Juncus Bulbosus Tissue Nutrient Concentrations and Stoichiometry in Oligotrophic Ecosystems: Variability with Seasons, Growth Forms, Organs and Habitats
Abstract
1. Introduction
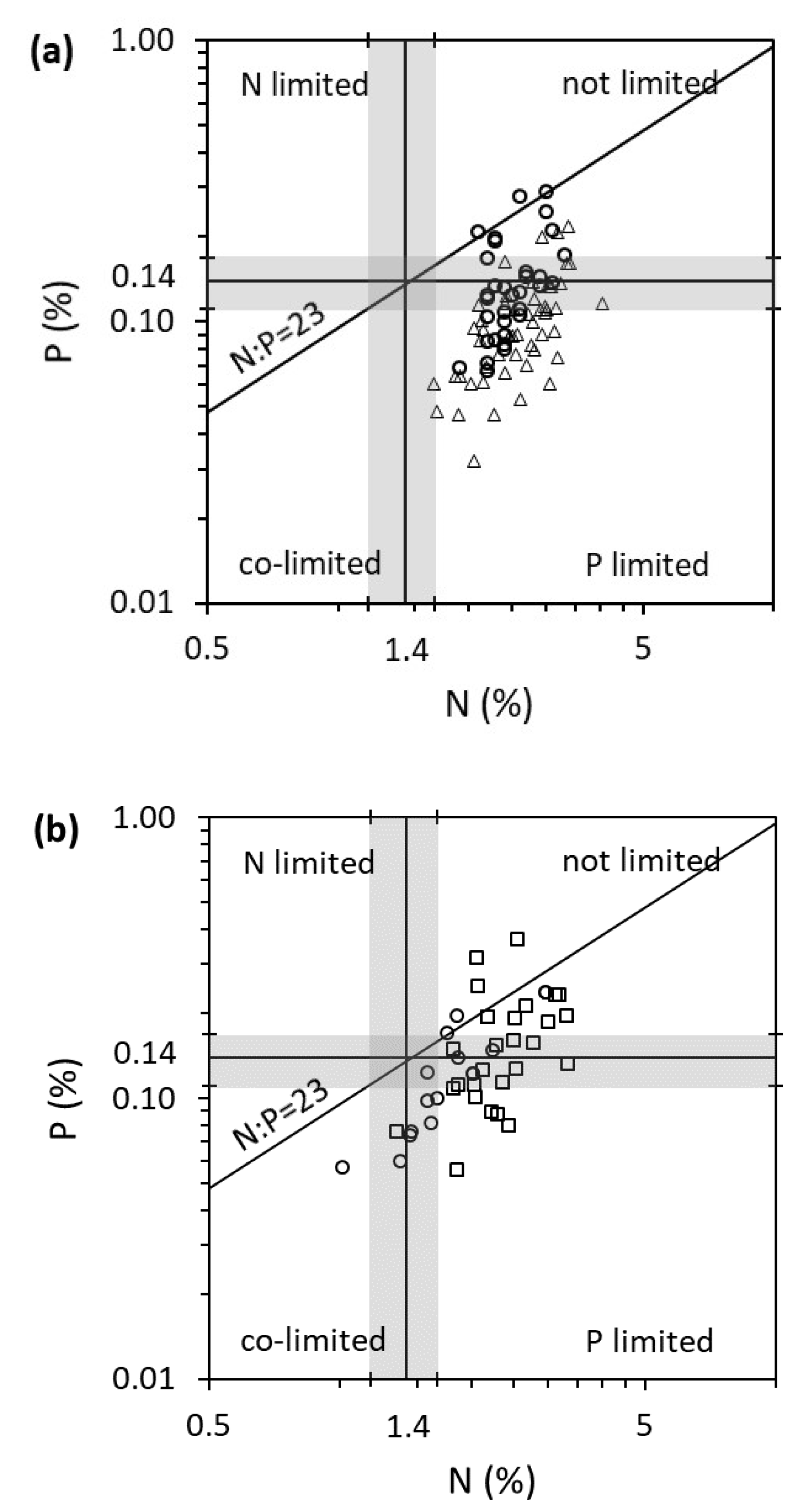
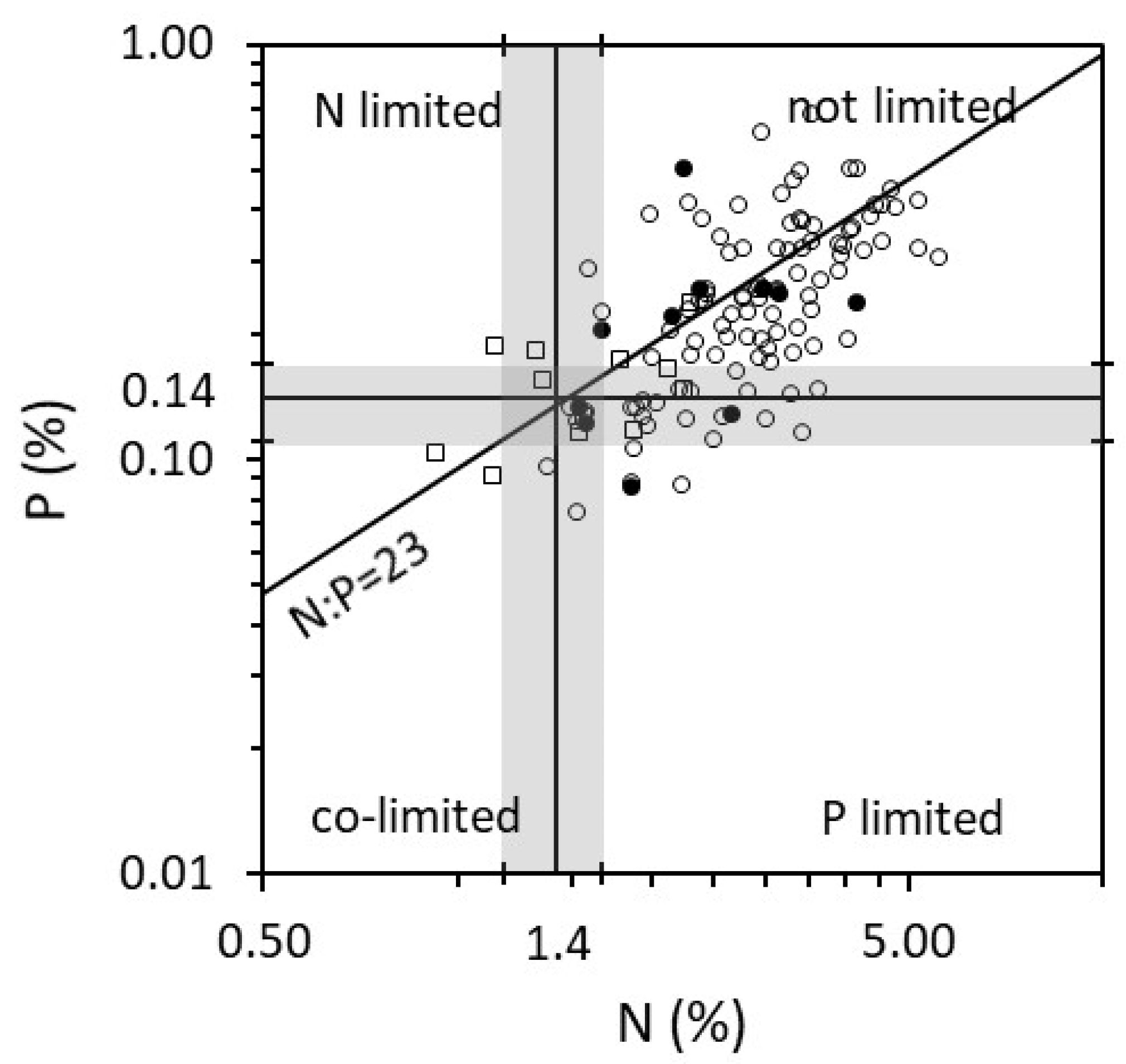
2. Results
2.1. Growth Forms and the Time of Year
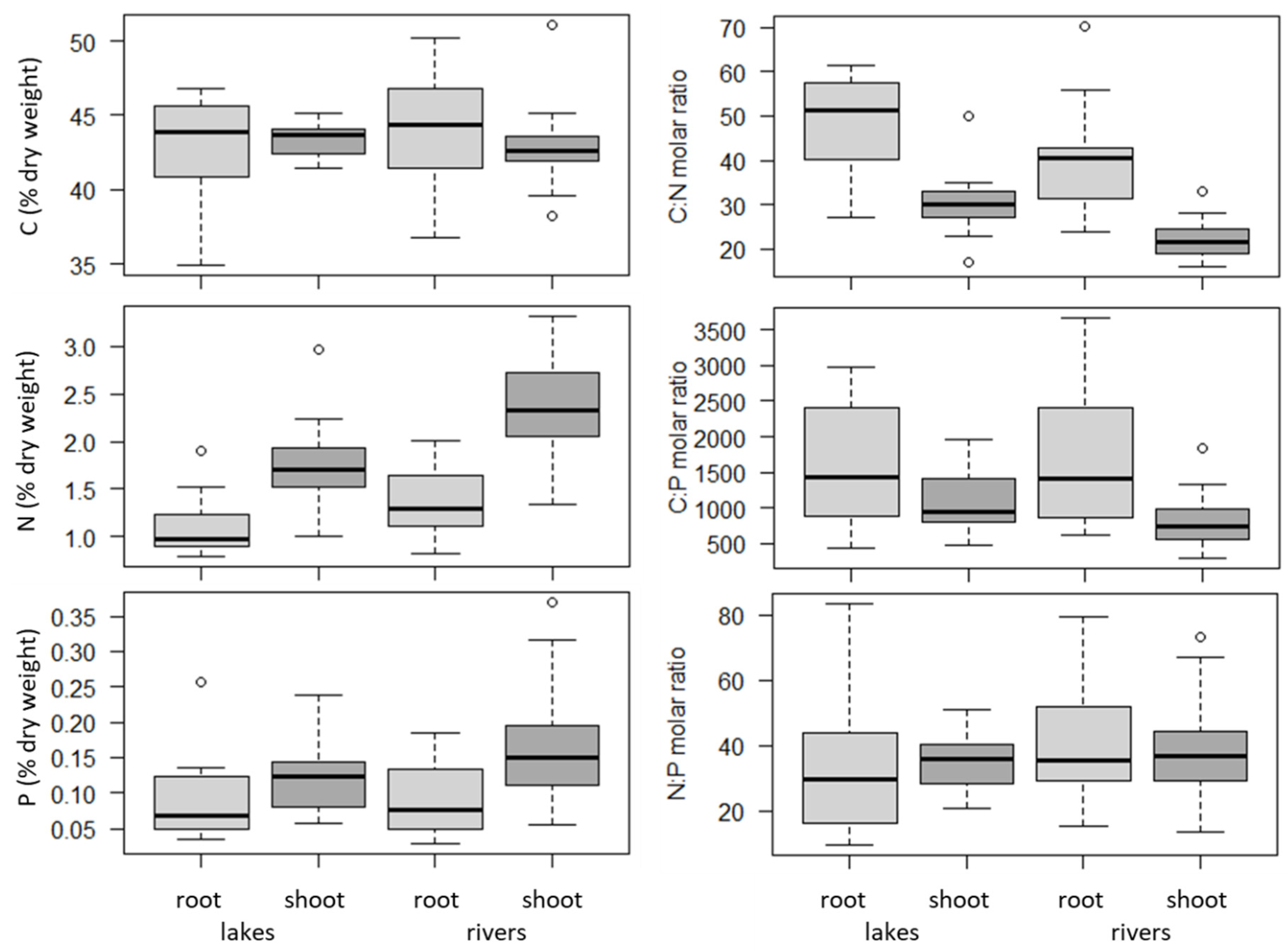
2.2. Plant Organs (Roots versus Shoots) and Habitats (Lakes versus Rivers)
3. Discussion
4. Materials and Methods
4.1. Juncus Bulbosus
4.2. Growth Form and the Time of Year
4.3. Plant Organs (Roots versus Shoots) and Habitats (Lakes versus Rivers)
4.4. Plant Tissue C, N, P Analyses
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cebrian, J. Patterns in the fate of production in plant communities. Am. Nat. 1999, 154, 449–468. [Google Scholar] [CrossRef]
- Bakker, E.S.; Wood, K.A.; Pages, J.F.; Veen, G.F.; Christianen, M.J.A.; Santamaria, L.; Nolet, B.A.; Hilt, S. Herbivory on freshwater and marine macrophytes: A review and perspective. Aquat. Bot. 2016, 135, 18–36. [Google Scholar] [CrossRef]
- Enriquez, S.; Duarte, C.M.; Sand-Jensen, K. Patterns in decomposition rates among photosynthetic organisms—The importance of detritus C-N-P content. Oecologia 1993, 94, 457–471. [Google Scholar] [CrossRef]
- Bernot, M.J.; Tank, J.L.; Royer, T.V.; David, M.B. Nutrient uptake in streams draining agricultural catchments of the midwestern United States. Freshw. Biol. 2006, 51, 499–509. [Google Scholar] [CrossRef]
- Levi, P.S.; Riis, T.; Alnøe, A.B.; Peipoch, M.; Maetzke, K.; Bruus, C.; Baattrup-Pedersen, A. Macrophyte complexity controls nutrient uptake in lowland streams. Ecosystems 2015, 18, 914–931. [Google Scholar] [CrossRef]
- O’Brien, J.M.; Lessard, J.L.; Plew, D.; Graham, S.E.; McIntosh, A.R. Aquatic macrophytes alter metabolism and nutrient cycling in lowland streams. Ecosystems 2014, 17, 405–417. [Google Scholar] [CrossRef]
