Estimating the Effects of a Hurricane on Carbon Storage in Mangrove Wetlands in Southwest Florida
Abstract
:1. Introduction
- Samples taken post-hurricane will have a thicker layer of inorganic carbon-rich, organic carbon-poor sediments compared to pre-hurricane samples.
- After a hurricane, fringe mangrove sites will have more sediment accumulation, but less organic carbon accretion compared to the further inland, riverine mangrove sites.
- Hurricanes and large storms will decrease carbon storage in mangrove swamps near open water.
2. Results
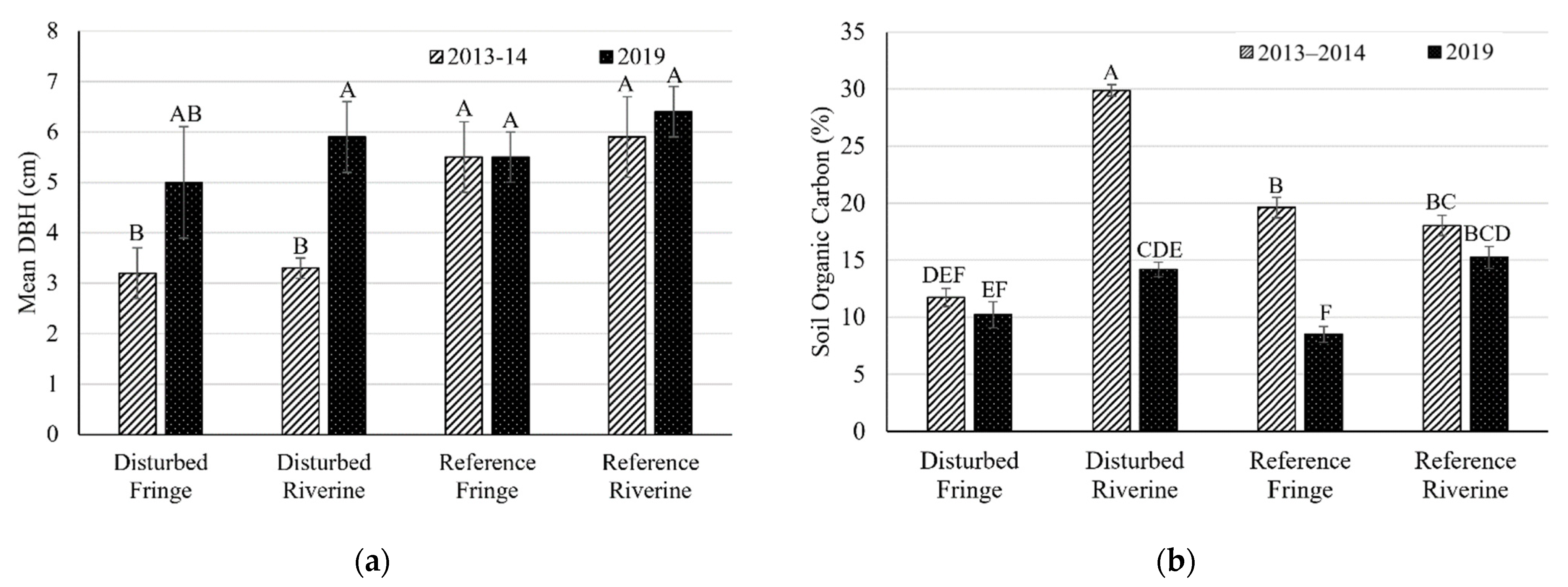
2.1. Mangrove Forest Community Structure
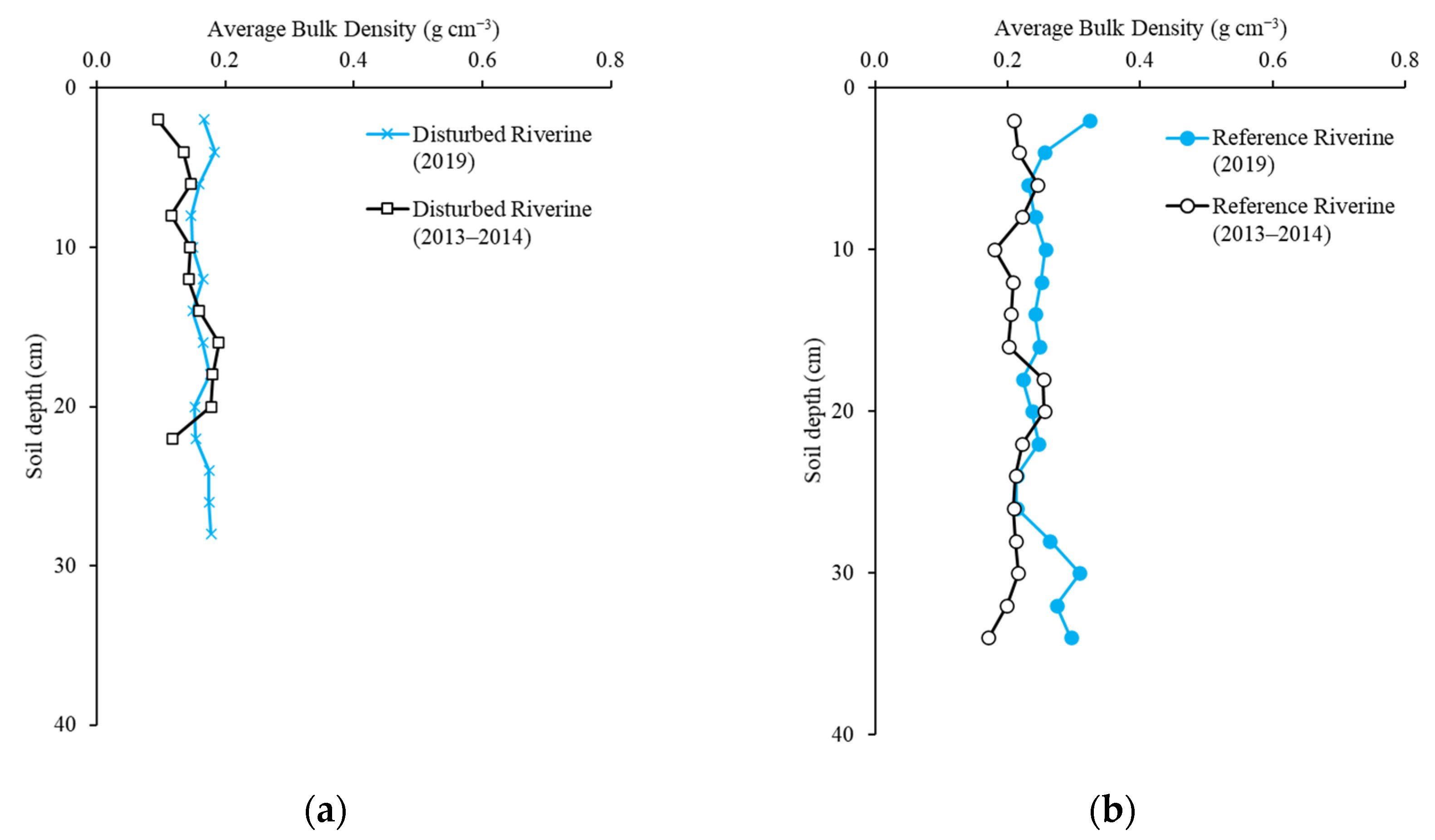
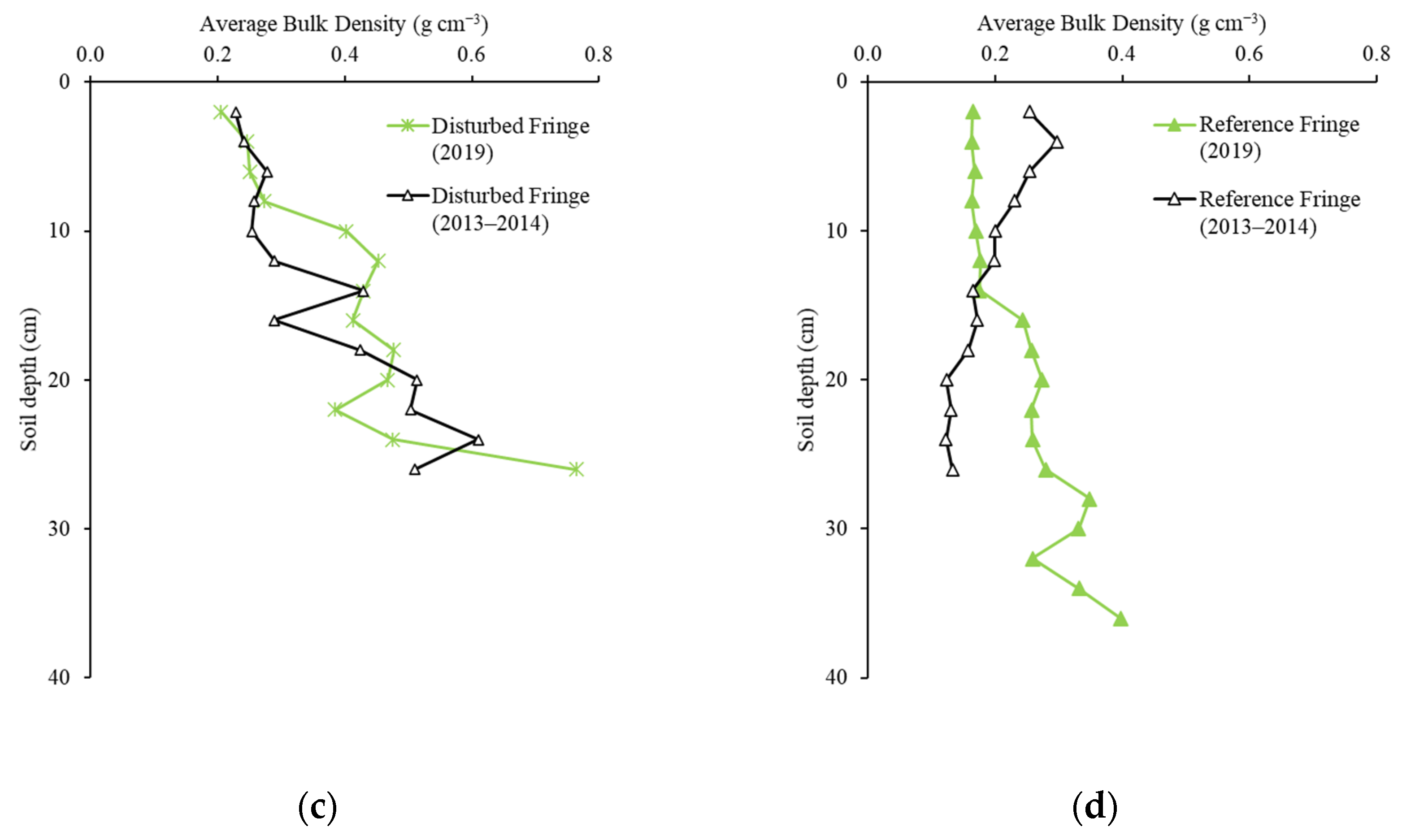
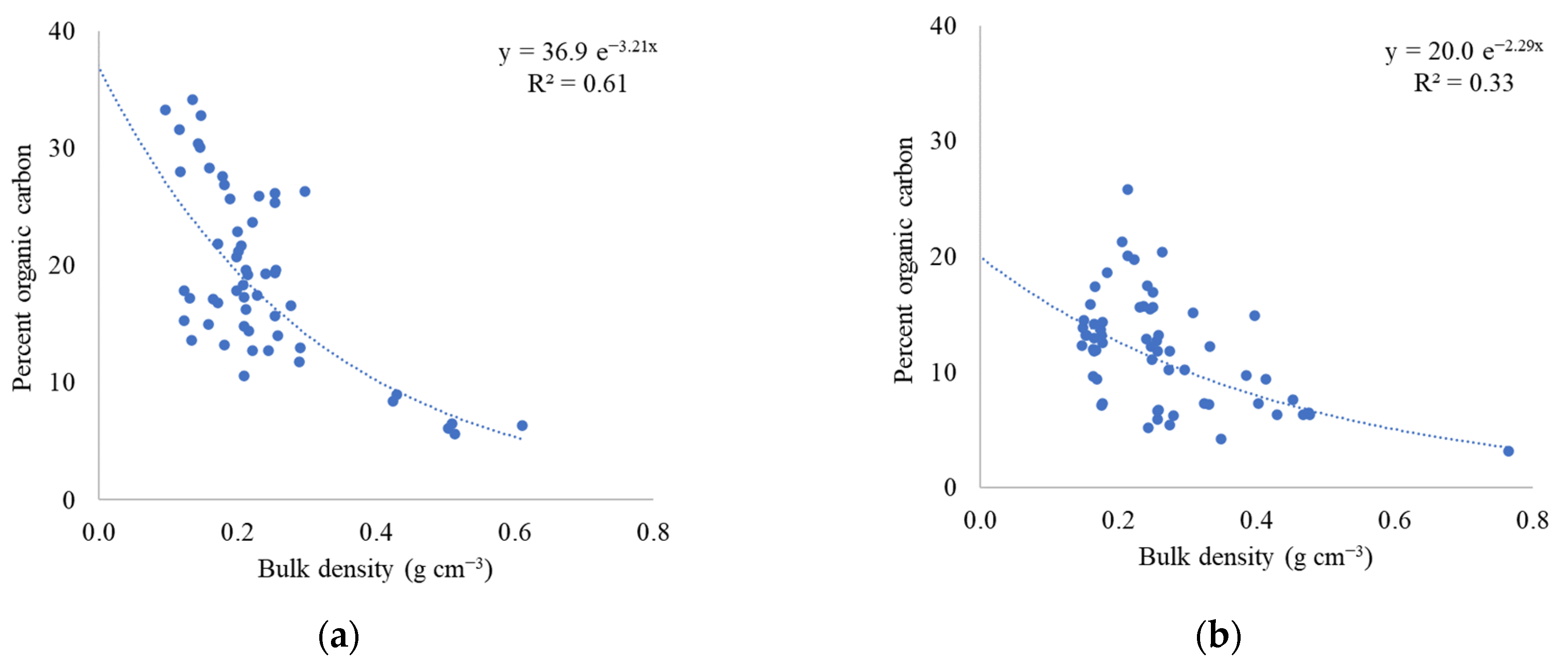
2.2. Soil Bulk Density
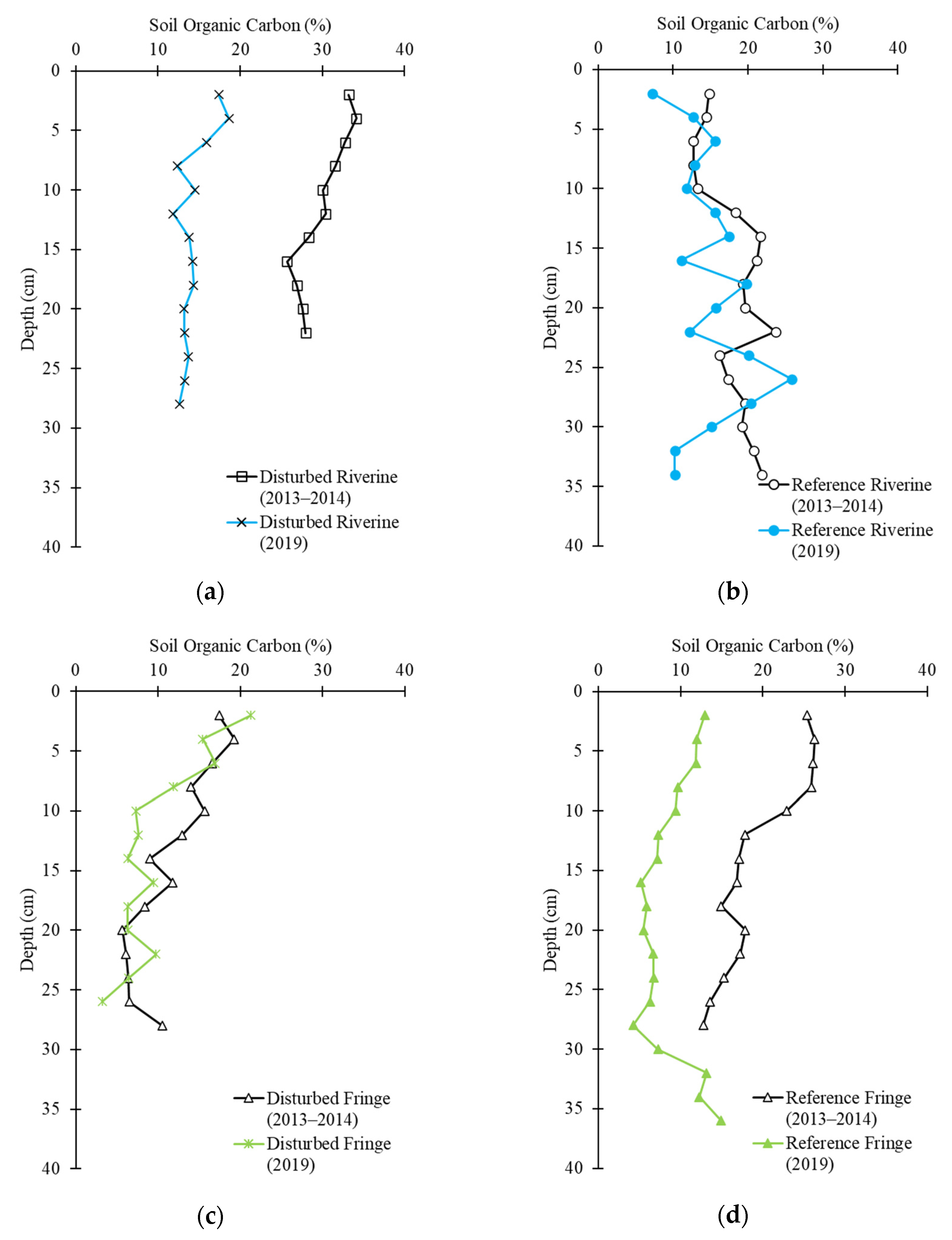
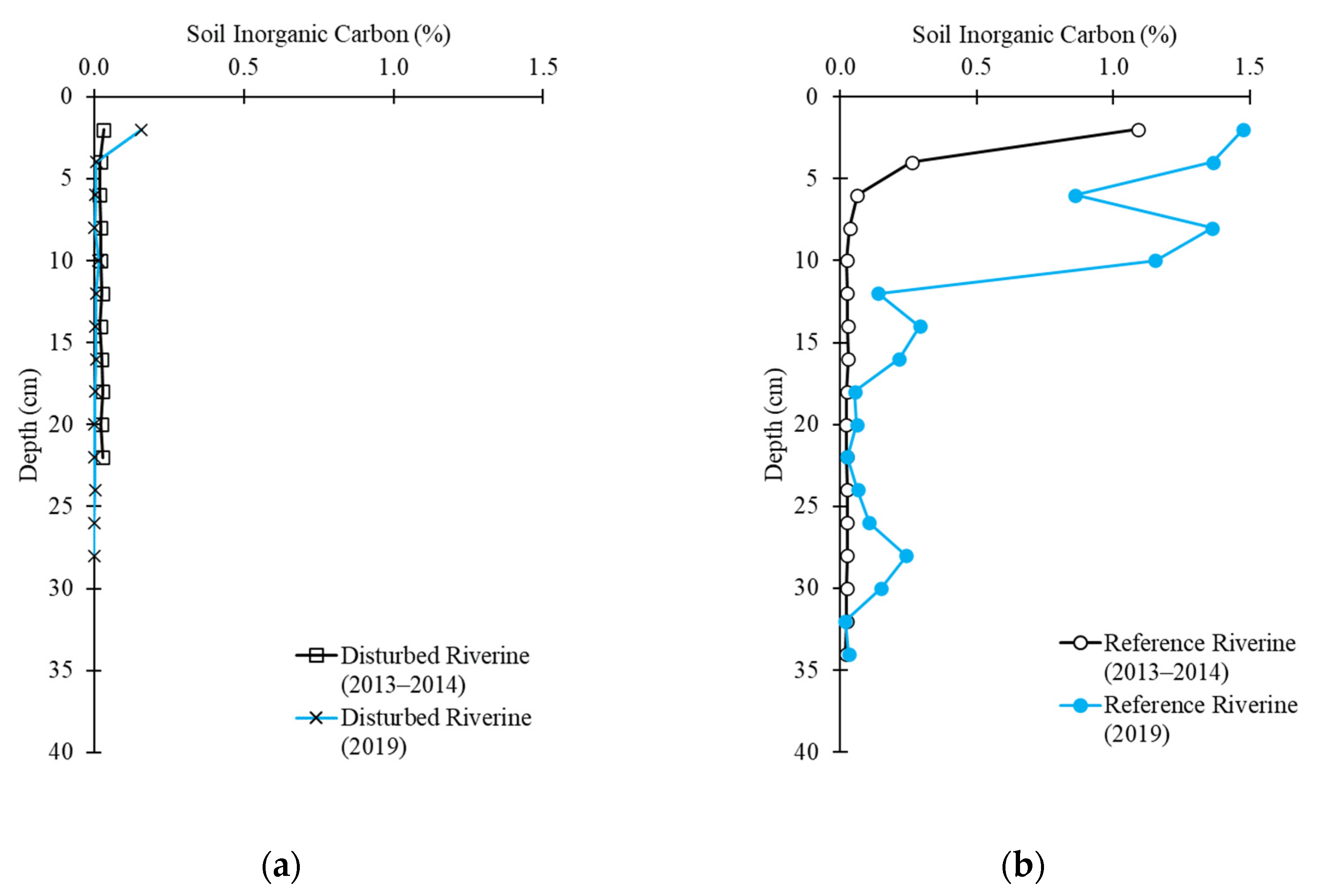
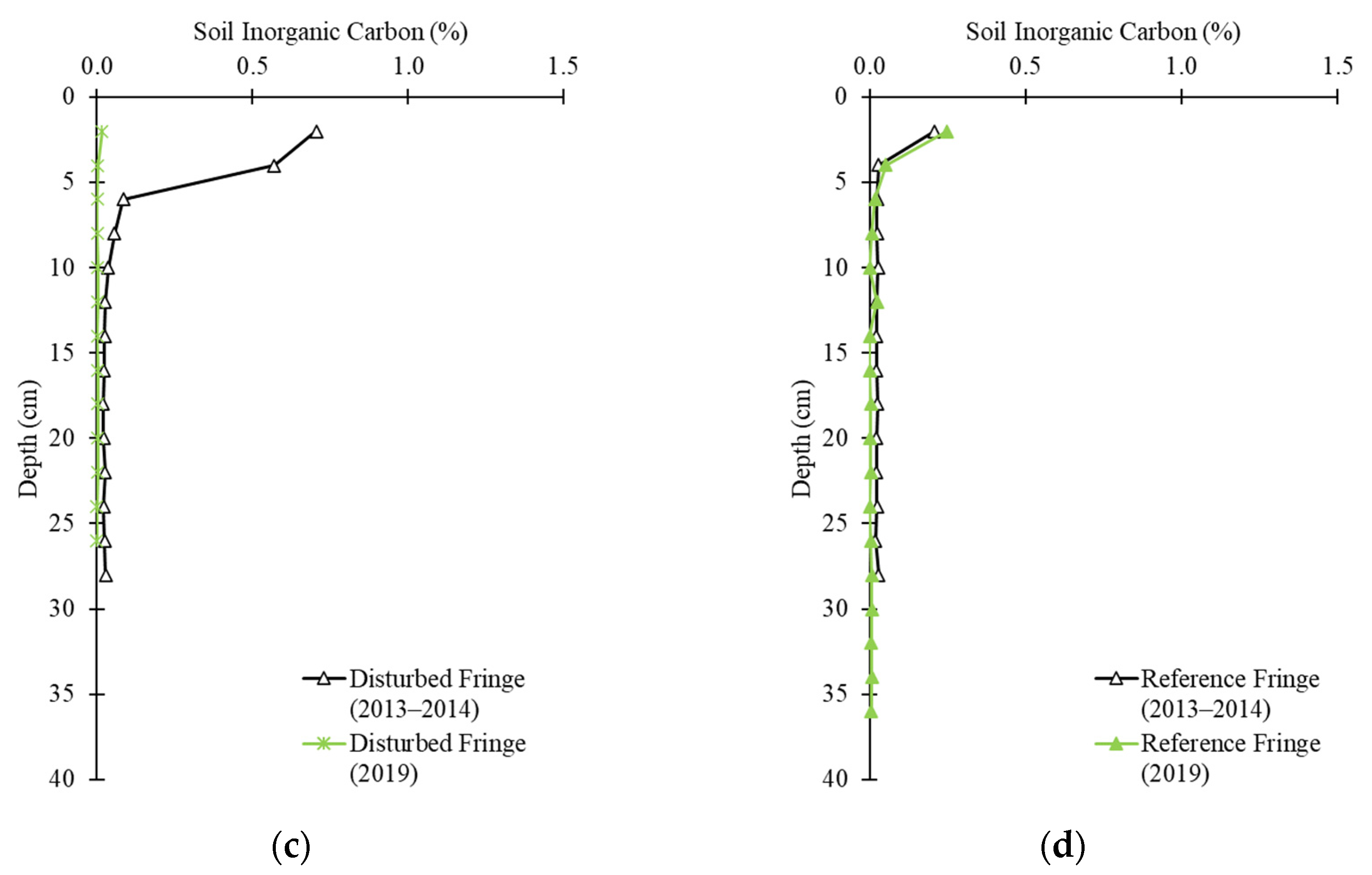
2.3. Soil Carbon Profile
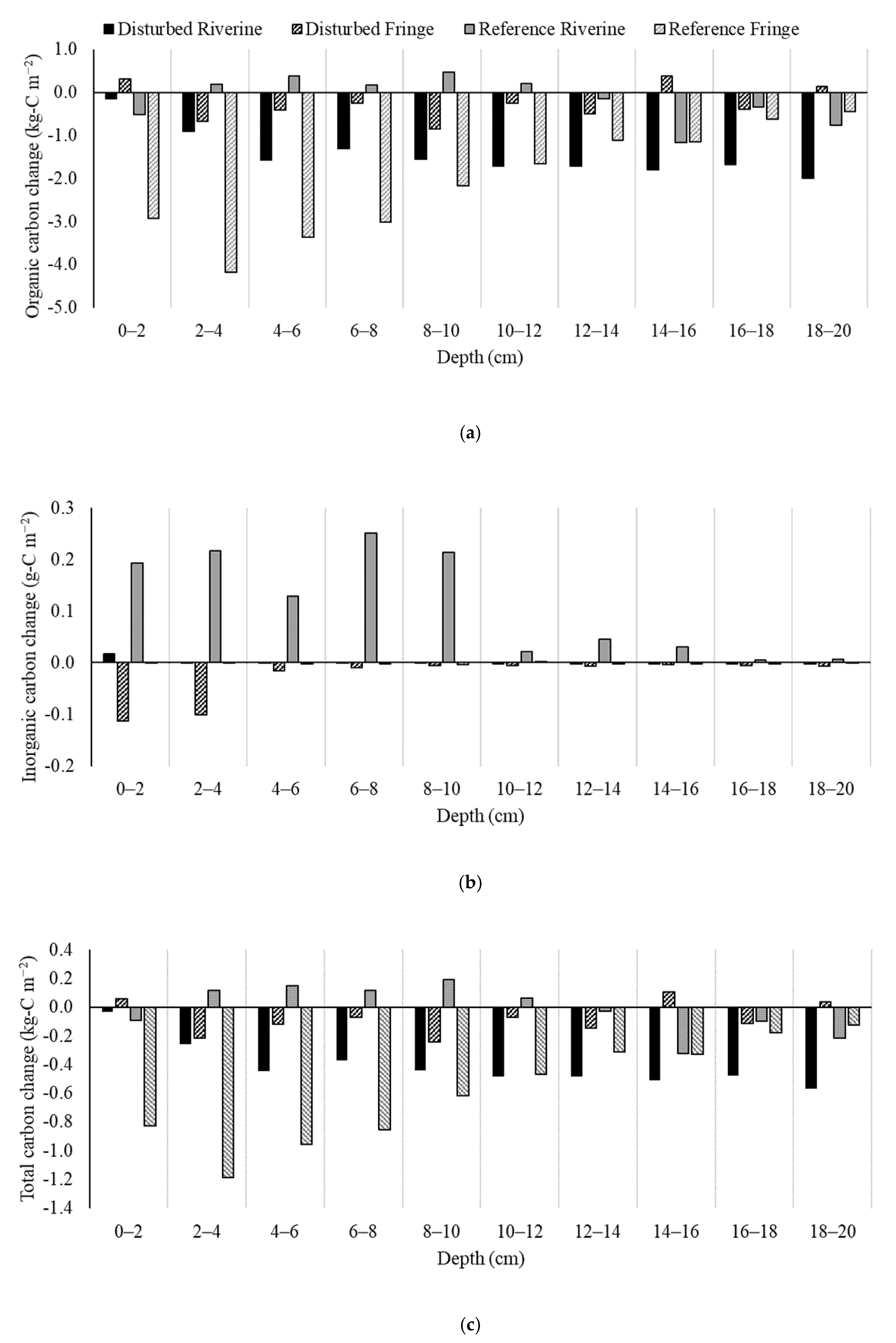
2.4. Soil Carbon Change
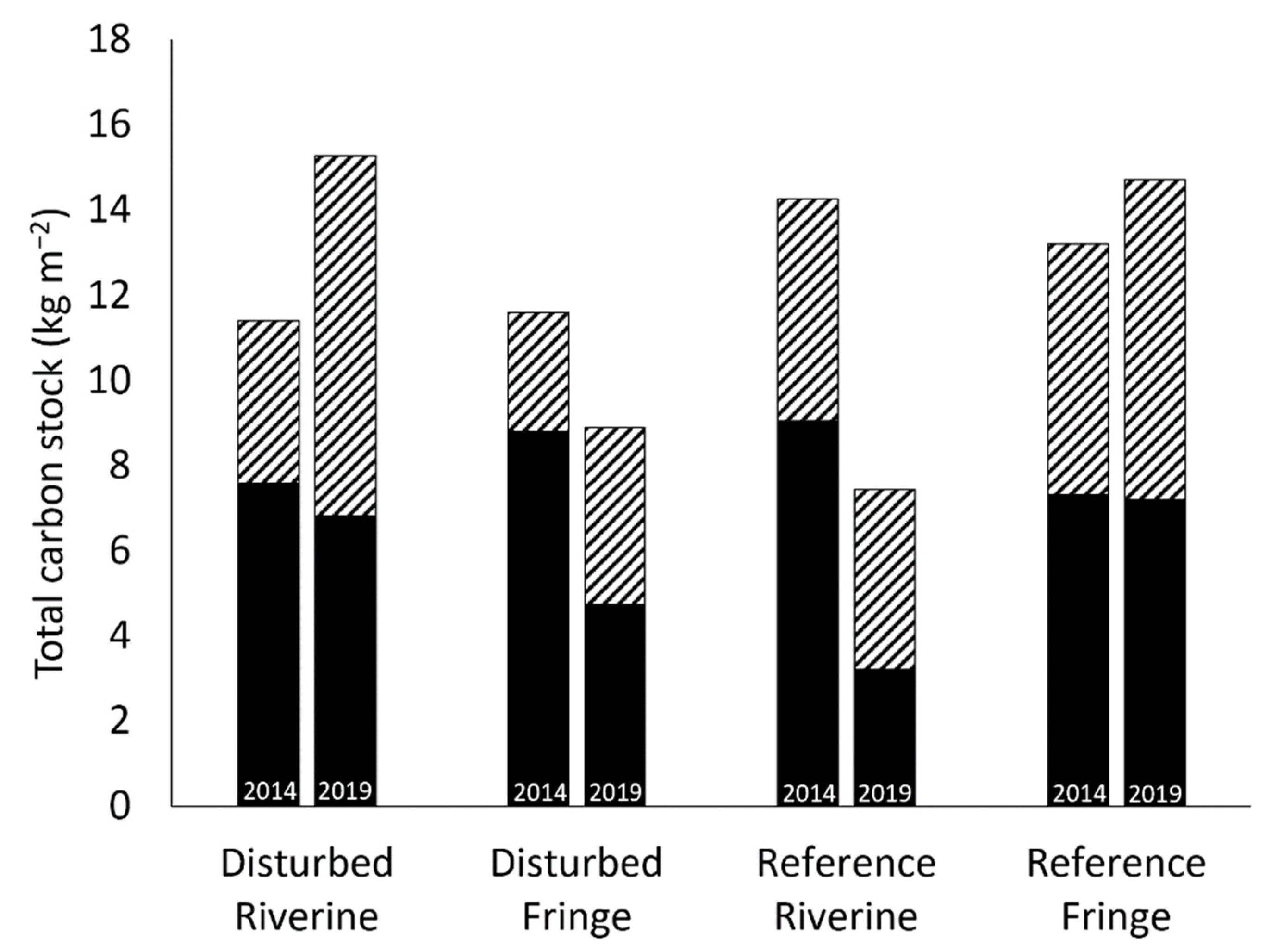
2.5. Total Carbon Stock
2.6. Carbon Sequestration
3. Discussion
3.1. Hurricane Effects on Mangrove Community Structure
3.2. Soil Carbon Change
3.3. Total Carbon Stock
3.4. Carbon Sequestration
3.5. Limitations
4. Materials and Methods
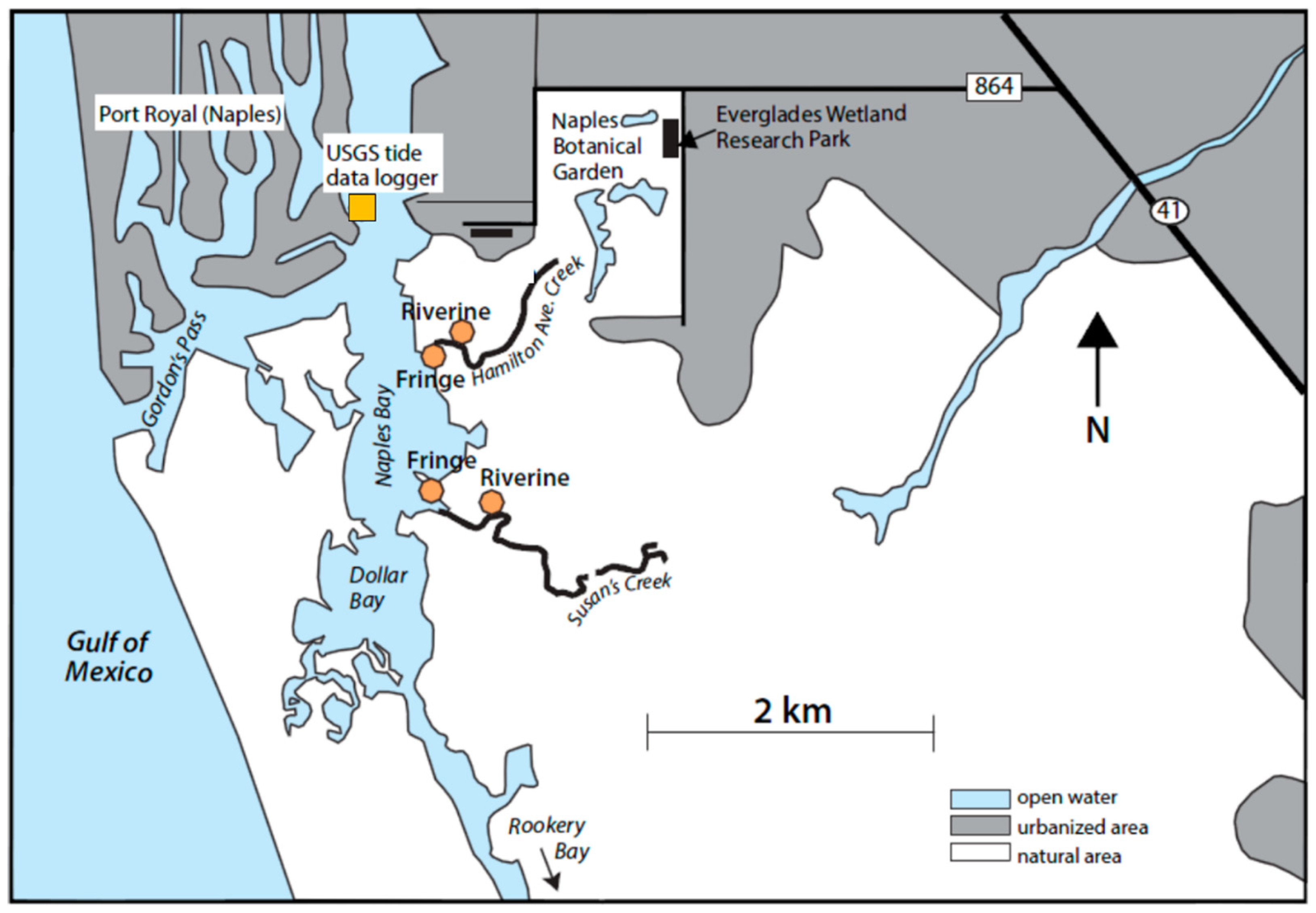
4.1. Study Site
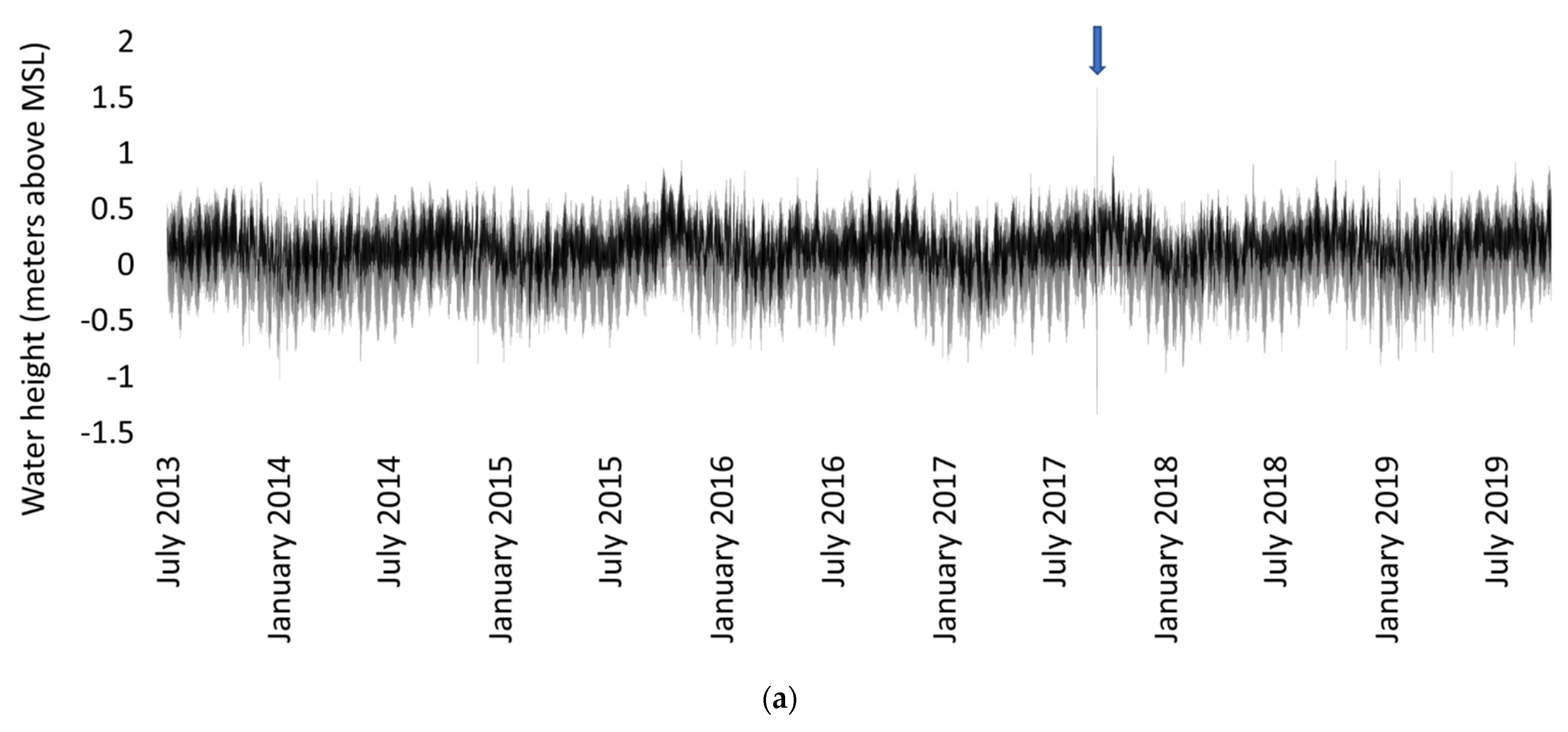
4.2. Sampling and Data Collection
4.3. Lab Work
