Effect of Sugarcane Cultivars Infected with Sugarcane Yellow Leaf Virus (ScYLV) on Feeding Behavior and Biological Performance of Melanaphis sacchari (Hemiptera: Aphididae)
Abstract
:1. Introduction
2. Results
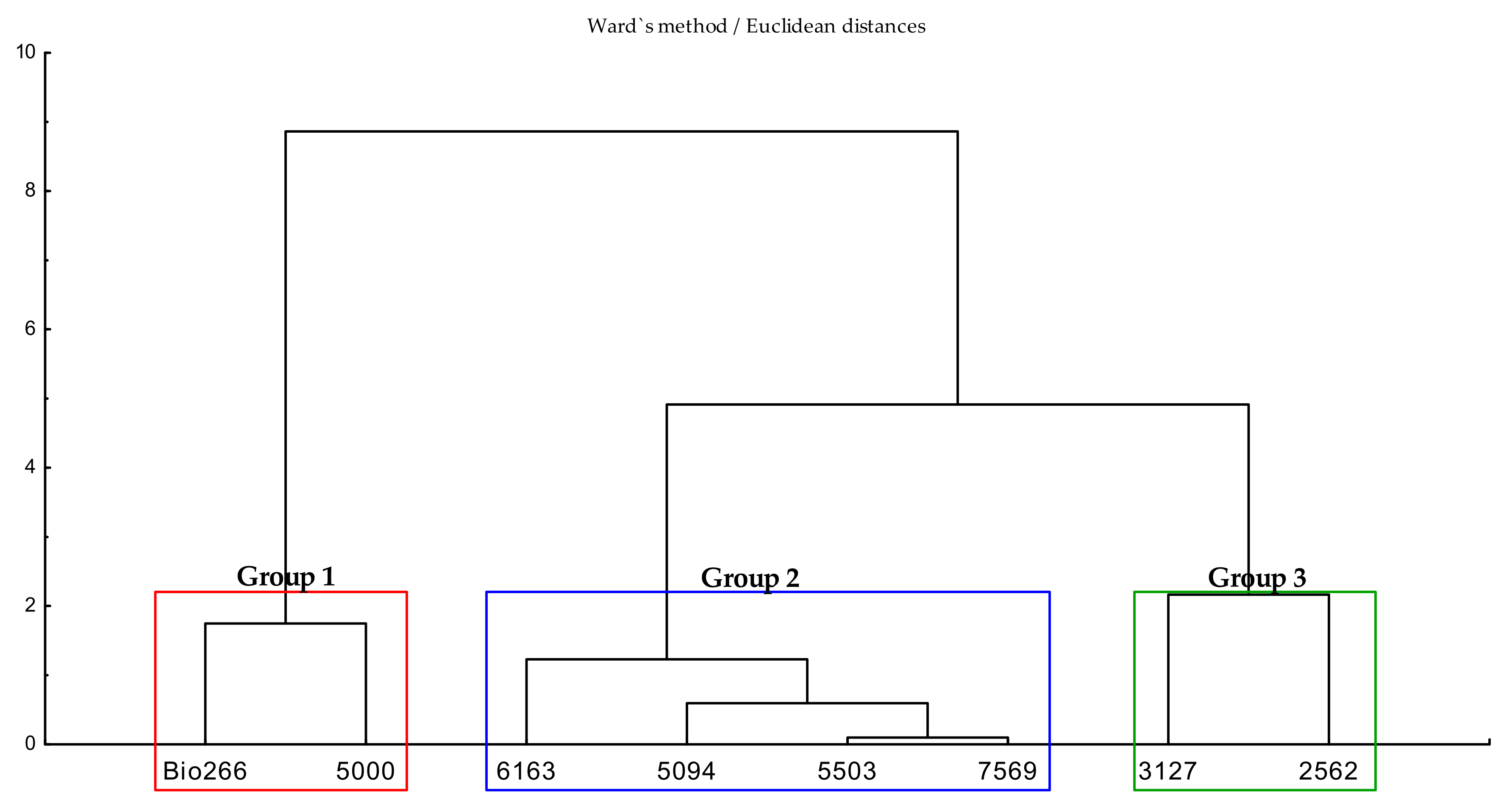
2.1. Effects of ScYLV-Infected Sugarcane Cultivars on the Life History of M. sacchari
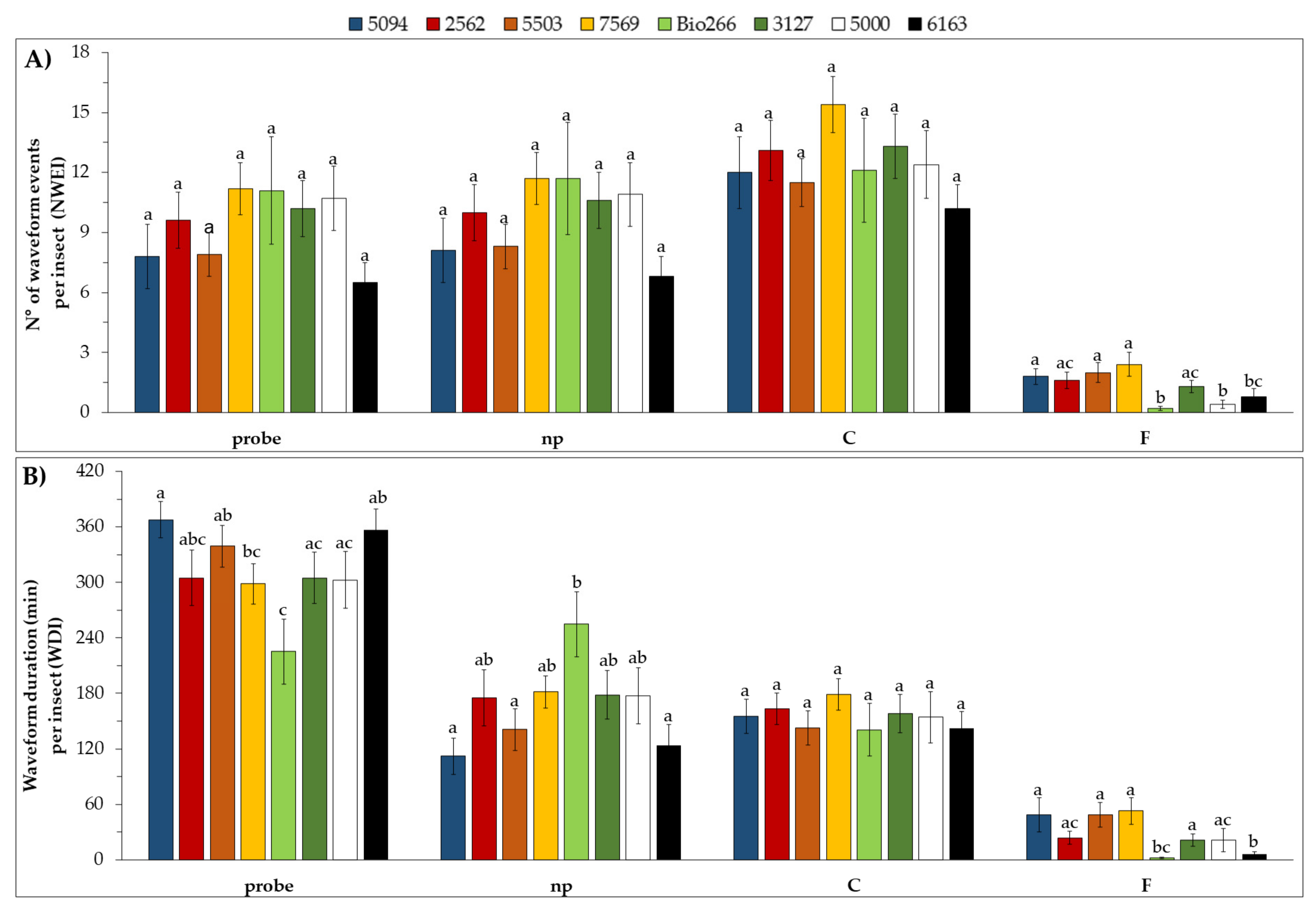
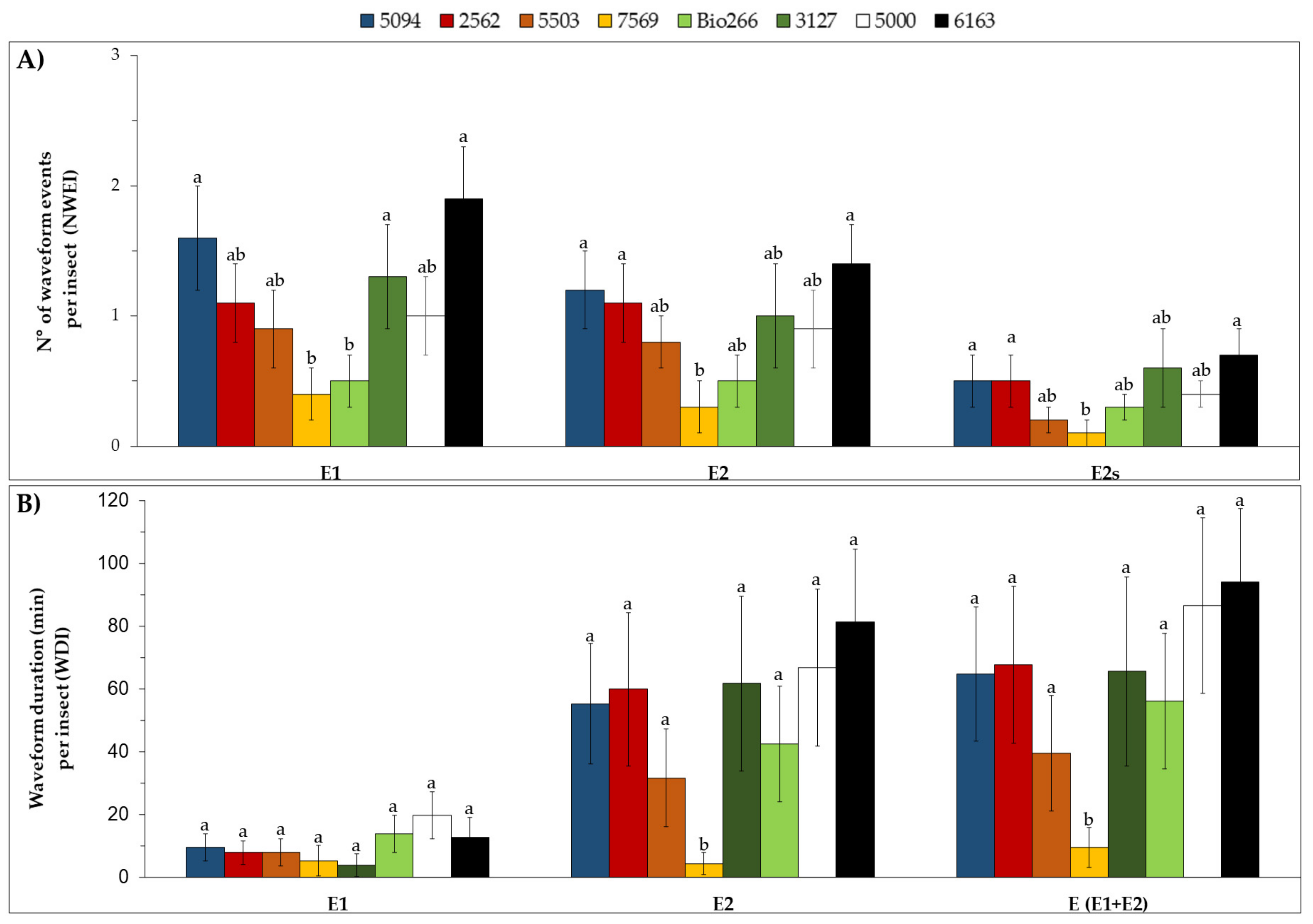
2.2. Stylet Activities and Probing Behavior of M. sacchari in ScYLV-Infected Sugarcane Cultivars
3. Discussion
4. Materials and Methods
4.1. Aphid Colony and Test Plants
4.2. Effects of ScYLV-Infected Sugarcane Cultivars on the Life History of M. sacchari
4.3. Stylet Activities and Probing Behavior of M. sacchari in ScYLV-Infected Sugarcane Cultivars
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chinnaraja, C.; Viswanathan, R. Quantification of sugarcane yellow leaf virus in sugarcane following transmission through aphid vector Melanaphis sacchari. Virus Dis. 2015, 26, 237–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garzo, E.; Moreno, A.; Plaza, M.; Fereres, A. Feeding behavior and virus-transmission ability of insect vectors exposed to systemic insecticides. Plants 2020, 9, 895. [Google Scholar] [CrossRef] [PubMed]
- Cronje, C.P.R.; Bailey, R.A.; McFarlane, K. Update on the occurrence of yellow leaf syndrome in the South African sugar industry and evidence for the causal agent. Proc. S. Afr. Sugar Technol. Ass. 1998, 72, 81–84. [Google Scholar]
- Comstock, J.C.; Irey, M.S.; Lockhart, B.E.L.; Wang, Z.K. Incidence of yellow leaf syndrome in CP cultivars based on polymerase chain reaction and serological techniques. Sugar Cane 1998, 4, 21–24. [Google Scholar]
- Gonçalves, M.C.; Vega, J.; Oliveira, J.G.; Gomes, M.M.A. Sugarcane yellow leaf virus infection leads to alterations in photosynthetic efficiency and carbohydrate accumulation in sugarcane leaves. Fitop. Brasil. 2005, 30, 10–16. [Google Scholar] [CrossRef]
- Gonçalves, M.C. Doenças causadas por vírus. In Cana-de-Açúcar; Dinardo-Miranda, L.L., Vasconcelos, A.C.M., Landell, M.G.A., Eds.; Instituto Agronômico de Campinas: São Paulo, Brasil, 2008; Volume 1, pp. 150–165. [Google Scholar]
