Plant Nitric Oxide Signaling under Drought Stress
Abstract
1. Introduction
2. Nitric Oxide: Background
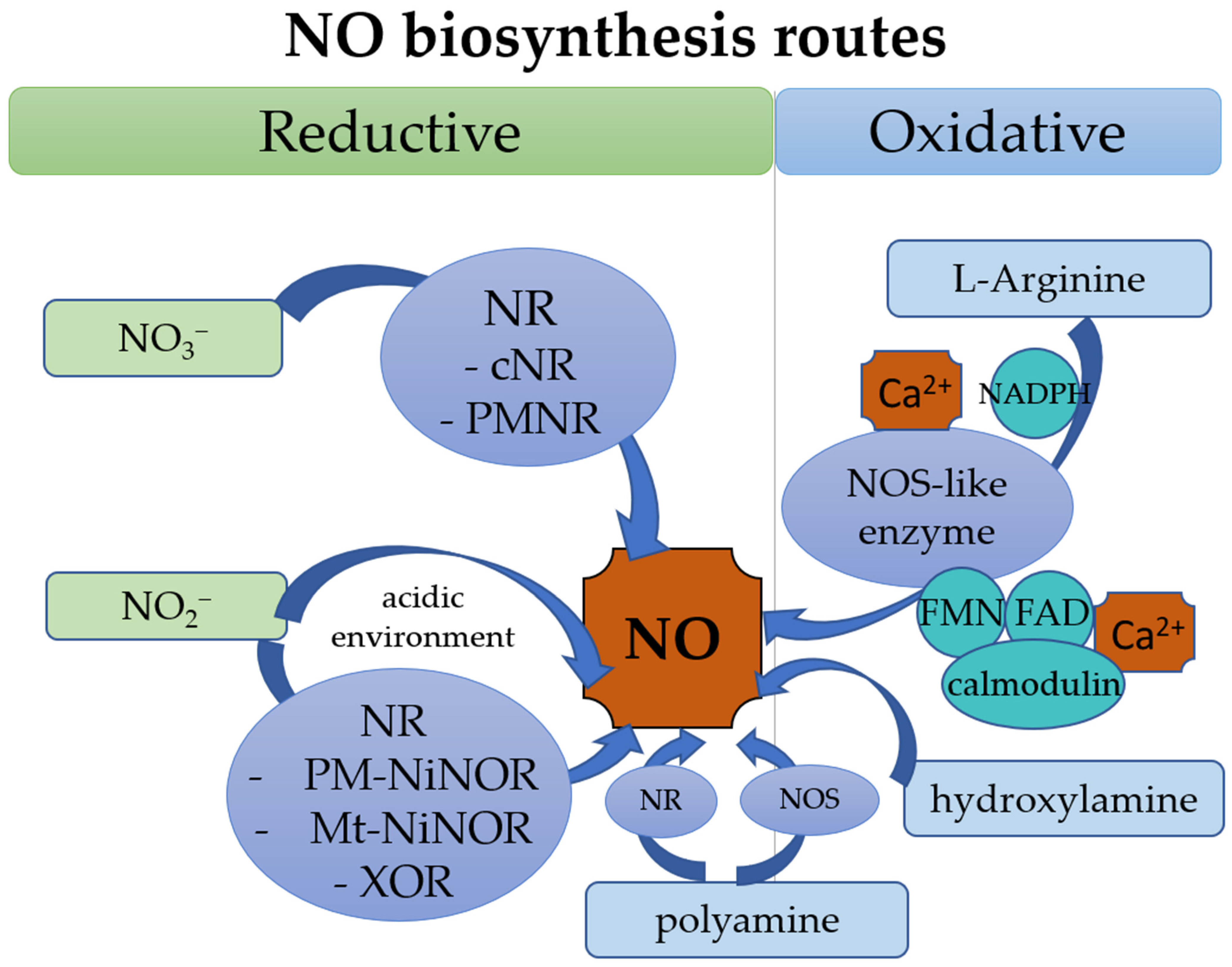
2.1. Source and Biosynthesis of NO in Plants
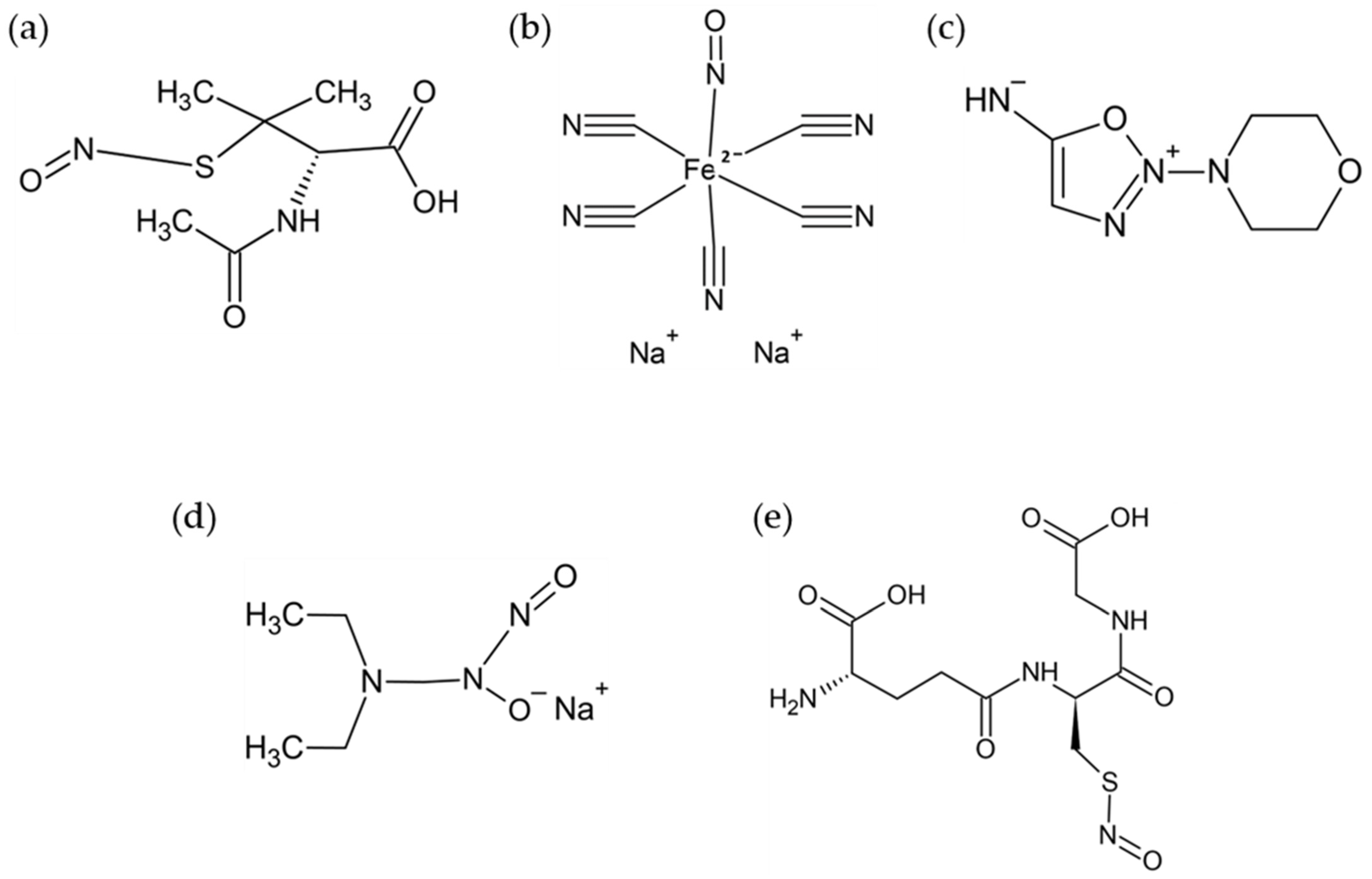
2.2. NO Donors
2.3. Functions of NO in Plants
3. NO and Drought Stress
3.1. Impacts of Drought on Plants
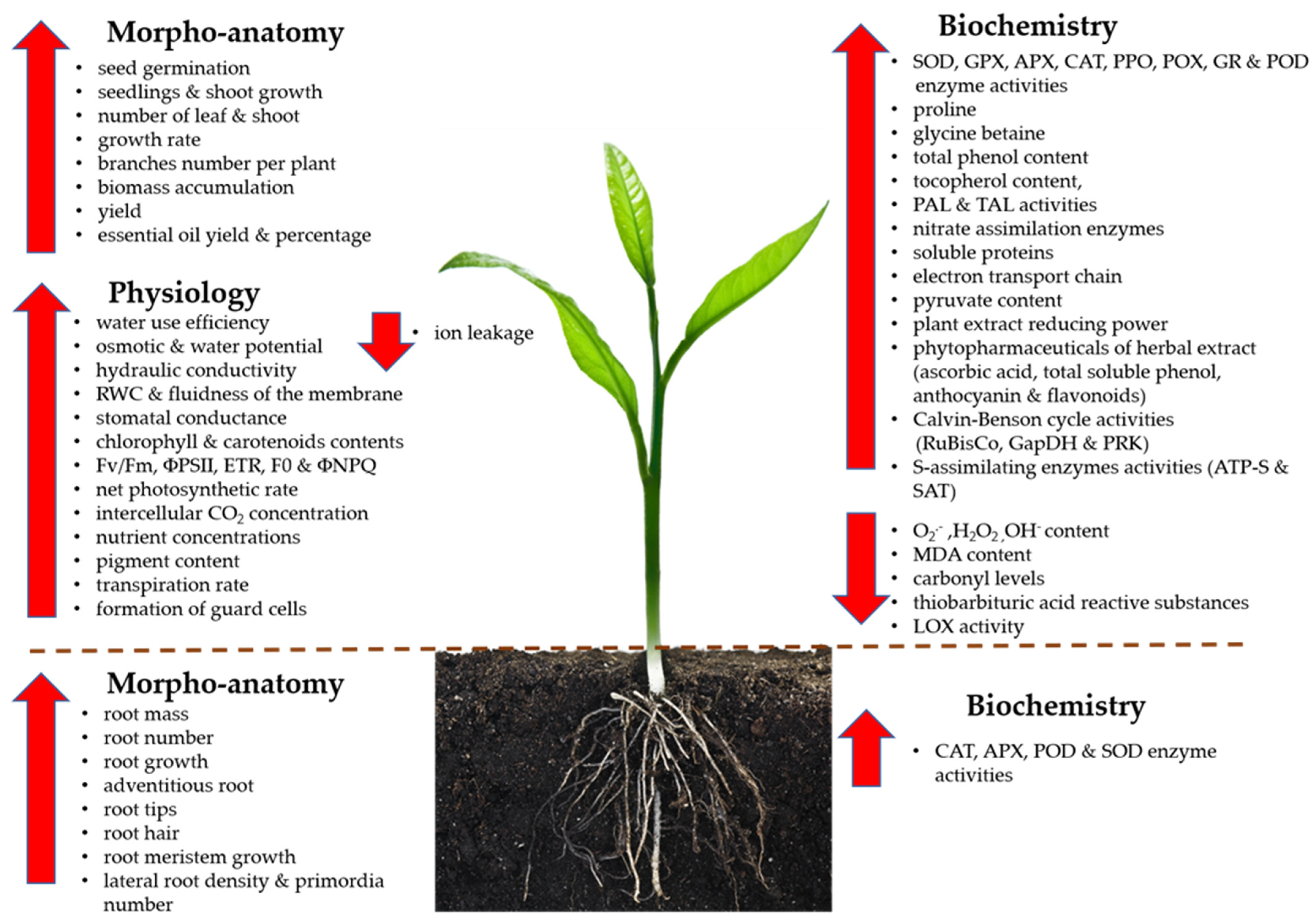
3.2. The Involvement of NO in Drought Tolerance
| Plant | Drought Imposition and Duration | Source of NO | Concentration (µmol/L) | Application Method | Response under Water Deficit Condition | Reference |
|---|---|---|---|---|---|---|
| Alfalfa (Medicago sativa L.) | 10% of PEG for 7 d | SNP | 100 | Seeds were germinated on filter papers containing treatment solutions (SNP and PEG) | Increment of the fresh weight, LRWC, chlorophyll content, proline content, soluble sugar contents and antioxidant enzyme activities (NR, SOD, POD, CAT, and APX), and reduction of root length, MDA level, differentially expressed genes involved in antioxidative defense system, photosynthesis, hormonal signal transduction, carbohydrate metabolism, and secondary metabolism | [46] |
| Withholding water for 6 d | NOSH or NOSH-A | 100 | Foliar spray | Improved acclimation to drought stress and improved the recovery after re-watering by reducing lipid peroxidation and proline accumulation levels | [21] | |
| Apple rootstocks (Malus spp.) | Withholding water for 7 d | SNP | 50, 100, 200, 300, and 400 | Foliar spray (5 times per d) | Protection of Malus seedlings from drought-induced oxidative damage by enhancing antioxidant enzyme activities and photosynthetic performance | [64] |
| Banana (Musa acuminata cv. Berangan) | 5% PEG for 9 d | SNP | 5 | SNP was supplied in liquid MS medium | Increment of the dry weight, number of roots formed, and antioxidant enzyme activities (SOD, CAT, APX, and GR); reduction of the percentage of yellow leaves | [65] |
| Broccoli (Brassica oleracea L.) | 60% field capacity for 21 d | SNP | 20 | Pre-sowing (seeds were soaked with SNP for 15 h) or foliar spray once | Enhancement of the fresh and dry biomass of shoot, shoot length, chlorophyll contents, GB, total phenolics, total soluble proteins, and activities of SOD and POD enzymes under water deficiency | [31] |
| Canola (Brassica napus L. Dunkeld and L. Cyclone) | 60% field capacity for 21 d | SNP | 20 | Foliar spray once | Upregulation of the oxidative defense system, osmoprotectant accumulation, and minimizing the lipid peroxidation. | [66] |
| Common bean (Phaseolus vulgaris L. cv. IAC Mileˆ- nio) | PEG (osmotic potential of - 0.3 MPa) for 12 and 17 d | GSNO | 50, 100, 500, 1000, and 2000 | Pre-sowing (seeds were soaked with GSNO for 1 min) | Improvement of seed germination and increment of initial root growth | [25] |
| Crambe (Crambe abyssinica) | 50% of the maximum water holding capacity for 32 and 136 h | SNP | 75, and 150 | Foliar spray (4 consecutive days at 24-h intervals) | Increment of water potential, osmotic potential, NR activity, photosynthetic rate, stomatal conductance, transpiration rate, photochemical efficiency of PSII (Fv/Fm), the effective quantum yield of photosystem II (ΦPSII), electron transport rate (ETR), initial fluorescence (F0), quantum yield of regulated energy dissipation (ΦNPQ), chlorophyll a and b content, antioxidant enzyme activities (SOD, CAT, APX, and GR) and reduction of MDA content, H2O2 level and O2 concentration in leaves | [67] |
| Cucumber (Cucumis sativus L.) | 0.05% of PEG for 6 d | SNP | 1, 10, 50, and 100 | Explants were placed on filter papers moistened with SNP | Increment of root number and length | [68] |
| European searocket (Cakile maritima Scop.) | Withholding water for 2, 7, and 14 d | SNP | 100 | Seedlings were pre-treated with Hoagland medium containing SNP for about 20 d | Improvement of growth activity, increment of chlorophyll and carotenoids contents, LRWC, proline content, P5CS protein accumulation, SOD and CAT enzyme activity, and reduction of osmotic potential, MDA content, and EL | [69] |
| Indian mustard (Brassica juncea cv. Varuna) | 10% of PEG for 4 d | SNP | 100 | Seedlings were treated with Hoagland medium containing SNP for 4 d | Increment of LRWC, chlorophyll content, net photosynthestic rate, internal CO2 concentration, stomatal conductance, transpiration rate, PSII efficiency, photochemical quenching, non-photochemical quenching, electron transport chain, RuBisCo, GAPDH, phosphoribulokinase, ATP-S, SAT activities, and genomic DNA template stability and reduction of thiobarbituric acid reactive substances, EL and OH− content | [56] |
