Effect of Paternal Genome Excess on the Developmental and Gene Expression Profiles of Polyspermic Zygotes in Rice
Abstract
1. Introduction
2. Results
2.1. Developmental Profiles of Polyspermic Rice Zygotes
2.2. Gene Expression Profiles of Polyspermic Zygotes
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Gamete Isolation
4.2. Production and Culture of Diploid Zygotes and Paternal Excess Polyspermic Zygotes
4.3. Microscopic Analysis
4.4. cDNA Synthesis, Library Preparation, and mRNA Sequencing
4.5. Analyses of Transcriptome Data
4.6. Semi-Quantitative RT-PCR
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raghavan, V. Some reflections on double fertilization, from its discovery to the present. New Phytol. 2003, 159, 565–583. [Google Scholar] [CrossRef]
- Nawaschin, S. Revision der Befruchtungsvorgange bei Lilium martagon und Fritillaria tenella. Bull. Sci. Acad. Imp. Sci. Saint Pétersbourg 1898, 9, 377–382. [Google Scholar]
- Guignard, M.L. Sur les antherozoides et la double copulation sexuelle chez les vegetaux angiosperms. Rev. Gén. Bot. 1899, 11, 129–135. [Google Scholar]
- Russell, S.D. Double fertilization. Int. Rev. Cytol. 1992, 140, 357–390. [Google Scholar]
- Haig, D.; Westoby, M. Genomic imprinting in endosperm: Its effect on seed development in crosses between species, and between different ploidies of the same species, and its implications for the evolution of apomixis. Philo. Trans. Biol. Sci. 1991, 333, 1–13. [Google Scholar]
- Scott, R.J.; Spielman, M.; Bailey, J.; Dickinson, H.G. Parent-of-origin effects on seed development in Arabidopsis thaliana. Development 1998, 125, 3329–3341. [Google Scholar]
- Tiwari, S.; Spielman, M.; Schulz, R.; Oakey, R.J.; Kelsey, G.; Salazar, A.; Zhang, K.; Pennell, R.; Scott, R.J. Transcriptional profiles underlying parent-of-origin effects in seeds of Arabidopsis thaliana. BMC Plant Biol. 2010, 10, 72. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, C.; Baulcombe, D.C.; Chen, Z.J. Maternal siRNAs as regulators of parental genome imbalance and gene expression in endosperm of Arabidopsis seeds. Proc. Natl. Acad. Sci. USA 2012, 109, 5529–5534. [Google Scholar] [CrossRef]
- Sekine, D.; Ohnishi, T.; Furuumi, H.; Ono, A.; Yamada, T.; Kurata, N.; Kinoshita, T. Dissection of two major components of the post-zygotic hybridization barrier in rice endosperm. Plant J. 2013, 76, 792–799. [Google Scholar] [CrossRef]
- Toda, E.; Ohnishi, Y.; Okamoto, T. Effects of an imbalanced parental genome ratio on development of rice zygotes. J. Exp. Bot. 2018, 69, 2609–2619. [Google Scholar] [CrossRef]
- Toda, E.; Okamoto, T. Polyspermy in angiosperms: Its contribution to polyploid formation and speciation. Mol. Reprod. Dev. 2020, 87, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Del Toro-De León, G.; García-Aguilar, M.; Gillmor, C.S. Non-equivalent contributions of maternal and paternal genomes to early plant embryogenesis. Nature 2014, 514, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; Shi, C.; Zhang, L.; Sun, M.-X. The expression and roles of parent-of-origin genes in early embryogenesis of angiosperms. Front. Plant Sci. 2014, 5, 729. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baroux, C.; Grossniklaus, U. The maternal-to-zygotic transition in flowering plants: Evidence, mechanisms, and plasticity. Curr. Top. Dev. Biol. 2015, 113, 351–371. [Google Scholar] [PubMed]
- Anderson, S.N.; Johnson, C.S.; Chesnut, J.; Jones, D.S.; Khanday, I.; Woodhouse, M.; Li, C.; Conrad, L.J.; Russell, S.D.; Sundaresan, V. The zygotic transition is initiated in unicellular plant zygotes with asymmetric activation of parental genomes. Dev. Cell 2017, 43, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Aichinger, E.; Gong, W.; Groot, E.; Verstraeten, I.; Vu, L.D.; De Smet, I.; Higashiyama, T.; Umeda, M.; Laux, T. Transcriptional integration of paternal and maternal factors in the Arabidopsis zygote. Genes Dev. 2017, 31, 617–627. [Google Scholar] [CrossRef]
- Zhao, P.; Begcy, K.; Dresselhaus, T.; Sun, M.-X. Does early embryogenesis in eudicots and monocots involve the same mechanism and molecular players? Plant Physiol. 2017, 173, 130–142. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, X.; Shen, K.; Liu, Z.; Cheng, T.; Liu, D.; Cheng, Y.; Peng, X.; Sun, M.-X. Two-step maternal-to-zygotic transition with two-phase parental genome contributions. Dev. Cell 2019, 49, 882–893. [Google Scholar] [CrossRef]
- Shen, W.H.; Parmentier, Y.; Hellmann, H.; Lechner, E.; Dong, A.; Masson, J.; Granier, F.; Lepiniec, L.; Estelle, M.; Genschik, P. Null mutation of AtCUL1 causes arrest in early embryogenesis in Arabidopsis. Mol. Biol. Cell 2002, 13, 1916–1928. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, H.Y.; Xie, C.H.; Xue, H.W.; Dijkhuis, P.; Liu, C.M. EMBRYONIC FACTOR 1 encodes an AMP deaminase and is essential for the zygote to embryo transition in Arabidopsis. Plant J. 2005, 42, 743–756. [Google Scholar] [CrossRef]