- Gonçalves, M.C.; Pinto, L.R.; Souza, S.C.; Landell, M.G.A. Virus Diseases of Sugarcane. A Constant Challenge to Sugarcane Breeding in Brazil. Funct. Plant Sci. Biotechnol. 2012, 6, 108–116. [Google Scholar]
- Zhu, Y.J.; Lim, S.T.; Schenck, S.; Arcinas, A.; Komor, E. RT-PCR and quantitative real-time RT-PCR detection of Sugarcane yellow leaf virus (SCYLV) in symptomatic and asymptomatic plants of Hawaiian sugarcane cultivars and the correlation of SCYLV titre to yield. Eur. J. Plant Pathol. 2010, 127, 263–273. [Google Scholar] [CrossRef]
- Ramouthar, P.V.; Berry, S.D.; Rutherford, R.S.; MCFarlane, S.A. Yield loss due to sugarcane yellow leaf virus and its prevalence in the South African sugar industry. Proc. S. Afr. Sugar Technol. Ass. 2013, 86, 244–254, ISSN 1028. [Google Scholar]
- Boukari, W.; Kaye, C.; Wei, C.; Hincapie, M.; LaBorde, C.; Irey, M.; Rott, P. Field infection of virus-free sugarcane by Sugarcane yellow leaf virus and effect of yellow leaf on sugarcane grown on organic and on mineral soils in Florida. Plant Dis. 2019, 103, 2367–2373. [Google Scholar] [CrossRef]
- Grisham, M.P.; Eggleston, G.; Hoy, J.W.; Viator, R.P. The effect of Sugarcane yellow leaf virus infection on yield of sugarcane in Louisiana. Sugar Cane Int. 2009, 27, 3–91, ISSN 0265-7406. [Google Scholar]
- Nault, L.R. Arthropod transmission of plant viruses: A new synthesis. Ann. Entomol. Soc. Am. 1997, 90, 521–541. [Google Scholar] [CrossRef]
- Ingwell, L.L.; Eigenbrode, S.D.; Bosque-Pérez, N.A. Plant viruses alter insect behaviour to enhance their spread. Sci. Rep. 2012, 2, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmo-Sousa, M.; Moreno, A.; Plaz, M.; Garzo, E.; Fereres, A. Cucurbit aphid-borne yellows vírus (CABYV) modifies the alighting, settling and probing behaviour of its vector Aphis gossypii favouring its own spread. Ann. Appl. Biol. 2016, 169, 284–297. [Google Scholar] [CrossRef]
