1. Introduction
Most of the steps leading to virus infection of a susceptible host are possible thanks to the hijacking of the host’s cellular machinery by the virus. Due to the obligate parasite status of viruses, the main challenge for these pathogens is to optimize their access to a host in order to complete their biological cycle, to ensure the production of viral populations and to be able to transfer infectious particles from plant to plant. This parasitism induces disturbances in the biology of the host that results in mild to severe physiological alterations (e.g., yellowing, dwarfing, reddening, leaf deformation, stunting, and necrosis). For plants of agronomic interest, viral infection generally leads to yield reduction. Therefore, strategies have been developed to limit or to prevent the entry of viral entities into the compatible cellular environment of a susceptible host, and thus to block the infectious process at its early steps. These methods include the modification of growing practices (e.g., optimization of sowing dates [
1,
2] and management of volunteers [
3]), the use of chemicals (e.g., insecticides for insect-borne viral diseases [
4]), mineral oils, or bio-control products (e.g., plant extracts and micro-organisms [
5]), and/or the use of genetic resources (i.e., resistant/tolerant cultivars [
6]).
The identification of genotype(s) with a reduced level of susceptibility among the available genetic resources is the first step of breeding for resistance/tolerance. Phenotypic screening, supplemented by serological and molecular virus diagnostics, has made it possible to describe different levels of plant-virus incompatibility corresponding to extreme resistance (ER, non-host plant), hyper-sensitivity (HR, the virus is contained by the host in a necrotic zone consisting of a few cells at the inoculation site), partial resistance (the viral cycle is altered in its dynamics and/or efficiency), and tolerance (the virus multiplies in the host without disturbing plant physiology) (for a review, [
7]). When a genetic resource presents such resistance/tolerance behavior, major efforts are made by breeders to introduce gene(s) involved in the phenotype into genetic background of cultivated susceptible varieties. However, this strategy could suffer from the scarcity of resistance/tolerance genes present in the available genetic resources.
Rapeseed (
Brassica napus), one of the most important oil crops [
8], can be infected by several viruses including turnip yellows virus (TuYV, Polerovirus genus, Luteoviridae family). TuYV is transmitted by aphids in a persistent, circulative, and non-propagative manner [
9,
10,
11]. The peach-potato aphid
Myzus persicae (Sulzer, 1776) is known to be the main vector of TuYV [
10] while the cabbage aphid Brevicoryne brassicae (Linnaeus, 1758), commonly found in rapeseed fields, has been reported to be a poorly efficient TuYV vector [
10]. The symptoms induced by TuYV include leaf reddening, interveinal chlorosis, and plant stunting. TuYV infection of susceptible rapeseed cultivars causes yield losses of up to 40% [
12,
13,
14,
15,
16]. Thus, turnip yellows virus is one of the major threats for rapeseed production. The scarcity of TuYV-resistance sources in rapeseed germplasm and the low efficiency of control methods based on growing practices led farmers to use chemical strategies (i.e., insecticides) to limit the incidence of TuYV on their crops. Indeed, the development in the early 1980s of chemical solutions made it possible, through seed and/or foliar treatments, to protect treated areas against direct (feeding) and indirect (vectoring of pathogens from plant-to-plant within the field) impacts of insects. For more than twenty years, the use of neonicotinoid insecticides (NNI) was the main strategy to control TuYV, with nearly 25% of rapeseed cultivated areas in France grown with treated seeds [
17]. However, insecticides could (i) induce side effects on the environment and on non-targeted organisms, and (ii) lead to the selection of insecticide-resistant individuals in aphid populations. In the context of the recent EU ban on the use of NNI in the field [
18,
19], which potentially increases crop exposure to insects and the viruses they transmit [
20,
21], the use of resistant/tolerant genotypes should be considered as the preferred solution to reduce the impacts of TuYV on rapeseed production.