- Barko, J.W.; Smart, R.M. Mobilization of sediment phosphorus by submersed freshwater macrophytes. Freshw. Biol. 1980, 10, 229–238. [Google Scholar] [CrossRef]
- Grutters, B.M.C.; Gross, E.M.; Bakker, E.S. Insect herbivory on native and exotic aquatic plants: Phosphorus and nitrogen drive insect growth and nutrient release. Hydrobiologia 2016, 778, 209–220. [Google Scholar] [CrossRef]
- Westlake, D.F.; Casey, H.; Dawson, F.H.; Ladle, M.; Mann, R.H.K.; Marker, A.F.H. The chalk-stream ecosystem. In Proceedings of the IBP-UNESCO Symposium on Productivity Problems of Freshwaters, Kazimiera Dolny, Poland, 6–12 May 1970; Kajak, Z., Hillbricht-Ilkowska, A., Eds.; Polish Scientific Publishers: Warsaw, Poland, 1972; pp. 615–635. [Google Scholar]
- Ladle, M.; Casey, H. Growth and nutrient relationships of Ranunculus penicillatus var calcareus in a small chalk stream. In Proceedings of the European Weed Research Council 3rd International Symposium on Aquatic Weeds; European Weed Research Society: Oxford, UK, 1971; pp. 53–62. [Google Scholar]
- Westlake, D.F. The biology of aquatic weeds in relation to their management. In Proceedings of the 9th British Weed Control Conference; British Weed Control Council: Brighton, UK, 1968; pp. 371–381. [Google Scholar]
- House, W.A.; Duplat, D.; Denison, F.H.; Henville, P.; Dawson, F.H.; Cooper, D.M.; May, L. The role of macrophytes in the retention of phosphorus in the river Thame, England. Chem. Ecol. 2001, 17, 271–291. [Google Scholar] [CrossRef]
- Desmet, N.J.S.; Van Belleghem, S.; Seuntjens, P.; Bouma, T.J.; Buis, K.; Meire, P. Quantification of the impact of macrophytes on oxygen dynamics and nitrogen retention in a vegetated lowland river. Phys. Chem. Earth 2011, 36, 479–489. [Google Scholar] [CrossRef]
- Demars, B.O.L.; Edwards, A.C. Tissue nutrient concentrations in freshwater aquatic macrophytes: High inter-taxon differences and low phenotypic response to nutrient supply. Freshw. Biol. 2007, 52, 2073–2086. [Google Scholar] [CrossRef]
