scholar]
- Sotáková, S.; Hanes, J.; Sisák, P.; Slovík, R.; Zaujec, A. Návody na Cvičenia z Geológie a Pôdoznalectva; Príroda: Bratislava, Slovakia, 1988; p. 181. [Google Scholar]
- US Salinity Laboratory Staff. Diagnosis and Improvement of Saline and Alkali Soils; Richards, L.A., Ed.; Soil and Water Conservation Research Branch, Agricultural Research Service: Washington, DC, USA, 1954; p. 30.
- Hennekens, S.M.; Schaminée, J.H.J. TURBOVEG, a comprehensive data base management system for vegetation data. J. Veg. Sci. 2001, 12, 589–591. [Google Scholar] [CrossRef]
- Tichý, L. JUICE, software for vegetation classification. J. Veg. Sci. 2002, 13, 451–453. [Google Scholar] [CrossRef]
- Krist, V. Halofytní vegetace jihozápadního Slovenska a severní části Malé uherské nížiny. Práce Morav. Přír. Společn. 1940, 12, 1–100. [Google Scholar]
- Borhidi, A. Social behaviour types, their naturalness and relative ecological indicator values of the higher plants of the Hungarian flora. Acta Bot. Hung. 1995, 39, 97–182. [Google Scholar]
- Chytrý, M.; Tichý, L.; Dřevojan, P.; Sádlo, J.; Zelený, D. Ellenberg-type indicator values for the Czech flora. Preslia 2018, 90, 83–103. [Google Scholar] [CrossRef]
- StatSoft Inc. STATISTICA (Data Analysis Software System), Version 7; StatSoft Inc.: Tulsa, OK, USA, 2004. [Google Scholar]