4.4. Statistical Analysis
5. Conclusions
- Mangroves are resilient and within two years of a hurricane, aboveground biomass production rebounds.
- Hurricanes cause a decrease in carbon in belowground stores. Aboveground carbon storage and biomass regeneration is important to buffer the overall carbon loss in mangroves.
- Due to carbon loss, post-hurricane mangroves may be a lower sink of carbon from the atmosphere for some number of years, thereby providing a positive feedback effect on climate change.
- With increased intensity of storms predicted in the tropics and subtropics due to climate change and with climate models projecting a steady increase in carbon dioxide in the atmosphere (1 percent per year) and tropical ocean surface temperatures rising by more than 2 °C by the end of the century [4], mangrove swamps are needed more than ever to provide a carbon sink while being resilient enough to continue to store carbon quickly after they are disturbed. It will be much more difficult, however, to restore mangroves to areas where humans have converted these natural ecosystems and their tidal creek watersheds. Current mangrove swamps must be protected so that they can continue to store carbon, protect humans from dangerous storms, and serve as nurseries for marine life.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hallegraeff, G.M. A Review of Harmful Algal Blooms and Their Apparent Global Increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef] [Green Version]
- Vitousek, P.M.; Mooney, H.A.; Lubchenco, J.; Melillo, J.M. Human Domination of Earth’s Ecosystems. Science 1997, 277, 494–499. [Google Scholar] [CrossRef] [Green Version]
- Webster, P.J.; Holland, G.J.; Curry, J.A.; Chang, H.R. Changes in Tropical Cyclone Number, Duration, and Intensity in a Warming Environment. Science 2005, 309, 1844–1846. [Google Scholar] [CrossRef] [Green Version]
- Aumann, H.H.; Behrangi, A.; Wang, Y. Increased Frequency of Extreme Tropical Deep Convection: AIRS Observations and Climate Model Predictions. Geophys. Res. Lett. 2018, 45, 13530–13537. [Google Scholar] [CrossRef] [Green Version]
- Mitsch, W.J.; Bernal, B.; Nahlik, A.M.; Mander, Ü.; Zhang, L.; Anderson, C.J.; Jørgensen, S.E.; Brix, H. Wetlands, Carbon, and Climate Change. Landsc. Ecol. 2013, 28, 583–597. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Gosselink, J.G. Wetlands, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A Blueprint for Blue Carbon: Toward an Improved Understanding of the Role of Vegetated Coastal Habitats in Sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Alongi, D.M. Carbon Sequestration in Mangrove Forests. Carbon Manag. 2012, 3, 313–322. [Google Scholar] [CrossRef]
