Both Allene Oxide Synthases Genes Are Involved in the Biosynthesis of Herbivore-Induced Jasmonic Acid and Herbivore Resistance in Rice
Abstract
1. Introduction
2. Results
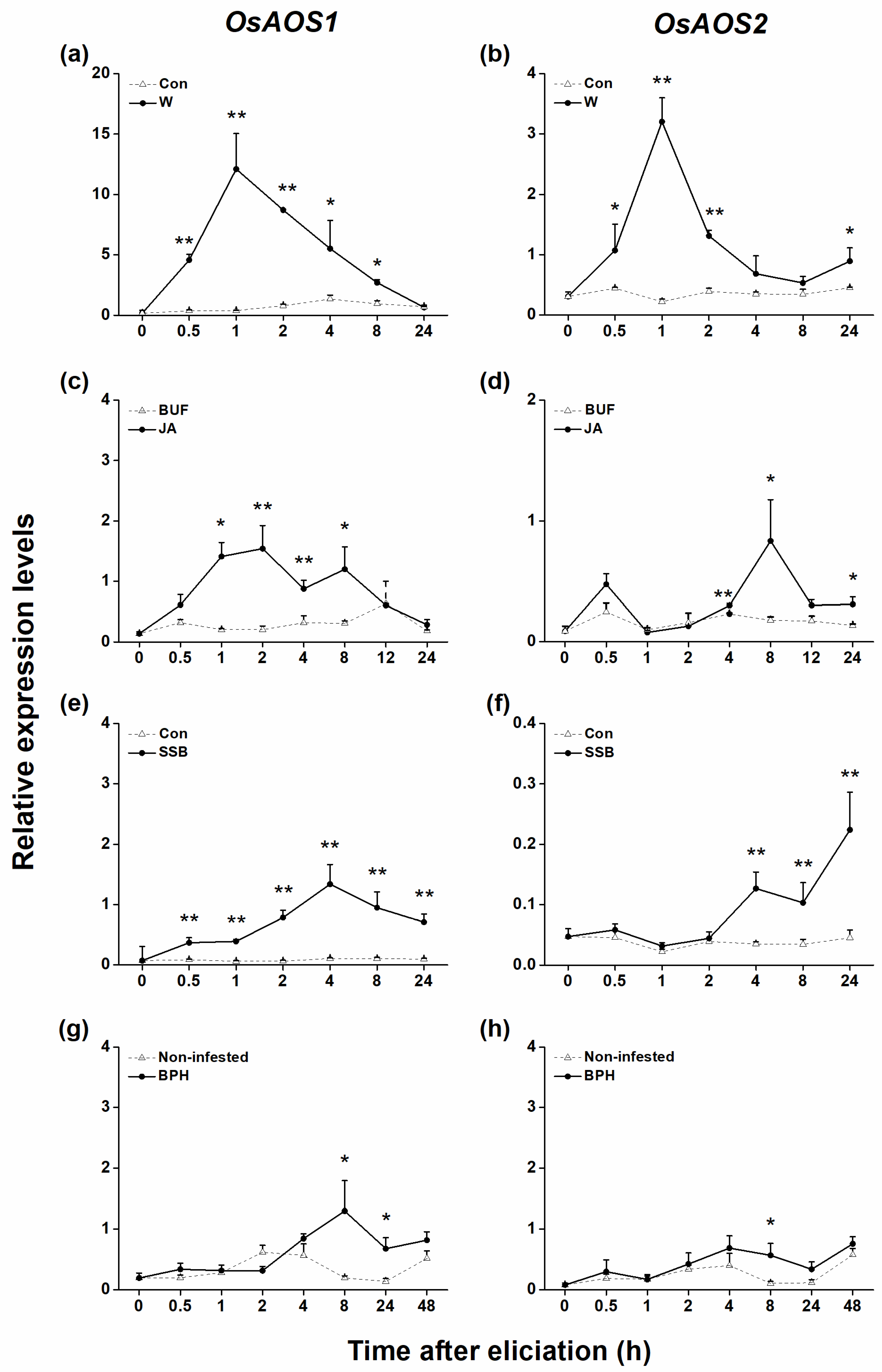
2.1. Both OsAOS1 and OsAOS2 Were Induced by Mechanical Wounding, Herbivore Infestation, and JA but Have Different Patterns
2.2. Silencing OsAOS1 and OsAOS2
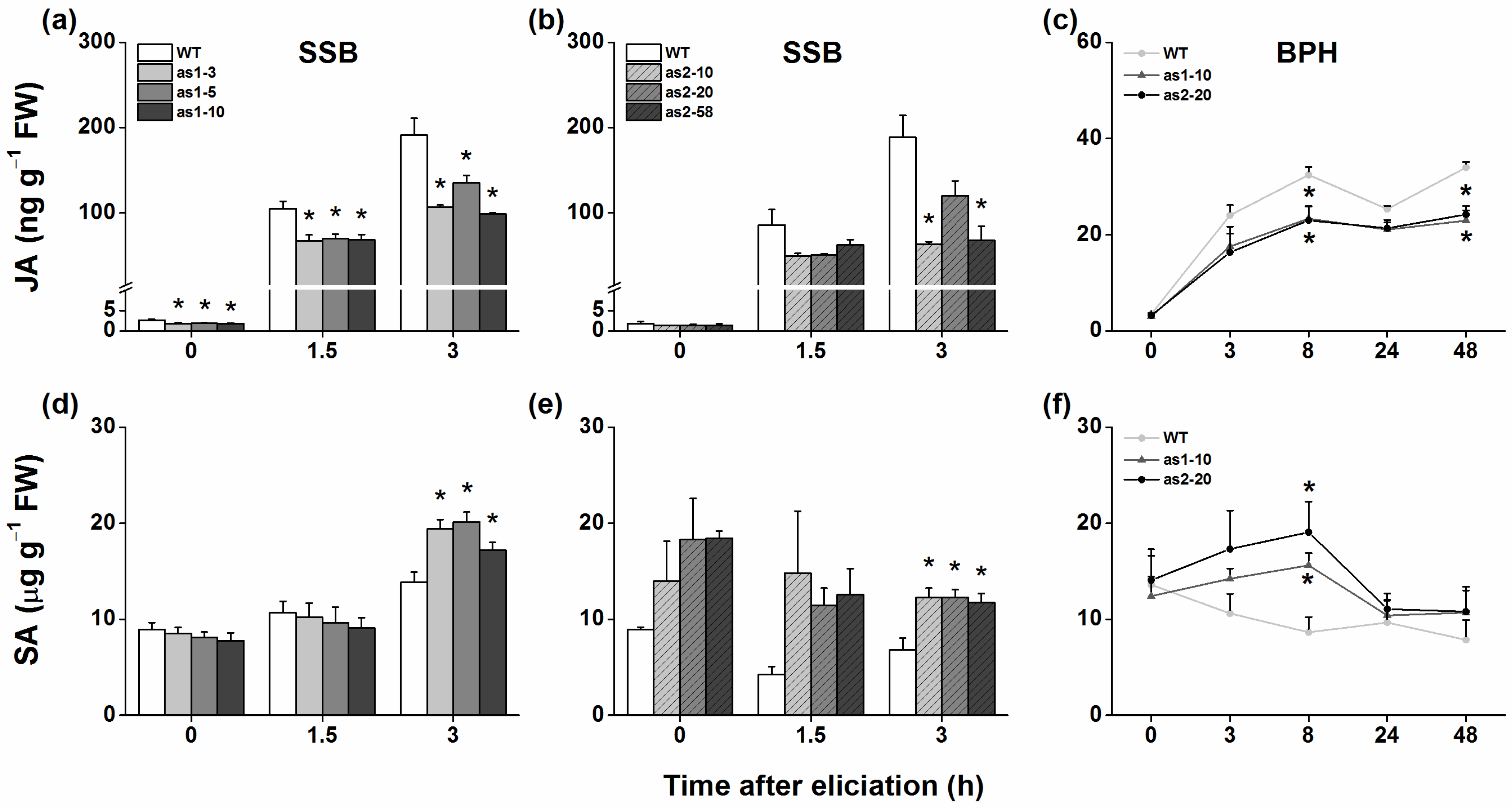
2.3. Both OsAOS1 and OsAOS2 Mediate Herbivore-Induced JA and SA Biosynthesis
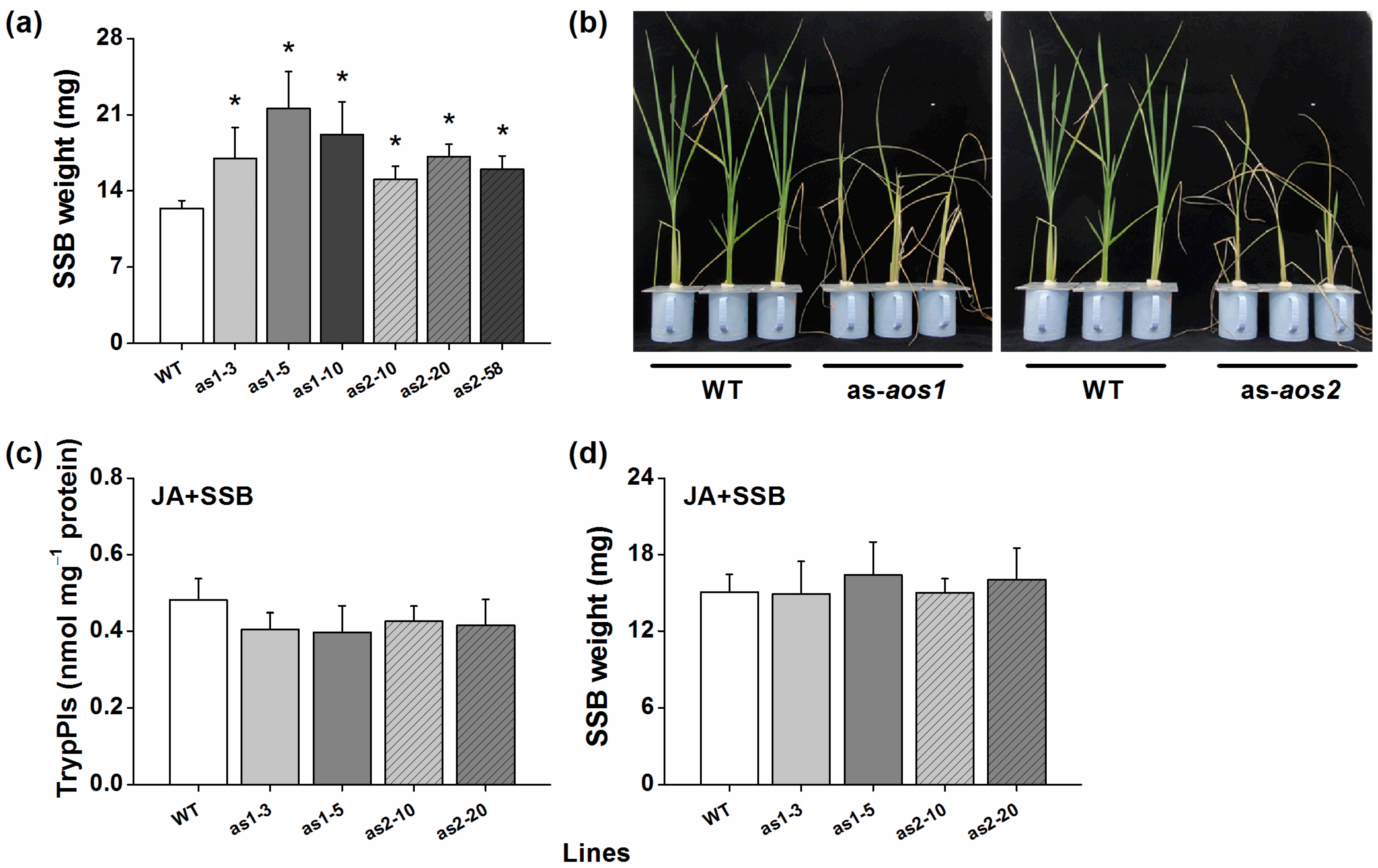
2.4. OsAOS1 and OsAOS2 Positively Regulates TrypPI Activity, Volatile Emmission, and Rice Resistance to SSB
2.5. OsAOS1 and OsAOS2 Negatively Modulate H2O2 Accumulation and Rice Resistance to BPH
3. Discussion
4. Materials and Methods
4.1. Plants and Insects
4.2. Cloning and Sequence Analysis of OsAOS1 and OsAOS2
4.3. Quantitative Real-Time PCR
4.4. Generation and Characterization of as-aos Transgenic Lines
4.5. Plant Treatments
4.6. JA, SA and H2O2 Analysis
4.7. Analysis of TrypPI Activity
4.8. Collection, Isolation and Identification of Rice Volatiles
4.9. Herbivore Bioassays
4.10. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.; Baldwin, I.T. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Chung, H.S.; Koo, A.J.K.; Gao, X.; Jayanty, S.; Thines, B.; Jones, A.D.; Howe, G.A. Regulation and Function of Arabidopsis JASMONATE ZIM-Domain Genes in Response to Wounding and Herbivory. Plant Physiol. 2008, 146, 952–964. [Google Scholar] [CrossRef]
- Erb, M.; Meldau, S.; Howe, G.A. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 2012, 17, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Schuman, M.C.; Baldwin, I.T. The Layers of Plant Responses to Insect Herbivores. Annu. Rev. Entomol. 2016, 61, 373–394. [Google Scholar] [CrossRef] [PubMed]