- Ronceret, A.; Gadea-Vacas, J.; Guilleminot, J.; Lincker, F.; Delorme, V.; Lahmy, S.; Pelletier, G.; Chabouté, M.E.; Devic, M. The first zygotic division in Arabidopsis requires de novo transcription of thymidylate kinase. Plant J. 2008, 53, 776–789. [Google Scholar] [CrossRef] [PubMed]
- Andreuzza, S.; Li, J.; Guitton, A.E.; Faure, J.E.; Casanova, S.; Park, J.S.; Choi, Y.; Chen, Z.; Berger, F. DNA LIGASE I exerts a maternal effect on seed development in Arabidopsis thaliana. Development 2010, 137, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Jiang, L.; Gong, H.; Liu, C.M. EMBRYONIC FACTOR 19 encodes a pentatricopeptide repeat protein that is essential for the initiation of zygotic embryogenesis in Arabidopsis. J. Integr. Plant Biol. 2012, 54, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Jiang, L.; Zhang, Y.; Lu, X.L.; Xie, Q.; Weijers, D.; Liu, C.M. The anaphase-promoting complex initiates zygote division in Arabidopsis through degradation of cyclin B1. Plant J. 2016, 86, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.Y.; Shi, D.Q.; Jia, P.F.; Tang, J.; Li, H.J.; Liu, J.; Yang, W.C. The Arabidopsis receptor kinase ZAR1 is required for zygote asymmetric division and its daughter cell fate. PLoS Genet. 2016, 12, e1005933. [Google Scholar] [CrossRef]
- Yang, K.J.; Guo, L.; Hou, X.L.; Gong, H.Q.; Liu, C.M. ZYGOTE-ARREST 3 that encodes the tRNA ligase is essential for zygote division in Arabidopsis. J. Integr. Plant Biol. 2017, 59, 680–692. [Google Scholar] [CrossRef]
- Uchiumi, T.; Uemura, I.; Okamoto, T. Establishment of an in vitro fertilization system in rice (Oryza sativa L.). Planta 2007, 226, 581–589. [Google Scholar] [CrossRef]
- Toda, E.; Ohnishi, Y.; Okamoto, T. Development of polyspermic rice zygotes. Plant Physiol. 2016, 171, 206–214. [Google Scholar] [CrossRef]
- Rahman, M.H.; Toda, E.; Kobayashi, M.; Kudo, T.; Koshimizu, S.; Takahara, M.; Iwami, M.; Watanabe, Y.; Sekimoto, H.; Yano, K.; et al. Expression of genes from paternal alleles in rice zygotes and involvement of OsASGR-BBML1 in initiation of zygotic development. Plant Cell Physiol. 2019, 60, 725–737. [Google Scholar] [CrossRef]
- Khanday, I.; Skinner, D.; Yang, B.; Mercier, R.; Sundaresan, V. A male-expressed rice embryogenic trigger redirected for asexual propagation through seeds. Nature 2019, 565, 91–95. [Google Scholar] [CrossRef]
- Ohnishi, Y.; Hoshino, R.; Okamoto, T. Dynamics of male and female chromatin during karyogamy in rice zygotes. Plant Physiol. 2014, 165, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Tu, X.; Chu, P.Y.; Lü, P.; Zhu, N.; Grierson, D.; Du, B.; Li, P.; Zhong, S. 3D chromatin architecture of large plant genomes determined by local A/B compartments. Mol. Plant 2017, 10, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cheng, Y.J.; Wang, J.W.; Weigel, D. Prominent topologically associated domains differentiate global chromatin packing in rice from Arabidopsis. Nat. Plants 2017, 3, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, P.; Lin, M.; Ye, Z.; Li, G.; Tu, L.; Shen, C.; Li, J.; Yang, Q.; Zhang, X. Evolutionary dynamics of 3D genome architecture following polyploidization in cotton. Nat. Plants 2018, 4, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Jiang, W.; Zhao, Y.; Zhou, D.X. Single-cell three-dimensional genome structures of rice gametes and unicellular zygotes. Nat. Plants 2019, 5, 795–800. [Google Scholar] [CrossRef]
- Kawashima, T.; Berger, F. Epigenetic reprogramming in plant sexual reproduction. Nat. Rev. Gen. 2014, 15, 613–624. [Google Scholar] [CrossRef]
- Gehring, M. Epigenetic dynamics during flowering plant reproduction: Evidence for reprogramming? New Phytol. 2019, 224, 91–96. [Google Scholar] [CrossRef]
- Conner, J.A.; Mookkan, M.; Huo, H.; Chae, K.; Ozias-Akins, P. A parthenogenesis gene of apomict origin elicits embryo formation from unfertilized eggs in a sexual plant. Proc. Natl. Acad. Sci. USA 2015, 112, 11205–11210. [Google Scholar] [CrossRef]
- Conner, J.A.; Podio, M.; Ozias-Akins, P. Haploid embryo production in rice and maize induced by PsASGR-BBML transgenes. Plant Reprod. 2017, 30, 41–52. [Google Scholar] [CrossRef]
- Boutilier, K.; Offringa, R.; Sharma, V.K.; Kieft, H.; Ouellet, T.; Zhang, L.; Hattori, J.; Liu, C.M.; Van Lammeren, A.A.; Miki, B.L.; et al. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 2002, 14, 1737–1749. [Google Scholar] [CrossRef]
- Abiko, M.; Maeda, H.; Tamura, K.; Hara-Nishimura, I.; Okamoto, T. Gene expression profiles in rice gametes and zygotes: Identification of gamete-enriched genes and up- or down-regulated genes in zygotes after fertilization. J. Exp. Bot. 2013, 64, 1927–1940. [Google Scholar] [CrossRef] [PubMed]
- Uchiumi, T.; Komatsu, S.; Koshiba, T.; Okamoto, T. Isolation of gametes and central cells from Oryza sativa L. Sex. Plant Reprod. 2006, 19, 37–45. [Google Scholar] [CrossRef]
- Rahman, M.H.; Toda, E.; Okamoto, T. In vitro production of zygotes by electro-fusion of rice gametes. Methods Mol. Biol. 2020, 2122, 257–267. [Google Scholar] [PubMed]
- Simon, A. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects (accessed on 17 December 2019).