- Whitfield, A.E.; Falk, B.W.; Rotenberg, D. Insect vector-mediated transmission of plant viruses. Virology 2015, 479, 278–289. [Google Scholar] [CrossRef] [Green Version]
- Fereres, A.; Raccah, B. Plant Virus Transmission by Insects. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 1–12. [Google Scholar] [CrossRef] [Green Version]
- Fereres, A. Aphid behavior and the transmission of noncirculative viruses. In Vector-Mediated Transmission of Plant Pathogens; Brown, J.K., Ed.; APS Press: St. Paul, MN, USA, 2016; pp. 31–45. [Google Scholar] [CrossRef]
- Hull, R. Plant Virology, 5th ed.; Elsevier Academic Press: San Diego, CA, USA, 2014; p. 1120. ISBN 978-0-12-384871-0. [Google Scholar]
- Su, Q.; Preisser, E.L.; Zhou, X.M.; Xie, W.; Liu, B.M.; Wang, S.L.; Wu, Q.J.; Zhang, Y.J. Manipulation of host quality and defence by a plant vírus improves performance of whitefly vectors. J. Econ. Entomol. 2015, 108, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Fereres, A.; Peñaflor, M.F.G.; Favaro, C.F.; Azevedo, K.E.X.; Landi, C.H.; Maluta, N.K.P.; Bento, J.M.S.; Lopes, J.R.S. Tomato infection by whitefly-transmitted circulative and non-circulative viruses induce contrasting changes in plant volatiles and vector behaviour. Viruses 2016, 8, 225. [Google Scholar] [CrossRef] [Green Version]
- Bosque-Pérez, N.A.; Eigenbrode, S.D. The influence of virus-induced changes in plants on aphid vectors: Insights from luteovirus pathosystems. Virus Res. 2011, 159, 201–205. [Google Scholar] [CrossRef]
- Fang, Y.; Jiao, X.; Xie, W.; Wang, S.; Wu, Q.; Shi, X.; Chen, G.; Su, Q.; Yang, H.; Pan, H.; et al. Tomato yellow leaf curl vírus alters the host preferences of its vector Bemisia tabaci. Sci. Rep. 2013, 3, 2876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mas, F.; Vereijssen, J.; Suckling, D.M. Influence of the pathogen Candidatus Liberibacter solanacearum on tomato host plant volatiles and psyllid vector settlement. J. Chem. Ecol. 2014, 40, 1197–1202. [Google Scholar] [CrossRef]