The first genetic resource with the TuYV-resistance phenotype described in Brassica was the re-synthetized
B. napus line ‘R54’ [
22]. The ‘R54’ TuYV-resistance phenotype has been introgressed into several commercial varieties. However, it has been reported to be sensitive to elevated ambient temperature [
23]. A recent work, carried out on accessions representing a subset of the
B. napus genetic diversity, allowed the identification in the TuYV-resistant rapeseed cv. Yudal of a single dominant QTL on chromosome A04 that does not segregate with markers linked to the ‘R54’ resistance, suggesting independent origins of the TuYV resistances present in ‘R54’ and in cv. Yudal [
24]. On the French market, the first rapeseed cultivar described for its TuYV-resistant phenotype was cv. Allison [Limagrain] in 2015. Since then, several cultivars (e.g., Architect [Limagrain], Angelico [Limagrain], Temptation [DSV], Coogan [RAGT], Smaragd [DSV], and Delice [DSV]) have been reported for their resistance to TuYV infection. However, no information is available on the impact of these resistant cultivars on the epidemiology of TuYV in fields. It is therefore necessary to study and describe steps of the infection process altered in a TuYV-resistant host in order to understand the impacts of resistant/tolerant cultivars on TuYV epidemics. Thus, different parameters that involved virus–host (infection rate, viral accumulation, and latency in infected plants) and vector–host (antibiosis and antixenosis) interactions have been identified as targets for this study. Using several experimental designs, these epidemiological parameters have been estimated for cv. Quizz ([RAGT], described to be tolerant to TuYV infection) and cv. Architect ([Limagrain], reported to be partially resistant to TuYV infection [
25]) to determine whether and how these genotypes can participate in future strategies to control TuYV in a neonicotinoid-free agriculture.
3. Discussion
In Europe, insecticides of the neonicotinoid family are no longer available to farmers [
18] and resistance to pyrethrinoids has been already described in numerous aphids species of economic importance including