| Indian mustard (Brassica juncea cv. Pusa Jagannath and cv. Varuna) | 10% of PEG for 4 d | SNP | 100 | Seedlings were treated with Hoagland medium containing SNP for 4 d | Brassica juncea cv. Pusa Jagannath had the antioxidant protection mainly through the accumulation of nonenzymatic antioxidants, whereas Brassica juncea cv. Varuna showed tolerance by the enhancement of both enzymatic and nonenzymatic antioxidant activities | [53] |
| Marjoram (Origanum majorana L. German type) | 70% depletion of available soil water for 95 d | SNP | 30 and 60 | Foliar spraying using a hand-atomizer on days 35, 50, 65, and 80 | Improved water use efficiency, increased plant biomass and essential oil yield and percentage, phytopharmaceuticals of herbal extract (ascorbic acid, total soluble phenol, anthocyanin, and flavonoid), antioxidant enzyme activities (CAT and POD), plant extract reducing power and reduction of H2O2, MDA, protein carbonyl group and percentage of EL | [70] |
| Milk thistle (Silybum marianum) | Withholding water for 14 d | SNP | 100 and 200 | Whole plants were sprayed with SNP 4 times using a wheeled plot sprayer | Increment of photosynthetic rate, chlorophyll a, b and carotenoid content, and seed yield | [71] |
| Perennial ryegrass (Lolium perenne L.) | Withholding water for 23 d | GSNO | 100 | Foliar spray every 2 d | Increment of total chlorophyll and carotenoids content, 1-SST activity associated with increased fructan content, GR activity, and S-nitrosothiols content and reduction of MDA content, H2O2 level, and ∙OH levels | [72] |
| Persian shallot (Allium hirtifolium) | 2, 4, 8 and 16 mmol/L of PEG for 16 weeks | SNP | 10, 40, and 70 | SNP was supplied in MS medium | Increment of regeneration rate, LRWC content, photosynthetic pigments, antioxidant enzyme activity (SOD and APX), proline and allicin accumulation, and reduction of H2O2, and MDA content in leaves | [73] |
| Physalis angulata | 80% and 20% field capacity for 20 d | SNP | 25, 50, 75, and 100 | Foliar spray with SNP twice per experiment | Low concentrations of SNP have shown to mitigate negative effects of water deficit, improving photosynthetic rates, maintenance of leaf water potential, and growth | [26] |
| Rapeseed (Brassica napus cv. BINA Sarisha 3) | 10% and 20% of PEG for 2 d | SNP | 500 | Seedlings were pre-treated with Hyponex solution containing SNP | Improvement of the levels of nonenzymatic antioxidant pool, glyoxalase system, and upregulation of antioxidant enzyme activities | [74] |
| Safflower (Carthamus tinctorius L.) | 20% of field capacity for 21 d | SNP | 25 | Whole plants were sprayed with SNP (1 d before and 7 d after drought stress treatments) | Improvement of the growth of aerial part, increment of chlorophyll content, and reduction of anthocyanin, flavonoid and phenol contents, and root length | [51] |
| Rice (Oryza sativa L. ‘Zhongzheyou No. 1’ hybrid indica) | 10% of PEG for 21 d | SNP | 20 | Seedlings were treated with SNP solution once | Increment of antioxidant activities (CAT, APX, and SOD), and reduction of O2•−, H2O2, OH−, and ONOO− content, MDA, and carbonyl levels in root | [11] |
| Soybean (Glycine max) | 20% water in the soil for 30 d | SNP | 100 | Foliar spray in 3 d intervals | Increment of photosynthesis and biomass accumulation | [37] |
| 5, 10, and 15% of PEG for 21 d | SNP | 100 | Whole plants were sprayed with SNP using an atomizer on alternate day | Increment of FW, SOD, CAT, POX, APX, PPO enzyme activities, phenylalanine ammonia-lyase, and tyrosine ammonia-lyase activities, total phenol, flavonol, and tocopherol content, and reduction of MDA content, aldehyde, H2O2 content, LOX activity, and EL | [55] | |
| 15% of PEG for 2 d | SNP | 50, 100, 200, 400, and 600 | SNP was supplied in Hoagland medium | Positively regulated the transcription of genes encoding Cyt-G6PD (GPD5, G6PD6, and G6PD7) | [75] | |
| Stevia rebaudiana Bertoni | 5%, 10% and 15% of PEG for 20, 40 and 60 d | SNP | 50, 100, 250, and 500 | SNP was supplied in MS medium | Increment of shoot length, shoot number, and leaf number | [52] |