- Ezcurra, P.; Ezcurra, E.; Garcillán, P.P.; Costa, M.T.; Aburto-Oropeza, O. Coastal Landforms and Accumulation of Mangrove Peat Increase Carbon Sequestration and Storage. Proc. Natl. Acad. Sci. USA 2016, 113, 4404–4409. [Google Scholar] [CrossRef] [Green Version]
- Marchio, D.A.; Savarese, M.; Bovard, B.; Mitsch, W.J. Carbon Sequestration and Sedimentation in Mangrove Swamps Influenced by Hydrogeomorphic Conditions and Urbanization in Southwest Florida. Forests 2016, 7, 116. [Google Scholar] [CrossRef] [Green Version]
- Cabezas, A.; Mitsch, W.J.; MacDonnell, C.; Zhang, L.; Bydałek, F.; Lasso, A. Methane Emissions from Mangrove Soils in Hydrologically Disturbed and Reference Mangrove Tidal Creeks in Southwest Florida. Ecol. Eng. 2018, 114, 57–65. [Google Scholar] [CrossRef]
- Marois, D.E.; Mitsch, W.J. Coastal Protection from Tsunamis and Cyclones Provided by Mangrove Wetlands—A Review. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2015, 11, 71–83. [Google Scholar] [CrossRef]
- Barbier, E.B. The Protective Service of Mangrove Ecosystems: A Review of Valuation Methods. Mar. Pollut. Bull. 2016, 109, 676–681. [Google Scholar] [CrossRef]
- Blankespoor, B.; Dasgupta, S.; Lange, G.M. Mangroves as a Protection from Storm Surges in a Changing Climate. Ambio 2017, 46, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, E.; Bouillon, S.; Dittmar, T.; Marchand, C. Organic Carbon Dynamics in Mangrove Ecosystems: A Review. Aquat. Bot. 2008, 89, 201–219. [Google Scholar] [CrossRef] [Green Version]
