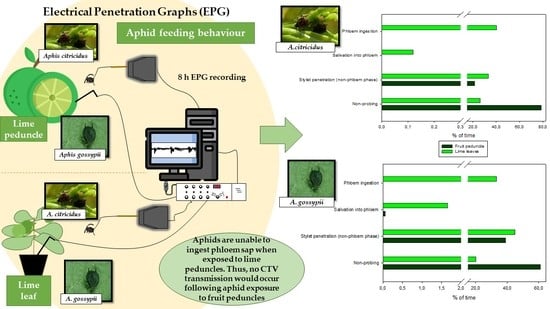
Aphids Are Unable to Ingest Phloem Sap from the Peduncles of Lime Fruits
Abstract
1. Introduction
2. Results
2.1. Probing Behaviour of Aphis citricidus on Fruit Peduncles and Lime Leaves
2.2. Probing Behaviour of Aphis gossypii on Fruit Peduncles and Lime Leaves
3. Discussion
4. Materials and Methods
4.1. Aphid Rearing and Test Plants
4.2. Stylet Activities of Aphis citricidus and Aphis gossypii on Lime Leaves and Fruit Peduncles
4.3. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO—Food and Agriculture Organization. FAOSTAT. Statistical Database. 2008. Available online: http://faostat.fao.org (accessed on 3 May 2020).
- FAO. Important Commodities in Agricultural Trade: Fruits and Vegetables. FAO Fact Sheets: Input for the WTO Ministerial Meeting in Cancún. No. 13. 2003. Available online: http://www.fao.org/tempref/docrep/fao/005/y4852e/y4852e12.pdf (accessed on 4 May 2020).
- Bar-Joseph, M.; Batuman, O.; Roistacher, C. The history of Citrus tristeza virus. In Citrus tristeza virus Complex and Tristeza Diseases; Karasev, A.V., Hilf, M.E., Eds.; APS Press: St. Paul, MN, USA, 2010; pp. 3–26. [Google Scholar]
- Karasev, V.A.; Bar-Joseph, M. Citrus tristeza virus and the taxonomy of Closteroviridae. In Citrus Tristeza Virus Complex and Tristeza Diseases; Karasev, A.V., Hilf, M.E., Eds.; APS Press: St. Paul, MN, USA, 2010; pp. 119–131. [Google Scholar]
- Martelli, G.P.; Agranovsky, A.A.; Bar-Joseph, M.; Boscia, D.; Candresse, T.; Coutts, R.H.A.; Dolja, V.V.; Hu, J.S.; Jelkmann, W.; Karasev, A.V.; et al. Family Closteroviridae. In Taxonomy, 9th Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2012; pp. 987–1001. [Google Scholar]
- Karasev, A.V. Genetic diversity and evolution of closteroviruses. Annu. Rev. Phytopathol. 2000, 38, 293–324. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Ayllón, M.A.; Kong, P.; Fernández, A.; Polek, M.L.; Guerri, J.; Moreno, P.; Falk, B.W. Genetic variation of Citrus tristeza virus isolates from California and Spain: Evidence for mixed infections and recombination. J. Virol. 2001, 75, 8054–8062. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ruiz, S.; Moreno, P.; Guerri, J.; Ambros, S. The complete nucleotide sequence of a severe stem pitting isolate of Citrus tristeza virus from Spain: Comparison with isolates from different origins. Arch. Virol. 2006, 151, 387–398. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Plant Health (PLH). Scientific opinion on the pest categorization of Citrus tristeza virus. EFSA J. 2014, 12, 3923. [Google Scholar] [CrossRef]
- EPPO (European and Mediterranean Plant Protection Organization). EPPO Global Database. 2017. Available online: https://gd.eppo.int (accessed on 3 May 2020).