M. persicae [
26]. Thus, the fine characterization of virus/aphid/rapeseed interactions involved in plant to plant transmission of TuYV appears to be essential for the implementation of future management strategies against this aphid-borne virus. The experiments carried out in this study allowed the analysis for different rapeseed cultivars of (i) viral infection key steps including infection rate, latency period, and quality of infected plants as sources for viral transmission, and (ii) aphid behavior including fecundity, antibiosis, and antixenosis phenomenon. The characterization of these important epidemiological parameters provides a better understanding of the processes that occur from the emergence of the first infected plant to the spread of TuYV in field, highlights the similarities between the cv. Quizz and the TuYV-susceptible cv. DK Exception (used as referent in this study) for their abilities to host aphids and virus, and accurately describes the partial TuYV-resistance phenotype carried by the rapeseed cv. Architect.
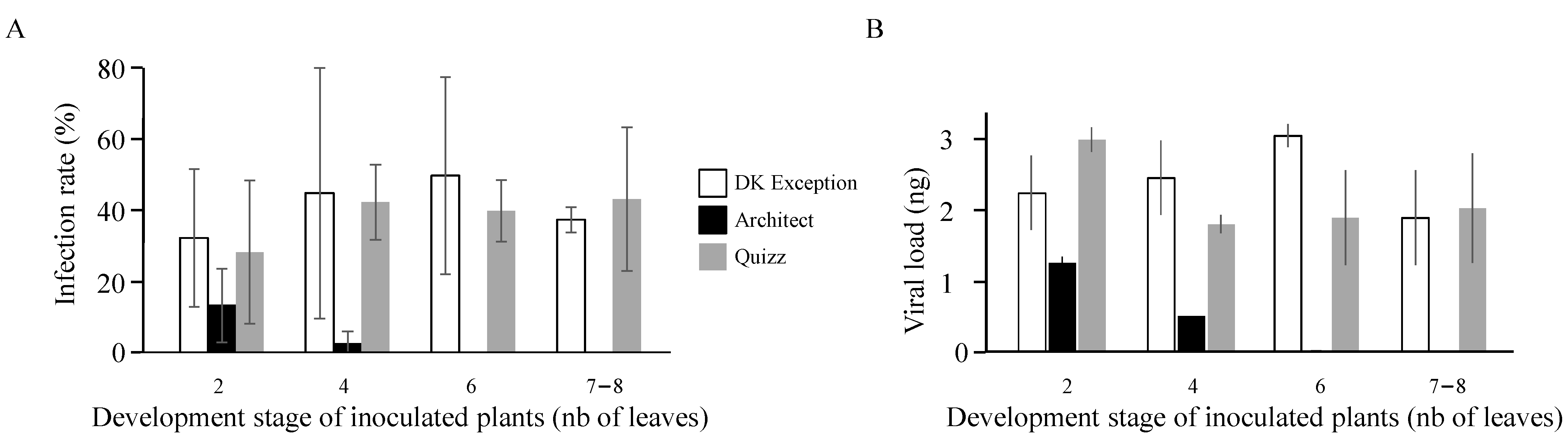
As turnip yellows virus is exclusively transmitted by aphids, the first step of the infection at the field scale corresponds to the introduction of the virus (primary infection) by viruliferous aphids flying from reservoirs. The low susceptibility of the cv. Architect compared to the TuYV-susceptible reference cv. DK Exception reduces the success of these primary infections by 2.4 and 18 times for inoculations occurring on plants at 2- and 4-leaves stages, respectively. Moreover, none of the cv. Architect plants inoculated at 6- and 7–8-leaves stages were infected under our experimental conditions, suggesting the expression of a resistance phenotype at these early growing stages. In France, the sowing period for rapeseed is from the end of August to the beginning of September. After sowing, four to fifteen days are needed for germinated seeds to produce cotyledons. Then, four to eight days later, the plantlets develop their first true leaf and quickly (at a rate of close to 2 new leaves per week) establish a rosette of about twenty-thirty