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Sakai, H.; Lee, S.S.; Tanaka, T.; Numa, H.; Kim, J.; Kawahara, Y.; Wakimoto, H.; Yang, C.C.; Iwamoto, M.; Abe, T.; et al. Rice Annotation Project Database (RAP-DB): An integrative and interactive database for rice genomics. Plant Cell Physiol. 2013, 54, e6. [Google Scholar] [CrossRef]
- Kawahara, Y.; De la Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.; Zhou, S.; et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 2013, 6, 4. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Sun, J.; Nishiyama, T.; Shimizu, K.; Kadota, K. TCC: An R package for comparing tag count data with robust normalization strategies. BMC Bioinform. 2013, 14, 219. [Google Scholar] [CrossRef]
- Kodama, Y.; Shumway, M.; Leinonen, R. The sequence read archive: Explosive growth of sequencing data. Nucleic Acids Res. 2012, 40, D54–D56. [Google Scholar] [CrossRef]

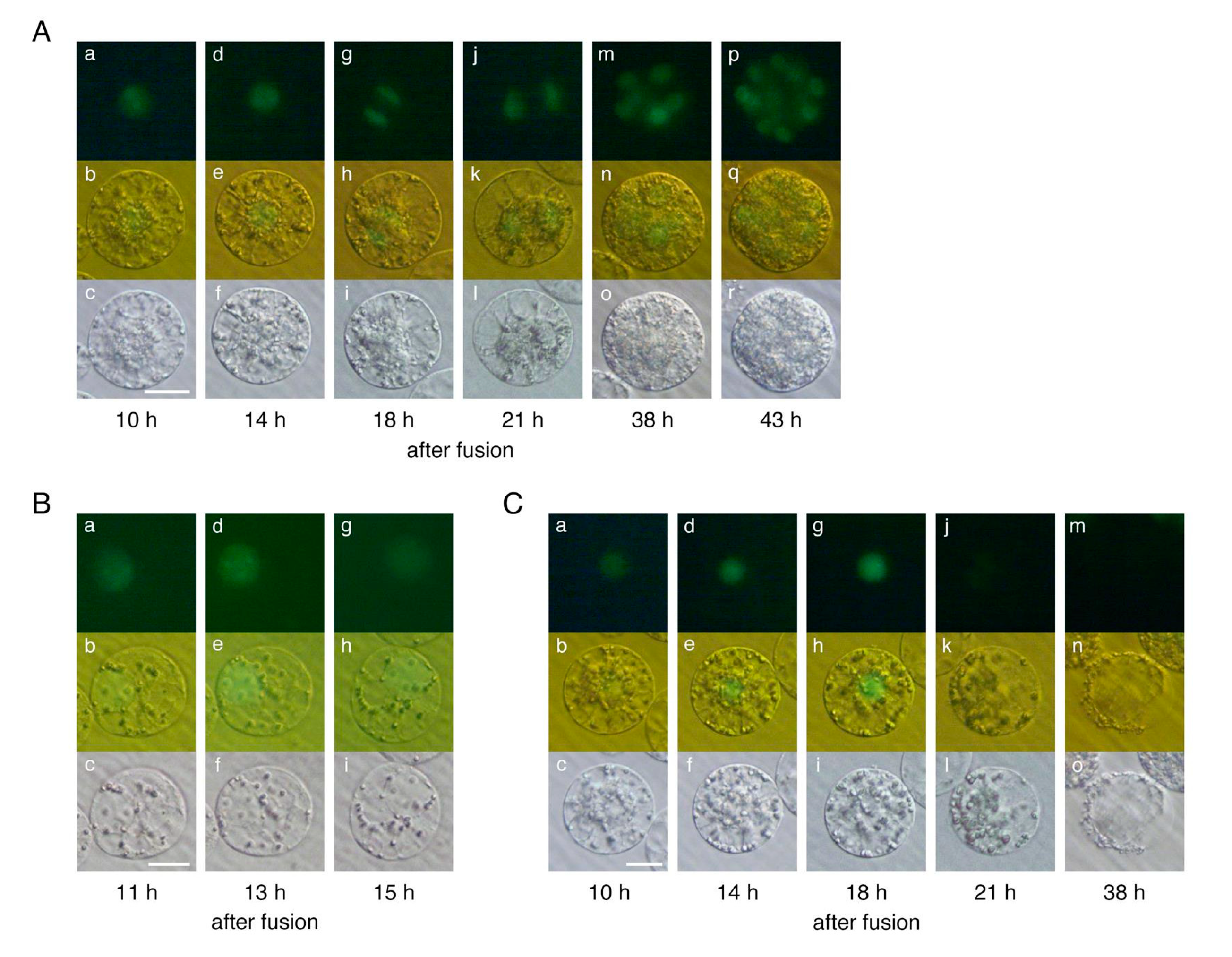
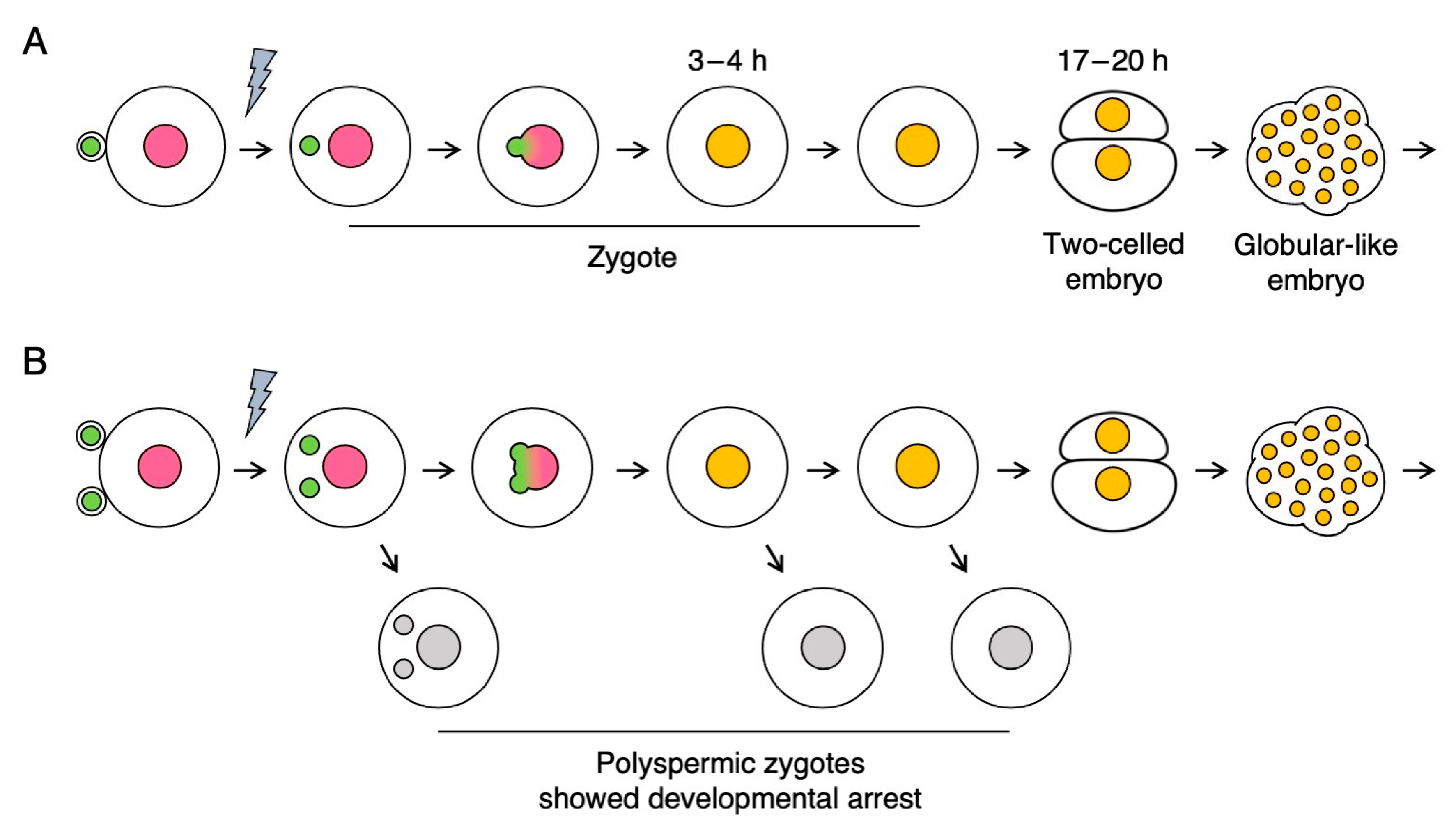
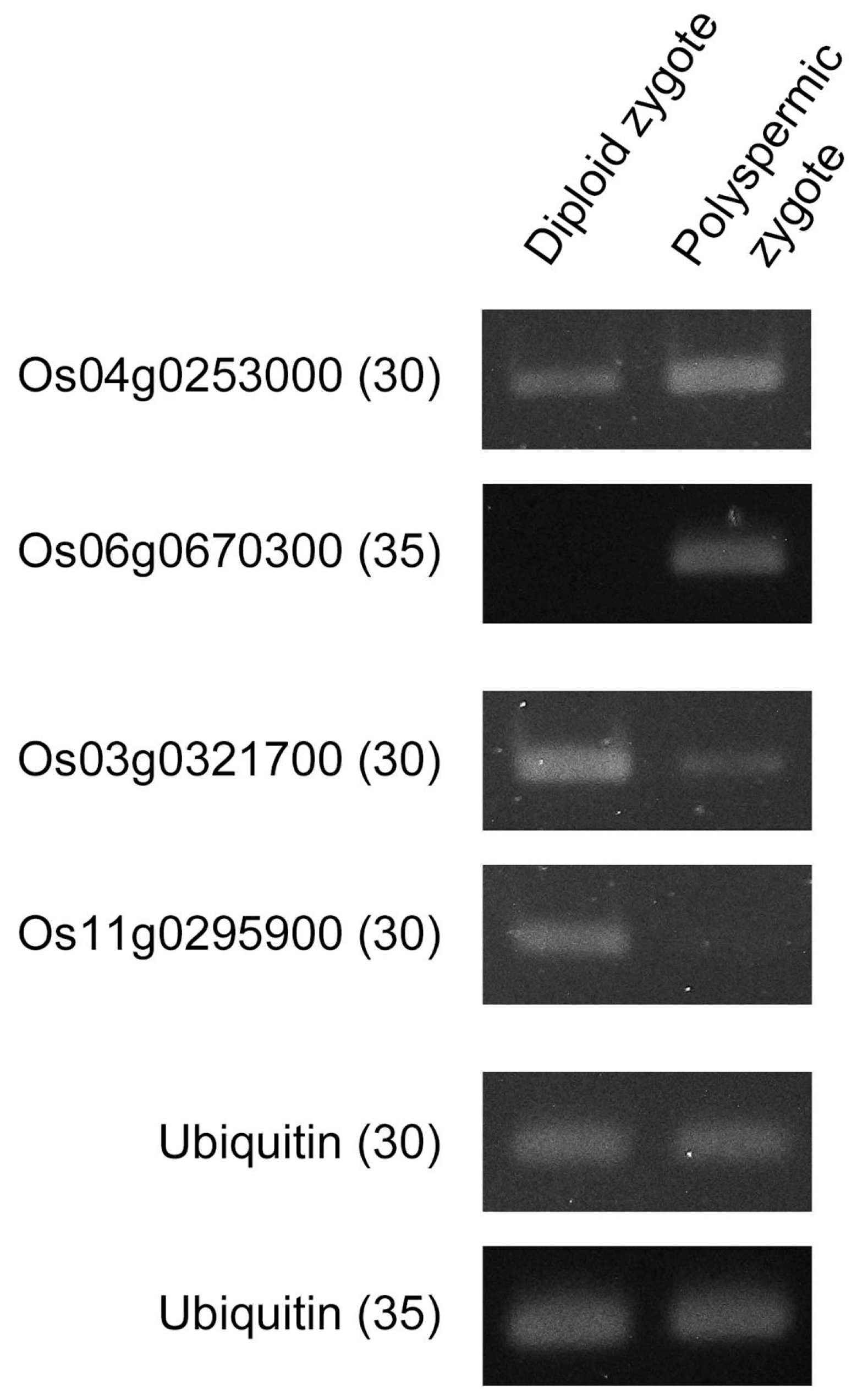
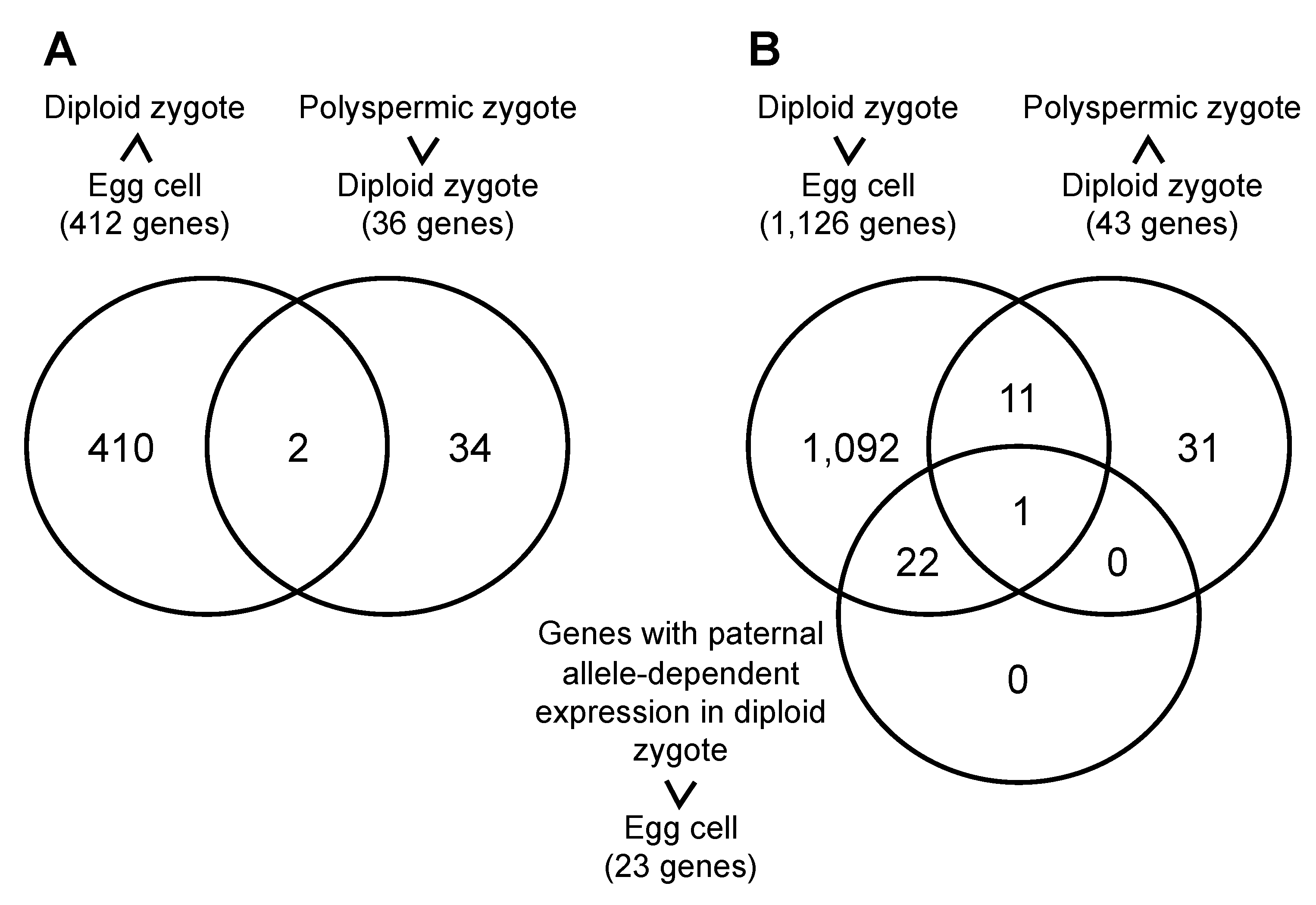
| Ploidy | Gametes Used for Fusion | No. of Zygotes Produced | No. of Zygotes That Developed to Specific Growth Stages | |||
|---|---|---|---|---|---|---|
| Karyogamy | Two-Celled Embryo | Globular-Like Embryo | Cell Mass | |||
| 2X | Egg + Sperm | 22 | 18 | 18 | 18 | 18 |
| 3X | Egg + Sperm + Sperm | 34 | 30 | 19 | 17 | 17 |
| Gene ID | Expression Level (Averaged TPM) | p Value | q Value | RAP-DB Description | |
|---|---|---|---|---|---|
| Diploid Zygotes | Poly spermic Zygotes | ||||
| Os01g0115600 | 0.0 | 99.8 | 2.17 × 10−5 | 0.01900855 | Similar to LRK14 |
| Os01g0136000 | 0.0 | 461.4 | 6.81 × 10−8 | 2.03 × 10−4 | Similar to cytosolic class I small heat-shock protein HSP17.5 |
| Os01g0149900 | 0.2 | 199.0 | 6.03 × 10−6 | 0.00748057 | Conserved hypothetical protein |