- Lyons, R.; Manners, J.M.; Kazan, K. Jasmonate biosynthesis and signaling in monocots: A comparative overview. Plant Cell Rep. 2013, 32, 815–827. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Sun, N.; Liu, H.; Zhao, Y.; Liang, Y.; Zhang, L.; Han, Y. Functional diversity of jasmonates in rice. Rice 2015, 8, 5. [Google Scholar] [CrossRef]
- Schaller, A.; Stintzi, A. Enzymes in jasmonate biosynthesis—Structure, function, regulation. Phytochemistry 2009, 70, 1532–1538. [Google Scholar] [CrossRef]
- Farmer, E.E.; Goossens, A. Jasmonates: What ALLENE OXIDE SYNTHASE does for plants. J. Exp. Biol. 2019, 70, 3373–3378. [Google Scholar] [CrossRef]
- Wasternack, C.; Feussner, I. The Oxylipin Pathways: Biochemistry and Function. Annu. Rev. Plant Biol. 2018, 69, 363–386. [Google Scholar] [CrossRef]
- Chehab, E.W.; Perea, J.V.; Gopalan, B.; Theg, S.; Dehesh, K. Oxylipin Pathway in Rice and Arabidopsis. J. Integr. Plant Biol. 2007, 49, 43–51. [Google Scholar] [CrossRef]
- Mosblech, A.; Feussner, I.; Heilmann, I. Oxylipins: Structurally diverse metabolites from fatty acid oxidation. Plant Physiol. Biochem. 2009, 47, 511–517. [Google Scholar] [CrossRef]
- Laudert, D.; Pfannschmidt, U.; Lottspeich, F.; Holländer-Czytko, H.; Weiler, E.W. Cloning, molecular and functional characterization of Arabidopsis thaliana allene oxide synthase (CYP 74), the first enzyme of the octadecanoid pathway to jasmonates. Plant Mol. Biol. 1996, 31, 323–335. [Google Scholar] [CrossRef]
- Maucher, H.; Hause, B.; Feussner, I.; Ziegler, J.; Wasternack, C. Allene oxide synthases of barley (Hordeum vulgare cv. Salome): Tissue specific regulation in seedling development. Plant J. 2000, 21, 199–213. [Google Scholar] [CrossRef]