| Sugarcane (Saccharum spp.) | PEG treatment for 7 d (−0.75 MPa) | NO3−:NH4+ ratios 100:0 and 70:30 | 5 | NO3−:NH4 was supplied in nutrient solution; plantlets were cultured in the medium for 2 weeks before drought stress treatment | Increment of photosynthetic rate, stomatal conductance, root growth, and reduction of ROS accumulation in plants treated with more nitrate | [32] |
| PEG treatment for 5 d (-0.75 MPa) | GSNO | 10, 100, 500, and 1000 | Foliar spray twice per day | Increment of photochemical activity, LRWC, leaf, and root dry matter | [76] | |
| Thyme (Thymus serpyllum Serpolet and T. Vulgaris L.). | 80, 60 and 40% field capacity | SNP | 50, 100, 150, and 200 | Whole plants were sprayed with SNP 3 times per experiment (prior to flowering stage, at 50% flowering, and at full bloom) | Increment of proline accumulation and reduction of some antioxidant activities which increased drought tolerance | [77] |
| Tomato (Lycopersicon esculentum Mill.) | Withholding water for 7 d | SNP | 50 and 100 | Foliar spray on alternate day after 1 d of water holding | Improvement of drought tolerance by increasing SOD activity, reducing H2O2, and other physiological processes (increased leaf number, average number of flower clusters per plant, LRWC, and lycopene content) | [78] |
| Watermelon (Citrullus lanatus var. lanatus KAR 98) | 15% of PEG for 10 d | SNP | 100 | SNP was supplied in Hoagland medium; the medium was replaced every 3 d | Increment of root length, APX, GR antioxidant activities, and reduction of MDA content, OH−, osmotic potential, and EL | [79] |
| Wheat (Triticum aestivum L. cv. Pishgam) | Soil moisture at 75% and 50% for 6 weeks | SNP | 100 | Foliar spray | Increment of seedling length, SOD enzyme activity, total soluble proteins, net photosynthetic rate, and intercellular carbon dioxide concentration and reduction of H2O2, and MDA contents | [23] |
| Wheat (Triticum aestivum L. cv. Prodip) | 15% and 30% of PEG for 9 d | SNP | 0.5 | SNP was supplied in Hyponex solution | Enhancement of the antioxidant defense system (both nonenzymatic and enzymatic components) in drought-stressed seedlings, endogenous NO content, glyoxalase system, and reduction of methylglyoxal content, which restored the LRWC, and further increased the proline content | [80] |
| Wheat (Triticum aestivum cv. Jing 852) | 7.5% of PEG for 5 d | SNP | 5 | SNP was supplied in Hoagland medium | Increment of lateral and primary root length | [81] |
| Wheat (Triticum aestivum cv. BARS-2009 and Triticum aestivum cv. Mairaj-2008) | 35% of water holding capacity till physiological maturity | SNP | 50, 100, and 150 | Foliar spray (7 d before drought stress treatment) using a hand sprayer | Increment of yield, chlorophyll contents, accumulation of soluble phenolics, proline, and GB, and reduction of MDA contents | [82] |
| Wheat (Triticum aestivum cv. 98SN146 and Triticum aestivum cv. Longchun22) | PEG for 24 h | SNP | 100 | Seedlings were pre-sprayed with SNP (12 h before drought stress treatment) | Increment of cyanide-resistant respiration, pyruvate content, Alternative oxidase gene (AOX1a), and alternative pathway | [83] |
| White clover (Trifolium repens) | PEG (-0.3 Mpa) for 8 d | SNP | 50 | SNP was supplied in Hoagland medium; plantlets were cultured in the medium for 3 d before drought stress treatment | Effectively mitigated water stress damage; changes of metabolic profiles, and associated metabolic pathways which could contribute to enhance stress tolerance | [84] |
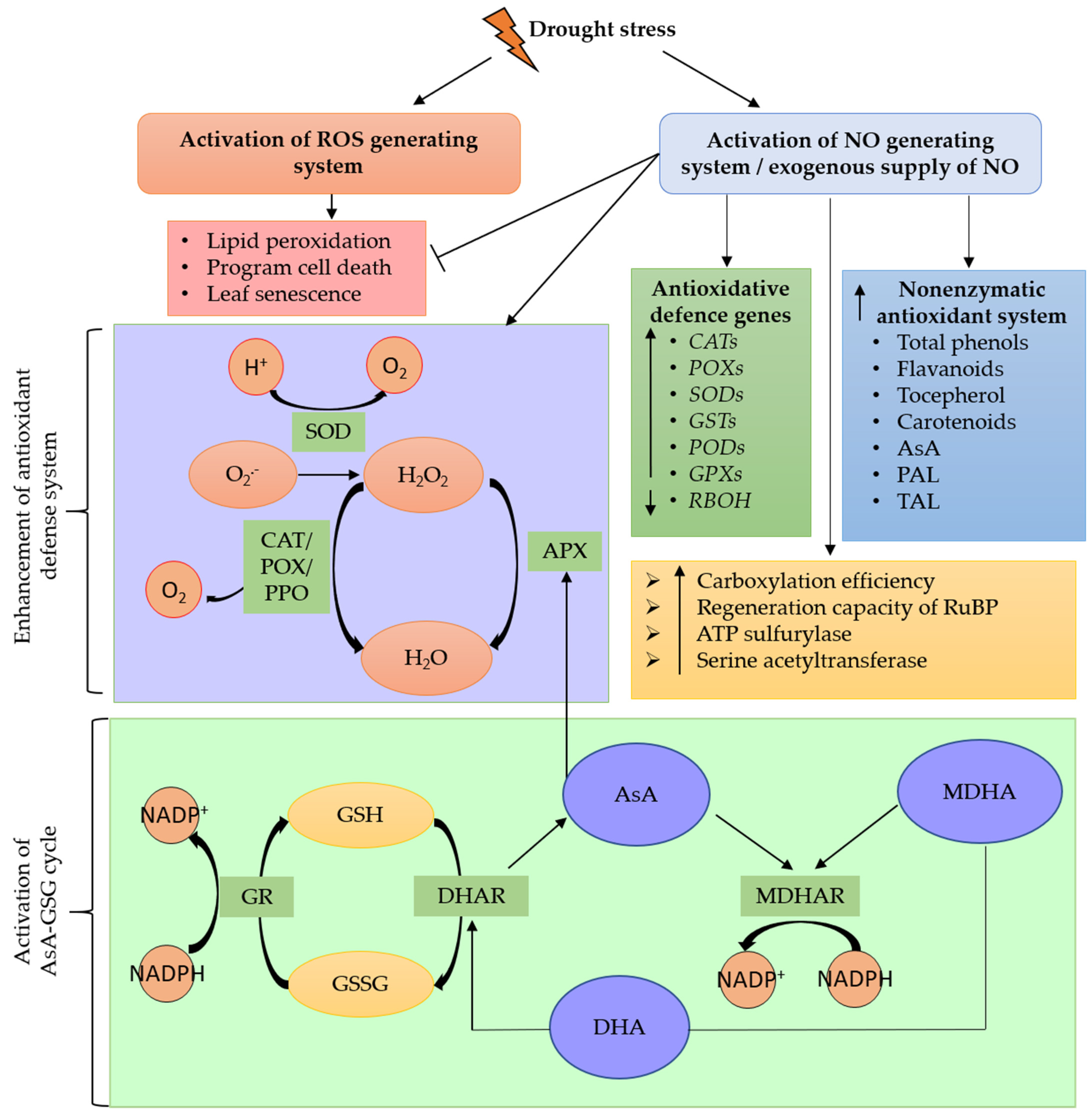
3.3. The Involvement of NO in the Antioxidant System
3.4. Synergistic Relationship of NO with Other Signaling Molecules
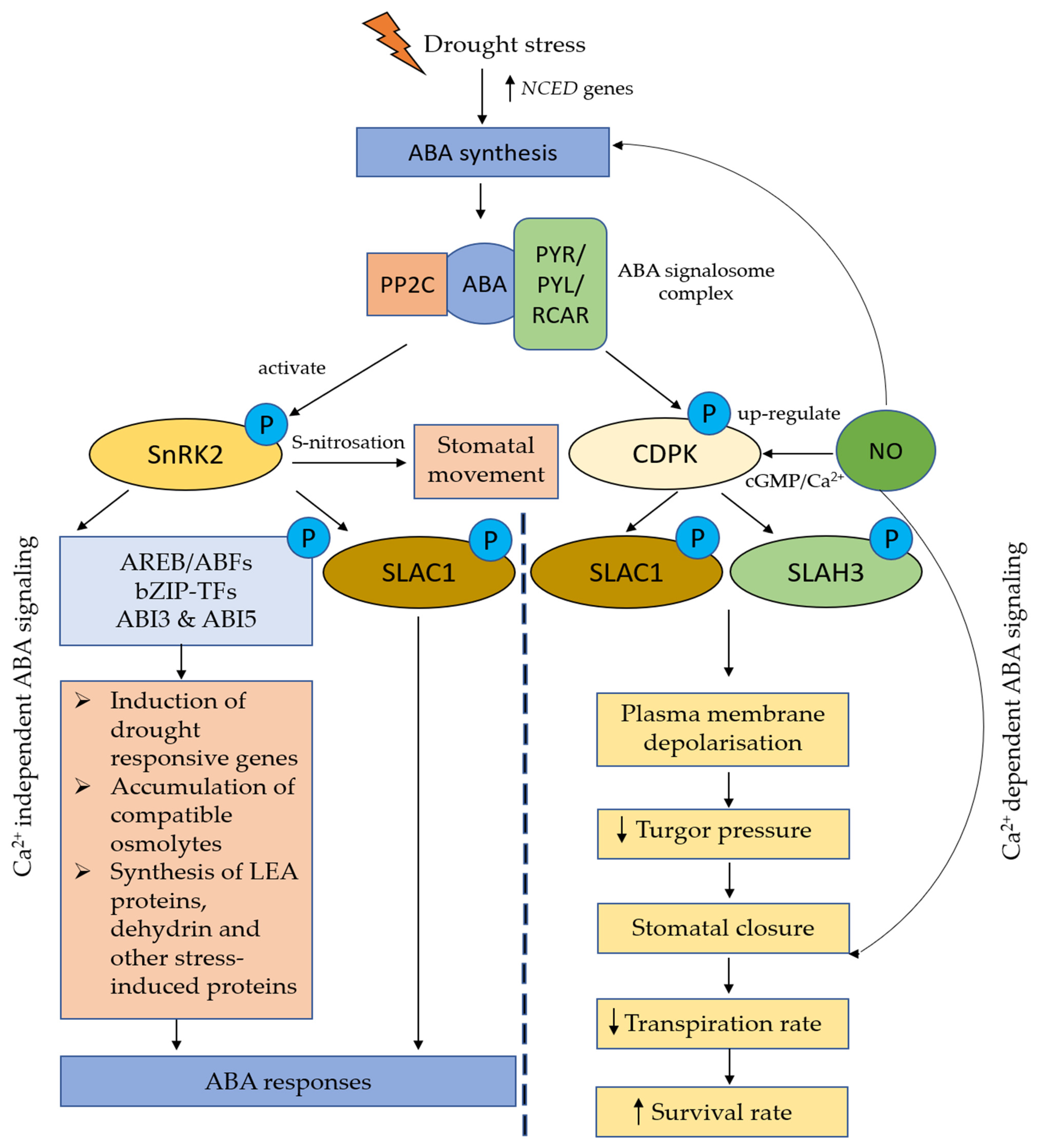
3.5. Crosstalk Between NO and Plant Hormones
4. NO-Responsive Proteins and Post-Translational Modifications
5. Detection of NO: Challenges
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waqas, M.A.; Kaya, C.; Riaz, A.; Farooq, M.; Nawaz, I.; Wilkes, A.; Li, Y. Potential mechanisms of abiotic stress tolerance in crop plants induced by thiourea. Front. Plant Sci. 2019, 10, 1336. [Google Scholar] [CrossRef]
- NOAA National Centers for Environmental Information: Billion-Dollar Weather and Climate Disasters: Overview. Available online: https://www.ncdc.noaa.gov/billions/ (accessed on 27 December 2020).