| Os01g0612500 | 0.2 | 430.2 | 2.16 × 10−5 | 0.01900855 | Phospholipase/carboxylesterase domain containing protein |
| Os01g0760000 | 0.2 | 137.8 | 5.66 × 10−6 | 0.00743199 | Similar to dynein light chain |
| Os01g0778500 | 0.0 | 194.2 | 1.56 × 10−6 | 0.00268023 | Similar to predicted protein |
| Os01g0794800 | 2.5 | 304.5 | 4.17 × 10−6 | 0.00564972 | Similar to subtilase |
| Os01g0866200 | 8033.4 | 28317.5 | 1.08 × 10−5 | 0.01179179 | Similar to histone H3 |
| Os02g0773200 | 98.0 | 3120.0 | 1.39 × 10−5 | 0.01409845 | UspA domain containing protein |
| Os03g0119900 | 5982.5 | 22237.0 | 6.93 × 10−6 | 0.00836748 | Similar to histone H4 |
| Os03g0217900 | 1420.4 | 14539.4 | 7.16 × 10−9 | 3.22 × 10−5 | Hypothetical protein |
| Os03g0227800 | 2108.4 | 9163.3 | 3.81 × 10−6 | 0.00549041 | Conserved hypothetical protein |
| Os03g0292100 | 384.8 | 1346.8 | 8.05 × 10−5 | 0.04732313 | Hypothetical conserved gene |
| Os03g0670700 | 756.9 | 3743.1 | 4.96 × 10−5 | 0.03357786 | Similar to glycine rich RNA binding protein |
| Os03g0675600 | 0.2 | 404.2 | 9.73 × 10−6 | 0.01086596 | Similar to phytosulfokines 3 precursor |
| Os04g0253000 | 461.5 | 6098.4 | 7.25 × 10−5 | 0.04321752 | Similar to histone H1 |
| Os04g0565500 | 0.0 | 263.3 | 2.93 × 10−7 | 6.88 × 10−4 | Similar to OSIGBa0158F05.8 protein |
| Os04g0668800 | 387.1 | 2053.6 | 2.68 × 10−6 | 0.00443244 | Putative thiol-disulphide oxidoreductase DCC family protein |
| Os05g0152201 | 1501.4 | 8510.0 | 5.84 × 10−6 | 0.00745909 | Conserved hypothetical protein |
| Os05g0475400 | 0.0 | 164.1 | 1.90 × 10−5 | 0.01728341 | Similar to alanine:glyoxylate aminotransferase-like protein |
| Os06g0597250 | 7031.8 | 18692.8 | 4.36 × 10−5 | 0.03172249 | Similar to B protein |