- Howe, G.A.; Lee, G.I.; Itoh, A.; Li, L.; DeRocher, A.E. Cytochrome P450-Dependent Metabolism of Oxylipins in Tomato. Cloning and Expression of Allene Oxide Synthase and Fatty Acid Hydroperoxide Lyase1. Plant Physiol. 2000, 123, 711–724. [Google Scholar] [CrossRef]
- Sivasankar, S.; Sheldrick, B.; Rothstein, S.J. Expression of Allene Oxide Synthase Determines Defense Gene Activation in Tomato1. Plant Physiol. 2000, 122, 1335–1342. [Google Scholar] [CrossRef]
- Stumpe, M.; Göbel, C.; Demchenko, K.; Hoffmann, M.; Klösgen, R.B.; Pawlowski, K.; Feussner, I. Identification of an allene oxide synthase (CYP74C) that leads to formation of α-ketols from 9-hydroperoxides of linoleic and linolenic acid in below-ground organs of potato. Plant J. 2006, 47, 883–896. [Google Scholar] [CrossRef]
- Agrawal, G.K.; Rakwal, R.; Jwa, N.; Han, K.; Agrawal, V.P. Molecular cloning and mRNA expression analysis of the first rice jasmonate biosynthetic pathway gene allene oxide synthase. Plant Physiol. Biochem. 2002, 40, 771–782. [Google Scholar] [CrossRef]
- Ha, S.B.; Lee, B.C.; Lee, D.E.; Kuk, Y.I.; Lee, A.Y.; Han, O.; Back, K. Molecular characterization of the gene encoding rice allene oxide synthase and its expression. Biosci. Biotechnol. Biochem. 2002, 66, 2719–2722. [Google Scholar] [CrossRef]
- Haga, K.; Iino, M. Phytochrome-mediated transcriptional up-regulation of ALLENE OXIDE SYNTHASE in rice seedlings. Plant Cell Physiol. 2004, 45, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.K.; Tamogami, S.; Han, O.; Iwahashi, H.; Rakwal, R. Rice octadecanoid pathway. Biochem. Biophys. Res. Commun. 2004, 317, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Zhang, G.; Zhang, W.; Hu, Y.; Zhang, J. Biological control of rice insect pests in China. Biol. Control 2013, 67, 8–20. [Google Scholar] [CrossRef]