- Dáder, B.; Gwynn-Jones, D.; Moreno, A.; Winters, A.; Fereres, A. Impact of UV-A radiation on the performance of aphids and whiteflies and on the leaf chemistry of their host plants. J. Photoch. Photobiol. B Biol. 2014, 138, 307–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Davis, T.S.; Eigenbrode, S.D. Aphid behavioral responses to vírus-infected plants are similar despite divergente fitness effects. Entomol. Exp. Appl. 2014, 153, 246–255. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Electronic recording of penetration behavior by aphids. Entomol. Exp. Appl. 1978, 24, 521–530. [Google Scholar] [CrossRef]
- Sun, M.; Voorrps, R.E.; Steenhuis-Broers, G.; van’t Westende, W.; Vosman, B. Reduced phloem uptake of Myzus persicae on an aphid resistant pepper accession. BMC Plant Biol. 2018, 18, 138. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Electrical nature of recorded signals during stylet penetration by aphids. Entomol. Exp. Appl. 1985, 38, 177–186. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Eletrical recording of stylet penetration activities. In Aphis, Their Biology, Natural Enemies and Control; Minks, A.K., Harrewijn, P., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1988; Volume 2, pp. 95–108. [Google Scholar]
- Van Helden, M.; Tjallingii, W.F. Experimental design and analysis in EPG experiments with emphasis on plant resistance research. In Principles and Applications of Electronic Monitoring and Other Techniques in the Study of Homopteran Feeding Behavior; Walker, G.P., Backus, E.A., Eds.; Thomas Say Publications in Entomology; The American Entomological Society: Annapolis, MD, USA, 2000; pp. 144–172. [Google Scholar]
- Jiménez, J.; Tjallingii, W.F.; Moreno, A.; Fereres, A. Newly distinguished cell punctures associated with transmission of the semipersistent phloem-limited Beet yellows virus. J. Virol. 2018, 92, e01076–e01118. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, J.; Garzo, E.; Alba-Tercedor, J.; Moreno, A.; Fereres, A.; Walker, G.P. The phloem-pd: A distinctive brief sieve element stylet puncture prior to sieve element phase of aphid feeding behavior. Arth. Plant Inter. 2019, 14, 67–78. [Google Scholar] [CrossRef]