leaves. Thus, under standard French growing conditions, the 6-leaves stage is reached by rapeseed plantlets at the 5th–6th weeks after sowing. Based on the resistance phenotype expressed from 6-leaves stage by cv. Architect, the latter is exposed to TuYV infections from the emergence of seedling to mid-October while the susceptible cv. DK Exception is exposed to virus infections for a longer period as illustrated by the infection rate (37.5–50%) obtained with plants inoculated at the 6- and 7–8-leaves stages. The intensity of aphid flights and the frequency of viruliferous aphids in autumn is known to vary in space and time [
27]. Winged
Myzus persicae migrate from the environment to rapeseed fields from few days after sowing (i.e., at the emergence of cotyledons) to the cold period of winter season. However, mild winter temperatures associated to climate change extend the period of aphid migration, increase the proportion of winged aphids in populations, stimulate aphid flight activity, and delay last winter flights [
28,
29]. Thus, growing the cv. Architect rapeseed with its reduced period of exposure to the risk of TuYV infection can be considered as a good strategy to limit viral incidence due to primary infections. However, primary infections usually represent only a small part of the overall epidemic process at the field scale. Indeed, the first infected plants (from primary infections) play important role in the spread (secondary infections) of the disease within the crop [
30]. Thus, in addition to the introduction of viruliferous aphids in fields, the spread of the disease depends on both the population dynamics of aphids and the latency period of infected plants. The measures related to the monitoring of rapeseed/
M. persicae interactions produced during this study did not reveal significant variation in the number of aphids produced after the maintenance of a single L
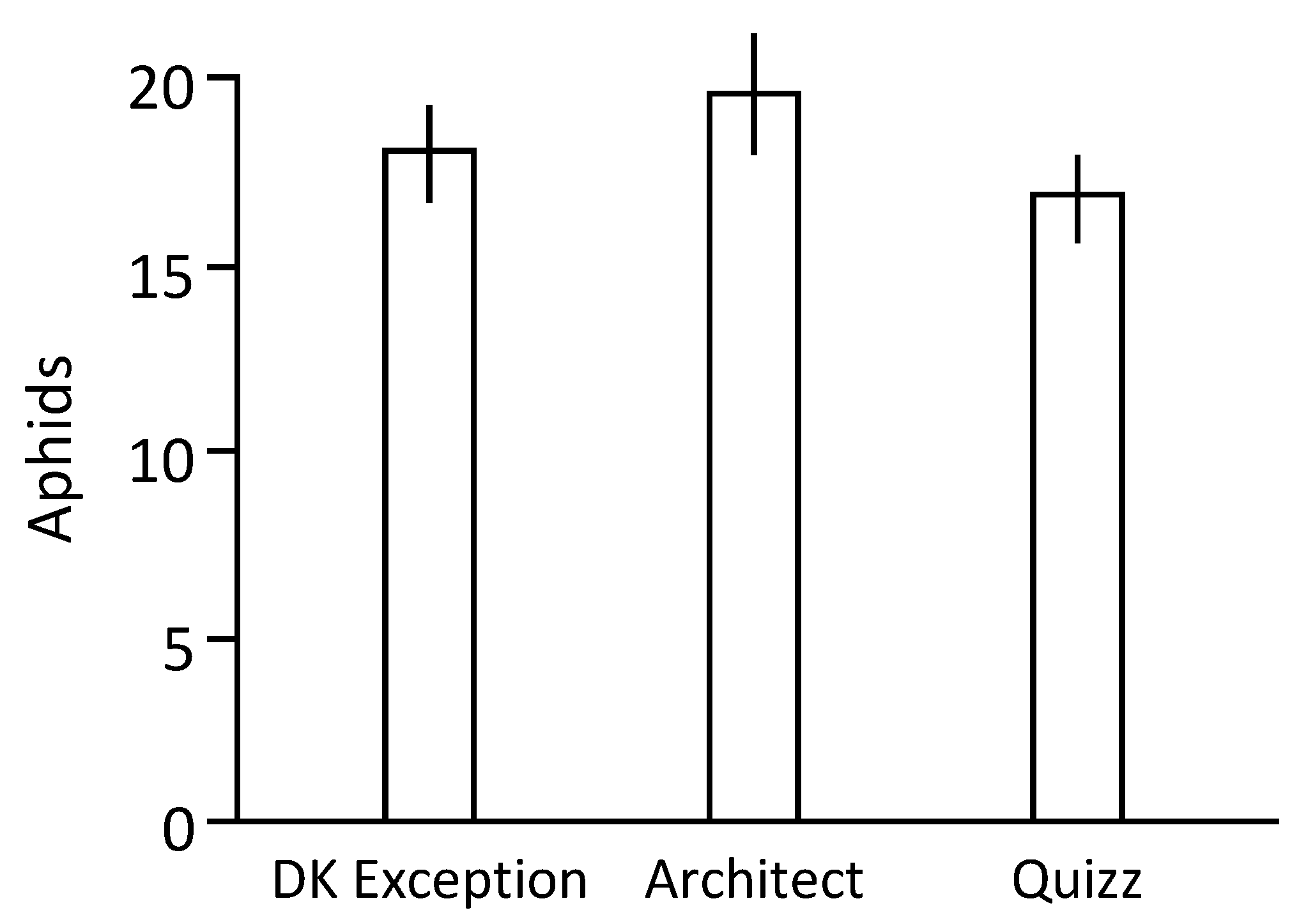