- Moe, T.F.; Brysting, A.K.; Andersen, T.; Schneider, S.C.; Kaste, O.; Hessen, D.O. Nuisance growth of Juncus bulbosus: The roles of genetics and environmental drivers tested in a large-scale survey. Freshw. Biol. 2013, 58, 114–127. [Google Scholar] [CrossRef]
- Preiner, S.; Dai, Y.; Pucher, M.; Reitsema, R.E.; Schoelynck, J.; Meire, P.; Hein, T. Effects of macrophytes on ecosystem metabolism and net nutrient uptake in a groundwater fed lowland river. Sci. Total Environ. 2020, 721, 137620. [Google Scholar] [CrossRef]
- Murphy, K.J.; Rørslett, B.; Springel, I. Strategy analysis of submerged lake macrophyte communities: An international example. Aquat. Bot. 1990, 36, 303–323. [Google Scholar] [CrossRef]
- Moeller, R.E. Seasonal changes in biomass, tissue chemistry, and net production of the evergreen hydrophyte, Lobelia dortmanna. Can. J. Bot. 1978, 56, 1425–1433. [Google Scholar] [CrossRef]
- Cronin, G.; Lodge, D.M. Effects of light and nutrient availability on the growth allocation, carbon/nitrogen balance, phenolic chemistry, and resistance to herbivory of two freshwater macrophytes. Oecologia 2003, 137, 32–41. [Google Scholar] [CrossRef]
- Guisan, A.; Tingley, R.; Baumgartner, J.B.; Naujokaitis-Lewis, I.; Sutcliffe, P.R.; Tulloch, A.I.T.; Regan, T.J.; Brotons, L.; McDonald-Madden, E.; Mantyka-Pringle, C.; et al. Predicting species distributions for conservation decisions. Ecol. Lett. 2013, 16, 1424–1435. [Google Scholar] [CrossRef]
- Garbey, C.; Murphy, K.J.; Thiébaut, G.; Muller, S. Variation in P-content in aquatic plant tissues offers an efficient tool for determining plant growth strategies along a resource gradient. Freshw. Biol. 2004, 49, 346–356. [Google Scholar] [CrossRef]
- Velle, G.; Skoglund, H.; Barlaup, B.T. Effects of nuisance submerged vegetation on the fauna in Norwegian rivers. Hydrobiologia 2021. [Google Scholar] [CrossRef]