- Bouillon, S.; Dahdouh-Guebas, F.; Rao, A.V.V.S.; Koedam, N.; Dehairs, F. Sources of Organic Carbon in Mangrove Sediments: Variability and Possible Ecological Implications. Hydrobiologia 2003, 495, 33–39. [Google Scholar] [CrossRef]
- Sussko, R.J.; Davis, R.A., Jr. Siliciclastic-to-Carbonate Transition on the Inner Shelf Embayment, Southwest Florida. Mar. Geol. 1992, 107, 51–60. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon Cycling and Storage in Mangrove Forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Balk, M.; Keuskamp, J.A.; Laanbroek, H.J. Potential for Sulfate Reduction in Mangrove Forest Soils: Comparison between Two Dominant Species of the Americas. Front. Microbiol. 2016, 7, 1855. [Google Scholar] [CrossRef] [Green Version]
- Smoak, J.M.; Breithaupt, J.L.; Smith, T.J.; Sanders, C.J. Sediment Accretion and Organic Carbon Burial Relative to Sea-Level Rise and Storm Events in Two Mangrove Forests in Everglades National Park. Catena 2013, 104, 58–66. [Google Scholar] [CrossRef]
- Cahoon, D.R.; Hensel, P.; Rybczyk, J.; McKee, K.L.; Proffitt, C.E.; Perez, B.C. Mass Tree Mortality Leads to Mangrove Peat Collapse at Bay Islands, Honduras after Hurricane Mitch. J. Ecol. 2003, 91, 1093–1105. [Google Scholar] [CrossRef]
- McKee, K.L.; Mendelssohn, I.A.; Hester, M.W. Reexamination of Pore Water Sulfide Concentrations and Redox Potentials near the Aerial Roots of Rhizophora Mangle and Avicennia Germinans. Am. J. Bot. 1988, 75, 1352–1359. [Google Scholar] [CrossRef]