1 larvae for 2 weeks on the tested rapeseed genotypes, suggesting that these genotypes are equivalent to host aphid populations. However, data indicate differences in the antixenosis phenomenon as denoted by the more efficient (1.14 times when compared to data associated to cv. DK Exception) colonization of cv. Architect plants by aphids in the arena-based experiments. This suggests that a field grown with cv. Architect can be associated to a higher proportion of plants infested by aphids, and consequently could be more exposed to plant-to-plant transmissions of aphid-borne virus.
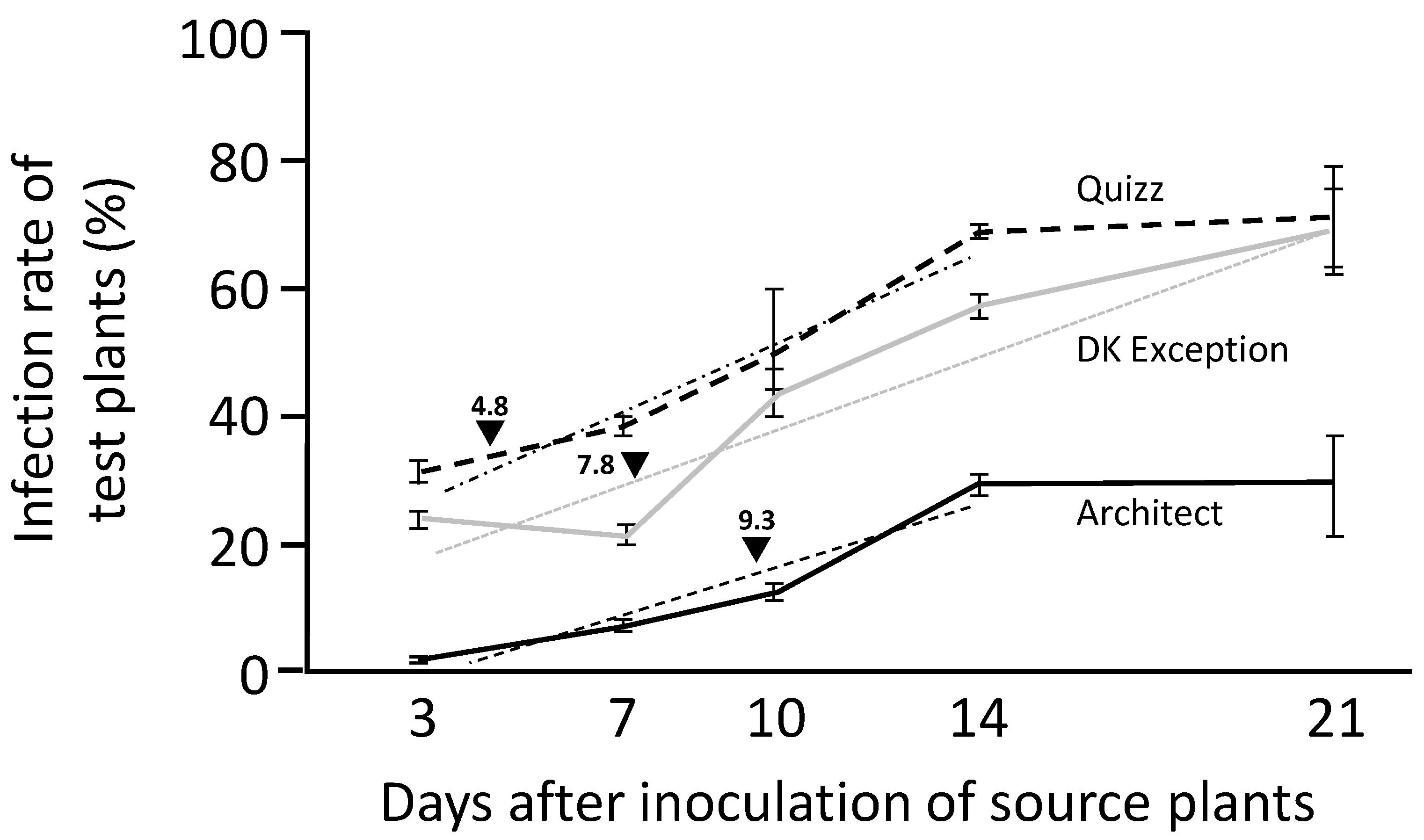
Viruliferous winged aphids are able to introduce TuYV into the field by feeding on visited plants. TuYV is transmitted in a persistent non-propagative manner [
11]. To initiate the infection, TuYV particle(s) must be introduced in the phloem of a susceptible host. Thus, success of TuYV transmission depends on feeding behavior of aphids (i.e., characteristics of phloem punctures) which could be different between host genotypes. Each individual of the progeny produced by an adult aphid (viruliferous or not) is virus-free until it has the opportunity to feed on an infectious plant. Thus, the quality of an infected plant as a source of virus for aphid-mediated transmission and the duration of the latency period (delay for an infected plant to acquire the infectious status) directly influence the ability of virus-free aphids (born in the field or flying from outside) to acquire and spread the disease from primary infected plants. Results showed that cv. Architect is a poor viral source for transmission (maximum source value of infected rapeseed plants is 2.3 times lower for cv. Architect than for cv. DK Exception) and infected cv. Architect plants require a longer period to acquire the infectious status (mid-duration of the latency period is 1.5 days longer when compared to cv. DK Exception). Taken together with the lower TuYV-susceptibility of cv. Architect described in this study, these epidemiological parameters can partly explain the low prevalence of infected cv. Architect plants reported in the arena test carried out in this work (3% (+/− 0.5%) infected cv. Architect plants vs. 38.5% (+/−3.2%) infected cv. DK Exception plants) and in data from field trials performed under low, standard and high virus pressures [
25].