- Kaiser, H.F. A note on Guttman’s lower bound for the number of common factors. Br. J. Stat. Psychol. 1961, 14, 1–12. [Google Scholar] [CrossRef]
- Roosien, B.K.; Gomulkiewicz, R.; Ingwell, L.L.; Bosque-Pérez, N.A.; Rajabaskar, D.; Eigenbrode, S.D. Conditional vector preference aids the spread of plant pathogens: Results from a model. Environ. Entomol. 2013, 42, 1299–1308. [Google Scholar] [CrossRef]
- Mauck, K.; Bosque-Pérez, N.A.; Eigenbrode, S.D.; De Moraes, C.M.; Mescher, M.C. Transmission mechanisms shape pathogen effects on host–vector interactions: Evidence from plant viruses. Funct. Ecol. 2012, 26, 1162–1175. [Google Scholar] [CrossRef]
- Eigenbrode, S.D.; Ding, H.; Shiel, P.; Berger, P.H. Volatiles from potato plants infected with potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera: Aphididae). Proc. R. Soc. Lond. B Biol. Sci. 2002, 269, 455–460. [Google Scholar] [CrossRef] [Green Version]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc. Natl. Acad. Sci. USA 2010, 107, 3600–3605. [Google Scholar] [CrossRef] [Green Version]
- Guarino, S.; Abbate, L.; Mercati, F.; Fatta Del Bosco, S.; Motisi, A.; Arif, M.A.; Cencetti, G.; Palagano, E.; Michelozzi, M. Citrus Varieties with Different Tolerance Grades to Tristeza Virus Show Dissimilar Volatile Terpene Profiles. Agronomy 2021, 11, 1120. [Google Scholar] [CrossRef]
- Chesnais, Q.; Couty, A.; Uzest, M.; Brault, V.; Ameline, A. Plant infection by two different viruses induce contrasting changes of vectors fitness and behavior. Insect Sci. 2017, 26, 86–96. [Google Scholar] [CrossRef] [Green Version]
- Maluta, N.K.P.; Garzo, E.; Moreno, A.; Lopes, J.R.S.; Fereres, A. Tomato yellow leaf curl virus benefits population growth of the Q biotype of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Neotrop. Entomol. 2014, 43, 385–392. [Google Scholar] [CrossRef]
- Maluta, N.K.P.; Fereres, A.; Lopes, J.R.S. Settling preferences of the whitefly vector Bemisia tabaci on infected plants varies with virus family and transmission mode. Entomol. Exp. Appl. 2017, 165, 138–147. [Google Scholar] [CrossRef]
