In Vitro and In Silico Studies of Soluble Epoxide Hydrolase Inhibitors from the Roots of Lycopus lucidus
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation and Identification
2.2. sEH Inhibitory Activity
2.3. Enzyme Kinetic on sEH
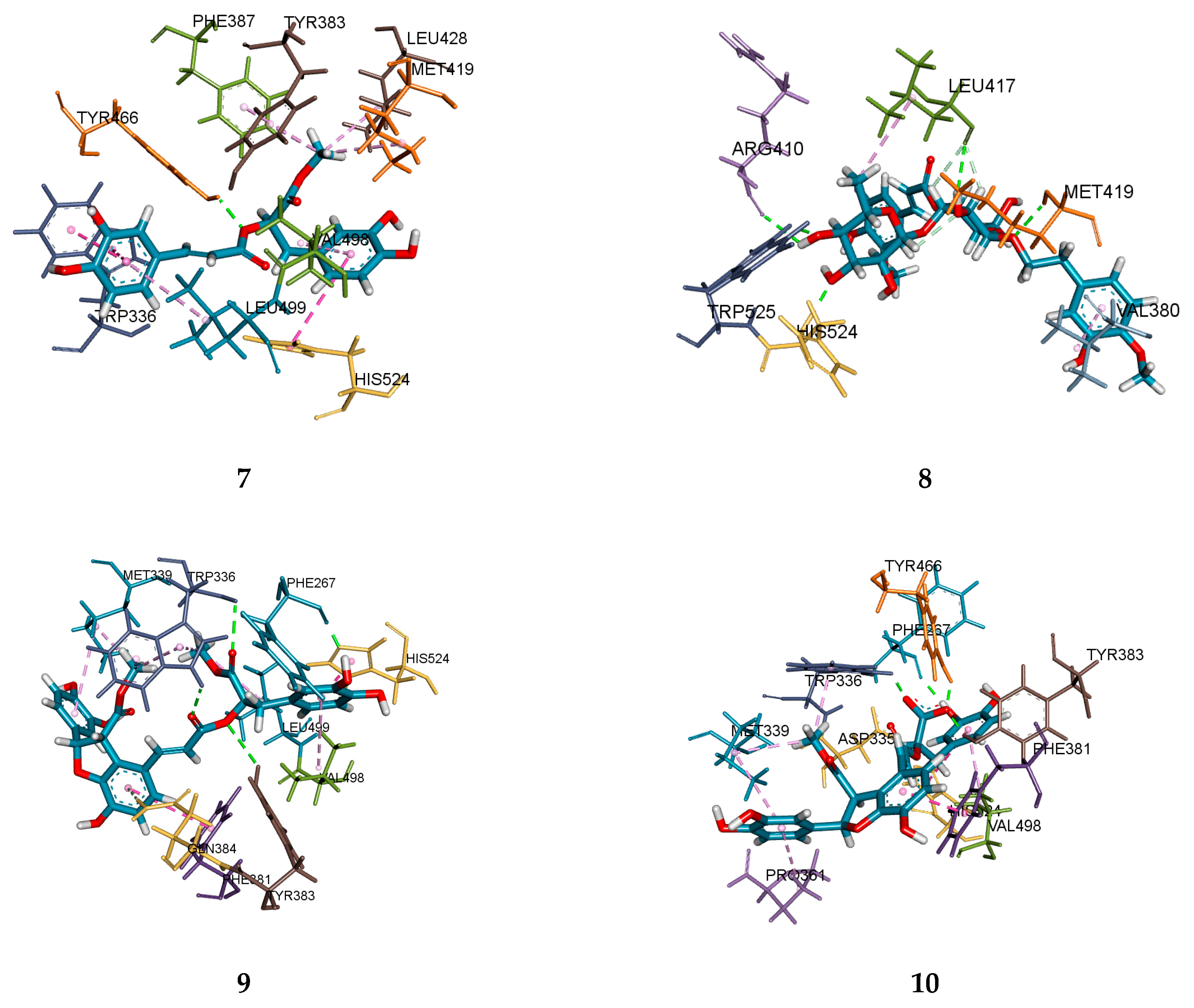
2.4. Molecular Docking Analysis
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. sEH Assay
3.5. Molecular Docking Studies
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fang, X.; Kaduce, T.L.; Weintraub, N.L.; Harmon, S.; Teesch, L.M.; Morisseau, C.; Thompson, D.A.; Hammock, B.D.; Spector, A.A. Pathways of epoxyeicosatrienoic acid metabolism in endothelial cells implications for the vascular effects of soluble epoxide hydrolase inhibition. J. Biol. Chem. 2001, 276, 14867–14874. [Google Scholar] [CrossRef]
- Newman, J.W.; Morisseau, C.; Harris, T.R.; Hammock, B.D. The soluble epoxide hydrolase encoded by EPXH2 is a bifunctional enzyme with novel lipid phosphate phosphatase activity. Proc. Natl. Acad. Sci. USA 2003, 100, 1558–1563. [Google Scholar] [CrossRef]
- Schmelzer, K.R.; Kubala, L.; Newman, J.W.; Kim, I.-H.; Eiserich, J.P.; Hammock, B.D. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc. Natl. Acad. Sci. USA 2005, 102, 9772–9777. [Google Scholar] [CrossRef]
- Seubert, J.M.; Sinal, C.J.; Graves, J.; DeGraff, L.M.; Bradbury, J.A.; Lee, C.R.; Goralski, K.; Carey, M.A.; Luria, A.; Newman, J.W. Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ. Res. 2006, 99, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Enayetallah, A.E.; French, R.A.; Thibodeau, M.S.; Grant, D.F. Distribution of soluble epoxide hydrolase and of cytochrome P450 2C8, 2C9, and 2J2 in human tissues. J. Histochem. Cytochem. 2004, 52, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Imig, J.D.; Zhao, X.; Capdevila, J.H.; Morisseau, C.; Hammock, B.D. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension 2002, 39, 690–694. [Google Scholar] [CrossRef]