| Os06g0670300 | 0.0 | 140.8 | 5.75 × 10−5 | 0.03626026 | MYB-like transcription factor |
| Os07g0483500 | 0.0 | 179.3 | 2.29 × 10−5 | 0.01934667 | Similar to phosphoribosyltransferase |
| Os08g0388300 | 0.2 | 123.7 | 7.68 × 10−6 | 0.00902684 | NB-ARC domain containing protein |
| Os08g0409900 | 0.2 | 156.2 | 1.63 × 10−5 | 0.01618585 | Major facilitator superfamily protein |
| Os09g0411500 | 1295.0 | 3925.1 | 8.55 × 10−5 | 0.04963598 | Similar to predicted protein |
| Os09g0433600 | 1651.2 | 5276.6 | 5.30 × 10−5 | 0.03536701 | Similar to histone H4 |
| Os09g0457100 | 0.8 | 184.3 | 4.83 × 10−5 | 0.03323256 | Cytochrome P450 family protein |
| Os09g0483400 | 560.5 | 8011.9 | 1.27 × 10−6 | 0.00227203 | Similar to ubiquitin/ribosomal fusion protein |
| Os09g0551600 | 8147.5 | 19900.5 | 5.76 × 10−5 | 0.03626026 | Similar to HMGd1 protein |
| Os10g0539500 | 8203.4 | 28218.2 | 1.23 × 10−6 | 0.00227203 | Similar to histone H4 |
| Os11g0222800 | 0.0 | 205.4 | 6.49 × 10−5 | 0.04028685 | Similar to LGC1 |
| Os11g0533400 | 0.0 | 426.6 | 5.85 × 10−11 | 6.54 × 10−7 | Conserved hypothetical protein |
| Os11g0550100 | 0.0 | 317.8 | 3.74 × 10−7 | 8.36 × 10−4 | Similar to NB-ARC domain containing protein |
| Os12g0127200 | 3.2 | 249.1 | 3.96 × 10−5 | 0.02999367 | Harpin-induced 1 domain containing protein |
| Os12g0438000 | 2594.6 | 8152.9 | 1.30 × 10−5 | 0.01384279 | Similar to histone H2A |
| Gene ID | Expression Level (Averaged TPM) | p Value | q Value | RAP-DB Description | |
|---|---|---|---|---|---|
| Diploid Zygotes | Polyspermic Zygotes | ||||
| Os01g0341200 | 612.4 | 2.2 | 3.77 × 10−6 | 0.005490414 | Tubulin, conserved site domain containing protein |
| Os01g0611900 | 107.1 | 0.0 | 2.24 × 10−5 | 0.019231324 | Pentatricopeptide repeat domain containing protein |
| Os01g0622033 | 368.3 | 1.8 | 8.79 × 10−5 | 0.049729216 | Hypothetical gene |