- Smith, C.M. Plant Resistance to Arthropods: Molecular and Conventional Approaches; Springer: Dordrecht, The Netherlands, 2005; p. 423. ISBN 978-1-4020-3702-3. [Google Scholar]
- Limaje, A.; Hayes, C.; Armstrong, J.S.; Hoback, W.; Zarrabi, A.; Paudyal, S.; Burke, J. Antibiosis and tolerance discovered in USDA-ARS sorghums resistant to the sugarcane aphid (Hemiptera: Aphididae). J. Entomol. Sci. 2018, 53, 230–241. [Google Scholar] [CrossRef]
- Paudyal, S.; Armstrong, J.S.; Giles, K.L.; Payton, M.E.; Opit, G.P.; Limaje, A. Categories of resistance to sugarcane aphid (Hemiptera: Aphididae) among sorghum genotypes. J. Econ. Entomol. 2019, 112, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Fartek, B.; Nibouche, S.; Turpin, P.; Costet, R.e.y.n.a.u.d.B. Resistance to M. sacchari in the sugarcane cultivar R 365. Entomol. Exp. Appl. 2012, 144, 270–278. [Google Scholar] [CrossRef]
- Nibouche, S.; Costet, L.; Holt, J.R.; Jacobson, A.; Pekarcik, A.; Sadeyen, J.; Armstrong, J.S.; Peterson, G.C.; McLaren, N.; Medina, R.F. Invasion of sorghum in the Americas by a new sugarcane aphid (Melanaphis sacchari) superclone. PLoS ONE 2018, 13, e0196124. [Google Scholar] [CrossRef]
- Park, J.; Thomasson, J.A.; Gorman, Z.; Brewer, M.J.; Rooney, W.L.; Kolomiets, M.V. Multivariate analysis of sorghum volatiles for the fast screening of sugarcane aphid infestation. J. Asia-Pac. Entomol. 2020, 23, 901–908. [Google Scholar] [CrossRef]
- Rodríguez-Vélez, J.M.; Uribe-Mu, C.A.; Sarmiento-Cordero, M.A.; Rodríguez-Vélez, B.; Cruz-Rodríguez, J.A.; Contreras-Ramos, A.; Huerta-Martínez, F.M.; Arredondo-Bernal, H.C. Relationships among Melanaphis sacchari, Its Predator Insect Communities, and Weather Conditions in Sorghum Crops at Colima, Mexico. Southwest. Entomol. 2021, 46, 47–58. [Google Scholar] [CrossRef]
- He, W.B.; Li, J.; Liu, S.S. Differential profiles of direct and indirect modification of vector feeding behavior by a plant virus. Sci. Rep. 2015, 5, 1–8, doi101038/srep07682. [Google Scholar]