- Tälle, M.; Deák, B.; Poschlod, P.; Valkó, O.; Westerberg, L.; Milberg, P. Grazing vs. mowing: A meta-analysis of biodiversity benefits for grassland management. Agric. Ecosyst. Environ. 2016, 222, 200–212. [Google Scholar] [CrossRef]
- Braak, C.J.F.T.; Šmilauer, P. CANOCO Reference Manual and Users Guide to Canoco for Windows. Software for Canonical Community Ordination (Version 4); Centre of Biometry: Wageningen, The Netherlands, 1998; pp. 1–351. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System; Open Source Geospatial Foundation: Chicago, IL, USA, 2018. [Google Scholar]
- Kelemen, A.; Török, P.; Valkó, O.; Deák, B.; Tóth, K.; Tóthmérész, B. Both facilitation and limiting similarity shape the species coexistence in dry alkali grasslands. Ecol. Complex. 2015, 21, 34–38. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties, 2nd ed.; John Wiley & Sons: New York, USA, 2001; p. 116. [Google Scholar] [CrossRef]
- Galvánek, D.; Dítě, D.; Eliáš, P.J.; Dítě, Z. Regeneration of threatened alkali steppe vegetation after a heavy disturbance by disk tillage. Plant Ecol. 2020, 221, 1177–1186. [Google Scholar] [CrossRef]
- Ungar, I.A. Are biotic factors significant in influencing the distribution of halophytes in saline habitats? Bot. Rev. 1998, 64, 176–199. [Google Scholar] [CrossRef]