- Smith, T.J.; Robblee, M.B.; Wanless, H.R.; Doyle, T.W. Mangroves, Hurricanes, and Lightning Strikes. BioScience 1994, 44, 256–262. [Google Scholar] [CrossRef]
- Radabaugh, K.R.; Powell, C.E.; Moyer, R.P. Coastal Habitat Integrated Mapping and Monitoring Program Report for the State of Florida; Florida Fish and Wildlife Conservation Commission: St. Petersburg, FL, USA, 2017. [Google Scholar]
- Ross, M.S.; Ruiz, P.L.; Telesnicki, G.J.; Meeder, J.F. Estimating Above-Ground Biomass and Production in Mangrove Communities of Biscayne National Park, Florida (U.S.A.). Wetl. Ecol. Manag. 2001, 9, 27–37. [Google Scholar] [CrossRef]
- Chambers, L.G.; Steinmuller, H.E.; Breithaupt, J.L. Toward a Mechanistic Understanding of “Peat Collapse” and Its Potential Contribution to Coastal Wetland Loss. Ecology 2019, 100, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Langley, J.A.; Drake, B.G.; Hungate, B.A. Extensive Belowground Carbon Storage Supports Roots and Mycorrhizae in Regenerating Scrub Oaks. Oecologia 2002, 131, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, G. Differential Effects of Nitrogen and Phosphorus Enrichment on Growth of Dwarf Avicennia Marina Mangroves. Aquat. Bot. 2009, 90, 184–190. [Google Scholar] [CrossRef]