- Limburg, D.D.; Mauck, P.A.; Godfrey, L.D. Characteristics of beet yellows closterovirus transmission to sugar beets by aphis fabae. Phytopathology 1997, 87, 766–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, K.; Li, F.; Liang, P.; Chen, X.; Liu, Y.; Tang, Q.; Gao, X. RNA interference of Dicer-1 and Argonaute-1 increasing the sensitivity of Aphis gossypii Glover (Hemiptera: Aphididae) to plant allelochemical. Pestic. Biochem. Physiol. 2017, 138, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Tetreault, H.M.; Grover, S.; Scully, E.D.; Gries, T.; Palmer, N.A.; Sarath, G.; Louis, J.; Sattler, S.E. Global responses of resistant and susceptible sorghum (Sorghum bicolor) to sugarcane aphid (Melanaphis sacchari). Front. Plant Sci. 2019, 10, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, M.C.; Klerks, M.M.; Verbeek, M.; Vega, J.; Van den Heuvel, J.F.J.M. The use of molecular beacons combined with NASBA for the sensitive detection of Sugarcane yellow leaf virus. Eur. J. Plant Pathol. 2002, 108, 401–407. [Google Scholar] [CrossRef]
- Carmo-Sousa, M.; Moreno, A.; Garzo, E.; Fereres, A. A non-persistently transmitted-virus induces a pull–push strategy in its aphid vector to optimize transmission and spread. Virus Res. 2014, 186, 38–46. [Google Scholar] [CrossRef]
- Sarria, E.; Cid, M.; Garzo, E.; Fereres, A. Excel workbook for automatic parameter calculation of EPG data. Computers and Electronics in Agriculture. Comput. Electr. Agric. 2009, 67, 34–42. [Google Scholar] [CrossRef]
- Backus, E.A.; Cline, A.R.; Ellerseick, M.R.; Serrano, M.S. Lygus hesperus (Hemiptera: Miridae) feeding on cotton: New methods and parameters for analysis of nonsequential electrical penetration graph data. Ann. Entomol. Soc. Am. 2007, 100, 296–310. [Google Scholar] [CrossRef] [Green Version]
- González-Estrada, E.; Cosmes, W. Shapiro-Wilk test for skew normal distributions based on data transformations. J. Stat. Comput. Simul. 2019, 89, 3258–3272. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, Version 22.0; IBM Corp: Armonk, NY, USA, 2013. [Google Scholar]
- Kaiser, H.F. The varimax criterion for analytic rotation in factor analysis. Psychometrika 1958, 23, 187–200. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT User’s Guide, Version 9.3; SAS: Cary, NC, USA, 2013. [Google Scholar]