- Grigore, M.N.; Villanueva, M.; Boscaiu, M.; Vicente, O. Do halophytes really require salts for their growth and development? An experimental approach. Not. Sci. Biol. 2012, 4, 23–29. [Google Scholar] [CrossRef]
- Zlatković, I.D.; Jenačković, D.D.; Ranđelović, V.D. Inland salt areas of Southeast Serbia: Ecological preferences of certain representatives of flora. Biologia 2019, 74, 1425–1440. [Google Scholar] [CrossRef]
- Melečková, Z.; Dítě, D.; Eliáš, P.J.; Píš, V.; Galvánek, D. Succession of saline vegetation in Slovakia after a large-scale disturbance. Ann. Bot. Fenn. 2014, 51, 285–296. [Google Scholar] [CrossRef]
- Hayward, H.E.; Wadleigh, C.H. Plant growth on saline and alkali soils. Adv. Agron. 1949, 1, 1–38. [Google Scholar]
- Brown, C.E.; Pezeshki, S.R.; DeLaune, R.D. The effects of salinity and soil drying on nutrient uptake and growth of Spartina alterniflora in a simulated tidal system. Environ. Exp. Bot. 2006, 58, 140–148. [Google Scholar] [CrossRef]
- Tóth, T.; Kertész, M. Application of soil-vegetation correlation to optimal resolution mapping of solonetzic rangeland. Arid. Soil Res. Rehabil. 1996, 10, 1–12. [Google Scholar] [CrossRef]
- Tóth, T.; Rajkai, K. Soil and plant correlations in a solonetzic grassland. Soil Sci. 1994, 157, 253–262. [Google Scholar] [CrossRef]