- Imig, J.D. Cardiovascular therapeutic aspects of soluble epoxide hydrolase inhibitors. Cardiovasc. Drug Rev. 2006, 24, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.C.; Davis, C.M.; Nelson, J.W.; Young, J.M.; Alkayed, N.J. Soluble epoxide hydrolase: Sex differences and role in endothelial cell survival. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1936–1942. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Xu, F.; Huse, L.M.; Morisseau, C.; Draper, A.J.; Newman, J.W.; Parker, C.; Graham, L.; Engler, M.M.; Hammock, B.D. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ. Res. 2000, 87, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Manhiani, M.; Quigley, J.E.; Knight, S.F.; Tasoobshirazi, S.; Moore, T.; Brands, M.W.; Hammock, B.D.; Imig, J.D. Soluble epoxide hydrolase gene deletion attenuates renal injury and inflammation with DOCA-salt hypertension. Am. J. Physiol. Renal. Physiol. 2009, 297, F740–F748. [Google Scholar] [CrossRef]
- Yang, X.; Lv, Y.; Tian, L.; Zhao, Y. Composition and systemic immune activity of the polysaccharides from an herbal tea (Lycopus lucidus Turcz). J. Agric. Food Chem. 2010, 58, 6075–6080. [Google Scholar] [CrossRef]
- Moon, H.-K.; Kim, Y.-C.; Hong, S.-P. Diagnostic characters and new populations of Lycopus lucidus var. hirtus (Lamiaceae). Kor. J. Plant Tax. 2013, 43, 99–102. [Google Scholar] [CrossRef]
- Woo, E.-R.; Piao, M.S. Antioxidative constituents from Lycopus lucidus. Arch. Pharm. Res. 2004, 27, 173–176. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.-L.; Wang, T.; Qian, S.-H. Chemical Constituents of the Aerial Parts of Lycopus lucidus var. hirtus. Chem. Nat. Compd. 2014, 50, 288–290. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kang, D.G.; Kim, J.S.; Lee, H.S. Lycopus lucidus inhibits high glucose-induced vascular inflammation in human umbilical vein endothelial cells. Vascul. Pharmacol. 2008, 48, 38–46. [Google Scholar] [CrossRef]
- Lu, Y.-H.; Huang, J.-H.; Li, Y.-C.; Ma, T.-T.; Sang, P.; Wang, W.-J.; Gao, C.-Y. Variation in nutritional compositions, antioxidant activity and microstructure of Lycopus lucidus Turcz. root at different harvest times. Food Chem. 2015, 183, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, Y.; He, N.; Croft, K.D. Isolation, characterization, and immunological effects of α-galacto-oligosaccharides from a new source, the herb Lycopus lucidus Turcz. J. Agric. Food Chem. 2010, 58, 8253–8258. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Fu, Q.; Li, F.; Zhou, W.; Xin, H.; Wang, H.; Jin, Y.; Liang, X. Hydrophilic interaction liquid chromatography for the separation, purification, and quantification of raffinose family oligosaccharides from Lycopus lucidus Turcz. J. Sep. Sci. 2015, 38, 2607–2613. [Google Scholar] [CrossRef] [PubMed]