- Hultén, E.; Fries, M. Atlas of North European Vascular Plants: North of the Tropic of Cancer; Koeltz: Königstein, Germany, 1986. [Google Scholar]
- Roelofs, J.G.M.; Brandrud, T.E.; Smolders, A.J.P. Massive expansion of Juncus bulbosus L. after liming of acidified SW Norwegian lakes. Aquat. Bot. 1994, 48, 187–202. [Google Scholar] [CrossRef]
- Rørslett, B.; Brandrud, T.E.; Johansen, S. Tilgroing i Terskelbasseng i Otra ved Valle. Problemanalyse og Forslag om Tiltak. Report No 2442; Norsk Institutt for Vannforskning: Oslo, Norway, 1990. [Google Scholar]
- Moe, T.F.; Hessen, D.O.; Demars, B.O.L. Functional biogeography: Stoichiometry and thresholds for interpreting nutrient limitation in aquatic plants. Sci. Total Environ. 2019, 677, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.C.; Moe, T.F.; Hessen, D.O.; Kaste, O. Juncus bulbosus nuisance growth in oligotrophic freshwater ecosystems: Different triggers for the same phenomenon in rivers and lakes? Aquat. Bot. 2013, 104, 15–24. [Google Scholar] [CrossRef]
- Maine, M.A.; Suñe, N.L.; Panigatti, M.C.; Pizarro, M.J.; Emiliani, F. Relationships between water chemistry and macrophyte chemistry in lotic and lentic environments. Arch. Für Hydrobiol. 1999, 145, 129–145. [Google Scholar] [CrossRef]
- Baldy, V.; Trémolières, M.; Andrieu, M.; Belliard, J. Changes in phosphorus content of two aquatic macrophytes according to water velocity, trophic status and time period in hardwater streams. Hydrobiologia 2007, 575, 343–351. [Google Scholar] [CrossRef]
- Thiébaut, G.; Muller, S. Linking phosphorus pools of water, sediment and macrophytes in running waters. Ann. Limnol. Int. J. Limnol. 2003, 39, 307–316. [Google Scholar] [CrossRef]
- Thiébaut, G. Does competition for phosphate supply explain the invasion pattern of Elodea species? Water Res. 2005, 39, 3385–3393. [Google Scholar] [CrossRef]