| Os01g0737700 | 415.1 | 0.6 | 7.21 × 10−9 | 3.22 × 10−5 | Similar to OSIGBa0101A01.4 protein |
| Os01g0804200 | 314.4 | 0.2 | 4.58 × 10−9 | 2.93 × 10−5 | Cytochrome P450 of the CYP94 subfamily, response to wounding and salt stress |
| Os01g0931400 | 719.8 | 60.2 | 8.76 × 10−5 | 0.049729216 | Thiamin pyrophosphokinase, eukaryotic domain containing protein |
| Os02g0281200 | 701.4 | 0.6 | 4.58 × 10−12 | 6.82 × 10−8 | Similar to NBS-LRR protein |
| Os02g0483500 | 3362.8 | 455.8 | 8.09 × 10−7 | 0.001643243 | Transferase family protein |
| Os02g0755900 | 194.4 | 0.2 | 4.46 × 10−5 | 0.031722495 | UDP-glucuronosyl/UDP-glucosyltransferase domain containing protein |
| Os02g0812000 | 208.8 | 0.2 | 5.71 × 10−5 | 0.036260256 | NAD(P)-binding domain containing protein |
| Os03g0299800 | 197.2 | 0.0 | 1.39 × 10−5 | 0.014098454 | Protein of unknown function Cys-rich family protein |
| Os03g0321700 | 2104.4 | 182.6 | 7.17 × 10−5 | 0.043217519 | Similar to WRKY transcription factor 55 |
| Os04g0177300 | 2108.4 | 13.6 | 1.40 × 10−10 | 1.25 × 10−6 | HIP116, Rad5p N-terminal domain containing protein |
| Os04g0403500 | 180.6 | 0.0 | 2.44 × 10−5 | 0.020228994 | NAD(P)-binding domain containing protein |
| Os04g0503600 | 3609.6 | 179.2 | 3.33 × 10−5 | 0.025674616 | Similar to OSIGBa0112M24.5 protein |
| Os04g0510600 | 454.0 | 0.0 | 8.17 × 10−6 | 0.009359965 | Tetratricopeptide-like helical domain containing protein |
| Os04g0584201 | 168.6 | 0.2 | 4.06 × 10−6 | 0.005649724 | Hypothetical protein |
| Os05g0164800 | 116.0 | 0.0 | 1.80 × 10−5 | 0.016782781 | Similar to zinc transporter 6, chloroplast precursor |
| Os05g0203800 | 564.3 | 0.0 | 5.27 × 10−8 | 1.68 × 10−4 | Transcription factor, floral organ development |
| Os05g0244700 | 1109.0 | 30.0 | 4.42 × 10−5 | 0.031722495 | Aminotransferase, class IV family protein |