- Cichewicz, R.H.; Kouzi, S.A. Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med. Res. Rev. 2004, 24, 90–114. [Google Scholar] [CrossRef]
- Ma, X.-L.; Lin, W.-B.; Zhang, G.-L. Chemical Constituents of Osmanthus yunnanensis. Nat. Prod. Res. Dev. 2009, 21, 593–599. [Google Scholar]
- Taketa, A.T.; Breitmaier, E.; Schenkel, E.P. Triterpenes and triterpenoidal glycosides from the fruits of Ilex paraguariensis (Maté). J. Braz. Chem. Soc. 2004, 15, 205–211. [Google Scholar] [CrossRef]
- Tan, J.-J.; Tan, C.-H.; Chen, Y.-L.; Jiang, S.-H.; Zhu, D.-Y. Chemical Constituents of Clerodendranthus spicatus. Nat. Prod. Res. Dev. 2009, 21, 592–608. [Google Scholar]
- Cheng, J.-J.; Zhang, L.-J.; Cheng, H.-L.; Chiou, C.-T.; Lee, I.-J.; Kuo, Y.-H. Cytotoxic hexacyclic triterpene acids from Euscaphis japonica. J. Nat. Prod. 2010, 73, 1655–1658. [Google Scholar] [CrossRef] [PubMed]
- Hwu, J.R.; Varadaraju, T.G.; Abd-Elazem, I.S.; Huang, R.C.C. First Total Syntheses of Oresbiusins A and B, Their Antipodes, and Racemates: Configuration Revision and Anti-HIV Activity. Eur. J. Org. Chem. 2012, 2012, 4684–4688. [Google Scholar] [CrossRef]
- Zhou, W.; Xie, H.; Xu, X.; Liang, Y.; Wei, X. Phenolic constituents from Isodon lophanthoides var. graciliflorus and their antioxidant and antibacterial activities. J. Funct. Foods 2014, 6, 492–498. [Google Scholar] [CrossRef]
- Yalcin, F.N.; Ersoez, T.; Akbay, P.; Çaliş, İ.; Dönmez, A.A.; STICHER, O. Iridoid and Phenylpropanoid Glycosides from Phlomis samia, P. monocephala and P. carica. Turk. J. Chem. 2003, 27, 295–306. [Google Scholar]
- Wang, L.; Li, X.; Zhang, S.; Lu, W.; Liao, S.; Liu, X.; Shan, L.; Shen, X.; Jiang, H.; Zhang, W. Natural products as a gold mine for selective matrix metalloproteinases inhibitors. Bioorg. Med. Chem. 2012, 20, 4164–4171. [Google Scholar] [CrossRef]
- Zhang, Z.-F.; Peng, Z.-G.; Gao, L.; Dong, B.; Li, J.-R.; Li, Z.-Y.; Chen, H.-S. Three new derivatives of anti-HIV-1 polyphenols isolated from Salvia yunnanensis. J. Asian Nat. Prod. Res. 2008, 10, 391–396. [Google Scholar] [CrossRef]