- Reitsema, R.E.; Preiner, S.; Meire, P.; Hein, T.; Dai, Y.R.; Schoelynck, J. Environmental control of macrophyte traits and interactions with metabolism and hydromorphology in a groundwater-fed river. River Res. Appl. 2021, 37, 294–306. [Google Scholar] [CrossRef]
- Christensen, K.K.; Sand-Jensen, K. Precipitated iron and manganese plaques restrict root uptake of phosphorus in Lobelia dortmanna. Can. J. Bot. 1998, 776, 2158–2163. [Google Scholar]
- Demars, B.O.L.; Edwards, A.C. Tissue nutrient concentrations in aquatic macrophytes: Comparison across biophysical zones, surface water habitats and plant life forms. Chem. Ecol. 2008, 24, 413–422. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry. The biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Fernándes-Aláez, M.; Fernándes-Aláez, C.; Bécares, E. Nutrient content in macrophytes in Spanish shallow lakes. Hydrobiologia 1999, 408–409, 317–326. [Google Scholar]
- Hegnauer, R.; Ruijgrok, H.W. Lilaea scilloides und Juncus bulbosus zwei neue cyanogene pflanzen. Phytochemistry 1971, 10, 2121–2124. [Google Scholar] [CrossRef]
- Ohlson, M.; Staaland, H. Mineral diversity in wild plants: Benefits and bane for moose. Oikos 2001, 94, 442–454. [Google Scholar] [CrossRef]
- Roelofs, J.G.M.; Schuurkes, J.A.A.R.; Smits, A.J.M. Impact of acidification and eutrophication on macrophyte communities in soft waters. II. Experimental studies. Aquat. Bot. 1984, 18, 389–411. [Google Scholar] [CrossRef]
- Svedang, M.U. Carbon dioxide as a factor regulating the growth dynamics of Juncus bulbosus. Aquat. Bot. 1992, 42, 231–240. [Google Scholar] [CrossRef]
- Lucassen, E.C.H.E.T.; Roelofs, J.G.M.; Schneider, S.C.; Smolders, A.J.P. Long-term effects of liming in Norwegian softwater lakes: The rise and fall of bulbous rush (Juncus bulbosus) and decline of isoetid vegetation. Freshw. Biol. 2016, 61, 769–782. [Google Scholar] [CrossRef]