- FAO 2018. 2017: The Impact of Disasters and Crises on Agriculture and Food Security. Available online: http://www.fao.org/3/I8656EN/i8656en.pdf (accessed on 27 December 2020).
- Zhou, H.; Chen, Y.; Zhai, F.; Zhang, J.; Zhang, F.; Yuan, X.; Xie, Y. Hydrogen sulfide promotes rice drought tolerance via reestablishing redox homeostasis and activation of ABA biosynthesis and signaling. Plant Physiol. Biochem. 2020, 155, 213–220. [Google Scholar] [CrossRef]
- Cao, Y.; Luo, Q.; Tian, Y.; Meng, F. Physiological and proteomic analyses of the drought stress response in Amygdalus mira (Koehne) Yü et Lu roots. BMC Plant Biol. 2017, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol. Plant. 2020, 168, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Del Castello, F.; Nejamkin, A.; Cassia, R.; Correa-Aragunde, N.; Fernández, B.; Foresi, N.; Lombardo, C.; Ramirez, L.; Lamattina, L. The era of nitric oxide in plant biology: Twenty years tying up loose ends. Nitric Oxide Biol. Chem. 2019, 85, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Tropea, T.; Wareing, M.; Greenwood, S.L.; Feelisch, M.; Sibley, C.P.; Cottrell, E.C. Nitrite mediated vasorelaxation in human chorionic plate vessels is enhanced by hypoxia and dependent on the NO-sGC-cGMP pathway. Nitric Oxide Biol. Chem. 2018, 80, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Astier, J.; Gross, I.; Durner, J. Nitric oxide production in plants: An update. J. Exp. Bot. 2018, 69, 3401–3411. [Google Scholar] [CrossRef]
- León, J.; Costa-Broseta, Á. Present knowledge and controversies, deficiencies, and misconceptions on nitric oxide synthesis, sensing, and signaling in plants. Plant Cell Environ. 2020, 43, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhu, C.; Zhong, C.; Zhang, J.; Wu, L.; Jin, Q.; Ma, Q. Nitric oxide synthase-mediated early nitric oxide burst alleviates water stress-induced oxidative damage in ammonium-supplied rice roots. BMC Plant Biol. 2019, 19, 108. [Google Scholar] [CrossRef]
- Wang, B.L.; Tang, X.Y.; Cheng, L.Y.; Zhang, A.Z.; Zhang, W.H.; Zhang, F.S.; Liu, J.Q.; Cao, Y.; Allan, D.L.; Vance, C.P.; et al. Nitric oxide is involved in phosphorus deficiency-induced cluster-root development and citrate exudation in white lupin. New Phytol. 2010, 187, 1112–1123. [Google Scholar] [CrossRef]
- Gupta, K.J.; Igamberdiev, A.U.; Manjunatha, G.; Segu, S.; Moran, J.F.; Neelawarne, B.; Bauwe, H.; Kaiser, W.M. The emerging roles of nitric oxide (NO) in plant mitochondria. Plant Sci. 2011, 181, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Bethke, P.C.; Badger, M.R.; Jones, R.L. Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell 2004, 16, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Barroso, J.B. Peroxisomal plant nitric oxide synthase (NOS) protein is imported by peroxisomal targeting signal type 2 (PTS2) in a process that depends on the cytosolic receptor PEX7 and calmodulin. FEBS Lett. 2014, 588, 2049–2054. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Wang, X.; Li, Z.; Zhang, Y.; Peng, Y.; Li, Y.; He, X.; Zhang, X.; Ma, X.; Huang, L.; et al. NO is involved in spermidine-induced drought tolerance in white clover via activation of antioxidant enzymes and genes. Protoplasma 2016, 253, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Rümer, S.; Gupta, K.J.; Kaiser, W.M. Plant cells oxidize hydroxylamines to NO. J. Exp. Bot. 2009, 60, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.C.; Chin, C.F.; Alderson, P. Effects of sodium nitroprusside on shoot multiplication and regeneration of Vanilla planifolia Andrews. Vitr. Cell. Dev. Biol. Plant 2013, 49, 626–630. [Google Scholar] [CrossRef]
- Floryszak-Wieczorek, J.; Milczarek, G.; Arasimowicz, M.; Ciszewski, A. Do nitric oxide donors mimic endogenous NO-related response in plants? Planta 2006, 224, 1363–1372. [Google Scholar] [CrossRef]
- Salahuddin, M.; Nawaz, F.; Shahbaz, M.; Naeem, M.; Zulfiqar, B.; Shabbir, R.N.; Hussain, R.A. Effect of exogenous nitric oxide (NO) supply on germination and seedling growth of mungbean (cv. Nm-54) under salinity stress. Legum. Res. 2017, 40, 846–852. [Google Scholar] [CrossRef]
- Antoniou, C.; Xenofontos, R.; Chatzimichail, G.; Christou, A.; Kashfi, K.; Fotopoulos, V. Exploring the potential of nitric oxide and hydrogen sulfide (NOSH)-releasing synthetic compounds as novel priming agents against drought stress in Medicago sativa plants. Biomolecules 2020, 10, 120. [Google Scholar] [CrossRef]
- Silveira, N.M.; Seabra, A.B.; Marcos, F.C.C.; Pelegrino, M.T.; Machado, E.C.; Ribeiro, R.V. Encapsulation of S-nitrosoglutathione into chitosan nanoparticles improves drought tolerance of sugarcane plants. Nitric Oxide Biol. Chem. 2019, 84, 38–44. [Google Scholar] [CrossRef]
- Faraji, J.; Sepehri, A. Exogenous Nitric oxide improves the protective effects of TiO2 nanoparticles on growth, antioxidant system, and photosynthetic performance of wheat seedlings under drought stress. J. Soil Sci. Plant Nutr. 2020, 20, 703–714. [Google Scholar] [CrossRef]
- Habib, N.; Ali, Q.; Ali, S.; Javed, M.T.; Zulqurnain Haider, M.; Perveen, R.; Shahid, M.R.; Rizwan, M.; Abdel-Daim, M.M.; Elkelish, A.; et al. Use of nitric oxide and hydrogen peroxide for better yield of wheat (Triticum aestivum L.) under water deficit conditions: Growth, osmoregulation, and antioxidative defense mechanism. Plants 2020, 9, 285. [Google Scholar] [CrossRef]
- Silveira, N.M.; Ribeiro, R.V.; Prataviera, P.J.C.; Pissolato, M.D.; Pieretti, J.C.; Seabra, A.B.; Machado, E.C. Germination and initial growth of common bean plants under water deficit as affected by seed treatment with S-nitrosoglutathione and calcium chloride. Theor. Exp. Plant Physiol. 2020, 32, 49–62. [Google Scholar] [CrossRef]
- da Silva Leite, R.; do Nascimento, M.N.; Tanan, T.T.; Gonçalves Neto, L.P.; da Silva Ramos, C.A.; da Silva, A.L. Alleviation of water deficit in Physalis angulata plants by nitric oxide exogenous donor. Agric. Water Manag. 2019, 216, 98–104. [Google Scholar] [CrossRef]
- Tian, X.; Lei, Y. Nitric oxide treatment alleviates drought stress in wheat seedlings. Biol. Plant. 2006, 50, 775–778. [Google Scholar] [CrossRef]
- Yamasaki, H. Nitrite-dependent nitric oxide production pathway: Implications for involvement of active nitrogen spicies in photoinhibition in vivo. Philos. Trans. R. Soc. B Biol. Sci. 2000, 355, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Clyde Hill, A.; Bennett, J.H. Inhibition of apparent photosynthesis by nitrogen oxides. Atmos. Environ. 1970, 4, 341–348. [Google Scholar] [CrossRef]
- Zottini, M.; Formentin, E.; Scattolin, M.; Carimi, F.; Lo Schiavo, F.; Terzi, M. Nitric oxide affects plant mitochondrial functionality in vivo. FEBS Lett. 2002, 515, 75–78. [Google Scholar] [CrossRef]
- Munawar, A.; Akram, N.A.; Ahmad, A.; Ashraf, M. Nitric oxide regulates oxidative defense system, key metabolites and growth of broccoli (Brassica oleracea L.) plants under water limited conditions. Sci. Hortic. 2019, 254, 7–13. [Google Scholar] [CrossRef]
- Pissolato, M.D.; Silveira, N.M.; Prataviera, P.J.C.; Machado, E.C.; Seabra, A.B.; Pelegrino, M.T.; Sodek, L.; Ribeiro, R.V. Enhanced nitric oxide synthesis through nitrate supply improves drought tolerance of sugarcane plants. Front. Plant Sci. 2020, 11, 970. [Google Scholar] [CrossRef]
- Senthil Kumar, R.; Shen, C.H.; Wu, P.Y.; Suresh Kumar, S.; Hua, M.S.; Yeh, K.W. Nitric oxide participates in plant flowering repression by ascorbate. Sci. Rep. 2016, 6, 35246. [Google Scholar] [CrossRef]
- Zuccarelli, R.; Rodríguez-Ruiz, M.; Lopes-Oliveira, P.J.; Pascoal, G.B.; Andrade, S.C.S.; Furlan, C.M.; Purgatto, E.; Palma, J.M.; Corpas, F.J.; Rossi, M.; et al. Multifaceted roles of nitric oxide in tomato fruit ripening: NO-induced metabolic rewiring and consequences for fruit quality traits. J. Exp. Bot. 2020, in press. [Google Scholar] [CrossRef]
- Majeed, S.; Nawaz, F.; Naeem, M.; Ashraf, M.Y.; Ejaz, S.; Ahmad, K.S.; Tauseef, S.; Farid, G.; Khalid, I.; Mehmood, K. Nitric oxide regulates water status and associated enzymatic pathways to inhibit nutrients imbalance in maize (Zea mays L.) under drought stress. Plant Physiol. Biochem. 2020, 155, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, S.; Zhao, B.; Li, C. Overexpression of TaLEA3 induces rapid stomatal closure under drought stress in Phellodendron amurense Rupr. Plant Sci. 2018, 277, 100–109. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, L.F.; de Menezes-Silva, P.E.; Lourenço, L.L.; Galmés, J.; Guimarães, A.C.; da Silva, A.F.; dos Reis Lima, A.P.; Henning, L.M.M.; Costa, A.C.; Silva, F.G.; et al. Improving water use efficiency by changing hydraulic and stomatal characteristics in soybean exposed to drought: The involvement of nitric oxide. Physiol. Plant. 2020, 168, 576–589. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, J.; Xing, D. Low concentrations of salicylic acid delay methyl jasmonate-induced leaf senescence by up-regulating nitric oxide synthase activity. J. Exp. Bot. 2016, 67, 5233–5245. [Google Scholar] [CrossRef] [PubMed]