- Castañeda-Moya, E.; Twilley, R.R.; Rivera-Monroy, V.H.; Zhang, K.; Davis, S.E.; Ross, M. Sediment and Nutrient Deposition Associated with Hurricane Wilma in Mangroves of the Florida Coastal Everglades. Estuaries Coasts 2010, 33, 45–58. [Google Scholar] [CrossRef]
- Lynch, J.C.; Meriwether, J.R.; McKee, B.A.; Vera-Herrera, F.; Twilley, R.R. Recent Accretion in Mangrove Ecosystems Based on 137 Cs and 210 Pb. Estuaries 1989, 12, 284–299. [Google Scholar] [CrossRef]
- Twilley, R.W.; Lugo, A.E.; Patterson-Zucca, C. Litter Production and Turnover in Basin Mangrove Forests in Southwest Florida. Ecology 1986, 67, 670–683. [Google Scholar] [CrossRef]
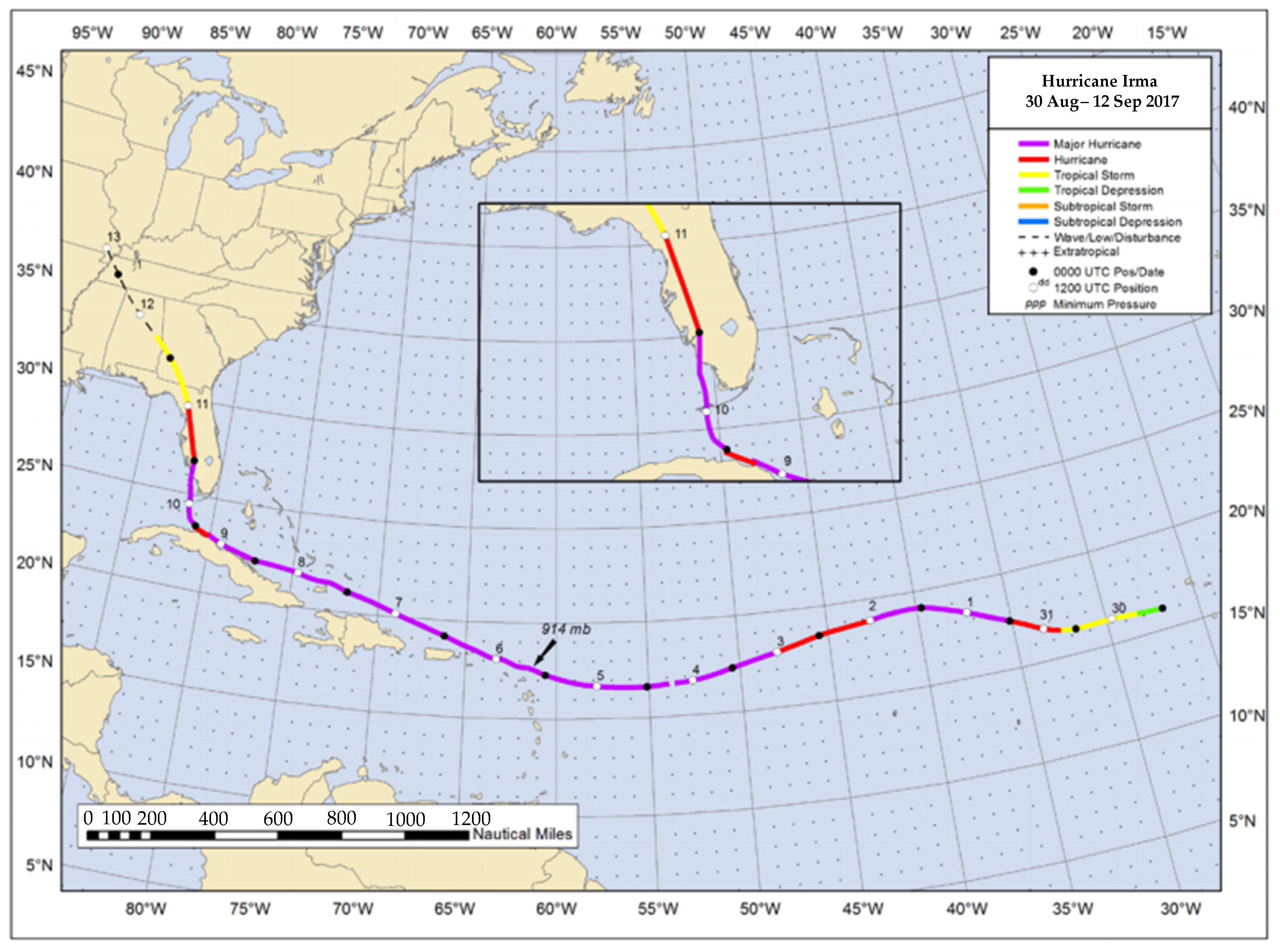
- Cangialosi, J.P.; Latto, A.S.; Berg, R. National Hurricane Center Tropical Cyclone Report: Hurricane Irma (AL112017); National Hurricane Center, National Oceanic and Atmospheric Administration: Miami, FL, USA, 2018. [Google Scholar]
- Marois, D.E.; Mitsch, W.J. A Mangrove Creek Restoration Plan Utilizing Hydraulic Modeling. Ecol. Eng. 2017, 108, 537–546. [Google Scholar] [CrossRef]
- MacDonnell, C.P.; Zhang, L.; Griffiths, L.; Mitsch, W.J. Nutrient Concentrations in Tidal Creeks as Indicators of the Water Quality Role of Mangrove Wetlands in Southwest Florida. Ecol. Indic. 2017, 80, 316–326. [Google Scholar] [CrossRef]
- NOAA Tides/Water Level, Naples, Gulf of Mexico, FL. Station ID: 8725110, NOAA. Available online: https://tidesandcurrents.noaa.gov/stationhome.html?id=8725110 (accessed on 29 September 2020).