- Dawson, W.O.; Garsey, S.M.; Tatineni, S.; Folimonova, S.Y.; Harper, S.J.; Gowda, S. Citrus tristeza virus–host interactions. Front. Microbiol. 2013, 4, 11–20. [Google Scholar] [CrossRef]
- Moreno, P.; Ambros, S.; Albiach-Martí, M.R.; Guerri, J.; Pena, L. Citrus tristeza virus: A pathogen that changed the course of the citrus industry. Mol. Plant Pathol. 2008, 9, 251–268. [Google Scholar] [CrossRef]
- Moreno, P.; Garnsey, S.M. Citrus tristeza diseases—A worldwide perspective. In Citrus Tristeza Virus Complex and Tristeza Diseases; Karasev, A.V., Hilf, M.E., Eds.; APS Press: St. Paul, MN, USA, 2016; pp. 27–49. [Google Scholar]
- Michaud, J.P. A review of the literature on Toxoptera citricida (Kirkaldi) (Homoptera: Aphididae). Fla. Entomol. 1998, 81, 37–61. [Google Scholar] [CrossRef]
- Yokomi, R.K.; Garnsey, S.M.; Civerolo, E.L.; Gumpf, D.J. Transmission of exotic Citrus tristeza virus isolates by a Florida colony of Aphis gossypii. Plant Dis. 1989, 73, 552–555. [Google Scholar] [CrossRef]
- Fereres, A. Aphid behavior and the transmission of noncirculative viruses. In Vector-Mediated Transmission of Plant Pathogens; APS Press: St. Paul, MN, USA, 2016; pp. 31–45. [Google Scholar] [CrossRef]
- Roistacher, C.N.; Moreno, P. The worldwide threat from destructive isolates of Citrus tristeza virus—A review. In International Organization of Citrus Virologists Conference Proceedings (1957–2010); University of California: Davis, CA, USA, 1991; Volume 11, p. 11. [Google Scholar]
- Gottwald, T.R. Concept in the epidemiology of Citrus Tristeza Virus. In Citrus Tristeza Virus Complex and Tristeza Diseases; Karasev, A.V., Hilf, M.E., Eds.; APS Press: St. Paul, MN, USA, 2010; pp. 119–131. [Google Scholar]
- Rocha-Peña, M.A.; Lee, R.F.; Lastra, R.; Niblett, C.L.; Ochoa-Corona, F.M.; Garnsey, S.M.; Yokomi, R.K. Citrus tristeza virus and its aphid vector Toxoptera citricida: Threats to citrus production in the Caribbean and Central and North America. Plant Dis. 1995, 79, 437–445. [Google Scholar] [CrossRef]
- Cambra, M.; Gorris, M.T.; Marroquín, C.; Román, M.P.; Olmos, A.; Martínez, M.C.; Hermoso de Mendoza, A.; López, A.; Navarro, L. Incidence and epidemiology of Citrus Tristeza Virus in the Valencian Community of Spain. Virus Res. 2000, 71, 85–95. [Google Scholar] [CrossRef]
- Marroquín, C.; Olmos, A.; Gorris, M.T.; Bertolini, E.; Martınez, M.C.; Carbonell, E.A.; de Mendoza, A.H.; Cambra, M. Estimation of the number of aphids carrying Citrus Tristeza Virus that visit adult citrus trees. Virus Res. 2004, 100, 101–108. [Google Scholar] [CrossRef]
- Yokomi, R.K.; Lastra, R.; Stoetzel, M.B.; Damsteegt, V.D.; Lee, R.F.; Garnsey, S.M.; Gottwald, T.R.; Rocha-Peña, M.A.; Niblett, C.L. Establishment of the brown citrus aphid (Homoptera: Aphididae) in Central America and the Caribbean Basin and transmission of Citrus Tristeza Virus. J. Econ. Entomol. 1994, 87, 1078–1085. [Google Scholar] [CrossRef]
- European Commission. Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community. Off. J. Eur. Commun. 2000, 169, 1–112. [Google Scholar]
