A Quarter Century History of ATML1 Gene Research
Abstract
1. Epidermis Formation and Its Role in Plant Development
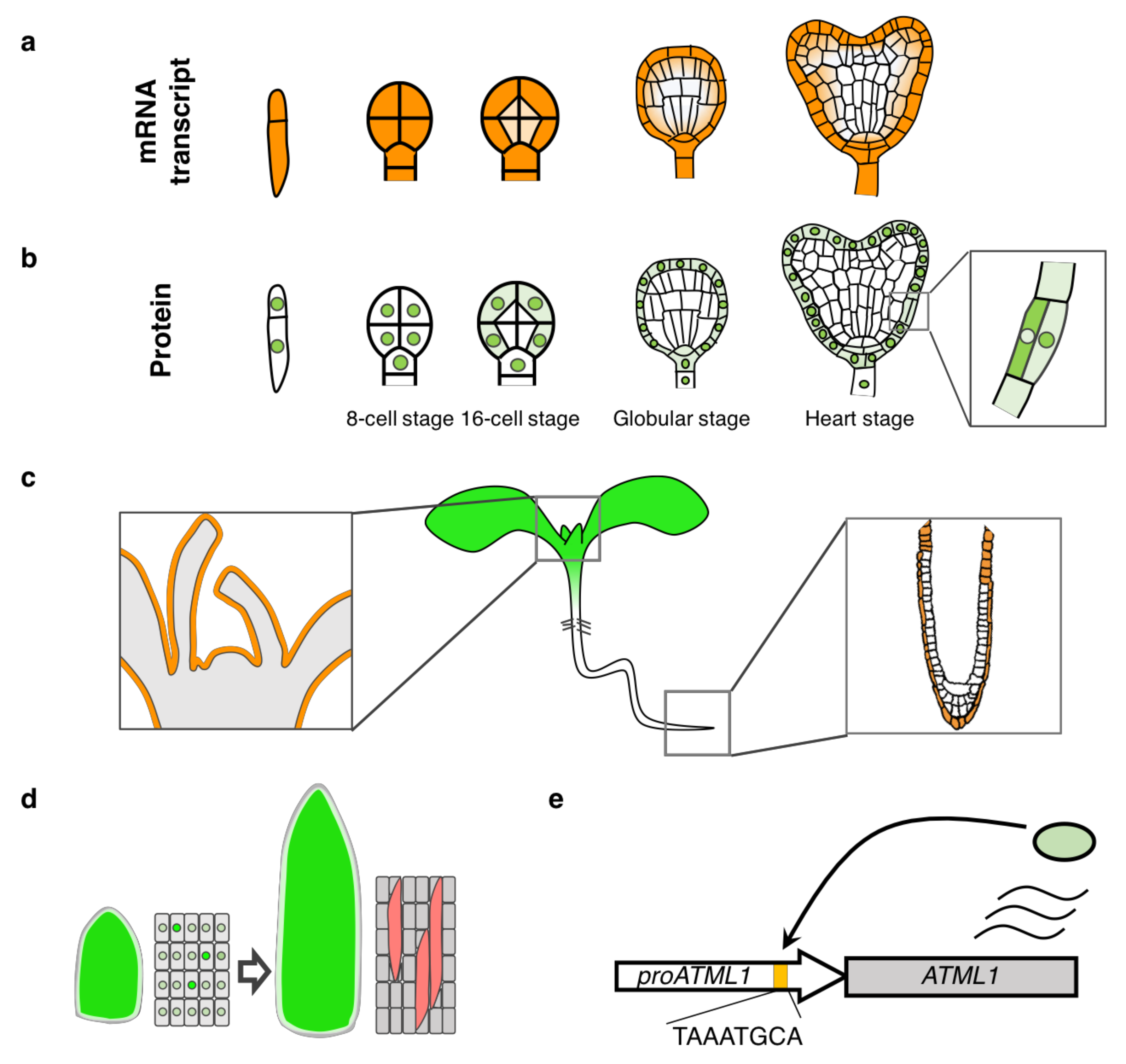
2. Cloning of the ATML1 Gene and Analysis of Its Expression Pattern
3. ATML1 Functions in Plant Development
4. Transcriptional Regulation of the ATML1 Gene
5. Post-transcriptional and Post-translational Regulation of ATML1
6. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pattanaik, S.; Patra, B.; Singh, S.K.; Yuan, L. An overview of the gene regulatory network controlling trichome development in the model plant, Arabidopsis. Front. Plant Sci. 2014, 5, 259. [Google Scholar] [CrossRef] [PubMed]
- Pillitteri, L.J.; Torii, K.U. Mechanisms of stomatal development. Annu. Rev. Plant Biol. 2012, 63, 591–614. [Google Scholar] [CrossRef] [PubMed]
- Takada, S.; Iida, H. Specification of epidermal cell fate in plant shoots. Front. Plant Sci. 2014, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Savaldi-Goldstein, S.; Peto, C.; Chory, J. The epidermis both drives and restricts plant shoot growth. Nature 2007, 446, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Nobusawa, T.; Okushima, Y.; Nagata, N.; Kojima, M.; Sakakibara, H.; Umeda, M. Synthesis of very-long-chain fatty acids in the epidermis controls plant organ growth by restricting cell proliferation. PLoS Biol. 2013, 11, e1001531. [Google Scholar] [CrossRef] [PubMed]
- Yephremov, A.; Wisman, E.; Huijser, P.; Huijser, C.; Wellesen, K.; Saedler, H. Characterization of the FIDDLEHEAD gene of Arabidopsis reveals a link between adhesion response and cell differentiation in the epidermis. Plant Cell 1999, 11, 2187–2201. [Google Scholar] [PubMed]
- Han, H.; Yan, A.; Li, L.; Zhu, Y.; Feng, B.; Liu, X.; Zhou, Y. A signal cascade originated from epidermis defines apical-basal patterning of Arabidopsis shoot apical meristems. Nat. Commun. 2020, 11, 1–17. [Google Scholar]
- Knauer, S.; Holt, A.L.; Rubio-Somoza, I.; Tucker, E.J.; Hinze, A.; Pisch, M.; Javelle, M.; Timmermans, M.C.; Tucker, M.R.; Laux, T. A protodermal miR394 signal defines a region of stem cell competence in the Arabidopsis shoot meristem. Dev. Cell 2013, 24, 125–132. [Google Scholar] [CrossRef]
- Ogawa, E.; Yamada, Y.; Sezaki, N.; Kosaka, S.; Kondo, H.; Kamata, N.; Abe, M.; Komeda, Y.; Takahashi, T. ATML1 and PDF2 play a redundant and essential role in Arabidopsis embryo development. Plant Cell Physiol. 2015, 56, 1183–1192. [Google Scholar] [CrossRef]
- Lu, P.; Porat, R.; Nadeau, J.A.; O’Neill, S.D. Identification of a meristem L1 layer-specific gene in Arabidopsis that is expressed during embryonic pattern formation and defines a new class of homeobox genes. Plant Cell 1996, 8, 2155–2168. [Google Scholar]
- Nadeau, J.A.; Zhang, X.S.; Li, J.; O’Neill, S.D. Ovule development: Identification of stage-specific and tissue-specific cDNAs. Plant Cell 1996, 8, 213–239. [Google Scholar] [PubMed]
- Rerie, W.G.; Feldmann, K.A.; Marks, M.D. The GLABRA2 gene encodes a homeo domain protein required for normal trichome development in Arabidopsis. Genes Dev. 1994, 8, 1388–1399. [Google Scholar] [CrossRef] [PubMed]