- Ozfidan-Konakci, C.; Yildiztugay, E.; Elbasan, F.; Kucukoduk, M.; Turkan, I. Hydrogen sulfide (H2S) and nitric oxide (NO) alleviate cobalt toxicity in wheat (Triticum aestivum L.) by modulating photosynthesis, chloroplastic redox and antioxidant capacity. J. Hazard. Mater. 2020, 388. [Google Scholar] [CrossRef] [PubMed]
- París, R.; Vazquez, M.M.; Graziano, M.; Terrile, M.C.; Miller, N.D.; Spalding, E.P.; Otegui, M.S.; Casalongué, C.A. Distribution of endogenous NO regulates early gravitropic response and PIN2 localization in Arabidopsis roots. Front. Plant Sci. 2018, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, T.; Zhang, C.; Hao, H.; Liu, P.; Zheng, M.; BaluÅka, F.; Åamaj, J.; Lin, J. Nitric oxide modulates the influx of extracellular Ca2+ and actin filament organization during cell wall construction in Pinus bungeana pollen tubes. New Phytol. 2009, 182, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Nasir, N.N.M.; Ho, C.L.; Lamasudin, D.U.; Saidi, N.B. Nitric oxide improves tolerance to Fusarium oxysporum f. sp. cubense Tropical Race 4 in banana. Physiol. Mol. Plant Pathol. 2020, 111, 4–9. [Google Scholar] [CrossRef]
- Verma, N.; Tiwari, S.; Singh, V.P.; Prasad, S.M. Nitric oxide in plants: An ancient molecule with new tasks. Plant Growth Regul. 2020, 90. [Google Scholar] [CrossRef]
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.G.; Yun, B.W. Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ. Exp. Bot. 2019, 161, 120–133. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wei, X.; Long, Y.; Ji, X. Transcriptional analysis reveals sodium nitroprusside affects alfalfa in response to PEG-induced osmotic stress at germination stage. Protoplasma 2020, 257, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lu, L.; Liu, L.; Liu, W.; Yu, Y.; Liu, X.; Hu, Y.; Jin, C.; Lin, X. Nitrate reductase-mediated early nitric oxide burst alleviates oxidative damage induced by aluminum through enhancement of antioxidant defenses in roots of wheat (Triticum aestivum). New Phytol. 2014, 201, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Mobin, M.; Mohammad, F.; Corpas, F.J. Nitric oxide and high temperature stress: A physiological perspective. In Nitric Oxide Action in Abiotic Stress Responses in Plants, 1st ed.; Khan, M.N., Mobin, M., Mohammad, F., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 77–93. [Google Scholar]
- Ahmad, P.; Rasool, S.; Gul, A.; Sheikh, S.A.; Akram, N.A.; Ashraf, M.; Kazi, A.M.; Gucel, S. Jasmonates: Multifunctional roles in stress tolerance. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Afrin, S.; Khan, M.K.; Hannan, M.A.; Skalicky, M.; Mortuza, M.G.; Brestic, M.; Hossain, M.A.; Murata, Y. Insights into nitric oxide-mediated water balance, antioxidant defence and mineral homeostasis in rice (Oryza sativa L.) under chilling stress. Nitric Oxide Biol. Chem. 2020, 100–101, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Chavoushi, M.; Najafi, F.; Salimi, A.; Angaji, S.A. Effect of salicylic acid and sodium nitroprusside on growth parameters, photosynthetic pigments and secondary metabolites of safflower under drought stress. Sci. Hortic. 2020, 259. [Google Scholar] [CrossRef]
- Pradhan, N.; Singh, P.; Dwivedi, P.; Pandey, D.K. Evaluation of sodium nitroprusside and putrescine on polyethylene glycol induced drought stress in Stevia rebaudiana Bertoni under in vitro condition. Ind. Crops Prod. 2020, 154, 112754. [Google Scholar] [CrossRef]
- Sahay, S.; Khan, E.; Gupta, M. Nitric oxide and abscisic acid protects against PEG-induced drought stress differentially in Brassica genotypes by combining the role of stress modulators, markers and antioxidants. Nitric Oxide Biol. Chem. 2019, 89, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Srivastava, D.; Tiwari, P.; Chakrabarty, D. ROS modulation in crop plants under drought stress. React. Oxyg. Nitrogen Sulfur Species Plants 2019, 311–336. [Google Scholar] [CrossRef]
- Rezayian, M.; Ebrahimzadeh, H.; Niknam, V. Nitric oxide stimulates antioxidant system and osmotic adjustment in soybean under drought stress. J. Soil Sci. Plant Nutr. 2020, 20, 1122–1132. [Google Scholar] [CrossRef]
- Sahay, S.; De La Cruz Torres, E.; Robledo-Arratia, L.; Gupta, M. Photosynthetic activity and RAPD profile of polyethylene glycol treated B. juncea L. under nitric oxide and abscisic acid application. J. Biotechnol. 2020, 313, 29–38. [Google Scholar] [CrossRef]
- Du, H.; Huang, F.; Wu, N.; Li, X.; Hu, H.; Xiong, L. Integrative regulation of drought escape through aba-dependent and -independent pathways in rice. Mol. Plant 2018, 11, 584–597. [Google Scholar] [CrossRef]
- Li, L.; Wei, S.; Shen, W. The role of methane in plant physiology: A review. Plant Cell Rep. 2020, 39, 171–179. [Google Scholar] [CrossRef]
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 2014, 21, 133–139. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Yi, J.; Yang, Y.; Lei, C.; Gong, M. Transcriptome response to drought, rehydration and re-dehydration in potato. Int. J. Mol. Sci. 2020, 21, 159. [Google Scholar] [CrossRef] [PubMed]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J.; Kubiś, J. Involvement of nitric oxide in water stress-induced responses of cucumber roots. Plant Sci. 2009, 177, 682–690. [Google Scholar] [CrossRef]
- García-Mata, C.; Lamattina, L. Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol. 2010, 188, 977–984. [Google Scholar] [CrossRef]
- Fu, Z.W.; Wang, Y.L.; Lu, Y.T.; Yuan, T.T. Nitric oxide is involved in stomatal development by modulating the expression of stomatal regulator genes in Arabidopsis. Plant Sci. 2016, 252, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Li, X.; Wei, Z.; Han, M.; Zhang, L.; Li, B. Exogenous nitric oxide protects against drought-induced oxidative stress in Malus rootstocks. Turk. J. Bot. 2016, 40, 17–27. [Google Scholar] [CrossRef]
- Amnan, M.A.M.; Pua, T.L.; Lau, S.E.; Tan, B.C.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Komatsu, S. Osmotic stress in banana is relieved by exogenous nitric oxide. PeerJ 2021, 9, e10879. [Google Scholar] [CrossRef]
- Akram, N.A.; Iqbal, M.; Muhammad, A.; Ashraf, M.; Al-Qurainy, F.; Shafiq, S. Aminolevulinic acid and nitric oxide regulate oxidative defense and secondary metabolisms in canola (Brassica napus L.) under drought stress. Protoplasma 2018, 255, 163–174. [Google Scholar] [CrossRef]
- Batista, P.F.; Costa, A.C.; Müller, C.; de Oliveira Silva-Filho, R.; da Silva, F.B.; Merchant, A.; Mendes, G.C.; Nascimento, K.J.T. Nitric oxide mitigates the effect of water deficit in Crambe abyssinica. Plant Physiol. Biochem. 2018, 129, 310–322. [Google Scholar] [CrossRef]
- Niu, L.; Yu, J.; Liao, W.; Yu, J.; Zhang, M.; Dawuda, M.M. Calcium and calmodulin are involved in nitric oxide-induced adventitious rooting of cucumber under simulated osmotic stress. Front. Plant Sci. 2017, 8, 1684. [Google Scholar] [CrossRef]
- Jday, A.; Ben Rejeb, K.; Slama, I.; Saadallah, K.; Bordenave, M.; Planchais, S.; Savouré, A.; Abdelly, C. Effects of exogenous nitric oxide on growth, proline accumulation and antioxidant capacity in Cakile maritima seedlings subjected to water deficit stress. Funct. Plant Biol. 2016, 43, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Farouk, S.; Al-Huqail, A.A. Sodium nitroprusside application regulates antioxidant capacity, improves phytopharmaceutical production and essential oil yield of marjoram herb under drought. Ind. Crops Prod. 2020, 158, 113034. [Google Scholar] [CrossRef]
- Zangani, E.; Zehtab-Salmasi, S.; Andalibi, B.; Zamani, A.A. Protective effects of nitric oxide on photosynthetic stability and performance of Silybum marianum under water deficit conditions. Agron. J. 2018, 110, 555–564. [Google Scholar] [CrossRef]
- Rigui, A.P.; Carvalho, V.; Wendt dos Santos, A.L.; Morvan-Bertrand, A.; Prud’homme, M.P.; Machado de Carvalho, M.A.; Gaspar, M. Fructan and antioxidant metabolisms in plants of Lolium perenne under drought are modulated by exogenous nitric oxide. Plant Physiol. Biochem. 2019, 145, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi-Golezani, K.; Farhadi, N.; Nikpour-Rashidabad, N. Responses of in vitro-cultured Allium hirtifolium to exogenous sodium nitroprusside under PEG-imposed drought stress. Plant Cell. Tissue Organ Cult. 2018, 133, 237–248. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Hossain, M.S.; Anee, T.I.; Parvin, K.; Fujita, M. Nitric oxide pretreatment enhances antioxidant defense and glyoxalase systems to confer peg-induced oxidative stress in rapeseed. J. Plant Interact. 2017, 12, 323–331. [Google Scholar] [CrossRef]