- European Union. Regulation 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants, amending Regulations (EU) No 228/2013, (EU) No 652/2014 and (EU) No 1143/2014 of the European Parliament and of the Council and repealing Council Directives 69/464/EEC, 74/647/EEC, 93/85/EEC, 98/57/EC, 2000/29/EC, 2006/91/EC and 2007/33/EC. Off. J. 2016, 317, 4–104. [Google Scholar]
- EFSA Panel on Plant Health (PLH); Jeger, M.; Bragard, C.; Caffier, D.; Dehnen-Schmutz, K.; Gilioli, G.; Gregoire, J.C.; Jaques Miret, J.A.; MacLeod, A.; Navajas Navarro, M.; et al. Pest categorisation of Citrus tristeza virus (non-European isolates). EFSA J. 2017, 15, e05031. [Google Scholar] [CrossRef]
- Fereres, A.; Moreno, A. Behavioural aspects influencing plant virus transmission by homopteran insects. Virus Res. 2009, 141, 158–168. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Electrical recording of stylet penetration activities. In Aphids: Their Biology, Natural Enemies and Control; Minks, A.K., Harrewjin, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; Volume 2B, pp. 95–108. [Google Scholar]
- Walker, G.P.; Backus, E.A. Principles and applications of electronic monitoring and other techniques in the study of homopteran feeding behavior. In Proceedings of the International Congress of Entomology 1992, Beijing, China, 28 June–4 July 1992; Entomological Society of America: Annapolis, MD, USA, 2000. [Google Scholar]
- Fereres, A.; Raccah, B. Plant Virus Transmission by Insects; eLS John Wiley and Sons Ltd.: Chiclester, UK, 2015. [Google Scholar] [CrossRef]
- Johnson, D.D.; Walker, G.P.; Creamer, R. Stylet penetration behavior resulting in inoculation of a semipersistently transmitted Closterovirus by the whitefly Bemisia argentifolii. Entomol. Exp. Appl. 2002, 102, 115–123. [Google Scholar] [CrossRef]
- Wayadande, A.C.; Nault, L.R. Leafhopper probing behavior associated with maize chlorotic dwarf virus transmission to maize. Phytopathology 1993, 83, 522–526. [Google Scholar] [CrossRef]
- Jiménez, J. Non-Circulative Virus Transmission by Aphids: New Insights into the Mechanisms of Transmission and the Interference for Retention Sites in the Vector. Ph.D. Thesis, E.T.S. de Ingeniería, Agronómica, Alimentaria y de Biosistemas (UPM), Madrid, Spain, 2019. [Google Scholar]
- Gray, S.M.; Power, A.G.; Smith, D.M.; Seaman, A.J.; Altman, N.S. Aphid transmission of barley yellow dwarf virus: Acquisition access periods and virus concentration requirements. Phytopathology 1991, 81, 539–545. [Google Scholar] [CrossRef]
- Bertolini, E.; Moreno, A.; Capote, N.; Olmos, A.; De Luis, A.; Vidal, E.; Pérez-Panadés, J.; Cambra, M. Quantitative detection of Citrus tristeza virus in plant tissues and single aphids by real-time RT-PCR. Eur. J. Plant Pathol. 2008, 120, 177–188. [Google Scholar] [CrossRef]
- Zhao, R.; He, Y.; Lu, Z.; Chen, W.; Zhou, C.; Wang, X.; Li, T. An analysis of the feeding behavior of three stages of Toxoptera citricida by DC electrical penetration graph waveforms. Entomol. Exp. Appl. 2019, 167, 370–376. [Google Scholar] [CrossRef]
- Bar-Joseph, M.; Marcus, R.; Lee, R.F. The continuous challenge of Citrus Tristeza Virus control. Annu. Rev. Phytopathol. 1989, 27, 291–316. [Google Scholar] [CrossRef]
- Gottwald, T.R.; Garnsey, S.M.; Cambra, M.; Moreno, P.; Irey, M.; Borbón, J. Comparative effects of aphid vector species on increase and spread of Citrus Tristeza Virus. Fruits 1997, 52, 397–404. [Google Scholar]