- Statsoft. Statistica: Data Analysis Software System, Version 7; Statsoft: Tulsa, OK, USA, 2007. [Google Scholar]

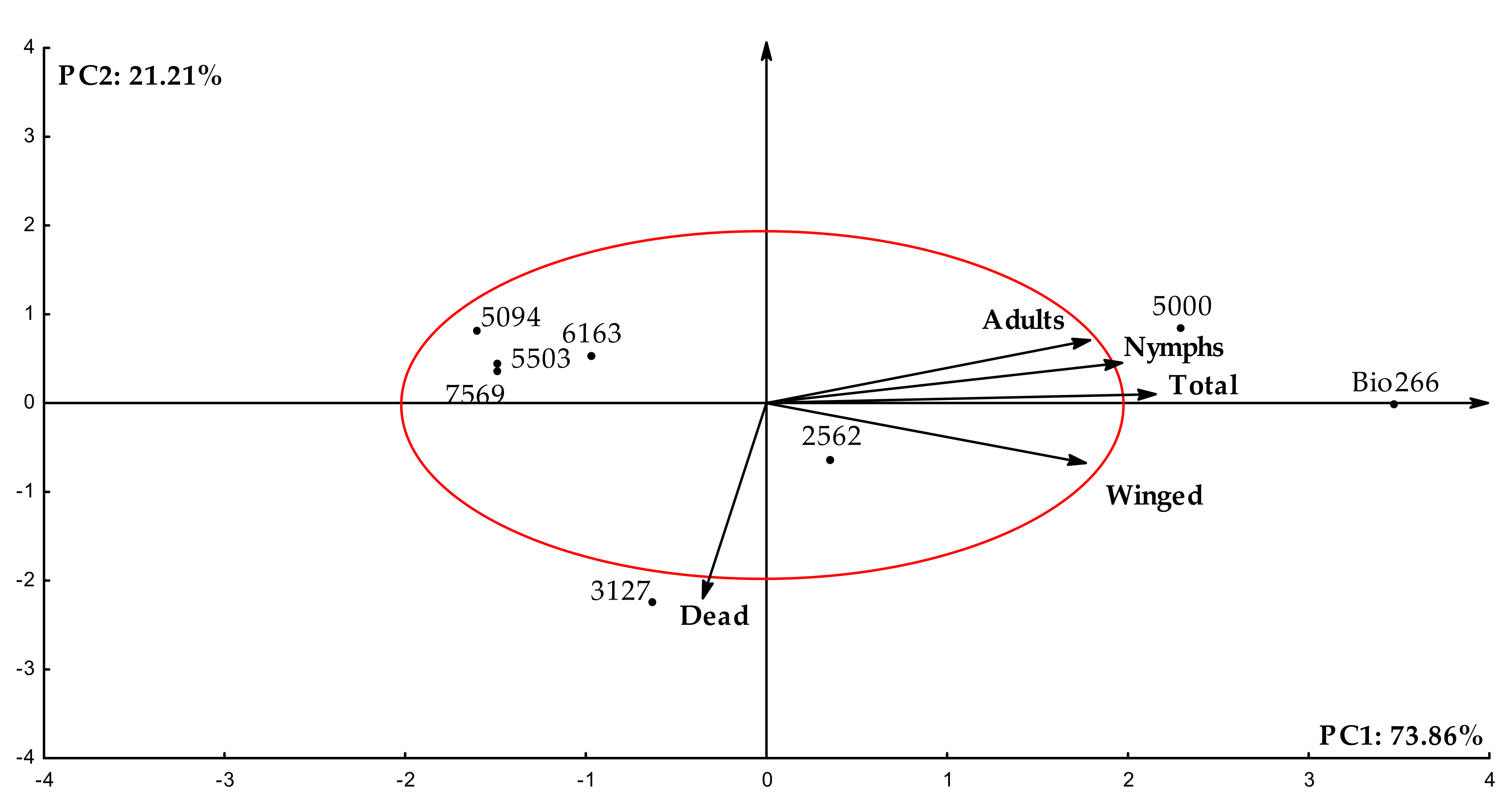
| Variables | PC1 | PC2 |
|---|---|---|
| Mean no. of nymphs | 0.98 | 0.04 |
| Mean no. of adults | 0.98 | 0.08 |
| No. winged | 0.86 | −0.29 |
| No. dead | −0.12 | −0.98 |
| Total | 0.99 | 0.01 |
| Eigenvalues | 3.69 | 1.06 |
| Variance (%) | 73.85 | 21.21 |
| Accumulated variance (%) | 73.85 | 95.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertasello, L.E.T.; Carmo-Sousa, M.; Prado Maluta, N.K.; Rossini Pinto, L.; Spotti Lopes, J.R.; Gonçalves, M.C. Effect of Sugarcane Cultivars Infected with Sugarcane Yellow Leaf Virus (ScYLV) on Feeding Behavior and Biological Performance of Melanaphis sacchari (Hemiptera: Aphididae). Plants 2021, 10, 2122. https://doi.org/10.3390/plants10102122
Bertasello LET, Carmo-Sousa M, Prado Maluta NK, Rossini Pinto L, Spotti Lopes JR, Gonçalves MC. Effect of Sugarcane Cultivars Infected with Sugarcane Yellow Leaf Virus (ScYLV) on Feeding Behavior and Biological Performance of Melanaphis sacchari (Hemiptera: Aphididae). Plants. 2021; 10(10):2122. https://doi.org/10.3390/plants10102122
Chicago/Turabian StyleBertasello, Luiz Eduardo Tilhaqui, Michele Carmo-Sousa, Nathalie K. Prado Maluta, Luciana Rossini Pinto, João R. Spotti Lopes, and Marcos Cesar Gonçalves. 2021. "Effect of Sugarcane Cultivars Infected with Sugarcane Yellow Leaf Virus (ScYLV) on Feeding Behavior and Biological Performance of Melanaphis sacchari (Hemiptera: Aphididae)" Plants 10, no. 10: 2122. https://doi.org/10.3390/plants10102122
APA StyleBertasello, L. E. T., Carmo-Sousa, M., Prado Maluta, N. K., Rossini Pinto, L., Spotti Lopes, J. R., & Gonçalves, M. C. (2021). Effect of Sugarcane Cultivars Infected with Sugarcane Yellow Leaf Virus (ScYLV) on Feeding Behavior and Biological Performance of Melanaphis sacchari (Hemiptera: Aphididae). Plants, 10(10), 2122. https://doi.org/10.3390/plants10102122