This fine characterization of interactions between members of the turnip yellows pathosystem allows to conclude that the partial TuYV-resistance of cv. Architect plants reduces the efficiency of both primary infections due to the migration of viruliferous aphids that occurs in autumn (lower infection rate and resistance phenotype expressed from the 6-leaves stage) and secondary spread of the disease in fields (longer latent period and lower source value for infected plants). These characteristics, which allow the maintenance of a greater proportion of healthy plants in the plots by targeting several steps of the epidemiological process at the field scale, make cv. Architect an interesting genetic support for the development and/or optimization of future strategies to control TuYV in rapeseed production. However, to complete the characterization of the partial resistance phenotype of cv. Architect, it remains important (i) to evaluate the behavior of this genotype against viral pressures differing in intensity, frequency, and duration (reflecting the multiple flights of winged aphids in autumn), and (ii) to test its durability against the genetic diversity and the evolution of TuYV. This obviously will be the next steps of our work on TuYV/M. persicae/rapeseed cv. Architect interactions.
4. Materials and Methods
4.1. Plants, Insects, and Virus
Rapeseed cvs. DK Exception (susceptible to TuYV), Architect (described for its TuYV-resistant phenotype), and Quizz (described for its TuYV-tolerant phenotype) were used in the experiments. Seeds were sown in N2 soil (Neuhaus® Huminsubstrat N2, Klasmann-Deilmann, Geeste, Germany) and maintained in a sowing chamber (day/night: 12 h/12 h, 23 °C/20 °C) for five days. Then, seedlings were transferred either individually in pots (TEKU® Pöppelmann France S.A.S., Rixheim, France) 7 × 7 cm and 9 × 9 cm or in pools of 30 plants in trays (L × W × H: 30 × 25 × 7 cm) depending on the experiments. Plantlets were grown in a growth chamber (day/night: 16 h/8 h, 25 °C/20 °C) for 7 days before being used in the experiments.
The
Myzus persicae clone Mp34 [
31] was used for virus transmissions. Virus-free Mp34 was maintained in a growth chamber in small plexiglass cages in the presence of healthy rapeseed plants cv. DK Exception.
Isolate PS of turnip yellows virus (TuYV-PS, collected in department of Ain, France, 2018) was maintained on rapeseed cv. DK Exception plants in small plexiglass cages in the presence of Mp34.
4.2. Infection Rates
Plantlets at different growing stages (2, 4, 6, and 7–8 leaves) were used to assess the susceptibility of rapeseed genotypes to TuYV-PS. Using a brush, viruliferous aphids (L2/L4 stages; 2 aphids/plant) were deposited on test plants. Then, plants were covered with micro-perforated plastic bags. At the end of the inoculation access period (i.e., 2 h), the viruliferous aphids were manually removed from plants and the latter were treated with an insecticide (Pirimor® 0.1% v/v, Syngenta®, Basel, Switzerland) before being transferred in a growth chamber. Three weeks later, plants were sampled and the presence of TuYV in each plant was assessed by enzyme-linked immuno-sorbent assay (ELISA). This complete procedure was replicated at least twice using sets of 20 plants/genotype/replication.
4.3. Production of Aphid Colonies
Larvae of M. persicae Mp34 were obtained from synchronized progenies produced on rapeseed cv. DK Exception. L1 larvae were deposited on test plants at 2-leaves stage (one larva/plant). Then, plants were individually covered by micro-perforated plastic bags and kept in a growth chamber for 9 days. At the end of this period, the number of aphids present on each plant was counted. This procedure was replicated 3 times using sets of 20 plants/genotype/replication.
4.4. Arena Tests
Thirty rapeseed plantlets (at 2-leaves stage), planted in 6 rows of 5 plants each (in trays (L × W × H: 30 × 25 × 7 cm)), constitute the arena design used in the experiment. L3/L4 larvae of viruliferous Mp34 were deposited (1 larva/arena) at the center of the arena. The whole arena (i.e., the 30 plants and the single aphid) was covered by insect proof net and maintained in a growth chamber for 14 days. At the end of this period, the number of aphids present on each plant was counted. Then, plants were sprayed with insecticide (Pirimor® 0.1% v/v), maintained in the growth chamber for 3 weeks, and individually tested for the presence of TuYV by ELISA. This experimental procedure was carried out with 2 arenas per genotype and replicated 4 times.