- Masucci, J.D.; Rerie, W.G.; Foreman, D.R.; Zhang, M.; Galway, M.E.; Marks, M.D.; Schiefelbein, J.W. The homeobox gene GLABRA2 is required for position-dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development 1996, 122, 1253–1260. [Google Scholar] [PubMed]
- Iida, H.; Yoshida, A.; Takada, S. ATML1 activity is restricted to the outermost cells of the embryo through post-transcriptional repressions. Development 2019, 146. [Google Scholar] [CrossRef] [PubMed]
- Sessions, A.; Weigel, D.; Yanofsky, M.F. The Arabidopsis thaliana MERISTEM LAYER 1 promoter specifies epidermal expression in meristems and young primordia. Plant J. 1999, 20, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Takada, S.; Jürgens, G. Transcriptional regulation of epidermal cell fate in the Arabidopsis embryo. Development 2007, 134, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Katsumata, H.; Komeda, Y.; Takahashi, T. Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development 2003, 130, 635–643. [Google Scholar] [CrossRef]
- San-Bento, R.; Farcot, E.; Galletti, R.; Creff, A.; Ingram, G. Epidermal identity is maintained by cell–cell communication via a universally active feedback loop in Arabidopsis thaliana. Plant J. 2014, 77, 46–58. [Google Scholar] [CrossRef]
- Peterson, K.M.; Shyu, C.; Burr, C.A.; Horst, R.J.; Kanaoka, M.M.; Omae, M.; Sato, Y.; Torii, K.U. Arabidopsis homeodomain-leucine zipper IV proteins promote stomatal development and ectopically induce stomata beyond the epidermis. Development 2013, 140, 1924–1935. [Google Scholar] [CrossRef]
- Takada, S.; Takada, N.; Yoshida, A. ATML1 promotes epidermal cell differentiation in Arabidopsis shoots. Development 2013, 140, 1919–1923. [Google Scholar] [CrossRef]
- Takada, S. Post-Embryonic induction of ATML1-SRDX alters the morphology of seedlings. PLoS ONE 2013, 8, e79312. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.M.; Teles, J.; Formosa-Jordan, P.; Refahi, Y.; San-Bento, R.; Ingram, G.; Jonsson, H.; Locke, J.C.W.; Roeder, A.H.K. Fluctuations of the transcription factor ATML1 generate the pattern of giant cells in the Arabidopsis sepal. eLife 2017, 6, e19131. [Google Scholar] [CrossRef] [PubMed]
- Roeder, A.H.; Cunha, A.; Ohno, C.K.; Meyerowitz, E.M. Cell cycle regulates cell type in the Arabidopsis sepal. Development 2012, 139, 4416–4427. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Takahashi, T.; Komeda, Y. Identification of a cis-regulatory element for L1 layer-specific gene expression, which is targeted by an L1-specific homeodomain protein. Plant J. 2001, 26, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Scheres, B. Plant cell identity. The role of position and lineage. Plant Physiol. 2001, 125, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.N.; Dermen, H. Flexibility in ontogeny as shown by the contribution of the shoot apical layers to leaves of periclinal chimeras. Am. J. Bot. 1975, 62, 935–947. [Google Scholar] [CrossRef]
- Johnson, K.L.; Degnan, K.A.; Ross Walker, J.; Ingram, G.C. AtDEK1 is essential for specification of embryonic epidermal cell fate. Plant J. 2005, 44, 114–127. [Google Scholar] [CrossRef]
- Nodine, M.D.; Yadegari, R.; Tax, F.E. RPK1 and TOAD2 are two receptor-like kinases redundantly required for Arabidopsis embryonic pattern formation. Dev. Cell 2007, 12, 943–956. [Google Scholar] [CrossRef]