- Wang, X.; Ruan, M.; Wan, Q.; He, W.; Yang, L.; Liu, X.; He, L.; Yan, L.; Bi, Y. Nitric oxide and hydrogen peroxide increase glucose-6-phosphate dehydrogenase activities and expression upon drought stress in soybean roots. Plant Cell Rep. 2020, 39, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Silveira, N.M.; Frungillo, L.; Marcos, F.C.C.; Pelegrino, M.T.; Miranda, M.T.; Seabra, A.B.; Salgado, I.; Machado, E.C.; Ribeiro, R.V. Exogenous nitric oxide improves sugarcane growth and photosynthesis under water deficit. Planta 2016, 244, 181–190. [Google Scholar] [CrossRef]
- Mohasseli, V.; Sadeghi, S. Exogenously applied sodium nitroprusside improves physiological attributes and essential oil yield of two drought susceptible and resistant specie of Thymus under reduced irrigation. Ind. Crops Prod. 2019, 130, 130–136. [Google Scholar] [CrossRef]
- Jangid, K.K.; Dwivedi, P. Physiological and biochemical changes by nitric oxide and brassinosteroid in tomato (Lycopersicon esculentum Mill.) under drought stress. Acta Physiol. Plant. 2017, 39. [Google Scholar] [CrossRef]
- Hamurcu, M.; Khan, M.K.; Pandey, A.; Ozdemir, C.; Avsaroglu, Z.Z.; Elbasan, F.; Omay, A.H.; Gezgin, S. Nitric oxide regulates watermelon (Citrullus lanatus) responses to drought stress. 3 Biotech 2020, 10, 494. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Inafuku, M.; Oku, H.; Fujita, M. Exogenous nitric oxide donor and arginine provide protection against short-term drought stress in wheat seedlings. Physiol. Mol. Biol. Plants 2018, 24, 993–1004. [Google Scholar] [CrossRef]
- Wu, S.; Sun, X.; Tan, Q.; Hu, C. Molybdenum improves water uptake via extensive root morphology, aquaporin expressions and increased ionic concentrations in wheat under drought stress. Environ. Exp. Bot. 2019, 157, 241–249. [Google Scholar] [CrossRef]
- Farooq, M.; Nawaz, A.; Chaudhary, M.A.M.; Rehman, A. Foliage-applied sodium nitroprusside and hydrogen peroxide improves resistance against terminal drought in bread wheat. J. Agron. Crop Sci. 2017, 203, 473–482. [Google Scholar] [CrossRef]
- Wang, H.; Huang, J.; Li, Y.; Li, C.; Hou, J.; Liang, W. Involvement of nitric oxide-mediated alternative pathway in tolerance of wheat to drought stress by optimizing photosynthesis. Plant Cell Rep. 2016, 35, 2033–2044. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yong, B.; Cheng, B.; Wu, X.; Zhang, Y.; Zhang, X.; Peng, Y. Nitric oxide, γ-aminobutyric acid, and mannose pretreatment influence metabolic profiles in white clover under water stress. J. Integr. Plant Biol. 2019, 61, 1255–1273. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ye, T.; Zhu, J.K.; Chan, Z. Constitutive production of nitric oxide leads to enhanced drought stress resistance and extensive transcriptional reprogramming in Arabidopsis. J. Exp. Bot. 2014, 65, 4119–4131. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Gaur, R.; Gupta, M. Comparative biochemical and RAPD analysis in two varieties of rice (Oryza sativa) under arsenic stress by using various biomarkers. J. Hazard. Mater. 2012, 217–218, 141–148. [Google Scholar] [CrossRef]
- Pandey, C.; Gupta, M. Selenium and auxin mitigates arsenic stress in rice (Oryza sativa L.) by combining the role of stress indicators, modulators and genotoxicity assay. J. Hazard. Mater. 2015, 287, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Wu, X.; Zhong, Y. Exogenously applied nitric oxide enhances the drought tolerance in hulless barley. Plant Prod. Sci. 2015, 18, 52–56. [Google Scholar] [CrossRef]
- Cai, W.; Liu, W.; Wang, W.S.; Fu, Z.W.; Han, T.T.; Lu, Y.T. Overexpression of rat neurons nitric oxide synthase in rice enhances drought and salt tolerance. PLoS ONE 2015, 10, e0131599. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Li, X.; Liu, X.; Chen, P.; Ren, C.; Dai, C. Enhanced drought tolerance in transgenic rice over-expressing of maize C4 phosphoenolpyruvate carboxylase gene via NO and Ca2+. J. Plant Physiol. 2015, 175, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zheng, Y.; Liu, J.; Zhang, H.; Chen, H. Heme oxygenase-1 delays gibberellin-induced programmed cell death of rice aleurone layers subjected to drought stress by interacting with nitric oxide. Front. Plant Sci. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Rodríguez-Serrano, M.; Bárány, I.; Prem, D.; Coronado, M.J.; Risueño, M.C.; Testillano, P.S. NO, ROS, and cell death associated with caspase-like activity increase in stress-induced microspore embryogenesis of barley. J. Exp. Bot. 2012, 63, 2007–2024. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ren, R.; Di, W.; Jia, M.; Li, Z.; Liu, Y.; Gao, R. Hydrogen peroxide and nitric oxide are involved in programmed cell death induced by cryopreservation in Dendrobium protocorm-like bodies. Plant Cell Tissue Organ Cult. 2019, 137, 553–563. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ros metabolism in plants under environmental stress: A review of recent experimental evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- Huang, D.; Huo, J.; Liao, W. Hydrogen sulfide: Roles in plant abiotic stress response and crosstalk with other signals. Plant Sci. 2021, 302, 110733. [Google Scholar] [CrossRef]
- Aroca, A.; Benito, J.M.; Gotor, C.; Romero, L.C. Persulfidation proteome reveals the regulation of protein function by hydrogen sulfide in diverse biological processes in Arabidopsis. J. Exp. Bot. 2017, 68, 4915–4927. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; Siddiqui, M.H. Nitric oxide-induced synthesis of hydrogen sulfide alleviates osmotic stress in wheat seedlings through sustaining antioxidant enzymes, osmolyte accumulation and cysteine homeostasis. Nitric Oxide Biol. Chem. 2017, 68, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Qi, C.; Ren, H.; Huang, A.; Hei, S.; She, X. Ethylene mediates brassinosteroid-induced stomatal closure via Gα protein-activated hydrogen peroxide and nitric oxide production in Arabidopsis. Plant J. 2015, 82, 280–301. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Yang, M.; Zhang, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Wollenweber, B.; Jiang, D. Crosstalk between hydrogen peroxide and nitric oxide mediates priming-induced drought tolerance in wheat. J. Agron. Crop Sci. 2020, in press. [Google Scholar] [CrossRef]
- Bright, J.; Desikan, R.; Hancock, J.T.; Weir, I.S.; Neill, S.J. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006, 45, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.B.; Huang, G.B.; Yu, J.H.; Zhang, M.L. Nitric oxide and hydrogen peroxide alleviate drought stress in marigold explants and promote its adventitious root development. Plant Physiol. Biochem. 2012, 58, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-B.; Zhang, M.-L.; Huang, G.-B.; Yu, J.-H. Ca2+ and CaM are involved in NO- and H2O2-induced adventitious root development in marigold. J. Plant Growth Regul. 2012, 31, 253–264. [Google Scholar] [CrossRef]
- Lamotte, O.; Gould, K.; Lecourieux, D.; Sequeira-Legrand, A.; Lebrun-Garcia, A.; Durner, J.; Pugin, A.; Wendehenne, D. Analysis of nitric oxide signaling functions in tobacco cells challenged by the elicitor cryptogein. Plant Physiol. 2004, 135, 516–529. [Google Scholar] [CrossRef]
- Lamotte, O.; Courtois, C.; Dobrowolska, G.; Besson, A.; Pugin, A.; Wendehenne, D. Mechanisms of nitric-oxide-induced increase of free cytosolic Ca2+ concentration in Nicotiana plumbaginifolia cells. Free Radic. Biol. Med. 2006, 40, 1369–1376. [Google Scholar] [CrossRef]
- Rodríguez-Serrano, M.; Romero-Puertas, M.C.; Pazmino, D.M.; Testillano, P.S.; Risueno, M.C.; Del Río, L.A.; Sandalio, L.M. Cellular response of pea plants to cadmium toxicity: Cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol. 2009, 150, 229–243. [Google Scholar] [CrossRef]
- Zou, J.J.; Wei, F.J.; Wang, C.; Wu, J.J.; Ratnasekera, D.; Liu, W.X.; Wu, W.H. Arabidopsis calcium-dependent protein kinase cpk10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiol. 2010, 154, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Foresi, N.; Mayta, M.L.; Lodeyro, A.F.; Scuffi, D.; Correa-Aragunde, N.; García-Mata, C.; Casalongué, C.; Carrillo, N.; Lamattina, L. Expression of the tetrahydrofolate-dependent nitric oxide synthase from the green alga Ostreococcus tauri increases tolerance to abiotic stresses and influences stomatal development in Arabidopsis. Plant J. 2015, 82, 806–821. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Reiter, R.J. Mitochondria: The birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2019, 2, 44–66. [Google Scholar] [CrossRef]
- Liang, B.; Ma, C.; Zhang, Z.; Wei, Z.; Gao, T.; Zhao, Q.; Ma, F.; Li, C. Long-term exogenous application of melatonin improves nutrient uptake fluxes in apple plants under moderate drought stress. Environ. Exp. Bot. 2018, 155, 650–661. [Google Scholar] [CrossRef]
- Antoniou, C.; Chatzimichail, G.; Xenofontos, R.; Pavlou, J.J.; Panagiotou, E.; Christou, A.; Fotopoulos, V. Melatonin systemically ameliorates drought stress-induced damage in Medicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J. Pineal Res. 2017, 62, e12401. [Google Scholar] [CrossRef]