- Sand-Jensen, K. Environmental control of bicarbonate use among freshwater and marine macrophytes. In Plant Life in Aquatic and Amphibious Habitats; Crawford, R.M.M., Ed.; Blackwell Scientific Publications: Oxford, UK, 1987; pp. 99–112. [Google Scholar]
- Hessen, D.O.; Ågren, G.I.; Anderson, T.R.; Elser, J.J.; De Ruiter, P.C. Carbon sequestration in ecosystems: The role of stoichiometry. Ecology 2004, 85, 1179–1192. [Google Scholar] [CrossRef]
- Titus, J.E.; Andorfer, J.H. Effects of CO2 enrichment on mineral accumulation and nitrogen relations in a submersed macrophyte. Freshw. Biol. 1996, 36, 661–671. [Google Scholar] [CrossRef]
- Reitsema, R.E.; Preiner, S.; Meire, P.; Hein, T.; De Boeck, G.; Blust, R.; Schoelynck, J. Implications of climate change for submerged macrophytes: Effects of CO2, flow velocity and nutrient concentration on Berula erecta. Aquat. Ecol. 2020, 54, 775–793. [Google Scholar] [CrossRef]
- Elser, J.J.; Andersen, T.; Baron, J.S.; Bergstrom, A.K.; Jansson, M.; Kyle, M.; Nydick, K.R.; Steger, L.; Hessen, D.O. Shifts in lake N:P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science 2009, 326, 835–837. [Google Scholar] [CrossRef]
- Ptacnik, R.; Solimini, A.G.; Andersen, T.; Tamminen, T.; Brettum, P.; Lepisto, L.; Willen, E.; Rekolainen, S. Diversity predicts stability and resource use efficiency in natural phytoplankton communities. Proc. Natl. Acad. Sci. USA 2008, 105, 5134–5138. [Google Scholar] [CrossRef]
- Smolders, A.; Lamers, L.; Lucassen, E.; Van der Velde, G.; Roelofs, J. Internal eutrophication: How it works and what to do about it—A review. Chem. Ecol. 2006, 22, 93–111. [Google Scholar] [CrossRef]
- Rørslett, B. Aquatic weed problems in a hydroelectric river: The R. Otra, Norway. Regul. Rivers Res. Manag. 1988, 2, 25–37. [Google Scholar]
- Moe, T.F.; Demars, B.O.L. Årsrapport Krypsivovervåking 2017; Report No L.NR. 7202-2017; NIVA: Oslo, Norway, 2017. [Google Scholar]