- Schmidt, D. (The vegetation of the saline areas around Győr). Flora Pannonica 2007, 5, 95–104. [Google Scholar]
- Dítě, D.; Dítětová, Z.; Eliáš, P.J.; Littera, P. Triglochin maritima—Rediscovered in southern Slovakia. Thaiszia J. Bot. 2017, 27, 129–136. [Google Scholar]
- European Commission. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Union 1992, 206, 7–50. [Google Scholar]
- Zlinszky, A.; Deák, B.; Kania, A.; Schroiff, A.; Pfeifer, N. Mapping Natura 2000 Habitat Conservation Status in a Pannonic Salt Steppe with Airborne Laser Scanning. Remote Sens. 2015, 7, 2991–3019. [Google Scholar] [CrossRef]
- Ventura, D.; Bonifazi, A.; Gravina, M.; Belluscio, A.; Ardizzone, G. Mapping and Classification of Ecologically Sensitive Marine Habitats Using Unmanned Aerial Vehicle (UAV) Imagery and Object-Based Image Analysis (OBIA). Remote Sens. 2018, 10, 1331. [Google Scholar] [CrossRef]

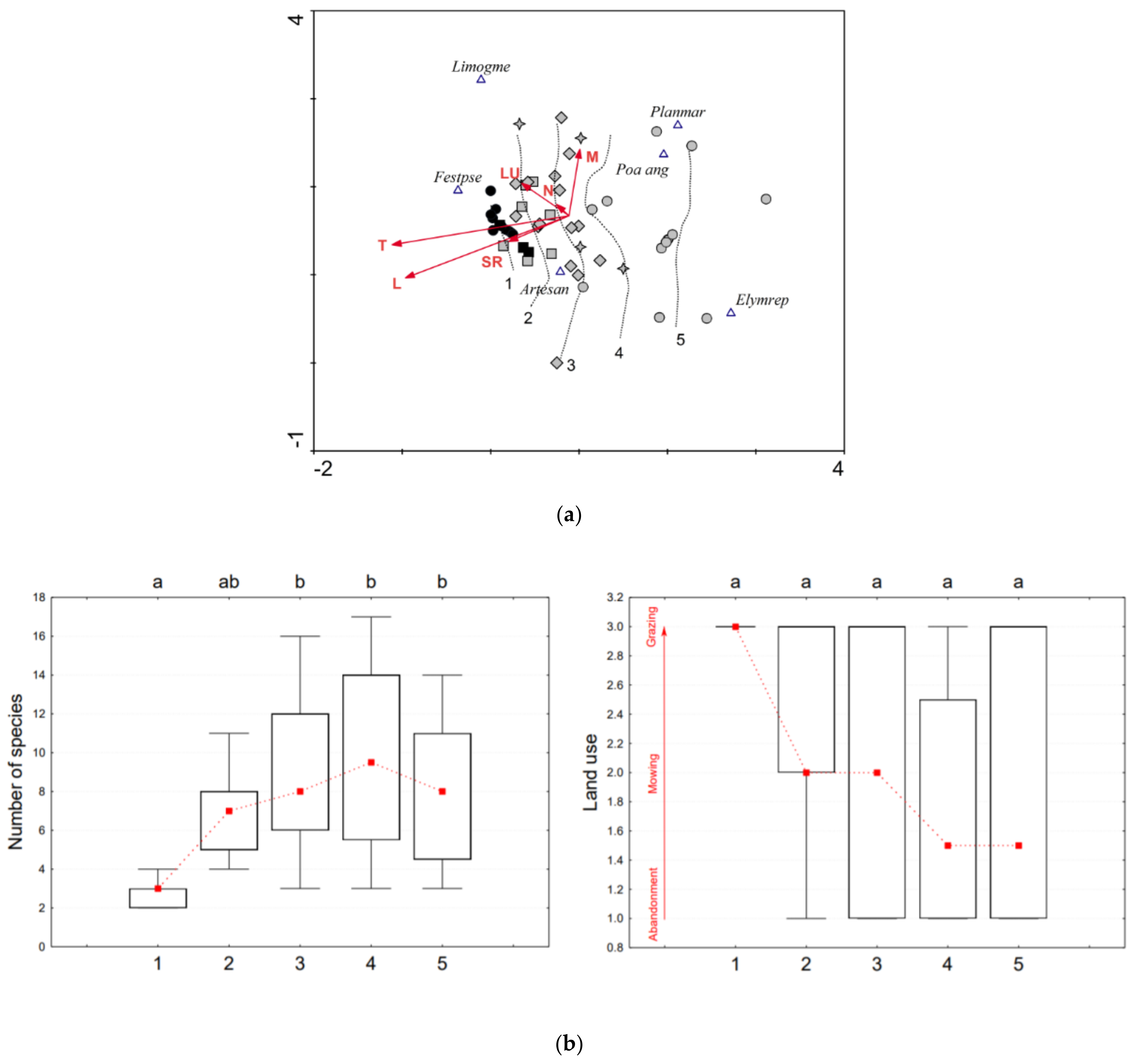
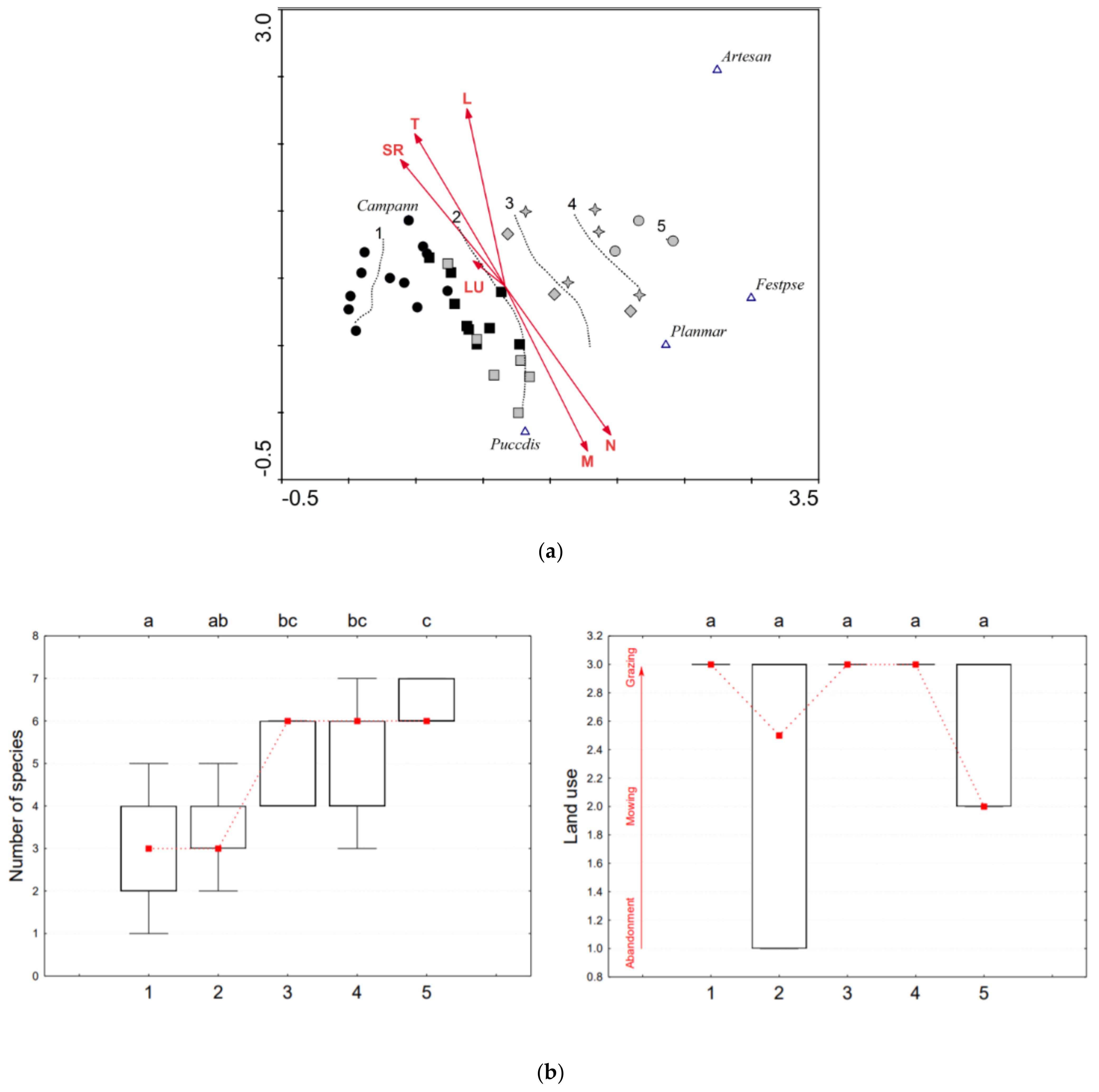
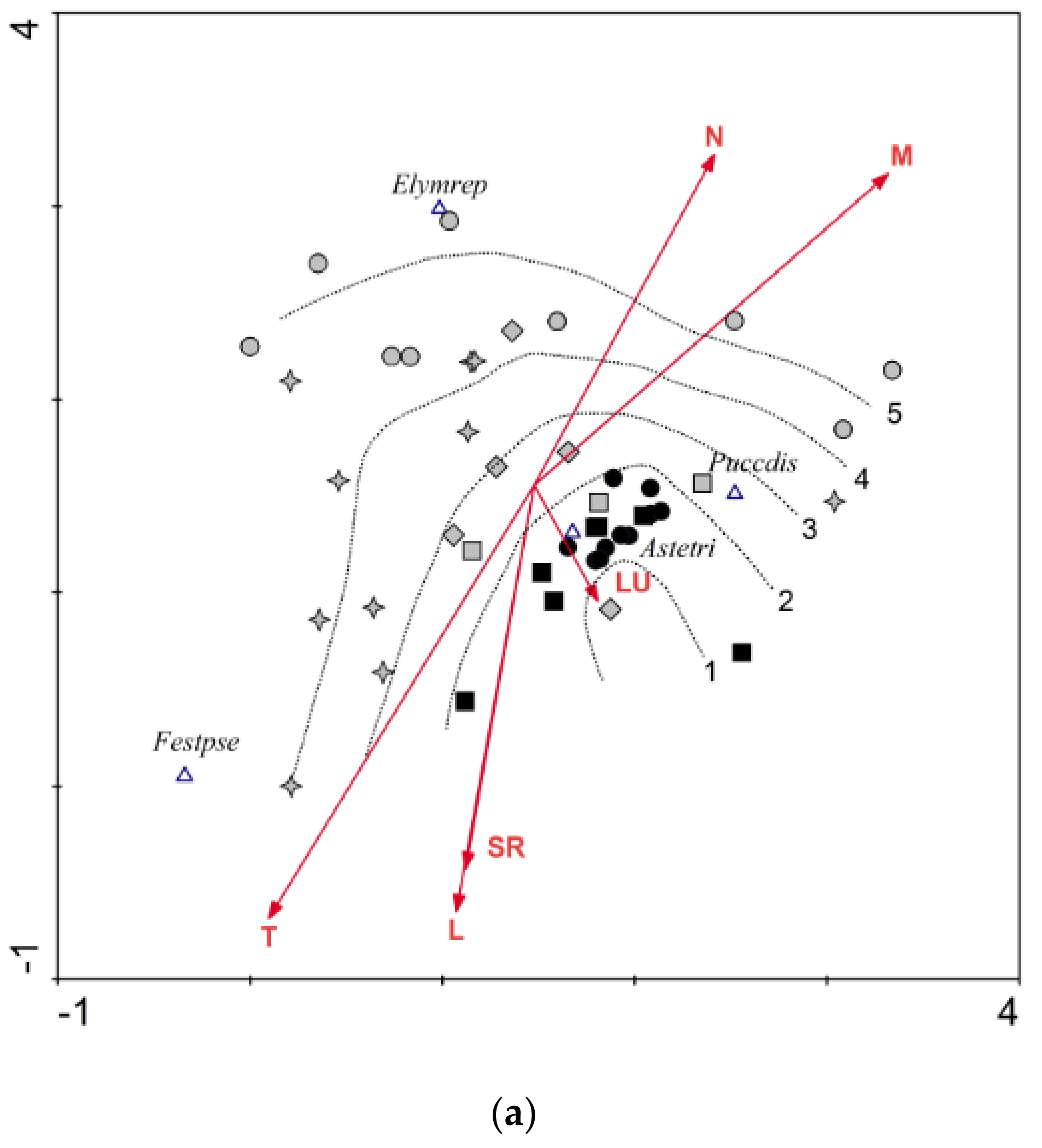


| % Cover of the 0.05 × 0.05 m Plot | Group No. and Its Symbol in Figure 2a, Figure 3a and Figure 4a | ||||
|---|---|---|---|---|---|
| 1 ● ■ | 2 ■ | 3 ♦ | 4 ✦ | 5 ● | |
| Characteristic species, including indicator species | ≥50% | ≥50% (min. two species) | ≥50% | (<50% *) ≥50% | |