- Chen, M.; Shelton, A.; Ye, G.Y. Insect-resistant genetically modified rice in China: From research to commercialization. Annu. Rev. Entomol. 2011, 56, 81–101. [Google Scholar] [CrossRef]
- Lu, J.; Li, J.; Ju, H.; Liu, X.; Erb, M.; Wang, X.; Lou, Y. Contrasting Effects of Ethylene Biosynthesis on Induced Plant Resistance against a Chewing and a Piercing-Sucking Herbivore in Rice. Mol. Plant 2014, 7, 1670–1682. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Qi, J.; Ren, N.; Cheng, J.; Erb, M.; Mao, B.; Lou, Y. Silencing OsHI-LOX makes rice more susceptible to chewing herbivores, but enhances resistance to a phloem feeder. Plant J. 2009, 60, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.; Qi, M.; Sheng, G.; Yang, Y. Inducible overexpression of a rice allene oxide synthase gene increases the endogenous jasmonic acid level, PR gene expression, and host resistance to fungal infection. Mol. Plant-Microbe Interact. 2006, 19, 1127–1137. [Google Scholar] [CrossRef]
- Qi, J.; Zhou, G.; Yang, L.; Erb, M.; Lu, Y.; Sun, X.; Cheng, J.; Lou, Y. The Chloroplast-Localized Phospholipases D α4 and α5 Regulate Herbivore-Induced Direct and Indirect Defenses in Rice. Plant Physiol. 2011, 157, 1987–1999. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ju, H.; Zhou, G.; Zhu, C.; Erb, M.; Wang, X.; Wang, P.; Lou, Y. An EAR-motif-containing ERF transcription factor affects herbivore-induced signaling, defense and resistance in rice. Plant J. 2011, 68, 583–596. [Google Scholar] [CrossRef]
- Hu, L.; Ye, M.; Li, R.; Lou, Y. OsWRKY53, a versatile switch in regulating herbivore-induced defense responses in rice. Plant Signal. Behav. 2016, 11, e1169357. [Google Scholar] [CrossRef] [PubMed]