4.5. Latency Period of Infected Plants
Viruliferous Mp34 aphids (L2/L4 stages) were deposited (5 aphids/plant) for 24 h on healthy rapeseed plantlets (cv. DK Exception, Architect and Quizz) at two-leaves stage. At the end of this inoculation access period (IAP), the aphids were manually removed from plants. These aphid-free TuYV-inoculated plants were considered in the experiment as ‘source’ plants. At 5 dates after inoculation of source plants (i.e., at 3, 7, 10, 14, and 21 days), non viruliferous Mp34 aphids (L1/L4 stage, at least 30 aphids/source plant) were contained for 24 h acquisition access period (AAP) on source plants. At the end of AAP, aphids from each source plant were transferred to healthy cv. DK Exception plants (2 aphids/plant) for 24 h IAP. These plants were considered as ‘test’ plants. At the end of IAP, test plants were treated with Pirimor® (0.1% v/v). All source and test plants were maintained in a growth chamber for 3 weeks after the end of their respective IAP. Then, plants were tested by ELISA (test plants) or RT-PCR (source plants) for the presence of TuYV (see below for diagnostic procedure). This procedure was carried out with 8 TuYV-infected source plants for each genotype/date combination and 10 test plants/source plant. The whole experiment was replicated 3 times.
4.6. Serological and Molecular Detection of TuYV in Plants
Each plants (i.e., all leaves) sampled for ELISA [
32] was ground using a Pollähne press (MEKU
®, Wennigsen, Germany). Plant material sampled for RT-PCR (i.e., pools of leaf discs from each leaf) was placed in sterile microtubes containing 1 metal ball (4 mm in diameter) and then ground in the presence of liquid nitrogen using the MM301 mill (Retsch, Hann. Münden, Germany).
For ELISA, wells of a microtiter plate (NUNC, Maxisorp) were incubated at 37 °C for 4 h with polyclonal anti-TuYV antibodies (LOEWE®, Sauerlach, Germany) previously diluted (1/400 (v/v)) in carbonate buffer (15 mM Na2CO3, 35 mM NaHCO3, pH = 9.6). Between each step of the ELISA protocol, the plates were washed 3 times with PBST buffer (137 mM NaCl, 8 mM Na2HPO4, 12H2O, 2,7 mM KCl, 1.5 mM KH2PO4, 0.05% (v/v) Tween 20), 2% (w/v) polyvinylpyrrolidone 40T). One hundred µl of plant sap was deposited in the wells and incubated overnight at 4 °C. Alkaline phosphatase coupled antibody (100 µL) diluted (1/400 (v/v)) in conjugated buffer (PBST buffer, 2% (w/v) ovalbumin) was deposited in wells and incubated for 4 h at 37 °C. After a final wash, 100 µL of diethanolamine (1N, pH = 9.8) containing p-nitrophenylphosphate (1 mg/mL) was deposited in wells and plate was incubated at room temperature and in the dark for 2 h. Then, optical density of each well was measured at 405 nm (OD405) using a spectrophotometer (Multiskan™ FC; Thermo Scientific™, Waltham, Massachusetts, USA). The positive threshold of the test was twice the OD405 value of healthy plant controls with a minimum value of OD405 = 0.1. A serial dilution of a semi-purified TuYV-PS fraction (749 ng/µL) was prepared and used in each plate to make it possible the transformation of raw OD405 values in viral load in tested samples.
Total RNA from rapeseed leaves was extracted using the Monarch
® Total RNA Miniprep Kit (New England Biolabs
®, Ipswich, MA, USA). The presence of TuYV in 1 µL RNA fraction was tested using the OneTaq
® One-step RT-PCR Kit (New England Biolabs
®) according to the manufacturer’s recommendations, a melting temperature of 55 °C, and the primers CPBM+ (5’-atgaatacggtcgtgggtaggag-3’) and CPBM- (5’-ccagctatcgatgaagaaccattg-3’) [
33]. After amplification, 5 µL of the reaction mixture was analyzed by agarose gel electrophoresis (1.5%
w/
v). Amplified products were revealed in the presence of ethidium bromide using a transilluminator.
4.7. Statistical Analyses
Statistical analyses were performed using R version 3.6.0 [
34]. The numbers of infected and healthy plants were analyzed using a quasibinomial generalized linear model (GLM). The effects were tested by comparing nested models using a Student test (drop1 function). The comparison of the viral loads was performed by using the Kruskal–Wallis test.