- Shi, H.T.; Li, R.J.; Cai, W.; Liu, W.; Wang, C.L.; Lu, Y.T. Increasing nitric oxide content in Arabidopsis thaliana by expressing rat neuronal nitric oxide synthase resulted in enhanced stress tolerance. Plant Cell Physiol. 2012, 53, 344–357. [Google Scholar] [CrossRef]
- He, H.; He, L.-F. Crosstalk between melatonin and nitric oxide in plant development and stress responses. Physiol. Plant. 2020, 170, 218–226. [Google Scholar] [CrossRef]
- Arese, M.; Magnifico, M.C.; Mastronicola, D.; Altieri, F.; Grillo, C.; Blanck, T.J.J.; Sarti, P. Nanomolar melatonin enhances nNOS expression and controls HaCaT-cells bioenergetics. IUBMB Life 2012, 64, 251–258. [Google Scholar] [CrossRef]
- Sarti, P.; Forte, E.; Giuffrè, A.; Mastronicola, D.; Magnifico, M.C.; Arese, M. The chemical interplay between nitric oxide and mitochondrial cytochrome c oxidase: Reactions, effectors and pathophysiology. Int. J. Cell Biol. 2012, 2012. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, X.; Zhang, Y.; Wang, J.; Yang, J.; Ishida, T.; Jiang, W.; Han, X.; Kang, J.; Wang, X.; et al. CLE9 peptide-induced stomatal closure is mediated by abscisic acid, hydrogen peroxide, and nitric oxide in Arabidopsis thaliana. Plant Cell Environ. 2019, 42, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Diao, Q.; Song, Y.; Shi, D.; Qi, H. Interaction of polyamines, abscisic acid, nitric oxide, and hydrogen peroxide under chilling stress in tomato (Lycopersicon esculentum mill.) seedlings. Front. Plant Sci. 2017, 8, 203. [Google Scholar] [CrossRef]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Hussain, S.S.; Ali, M.; Ahmad, M.; Siddique, K.H.M. Polyamines: Natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol. Adv. 2011, 29, 300–311. [Google Scholar] [CrossRef]
- Yang, B.; Wu, J.; Gao, F.; Wang, J.; Su, G. Polyamine-induced nitric oxide generation and its potential requirement for peroxide in suspension cells of soybean cotyledon node callus. Plant Physiol. Biochem. 2014, 79, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Tun, N.N.; Santa-Catarina, C.; Begum, T.; Silveira, V.; Handro, W.; Segal Floh, E.I.; Scherer, G.F.E. Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol. 2006, 47, 346–354. [Google Scholar] [CrossRef]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J.; Kubiś, J. Interaction between polyamine and nitric oxide signaling in adaptive responses to drought in cucumber. J. Plant Growth Regul. 2009, 28, 177–186. [Google Scholar] [CrossRef]
- Montilla-Bascón, G.; Rubiales, D.; Hebelstrup, K.H.; Mandon, J.; Harren, F.J.M.; Cristescu, S.M.; Mur, L.A.J.; Prats, E. Reduced nitric oxide levels during drought stress promote drought tolerance in barley and is associated with elevated polyamine biosynthesis. Sci. Rep. 2017, 7, 13311. [Google Scholar] [CrossRef] [PubMed]
- Prakash, V.; Singh, V.P.; Tripathi, D.K.; Sharma, S.; Corpas, F.J. Crosstalk between nitric oxide (NO) and abscisic acid (ABA) signalling molecules in higher plants. Environ. Exp. Bot. 2019, 161, 41–49. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, W.; Yang, J.; Ismail, A.M. Role of ABA in integrating plant responses to drought and salt stresses. Field Crop. Res. 2006, 97, 111–119. [Google Scholar] [CrossRef]
- Freschi, L. Nitric oxide and phytohormone interactions: Current status and perspectives. Front. Plant Sci. 2013, 4, 398. [Google Scholar] [CrossRef]
- Liu, Y.-c.; Ma, W.; Niu, J.-f.; Li, B.; Zhou, W.; Liu, S.; Yan, Y.-p.; Ma, J.; Wang, Z. zhi Systematic analysis of SmWD40s, and responding of SmWD40-170 to drought stress by regulation of ABA- and H2O2-induced stomal movement in Salvia miltiorrhiza bunge. Plant Physiol. Biochem. 2020, 153, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.; Griffiths, R.; Hancock, J.; Neill, S. A new role for an old enzyme: Nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2002, 99, 16314–16318. [Google Scholar] [CrossRef] [PubMed]
- Dubovskaya, L.V.; Bakakina, Y.S.; Kolesneva, E.V.; Sodel, D.L.; Mcainsh, M.R.; Hetherington, A.M.; Volotovski, I.D. cGMP-dependent ABA-induced stomatal closure in the ABA-insensitive Arabidopsis mutant abi1-1. New Phytol. 2011, 191, 57–69. [Google Scholar] [CrossRef]
- Wang, P.; Du, Y.; Hou, Y.J.; Zhao, Y.; Hsu, C.C.; Yuan, F.; Zhu, X.; Tao, W.A.; Song, C.P.; Zhu, J.K. Nitric oxide negatively regulates abscisic acid signaling in guard cells by S-nitrosylation of OST1. Proc. Natl. Acad. Sci. USA 2015, 112, 613–618. [Google Scholar] [CrossRef]
- Khan, M.; Imran, Q.M.; Shahid, M.; Mun, B.G.; Lee, S.U.; Khan, M.A.; Hussain, A.; Lee, I.J.; Yun, B.W. Nitric oxide- induced AtAO3 differentially regulates plant defense and drought tolerance in Arabidopsis thaliana. BMC Plant Biol. 2019, 19, 602. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chen, Z.; Liu, Y.; Zhang, H.; Zhang, M.; Liu, Q.; Hong, X.; Zhu, J.K.; Gong, Z. ABO3, a WRKY transcription factor, mediates plant responses to abscisic acid and drought tolerance in Arabidopsis. Plant J. 2010, 63, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Imran, Q.M.; Hussain, A.; Mun, B.G.; Lee, S.U.; Asaf, S.; Ali, M.A.; Lee, I.J.; Yun, B.W. Transcriptome wide identification and characterization of NO-responsive WRKY transcription factors in Arabidopsis thaliana L. Environ. Exp. Bot. 2018, 148, 128–143. [Google Scholar] [CrossRef]
- Dar, T.A.; Uddin, M.; Khan, M.M.A.; Hakeem, K.R.; Jaleel, H. Jasmonates counter plant stress: A review. Environ. Exp. Bot. 2015, 115, 49–57. [Google Scholar] [CrossRef]
- Liu, X.; Shi, W.; Zhang, S.; Lou, C. Nitric oxide involved in signal transduction of Jasmonic acid-induced stomatal closure of Vicia faba L. Chinese Sci. Bull. 2005, 50, 520–525. [Google Scholar] [CrossRef]
- Palmieri, M.C.; Sell, S.; Huang, X.; Scherf, M.; Werner, T.; Durner, J.; Lindermayr, C. Nitric oxide-responsive genes and promoters in Arabidopsis thaliana: A bioinformatics approach. J. Exp. Bot. 2008, 59, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.W.; Wu, J.Y. Nitric oxide is involved in methyl jasmonate-induced defense responses and secondary metabolism activities of Taxus cells. Plant Cell Physiol. 2005, 46, 923–930. [Google Scholar] [CrossRef]
- Shan, C.; Zhou, Y.; Liu, M. Nitric oxide participates in the regulation of the ascorbate-glutathione cycle by exogenous jasmonic acid in the leaves of wheat seedlings under drought stress. Protoplasma 2015, 252, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Zottini, M.; Costa, A.; De Michele, R.; Ruzzene, M.; Carimi, F.; Lo Schiavo, F. Salicylic acid activates nitric oxide synthesis in Arabidopsis. J. Exp. Bot. 2007, 58, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Freschi, L.; Corpas, F.J.; Khan, N.A. Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ. Sci. Pollut. Res. 2017, 24, 2273–2285. [Google Scholar] [CrossRef]
- Gémes, K.; Poór, P.; Horváth, E.; Kolbert, Z.; Szopkó, D.; Szepesi, Á.; Tari, I. Cross-talk between salicylic acid and NaCl-generated reactive oxygen species and nitric oxide in tomato during acclimation to high salinity. Physiol. Plant. 2011, 142, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Zhao, S.; Dong, H.; Zhang, H.; Sun, L.; Miao, C. Nia1 and Nia2 are involved in exogenous salicylic acid-induced nitric oxide generation and stomatal closure in Arabidopsis. J. Integr. Plant Biol. 2010, 52, 298–307. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Scherer, G.F.E.; Holk, A. NO donors mimic and NO inhibitors inhibit cytokinin action in betalaine accumulation in Amaranthus caudatus. Plant Growth Regul. 2000, 32, 345–350. [Google Scholar] [CrossRef]
- Shao, R.; Wang, K.; Shangguan, Z. Cytokinin-induced photosynthetic adaptability of Zea mays L. to drought stress associated with nitric oxide signal: Probed by ESR spectroscopy and fast OJIP fluorescence rise. J. Plant Physiol. 2010, 167, 472–479. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, Y.T.; Tian, H.; Guo, F.Q. Nitric oxide mediates cytokinin functions in cell proliferation and meristem maintenance in Arabidopsis. Mol. Plant 2013, 6, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Xiao-Ping, S.; Xi-Gui, S. Cytokinin- and auxin-induced stomatal opening is related to the change of nitric oxide levels in guard cells in broad bean. Physiol. Plant. 2006, 128, 569–579. [Google Scholar] [CrossRef]
- Kong, X.; Wang, T.; Li, W.; Tang, W.; Zhang, D.; Dong, H. Exogenous nitric oxide delays salt-induced leaf senescence in cotton (Gossypium hirsutum L.). Acta Physiol. Plant. 2016, 38, 61. [Google Scholar] [CrossRef]