- National Oceanic and Atmospheric Administration (NOAA) National Centers for Environmental Information (NCEI). Available online: https://www.ncdc.noaa.gov/ (accessed on 25 May 2021).
- Imbert, D.; Rollet, B. Phytmassaerienne et Production Primaire Dans La Mangrove Du Grand Cul-de-Sac Marine (Guadeloupe, Antilles Francaises). Bull. Ecol. 1989, 20, 27–39. [Google Scholar]
- Komiyama, A.; Ong, J.E.; Poungparn, S. Allometry, Biomass, and Productivity of Mangrove Forests: A Review. Aquat. Bot. 2008, 89, 128–137. [Google Scholar] [CrossRef]
- Appleby, P.G.; Oldfield, F. The Calculation of Lead-210 Dates Assuming a Constant Rate of Supply of Unsupported 210Pb to the Sediment. Catena 1978, 5, 1–8. [Google Scholar] [CrossRef]
| Hydrologically Disturbed Creek | Reference Creek | |||||
|---|---|---|---|---|---|---|
| Riverine | Fringe | Riverine | Fringe | |||
| Tree density (stems m−2) | R. mangle | 2013–2014 | 0.77 | 0.46 | 0.51 | 0.39 |
| 2019 | 0.56 | 0.48 | 0.48 | 0.76 | ||
| A. germinans | 2013–2014 | - | - | - | 0.01 | |
| 2019 | - | - | - | - | ||
| L. racemosa | 2013–2014 | 0.21 | 0.18 | 0.01 | 0.03 | |
| 2019 | 0.48 | 0.04 | - | 0.08 | ||
| Mean DBH (cm) ± SE | R. mangle | 2013–2014 | 3.3 ± 0.2 (32) | 3.2 ± 0.5 (38) | 5.9 ± 0.8 (52) | 5.5 ± 0.7 (39) |
| 2019 | 5.9 ± 0.7 (14) | 5.0 ± 1.1 (12) | 6.4 ± 0.5 (12) | 5.5 ± 0.5 (19) | ||
| A. germinans | 2013–2014 | - | - | - | 24.4 (1) | |
| 2019 | - | - | - | - | ||
| L. racemosa | 2013–2014 | 7.0 ± 0.4 (10) | 1.4 ± 0.2 (8) | 25 (1) | 18.2 ± 1.1 (3) | |
| 2019 | 6.4 ± 0.9 (12) | 0.9 (1) | - | 12.4 ± 3.7 (2) | ||
| Aboveground biomass (kg m−2) | R. mangle | 2013–2014 | 3.55 ± 0.02 (32) | 6.01 ± 0.05 (38) | 8.66 ± 0.02 (52) | 5.39 ± 0.02 (39) |
| 2019 | 10.48 ± 0.21 (14) | 9.18 ± 0.29 (12) | 9.34 ± 0.15 (12) | 11.46 ± 0.14 (19) | ||
| A. germinans | 2013–2014 | - | - | - | 3.13 (1) | |
| 2019 | - | - | - | - | ||
| L. racemosa | 2013–2014 | 4.88 ± 0.09 (10) | 0.12 ± 0.01 (8) | 2.83 (1) | 4.55 ± 0.61 (3) | |
| 2019 | 8.28 ± 0.21 (12) | 0.01 (1) | - | 5.23 ± 1.55 (2) | ||
| Total | 2013–2014 | 8.43 ± 0.03 (42) | 6.14 ± 0.04 (46) | 11.49 ± 0.05 (53) | 13.08 ± 0.09 (43) | |
| 2019 | 18.77 ± 0.15 (26) | 9.18 ± 0.27 (21) | 9.34 ± 0.15 (12) | 16.69 ± 0.21 (21) | ||
| Site | Inorganic Carbon Change (g-C m−2) | Organic Carbon Change (g-C m−2) |
|---|---|---|
| Disturbed Riverine | +0.21 | −4071 |
| Disturbed Fringe | −77 | −706 |
| Reference Riverine | +315 | −433 |
| Reference Fringe | −5.23 | −5854 |
| Average | +58 | −2766 |
| Site | Sediment Accretion Rate (mm yr−1) | Carbon Sequestration Rate (g-C m−2 yr−1) | ||
|---|---|---|---|---|
| 2013–2014 | 2019 | 2013–2014 | 2019 | |
| Disturbed Riverine | 3.04 | 1.91 | 126 | 44.9 |
| Disturbed Fringe | 2.23 | 1.79 | 74 | 62.6 |
| Reference Riverine | 5.43 | 4.02 | 162 | 154 |
| Reference Fringe | 2.28 | 3.69 | 127 | 70.0 |
| Average | 3.25 ± 0.75 | 2.85 ± 0.58 | 122.3 ± 18.1 | 82.9 ± 24.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griffiths, L.N.; Mitsch, W.J. Estimating the Effects of a Hurricane on Carbon Storage in Mangrove Wetlands in Southwest Florida. Plants 2021, 10, 1749. https://doi.org/10.3390/plants10081749
Griffiths LN, Mitsch WJ. Estimating the Effects of a Hurricane on Carbon Storage in Mangrove Wetlands in Southwest Florida. Plants. 2021; 10(8):1749. https://doi.org/10.3390/plants10081749
Chicago/Turabian StyleGriffiths, Lauren N., and William J. Mitsch. 2021. "Estimating the Effects of a Hurricane on Carbon Storage in Mangrove Wetlands in Southwest Florida" Plants 10, no. 8: 1749. https://doi.org/10.3390/plants10081749
APA StyleGriffiths, L. N., & Mitsch, W. J. (2021). Estimating the Effects of a Hurricane on Carbon Storage in Mangrove Wetlands in Southwest Florida. Plants, 10(8), 1749. https://doi.org/10.3390/plants10081749