- Tanaka, H.; Watanabe, M.; Sasabe, M.; Hiroe, T.; Tanaka, T.; Tsukaya, H.; Ikezaki, M.; Machida, C.; Machida, Y. Novel receptor-like kinase ALE2 controls shoot development by specifying epidermis in Arabidopsis. Development 2007, 134, 1643–1652. [Google Scholar] [CrossRef]
- Nodine, M.D.; Bartel, D.P. MicroRNAs prevent precocious gene expression and enable pattern formation during plant embryogenesis. Genes Dev. 2010, 24, 2678–2692. [Google Scholar] [CrossRef]
- Ariel, F.D.; Manavella, P.A.; Dezar, C.A.; Chan, R.L. The true story of the HD-Zip family. Trends Plant Sci. 2007, 12, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zuo, K.; Zhang, J.; Liu, X.; Zhang, L.; Sun, X.; Tang, K. An L1 box binding protein, GbML1, interacts with GbMYB25 to control cotton fibre development. J. Exp. Bot. 2010, 61, 3599–3613. [Google Scholar] [CrossRef] [PubMed]
- Schrick, K.; Nguyen, D.; Karlowski, W.M.; Mayer, K.F. START lipid/sterol-binding domains are amplified in plants and are predominantly associated with homeodomain transcription factors. Genome Biol. 2004, 5, R41. [Google Scholar] [CrossRef] [PubMed]
- Schrick, K.; Bruno, M.; Khosla, A.; Cox, P.N.; Marlatt, S.A.; Roque, R.A.; Nguyen, H.C.; He, C.; Snyder, M.P.; Singh, D.; et al. Shared functions of plant and mammalian StAR-related lipid transfer (START) domains in modulating transcription factor activity. BMC Biol. 2014, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Rombolá-Caldentey, B.; Rueda-Romero, P.; Iglesias-Fernández, R.; Carbonero, P.; Oñate-Sánchez, L. Arabidopsis DELLA and two HD-ZIP transcription factors regulate GA signaling in the epidermis through the L1 box cis-element. Plant Cell 2014, 26, 2905–2919. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Sentoku, N.; Nishimura, A.; Hong, S.-K.; Sato, Y.; Matsuoka, M. Position dependent expression of GL2-type homeobox gene, Roc1: Significance for protoderm differentiation and radial pattern formation in early rice embryogenesis. Plant J. 2002, 29, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Javelle, M.; Klein-Cosson, C.; Vernoud, V.; Boltz, V.; Maher, C.; Timmermans, M.; Depège-Fargeix, N.; Rogowsky, P.M. Genome-wide characterization of the HD-ZIP IV transcription factor family in maize: Preferential expression in the epidermis. Plant Physiol. 2011, 157, 790–803. [Google Scholar] [CrossRef]
- Gao, L.; Tian, Y.; Chen, M.-C.; Wei, L.; Gao, T.-G.; Yin, H.-J.; Zhang, J.-L.; Kumar, T.; Liu, L.-B.; Wang, S.-M. Cloning and functional characterization of epidermis-specific promoter MtML1 from Medicago truncatula. J. Biotechnol. 2019, 300, 32–39. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iida, H.; Takada, S. A Quarter Century History of ATML1 Gene Research. Plants 2021, 10, 290. https://doi.org/10.3390/plants10020290
Iida H, Takada S. A Quarter Century History of ATML1 Gene Research. Plants. 2021; 10(2):290. https://doi.org/10.3390/plants10020290
Chicago/Turabian StyleIida, Hiroyuki, and Shinobu Takada. 2021. "A Quarter Century History of ATML1 Gene Research" Plants 10, no. 2: 290. https://doi.org/10.3390/plants10020290
APA StyleIida, H., & Takada, S. (2021). A Quarter Century History of ATML1 Gene Research. Plants, 10(2), 290. https://doi.org/10.3390/plants10020290