- Thuong, P.T.; Kang, K.W.; Kim, J.K.; Seo, D.B.; Lee, S.J.; Kim, S.H.; Oh, W.K. Lithospermic acid derivatives from Lithospermum erythrorhizon increased expression of serine palmitoyltransferase in human HaCaT cells. Bioorg. Med. Chem. Lett. 2009, 19, 1815–1817. [Google Scholar] [CrossRef]
- Khanh, P.N.; Duc, H.V.; Huong, T.T.; Son, N.T.; Ha, V.T.; Van, D.T.; Tai, B.H.; Kim, J.E.; Jo, A.R.; Kim, Y.H. Alkylphloroglucinol derivatives and triterpenoids with soluble epoxide hydrolase inhibitory activity from Callistemon citrinus. Fitoterapia 2016, 109, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.S.; Kim, J.H.; Lin, Y.; Su, X.D.; Kang, J.S.; Yang, S.Y.; Kim, Y.H. Inhibitory Activity of Quercetin 3-O-Arabinofuranoside and 2-Oxopomolic Acid Derived from Malus domestica on Soluble Epoxide Hydrolase. Molecules 2020, 25, 4352. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.-Y.; Bai, M.-M.; Tian, J.-M.; Pescitelli, G.; Ivšić, T.; Huang, X.-H.; Lee, H.; Son, Y.N.; Kim, J.H.; Kim, Y.H. Chemical components from the seeds of Catalpa bungei and their inhibitions of soluble epoxide hydrolase, cholinesterase and nuclear factor kappa B activities. RSC Adv. 2016, 6, 40706–40716. [Google Scholar] [CrossRef]
- Li, W.; Kim, J.H.; Zhou, W.; Shim, S.H.; Ma, J.Y.; Kim, Y.H. Soluble epoxide hydrolase inhibitory activity of phenolic components from the rhizomes and roots of Gentiana scabra. Biosci. Biotechnol. Biochem. 2015, 79, 907–911. [Google Scholar] [CrossRef]
- Su, X.D.; Guo, R.H.; Yang, S.Y.; Kim, Y.H.; Kim, Y.R. Anti-bacterial effects of components from Sanguisorba officinalis L. on Vibrio vulnificus and their soluble epoxide hydrolase inhibitory activity. Nat. Prod. Res. 2019, 33, 3445–3449. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Morgan, A.M.; Tai, B.H.; Van, D.T.; Cuong, N.M.; Kim, Y.H. Inhibition of soluble epoxide hydrolase activity by compounds isolated from the aerial parts of Glycosmis stenocarpa. J. Enzyme Inhib. Med. Chem. 2016, 31, 640–644. [Google Scholar] [CrossRef]
- Lee, G.Y.; Kim, J.H.; Choi, S.-K.; Kim, Y.H. Constituents of the seeds of Cassia tora with inhibitory activity on soluble expoxide hydrolease. Bioorg. Med. Chem. Lett. 2015, 25, 5097–5101. [Google Scholar] [CrossRef]
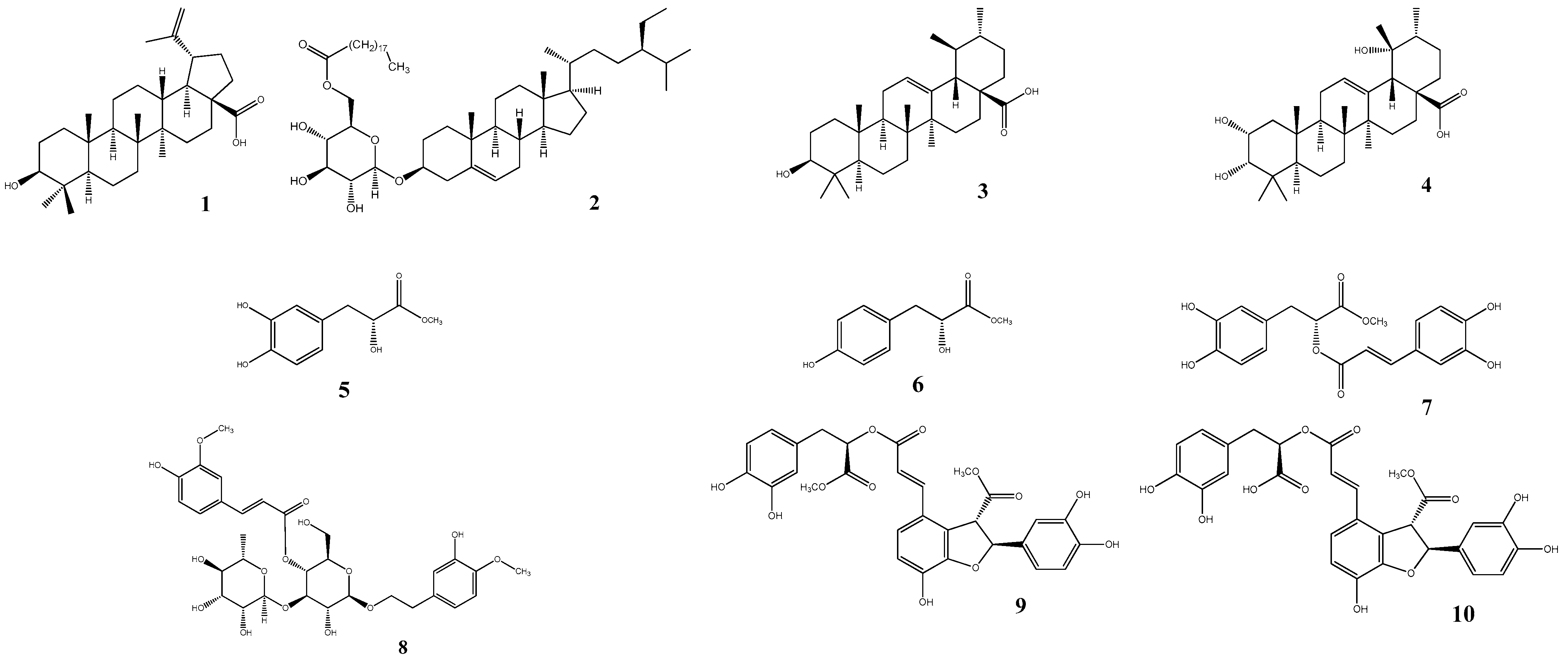