- Mengel, A.; Chaki, M.; Shekariesfahlan, A.; Lindermayr, C. Effect of nitric oxide on gene transcription—S-nitrosylation of nuclear proteins. Front. Plant Sci. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Whiteman, M.; Li, L.; Kostetski, I.; Chu, S.H.; Siau, J.L.; Bhatia, M.; Moore, P.K. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem. Biophys. Res. Commun. 2006, 343, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Belenghi, B.; Romero-Puertas, M.C.; Vercammen, D.; Brackenier, A.; Inzé, D.; Delledonne, M.; Van Breusegem, F. Metacaspase activity of Arabidopsis thaliana is regulated by S-nitrosylation of a critical cysteine residue. J. Biol. Chem. 2007, 282, 1352–1358. [Google Scholar] [CrossRef]
- Ortega-Galisteo, A.P.; Rodríguez-Serrano, M.; Pazmiño, D.M.; Gupta, D.K.; Sandalio, L.M.; Romero-Puertas, M.C. S-Nitrosylated proteins in pea (Pisum sativum L.) leaf peroxisomes: Changes under abiotic stress. J. Exp. Bot. 2012, 63, 2089–2103. [Google Scholar] [CrossRef]
- Feechan, A.; Kwon, E.; Yun, B.W.; Wang, Y.; Pallas, J.A.; Loake, G.J. A central role for S-nitrosothiols in plant disease resistance. Proc. Natl. Acad. Sci. USA 2005, 102, 8054–8059. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, J.K.; Lang, Z. Nitric oxide suppresses the inhibitory effect of abscisic acid on seed germination by S-nitrosylation of SnRK2 proteins. Plant Signal. Behav. 2015, 10, 2–5. [Google Scholar] [CrossRef]
- Correa-Aragunde, N.; Foresi, N.; Delledonne, M.; Lamattina, L. Auxin induces redox regulation of ascorbate peroxidase 1 activity by S-nitrosylation/denitrosylation balance resulting in changes of root growth pattern in Arabidopsis. J. Exp. Bot. 2013, 64, 3339–3349. [Google Scholar] [CrossRef]
- Feng, J.; Wang, C.; Chen, Q.; Chen, H.; Ren, B.; Li, X.; Zuo, J. S-nitrosylation of phosphotransfer proteins represses cytokinin signaling. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Valderrama, R.; Mata-Pérez, C.; Padilla, M.N.; Corpas, F.J.; Barroso, J.B. Antioxidant systems are regulated by nitric oxide-mediated post-translational modifications (NO-PTMs). Front. Plant Sci. 2016, 7, 152. [Google Scholar] [CrossRef] [PubMed]
- Chaki, M.; Valderrama, R.; Fernández-Ocaña, A.M.; Carreras, A.; Gómez-Rodríguez, M.V.; López-Jaramillo, J.; Begara-Morales, J.C.; Sánchez-Calvo, B.; Luque, F.; Leterrier, M.; et al. High temperature triggers the metabolism of S-nitrosothiols in sunflower mediating a process of nitrosative stress which provokes the inhibition of ferredoxin-NADP reductase by tyrosine nitration. Plant Cell Environ. 2011, 34, 1803–1818. [Google Scholar] [CrossRef] [PubMed]
- Chaki, M.; Valderrama, R.; Fernández-Ocaña, A.M.; Carreras, A.; Gómez-Rodríguez, M.V.; Pedrajas, J.R.; Begara-Morales, J.C.; Sánchez-Calvo, B.; Luque, F.; Leterrier, M.; et al. Mechanical wounding induces a nitrosative stress by down-regulation of GSNO reductase and an increase in S-nitrosothiols in sunflower (Helianthus annuus) seedlings. J. Exp. Bot. 2011, 62, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.W.; Feechan, A.; Yin, M.; Saidi, N.B.B.; Le Bihan, T.; Yu, M.; Moore, J.W.; Kang, J.G.; Kwon, E.; Spoel, S.H.; et al. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 2011, 478, 264–268. [Google Scholar] [CrossRef]
- Feng, J.; Chen, L.; Zuo, J. Protein S-Nitrosylation in plants: Current progresses and challenges. J. Integr. Plant Biol. 2019, 61, 1206–1223. [Google Scholar] [CrossRef]
- Vandelle, E.; Delledonne, M. Peroxynitrite formation and function in plants. Plant Sci. 2011, 181, 534–539. [Google Scholar] [CrossRef]
- Holzmeister, C.; Gaupels, F.; Geerlof, A.; Sarioglu, H.; Sattler, M.; Durner, J.; Lindermayr, C. Differential inhibition of Arabidopsis superoxide dismutases by peroxynitrite-mediated tyrosine nitration. J. Exp. Bot. 2015, 66, 989–999. [Google Scholar] [CrossRef]
- Castillo, M.-C.; Lozano-Juste, J.; González-Guzmán, M.; Rodriguez, L.; Rodriguez, P.L.; León, J. Inactivation of PYR/PYL/RCAR ABA receptors by tyrosine nitration may enable rapid inhibition of ABA signaling by nitric oxide in plants. Sci. Signal. 2015, 8, ra89. [Google Scholar] [CrossRef]
- Zhang, S.; Melzer, M.M.; Sen, S.N.; Çelebi-Ölçüm, N.; Warren, T.H. A motif for reversible nitric oxide interactions in metalloenzymes. Nat. Chem. 2016, 8, 663–669. [Google Scholar] [CrossRef]
- Hebelstrup, K.H.; van Zanten, M.; Mandon, J.; Voesenek, L.A.C.J.; Harren, F.J.M.; Cristescu, S.M.; Møller, I.M.; Mur, L.A.J. Haemoglobin modulates NO emission and hyponasty under hypoxia-related stress in Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 5581–5591. [Google Scholar] [CrossRef] [PubMed]
- Zaffagnini, M.; De Mia, M.; Morisse, S.; Di Giacinto, N.; Marchand, C.H.; Maes, A.; Lemaire, S.D.; Trost, P. Protein S-nitrosylation in photosynthetic organisms: A comprehensive overview with future perspectives. Biochim. Biophys. Acta Proteins Proteom. 2016, 1864, 952–966. [Google Scholar] [CrossRef] [PubMed]
- Griveau, S.; Besson-Bard, A.; Bedioui, F.; Wendehenne, D. Electrochemical Detection of Nitric Oxide in Plant Cell Suspensions. In Plant Nitric Oxide: Methods and Protocols, 1st ed.; Gupta, K.J., Ed.; Springer: New York, NY, USA, 2016; pp. 127–137. [Google Scholar]
- Mur, L.A.J.; Mandon, J.; Cristescu, S.M.; Harren, F.J.M.; Prats, E. Methods of nitric oxide detection in plants: A commentary. Plant Sci. 2011, 181, 509–519. [Google Scholar] [CrossRef]
- Ruemer, S.; Krischke, M.; Fekete, A.; Lesch, M.; Mueller, M.J.; Kaiser, W.M. Methods to Detect Nitric Oxide in Plants: Are DAFs Really Measuring NO? In Plant Nitric Oxide: Methods and Protocols, 1st ed.; Gupta, K.J., Ed.; Springer: New York, NY, USA, 2016; pp. 57–68. [Google Scholar]
- Planchet, E.; Kaiser, W.M. Nitric oxide (NO) detection by DAF fluorescence and chemiluminescence: A comparison using abiotic and biotic NO sources. J. Exp. Bot. 2006, 57, 3043–3055. [Google Scholar] [CrossRef] [PubMed]
- Wany, A.; Gupta, K.J. Localization of Nitric Oxide in Wheat Roots by DAF Fluorescence. In Plant Nitric Oxide: Methods and Protocols, 1st ed.; Gupta, K.J., Ed.; Springer: New York, NY, USA, 2016; pp. 39–47. [Google Scholar]
- Ren, Q.Q.; Huang, X.R.; Liu, G.C.; Jun, O.Y.; Li, M.T.; Chen, H.; Zhao, Y.-D.; Chen, W. A field-compatible technique using an electrochemical sensing microbundle for real-time and simultaneous in vivo measurement of hydrogen peroxide, nitric oxide, and pH under drought stress. Sens. Actuators B Chem. 2015, 220, 743–748. [Google Scholar] [CrossRef]
- Makishima, A. Topics of Bioluminescence and Chemoluminescence. In Biochemistry for Materials Science: Catalysis, Complexes and Proteins, 1st ed.; Gifford, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 77–83. [Google Scholar]
- Hetrick, E.M.; Schoenfisch, M.H. Analytical chemistry of nitric oxide. Annu. Rev. Anal. Chem. 2009, 2, 409–433. [Google Scholar] [CrossRef]
- Davies, M.J. Detection and characterisation of radicals using electron paramagnetic resonance (EPR) spin trapping and related methods. Methods 2016, 109, 21–30. [Google Scholar] [CrossRef]
- Csonka, C.; Páli, T.; Bencsik, P.; Görbe, A.; Ferdinandy, P.; Csont, T. Measurement of NO in biological samples. Br. J. Pharmacol. 2015, 172, 1620–1632. [Google Scholar] [CrossRef]
- Kohno, M. Applications of electron spin resonance spectrometry for reactive oxygen species and reactive nitrogen species research. J. Clin. Biochem. Nutr. 2010, 47, 1–11. [Google Scholar] [CrossRef]
- Griendling, K.K.; Touyz, R.M.; Zweier, J.L.; Dikalov, S.; Chilian, W.; Chen, Y.R.; Harrison, D.G.; Bhatnagar, A. Measurement of reactive oxygen species, reactive nitrogen species, and redox-dependent signaling in the cardiovascular system: A scientific statement from the American heart association. Circ. Res. 2016, 119, e39–e75. [Google Scholar] [CrossRef] [PubMed]
- Bright, J.; Hiscock, S.J.; James, P.E.; Hancock, J.T. Pollen generates nitric oxide and nitrite: A possible link to pollen-induced allergic responses. Plant Physiol. Biochem. 2009, 47, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Besson-Bard, A.; Griveau, S.; Bedioui, F.; Wendehenne, D. Real-time electrochemical detection of extracellular nitric oxide in tobacco cells exposed to cryptogein, an elicitor of defence responses. J. Exp. Bot. 2008, 59, 3407–3414. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, S.-E.; Hamdan, M.F.; Pua, T.-L.; Saidi, N.B.; Tan, B.C. Plant Nitric Oxide Signaling under Drought Stress. Plants 2021, 10, 360. https://doi.org/10.3390/plants10020360
Lau S-E, Hamdan MF, Pua T-L, Saidi NB, Tan BC. Plant Nitric Oxide Signaling under Drought Stress. Plants. 2021; 10(2):360. https://doi.org/10.3390/plants10020360
Chicago/Turabian StyleLau, Su-Ee, Mohd Fadhli Hamdan, Teen-Lee Pua, Noor Baity Saidi, and Boon Chin Tan. 2021. "Plant Nitric Oxide Signaling under Drought Stress" Plants 10, no. 2: 360. https://doi.org/10.3390/plants10020360
APA StyleLau, S.-E., Hamdan, M. F., Pua, T.-L., Saidi, N. B., & Tan, B. C. (2021). Plant Nitric Oxide Signaling under Drought Stress. Plants, 10(2), 360. https://doi.org/10.3390/plants10020360