| Generalists indicating degradation | 0% | >0% to <5% | >0% to <25% | (>0%*) ≥25% to <50% | ≥50% |
| Accompanying halophytic species | ≥30% * | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dítě, Z.; Šuvada, R.; Tóth, T.; Jun, P.E.; Píš, V.; Dítě, D. Current Condition of Pannonic Salt Steppes at Their Distribution Limit: What Do Indicator Species Reveal about Habitat Quality? Plants 2021, 10, 530. https://doi.org/10.3390/plants10030530
Dítě Z, Šuvada R, Tóth T, Jun PE, Píš V, Dítě D. Current Condition of Pannonic Salt Steppes at Their Distribution Limit: What Do Indicator Species Reveal about Habitat Quality? Plants. 2021; 10(3):530. https://doi.org/10.3390/plants10030530
Chicago/Turabian StyleDítě, Zuzana, Róbert Šuvada, Tibor Tóth, Pavol Eliáš Jun, Vladimír Píš, and Daniel Dítě. 2021. "Current Condition of Pannonic Salt Steppes at Their Distribution Limit: What Do Indicator Species Reveal about Habitat Quality?" Plants 10, no. 3: 530. https://doi.org/10.3390/plants10030530
APA StyleDítě, Z., Šuvada, R., Tóth, T., Jun, P. E., Píš, V., & Dítě, D. (2021). Current Condition of Pannonic Salt Steppes at Their Distribution Limit: What Do Indicator Species Reveal about Habitat Quality? Plants, 10(3), 530. https://doi.org/10.3390/plants10030530