- Rørslett, B. Tilgroing i Otra Nedstrøms Brokke. Problemanalyse og Forslag om Tiltak; Report no: O-86130; Norsk Institutt for Vannforskning: Oslo, Norway, 1987. [Google Scholar]
- Murphy, K.J. Plant communities and plant diversity in softwater lakes of northern Europe. Aquat. Bot. 2002, 73, 287–324. [Google Scholar] [CrossRef]
- Roelofs, J.G.M. Impact of acidification and eutrophication on macrophyte communities in soft waters in the Netherlands. I. Field observations. Aquat. Bot. 1983, 17, 139–155. [Google Scholar] [CrossRef]
- Aulio, K. Elemental composition of Juncus bulbosus in an acidified freshwater reservoir. Environ. Pollut. 1987, 44, 1–11. [Google Scholar] [CrossRef]
- Svedäng, M.U. The growth dynamics of Juncus bulbosus L.—A strategy to avoid competition? Aquat. Bot. 1990, 37, 123–138. [Google Scholar] [CrossRef]
- Brandrud, T.E. Effects of liming on aquatic macrophytes, with emphasis on Scandinavia. Aquat. Bot. 2002, 73, 395–404. [Google Scholar] [CrossRef]
- Preston, C.D.; Pearman, D.A.; Dines, T.D. New Atlas of the British & Irish Flora; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Kaste, Ø.; Johansen, S.W.; Mjelde, M.; Andersen, T.; Hessen, D.; Holm, T.M.; Rangberg, A. Kan Næringsubalanse i Vann Føre til Problemvekst av Krypsiv? Resultater fra Forprosjekt i 2006; Report No 5341; Norsk Institutt for Vannforskning: Oslo, Norway, 2007. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing Vienna, Austria: R Foundation for Statistical Computing. 2018. Available online: http://www.R-project.org/ (accessed on 29 January 2021).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Barton, K. Package ‘MuMIn’. ‘R’ Package Version 1.15.62016. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 29 January 2021).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest: Tests in Linear Mixed Effects Models. R Package Version 2.0-292015. Available online: http://CRAN.R-project.org/package=lmerTest (accessed on 29 January 2021).
- Ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]

| Time of Sampling | Growth Form | |||
|---|---|---|---|---|
| P | P | |||
| C | 0.2 | 0.68 | 1.8 | 1.41 |
| N | 2.3 | 0.13 | 9.9 | 0.007 |
| P | 20.1 | 7 × 10−6 | 1.5 | 0.47 |
| C:N | 1.9 | 0.17 | 8.1 | 0.02 |
| C:P | 20.2 | 7 × 10−6 | 1.2 | 0.55 |
| N:P | 33.1 | 9 × 10−9 | 7.2 | 0.03 |
| Time of Sampling | Growth Form | |||
|---|---|---|---|---|
| P | P | |||
| C | 0.03 | 0.87 | 2.1 | 0.15 |
| N | 1.1 | 0.29 | 0.7 | 0.41 |
| P | 0.1 | 0.77 | 0.7 | 0.39 |
| C:N | 1.1 | 0.30 | 1.7 | 0.20 |
| C:P | 0.1 | 0.78 | 0.4 | 0.51 |
| N:P | 0.4 | 0.51 | 1.3 | 0.25 |
| Percentage of Explained Variance | |||||
|---|---|---|---|---|---|
| Singly | P | After Stepwise Regression | P | Selection Order | |
| Organs | 20.8 | <0.001 | 20.8 | <0.001 | 1 |
| Habitats | 4.6 | 0.004 | 4.1 | 0.028 | 3 |
| TP | 4.6 | 0.006 | 3.9 | 0.040 | 4 |
| lnEC | 3.0 | 0.027 | 4.3 | 0.019 | 2 |
| Datasets | Year | Description |
|---|---|---|
| 6 lakes | 2006 | Replicate J. bulbosus sampling of different growth forms in 6 lakes at two seasons (spring and autumn), n = 86 |
| 10 lakes | 2008 | Single J. bulbosus samples (roots and shoots together), two sampling times (early and late summer) and two growth forms, n = 40 |
| 16 lakes and 28 river sites | 2010 | Single J. bulbosus samples, two habitats (lakes and rivers) and two organs (roots versus shoots), n = 80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moe, T.F.; Hessen, D.O.; Demars, B.O.L. Juncus Bulbosus Tissue Nutrient Concentrations and Stoichiometry in Oligotrophic Ecosystems: Variability with Seasons, Growth Forms, Organs and Habitats. Plants 2021, 10, 441. https://doi.org/10.3390/plants10030441
Moe TF, Hessen DO, Demars BOL. Juncus Bulbosus Tissue Nutrient Concentrations and Stoichiometry in Oligotrophic Ecosystems: Variability with Seasons, Growth Forms, Organs and Habitats. Plants. 2021; 10(3):441. https://doi.org/10.3390/plants10030441
Chicago/Turabian StyleMoe, Therese F., Dag O. Hessen, and Benoît O. L. Demars. 2021. "Juncus Bulbosus Tissue Nutrient Concentrations and Stoichiometry in Oligotrophic Ecosystems: Variability with Seasons, Growth Forms, Organs and Habitats" Plants 10, no. 3: 441. https://doi.org/10.3390/plants10030441
APA StyleMoe, T. F., Hessen, D. O., & Demars, B. O. L. (2021). Juncus Bulbosus Tissue Nutrient Concentrations and Stoichiometry in Oligotrophic Ecosystems: Variability with Seasons, Growth Forms, Organs and Habitats. Plants, 10(3), 441. https://doi.org/10.3390/plants10030441