- Jimenez, J.; Tjallingii, W.F.; Moreno, A.; Fereres, A. Newly distinguished cell punctures associated with transmission of the semipersistent phloem-limited beet yellows virus. J. Virol. 2018, 2, e01076-18. [Google Scholar] [CrossRef]
- Horbens, M.; Feldner, A.; Höfer, M.; Neinhuis, C. Ontogenetic tissue modification in Malus fruit peduncles: The role of sclereids. Ann. Biol. 2014, 113, 105–118. [Google Scholar] [CrossRef]
- Bar-Joseph, M.; Roistacher, C.N.; Garnsey, S.M.; Gumpf, D.J. A review on tristeza, an ongoing threat to citriculture. Proc. Int. Soc. Citric. 1981, 1, 419–423. [Google Scholar]
- Harris, K.F.; Harris, L.J. Ingestion-egestion theory of cuticula-borne virus transmission. In Virus-Insect-Plant Interactions; Harris, K.F., Smith, O.P., Duffus, J.E., Eds.; Academic Press: Cambridge, MA, USA, 2001; pp. 111–132. [Google Scholar] [CrossRef]
- Campolo, O.; Chiera, E.; Malacrinò, A.; Laudani, F.; Fontana, A.; Albanese, G.R.; Palmeri, V. Acquisition and transmission of selected CTV isolates by Aphis gossypii. J. Asia Pac. Entomol. 2014, 17, 493–498. [Google Scholar] [CrossRef]
- Jiménez, J.; Garzo, E.; Alba-Tercedor, J.; Moreno, A.; Fereres, A.; Walker, G.P. The phloem-pd: A distinctive brief sieve element stylet puncture prior to sieve element phase of aphid feeding behavior. Arthropod Plant. Interact. 2020, 14, 67–78. [Google Scholar] [CrossRef]
- Killiny, N.; Harper, S.J.; Alfaress, S.; El Mohtar, C.; Dawson, W.O. Minor coat and heat shock proteins are involved in the binding of Citrus Tristeza Virus to the foregut of its aphid vector, Toxoptera citricida. Appl. Environ. Microbiol. 2016, 82, 6294–6302. [Google Scholar] [CrossRef]
- Garnsey, S.M.; Cambra, M. Enzyme-linked immunosorbent assay (ELISA). In Graft Transmissible Diseases of Grapevines; Martelli, G.P., Ed.; Distribution and Sales Section, FAO: Roma, Italy, 1993; pp. 169–192. [Google Scholar]
- Tjallingii, W.F. Membrane potentials as an indication for plant cell penetration by aphid stylets. Entomol. Exp. Appl. 1985, 38, 187–193. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Electronic recording of penetration behaviour by aphids. Entomol. Exp. Appl. 1978, 24, 721–730. [Google Scholar] [CrossRef]
- Sarriá, E.V.; Cid, M.; Garzo, E.; Fereres, A. Excel workbook for automatic parameter calculation of EPG data. Comput. Electron. Agric. 2009, 67, 35–42. [Google Scholar] [CrossRef]
| Aphid Species | Waveforms * | Fruit Peduncles | Lime Leaves | χ2 | p |
|---|---|---|---|---|---|
| Aphis citricidus | C | 100 (15/15) a | 100 (14/14) a | - | - |
| E1 | 0 (0/15) b | 64.3 (9/14) a | 13.982 | 0.0002 | |
| E2 | 0 (0/15) b | 64.3 (9/14) a | 13.982 | 0.0002 | |
| Aphis gossypii | C | 100 (16/16) a | 100 (16/16) a | - | - |
| E1 | 6.25 (1/16) b | 93.75 (15/16) a | 24.500 | 0.0001 | |
| E2 | 0 (0/16) b | 93.75 (15/16) a | 28.240 | 0.0001 |
| Aphid Species | EPG Variables | Fruit Peduncles | Lime Leaves | p |
|---|---|---|---|---|
| Aphis citricidus | Number of Waveform Events | |||
| np | 11.60 ± 2.07 a | 3.29 ± 0.49 b | <0.0001 | |
| C | 10.73 ± 2.11 a | 3.50 ± 0.49 b | 0.0007 | |