| Os05g0594200 | 414.3 | 0.2 | 1.82 × 10−7 | 4.78 × 10−4 | Similar to cation/proton exchanger 1a |
| Os06g0520600 | 8126.6 | 1677.2 | 1.71 × 10−5 | 0.016639221 | Similar to zinc finger CCCH type domain containing protein ZFN-like 1 |
| Os06g0591200 | 180.6 | 0.0 | 4.55 × 10−5 | 0.031797243 | Conserved hypothetical protein |
| Os07g0170000 | 944.2 | 70.8 | 6.90 × 10−5 | 0.042206892 | Similar to Brn1-like protein |
| Os07g0668900 | 87.4 | 0.0 | 5.52 × 10−5 | 0.036260256 | Similar to serine/threonine-protein kinase PBS1 |
| Os08g0191300 | 4671.0 | 468.6 | 6.61 × 10−9 | 3.22 × 10−5 | Conserved hypothetical protein |
| Os08g0197300 | 160.8 | 0.4 | 2.59 × 10−5 | 0.021001501 | F-box domain, cyclin-like domain containing protein |
| Os08g0224700 | 3489.6 | 787.8 | 3.25 × 10−5 | 0.02549321 | Similar to 26S proteasome subunit RPN2a |
| Os08g0406900 | 989.1 | 0.6 | 1.39 × 10−9 | 1.03 × 10−5 | Hypothetical protein |
| Os09g0133600 | 1001.5 | 0.0 | 3.80 × 10−20 | 1.70 × 10−15 | Fibrillin, plastoglobule (PG) formation and lipid metabolism in chloroplasts |
| Os09g0433650 | 2942.0 | 197.4 | 3.16 × 10−5 | 0.025214678 | Tobacco mosaic virus coat protein family protein |
| Os09g0498700 | 241.0 | 1.6 | 1.78 × 10−5 | 0.016782781 | F-box domain, cyclin-like domain containing protein |
| Os09g0549300 | 214.2 | 0.0 | 4.47 × 10−5 | 0.031722495 | Flavin-containing monooxygenase FMO family protein |
| Os09g0552600 | 2250.3 | 264.8 | 1.20 × 10−7 | 3.36 × 10−4 | RmlC-like jelly roll fold domain containing protein |
| Os10g0552400 | 1476.6 | 1.8 | 1.82 × 10−16 | 4.07 × 10−12 | U-box E3 ubiquitin ligase |
| Os11g0213000 | 791.6 | 2.6 | 4.66 × 10−7 | 9.91 × 10−4 | Similar to protein kinase domain containing protein, expressed |