- Van Poecke, R.M.; Posthumus, M.A.; Dicke, M. Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: Chemical, behavioral, and gene-expression analysis. J. Chem. Ecol. 2001, 27, 1911–1928. [Google Scholar] [CrossRef]
- Laudert, D.; Weiler, E.W. Allene oxide synthase: A major control point in Arabidopsis thaliana octadecanoid signalling. Plant J. 1998, 15, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, J.; Keinänen, M.; Baldwin, I.T. Herbivore-induced allene oxide synthase transcripts and jasmonic acid in Nicotiana attenuata. Phytochemistry 2001, 58, 729–738. [Google Scholar] [CrossRef]
- Lee, A.; Cho, K.; Jang, S.; Rakwal, R.; Iwahashi, H.; Agrawal, G.K.; Shim, J.; Han, O. Inverse correlation between jasmonic acid and salicylic acid during early wound response in rice. Biochem. Biophys. Res. Commun. 2004, 318, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ye, M.; Kuai, P.; Ye, M.; Erb, M.; Lou, Y. OsLRR-RLK1, an early responsive leucine-rich repeat receptor-like kinase, initiates rice defense responses against a chewing herbivore. New Phytol. 2018, 219, 1097–1111. [Google Scholar] [CrossRef]
- Du, B.; Zhang, W.; Liu, B.; Hu, J.; Wei, Z.; Shi, Z.; He, R.; Zhu, L.; Chen, R.; Han, B.; et al. Identification and characterization of Bph14, a gene conferring resistance to brown planthopper in rice. Proc. Natl. Acad. Sci. USA 2009, 106, 22163–22168. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice, 3rd ed.; International Rice Research Institute: Los Baños, PH, USA, 1976. [Google Scholar]
- Lu, J.; Robert, C.A.M.; Riemann, M.; Cosme, M.; Mène-Saffrané, L.; Massana, J.; Stout, M.J.; Lou, Y.; Gershenzon, J.; Erb, M. Induced Jasmonate Signaling Leads to Contrasting Effects on Root Damage and Herbivore Performance. Plant Physiol. 2015, 167, 1100–1116. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Baldwin, I.T. Silencing of a Germin-Like Gene in Nicotiana attenuata Improves Performance of Native Herbivores. Plant Physiol. 2006, 140, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- van Dam, N.M.; Horn, M.; Mares, M.; Baldwin, I.T. Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata. J. Chem. Ecol. 2001, 27, 547–568. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Du, M.; Turlings, T.C.J.; Cheng, J.; Shan, W. Exogenous application of jasmonic acid induces volatile emissions in rice and enhances parasitism of Nilaparvata lugens eggs by the parasitoid Anagrus nilaparvatae. J. Chem. Ecol. 2005, 31, 1985–2002. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, J.; Zhang, T.; Huangfu, J.; Li, R.; Lou, Y. Both Allene Oxide Synthases Genes Are Involved in the Biosynthesis of Herbivore-Induced Jasmonic Acid and Herbivore Resistance in Rice. Plants 2021, 10, 442. https://doi.org/10.3390/plants10030442
Zeng J, Zhang T, Huangfu J, Li R, Lou Y. Both Allene Oxide Synthases Genes Are Involved in the Biosynthesis of Herbivore-Induced Jasmonic Acid and Herbivore Resistance in Rice. Plants. 2021; 10(3):442. https://doi.org/10.3390/plants10030442
Chicago/Turabian StyleZeng, Jiamei, Tongfang Zhang, Jiayi Huangfu, Ran Li, and Yonggen Lou. 2021. "Both Allene Oxide Synthases Genes Are Involved in the Biosynthesis of Herbivore-Induced Jasmonic Acid and Herbivore Resistance in Rice" Plants 10, no. 3: 442. https://doi.org/10.3390/plants10030442
APA StyleZeng, J., Zhang, T., Huangfu, J., Li, R., & Lou, Y. (2021). Both Allene Oxide Synthases Genes Are Involved in the Biosynthesis of Herbivore-Induced Jasmonic Acid and Herbivore Resistance in Rice. Plants, 10(3), 442. https://doi.org/10.3390/plants10030442