| E1 | 0 ± 0 b | 0.93 ± 0.25 a | 0.0032 | |
| E2 | 0 ± 0 b | 0.93 ± 0.25 a | 0.0032 | |
| Total Waveform Duration (min) | ||||
| np | 376.93 ± 20.87 a | 126.54 ± 35.69 b | <0.0001 | |
| C | 99.91 ± 21.57 a | 159.57 ± 35.16 a | 0.1761 | |
| E1 | 0 ± 0 b | 0.57 ± 0.15 a | 0.0032 | |
| E2 | 0 ± 0 b | 193.31 ± 49.16 a | 0.0032 | |
| Mean Duration of Waveform Events (min) | ||||
| np | 32.49 ± 6.04 a | 30.26 ± 8.69 a | 0.696 | |
| C | 9.31 ± 0.98 a | 45.59 ± 11.31 b | <0.0001 | |
| E1 | 0 ± 0 a | 0.62 ± 0.05 b | <0.0001 | |
| E2 | 0 ± 0 a | 208.18 ± 45.95 b | <0.0001 | |
| Sequential a (min) | ||||
| Start EPG—1st Probe | 8.30 ± 4.78 a | 10.51 ± 3.29 a | 0.2752 | |
| Start EPG—1st E | 480.00 ± 0.00 a | 225.67 ± 53.84 b | 0.0094 | |
| Number of Waveform Events | ||||
| np | 15.06 ± 2.13 a | 11.63 ± 1.59 a | 0.178 | |
| C | 14.50 ± 2.09 a | 14.13 ± 1.98 a | 0.844 | |
| E1 | 0.13 ± 0.13 a | 3.31 ± 0.74 b | <0.0001 | |
| E2 | 0 ± 0 a | 3.00 ± 0.68 b | <0.0001 | |
| Total Waveform Duration (min) | ||||
| np | 292.57 ± 17.99 a | 93.31 ± 21.07 b | <0.0001 | |
| C | 187.16 ± 18.00 a | 169.94 ± 25.57 a | 0.586 | |
| Aphis gossypii | E1 | 0.27 ± 0.27 a | 7.93 ± 2.27 b | <0.0001 |
| E2 | 0 ± 0 a | 159.40 ± 32.18 b | <0.0001 | |
| Mean Duration of Waveform Events (min) | ||||
| np | 25.07 ± 4.11 a | 9.39 ± 2.81 b | <0.0001 | |
| C | 15.51 ± 2.09 a | 15.30 ± 4.59 a | 0.563 | |
| E1 | 2.18 a | 3.01 ± 0.98 a | 0.875 | |
| E2 | 0 ± 0 a | 98.82 ± 32.96 b | <0.0001 | |
| Sequential a (min) | ||||
| Start EPG—1st Probe | 19.83 ± 2.32 a | 3.57 ±1.36 b | <0.0001 | |
| Start EPG—1st E | 463.61 ± 16.38 a | 175.84 ± 34.26 b | <0.0001 |
| Aphid Species | Aphid Behaviour * | Fruit Peduncles | Lime Leaves | χ2 | p |
|---|---|---|---|---|---|
| Aphis citricidus | Non-Probing | 78.53 a | 26.36 a | 56.321 | <0.0001 |
| Stylet Penetration (Non-phloem Phase) | 21.47 a | 33.25 a | 3.653 | 0.585 | |
| Salivation into Phloem (E1) | 0 a | 0.12 a | - | >0.999 | |
| Phloem Ingestion (E2) | 0 a | 40.27 b | 50.000 | <0.0001 | |
| Aphis gossypii | Non-Probing | 61.01 a | 20.21 b | 34.879 | <0.0001 |
| Stylet Penetration (Non-phloem Phase) | 38.94 a | 44.92 a | 0.739 | 0.395 | |
| Salivation into Phloem (E1) | 0.05 a | 1.65 a | 2.020 | 0.249 | |
| Phloem Ingestion (E2) | 0 a | 33.21 b | 39.521 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez, C.; Carmo-Sousa, M.; Lopes, J.R.S.; Fereres, A.; Moreno, A. Aphids Are Unable to Ingest Phloem Sap from the Peduncles of Lime Fruits. Plants 2020, 9, 1528. https://doi.org/10.3390/plants9111528
Vázquez C, Carmo-Sousa M, Lopes JRS, Fereres A, Moreno A. Aphids Are Unable to Ingest Phloem Sap from the Peduncles of Lime Fruits. Plants. 2020; 9(11):1528. https://doi.org/10.3390/plants9111528
Chicago/Turabian StyleVázquez, Carolina, Michele Carmo-Sousa, Joao Roberto Spotti Lopes, Alberto Fereres, and Aranzazu Moreno. 2020. "Aphids Are Unable to Ingest Phloem Sap from the Peduncles of Lime Fruits" Plants 9, no. 11: 1528. https://doi.org/10.3390/plants9111528
APA StyleVázquez, C., Carmo-Sousa, M., Lopes, J. R. S., Fereres, A., & Moreno, A. (2020). Aphids Are Unable to Ingest Phloem Sap from the Peduncles of Lime Fruits. Plants, 9(11), 1528. https://doi.org/10.3390/plants9111528