| Compounds | sEH a | |
|---|---|---|
| IC50 (μM) | Type (Ki,μM) | |
| 1 | 67.8 ± 3.2 | N.D. |
| 2 | N.D. c | N.D. |
| 3 | 42.1 ± 4.0 | N.D. |
| 4 | N.D. | N.D. |
| 5 | N.D. | N.D. |
| 6 | 52.0 ± 1.9 | N.D. |
| 7 | 16.8 ± 0.6 | Competitive (9.2) |
| 8 | 19.5 ± 4.1 | Mixed (5.7) |
| 9 | 10.6 ± 3.2 | Competitive (1.9) |
| 10 | 35.7 ± 2.1 | Competitive (7.6) |
| AUDA b (nM) | 2.0 ± 0.2 | |
| Compounds | Docking Analysis (Total Score) a | Hydrogen Bond | Hydrogen Bonds (Å) | Interacting Amino Acids Residues |
|---|---|---|---|---|
| 7 | 8.6026 | 1 | Tyr466 (2.09) | Tyr466, Trp336, His524, Met419, Leu428, Tyr383, Phr387, Val498, Leu499 |
| 8 | 7.6157 | 7 | His524 (1.91), Trp525 (1.99), Met419 (2.31), Leu417 (2.34, 2.75, 2.85), Arg410 (2.58) | Met419, Trp525, His524, Leu417, Arg410, Val380 |
| 9 | 8.7626 | 5 | Phe267 (1.88), Trp336 (2.36, 2.51), Gln384 (2.71), Tyr383 (3.06) | Trp336, Tyr383, Phe267, Gln384, His524, Phe381. Leu499, Met339, Val498 |
| 10 | 5.9275 | 5 | Tyr466 (1.70), Tyr383 (1.85), Trp336(1.90), Phe267 (1.98), Asp335 (2.84) | Trp336, Tyr383, Tyr466, Phe267, Asp335, Phe381, His524, Met339, Val498, Pro361 |
| AUDA b | 11.3220 | 4 | Tyr343 (1.96), Tyr383 (1.82), Asp335(1.93, 2.83) | Tyr343, Tyr383, Asp335, Trp336, Met419, Pro268, Leu408, Trp525 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.K.; Lee, J.S.; Yang, S.Y.; Lee, K.Y.; Kim, Y.H. In Vitro and In Silico Studies of Soluble Epoxide Hydrolase Inhibitors from the Roots of Lycopus lucidus. Plants 2021, 10, 356. https://doi.org/10.3390/plants10020356
Han YK, Lee JS, Yang SY, Lee KY, Kim YH. In Vitro and In Silico Studies of Soluble Epoxide Hydrolase Inhibitors from the Roots of Lycopus lucidus. Plants. 2021; 10(2):356. https://doi.org/10.3390/plants10020356
Chicago/Turabian StyleHan, Yoo Kyong, Ji Sun Lee, Seo Young Yang, Ki Yong Lee, and Young Ho Kim. 2021. "In Vitro and In Silico Studies of Soluble Epoxide Hydrolase Inhibitors from the Roots of Lycopus lucidus" Plants 10, no. 2: 356. https://doi.org/10.3390/plants10020356
APA StyleHan, Y. K., Lee, J. S., Yang, S. Y., Lee, K. Y., & Kim, Y. H. (2021). In Vitro and In Silico Studies of Soluble Epoxide Hydrolase Inhibitors from the Roots of Lycopus lucidus. Plants, 10(2), 356. https://doi.org/10.3390/plants10020356