| Os11g0295900 | 2590.4 | 134.4 | 3.26 × 10−6 | 0.005025349 | AP2-transcription factor, initiation of zygotic development |
| Os11g0437600 | 612.6 | 0.2 | 1.21 × 10−8 | 4.17 × 10−5 | Protein of unknown function DUF506, plant family protein |
| Os11g0619800 | 220.4 | 0.0 | 2.60 × 10−7 | 6.45 × 10−4 | Kelch related domain containing protein |
| Os12g0135800 | 316.2 | 0.0 | 9.14 × 10−9 | 3.40 × 10−5 | Alpha/beta hydrolase fold-3 domain containing protein |
| Os12g0268000 | 585.3 | 1.7 | 1.17 × 10−6 | 0.002272031 | Cytochrome P450 monooxygenase, tryptamine 5-hydroxylase |
| Os12g0283400 | 1103.2 | 0.2 | 2.98 × 10−6 | 0.004756237 | Pectinesterase inhibitor domain containing protein |
| Os12g0637100 | 619.8 | 6.4 | 8.01 × 10−9 | 3.25 × 10−5 | Similar to purple acid phosphatase |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deushi, R.; Toda, E.; Koshimizu, S.; Yano, K.; Okamoto, T. Effect of Paternal Genome Excess on the Developmental and Gene Expression Profiles of Polyspermic Zygotes in Rice. Plants 2021, 10, 255. https://doi.org/10.3390/plants10020255
Deushi R, Toda E, Koshimizu S, Yano K, Okamoto T. Effect of Paternal Genome Excess on the Developmental and Gene Expression Profiles of Polyspermic Zygotes in Rice. Plants. 2021; 10(2):255. https://doi.org/10.3390/plants10020255
Chicago/Turabian StyleDeushi, Ryouya, Erika Toda, Shizuka Koshimizu, Kentaro Yano, and Takashi Okamoto. 2021. "Effect of Paternal Genome Excess on the Developmental and Gene Expression Profiles of Polyspermic Zygotes in Rice" Plants 10, no. 2: 255. https://doi.org/10.3390/plants10020255
APA StyleDeushi, R., Toda, E., Koshimizu, S., Yano, K., & Okamoto, T. (2021). Effect of Paternal Genome Excess on the Developmental and Gene Expression Profiles of Polyspermic Zygotes in Rice. Plants, 10(2), 255. https://doi.org/10.3390/plants10020255







