Effect of Rice Grain (Oryza sativa L.) Enrichment with Selenium on Foliar Leaf Gas Exchanges and Accumulation of Nutrients
Abstract
1. Introduction
2. Results
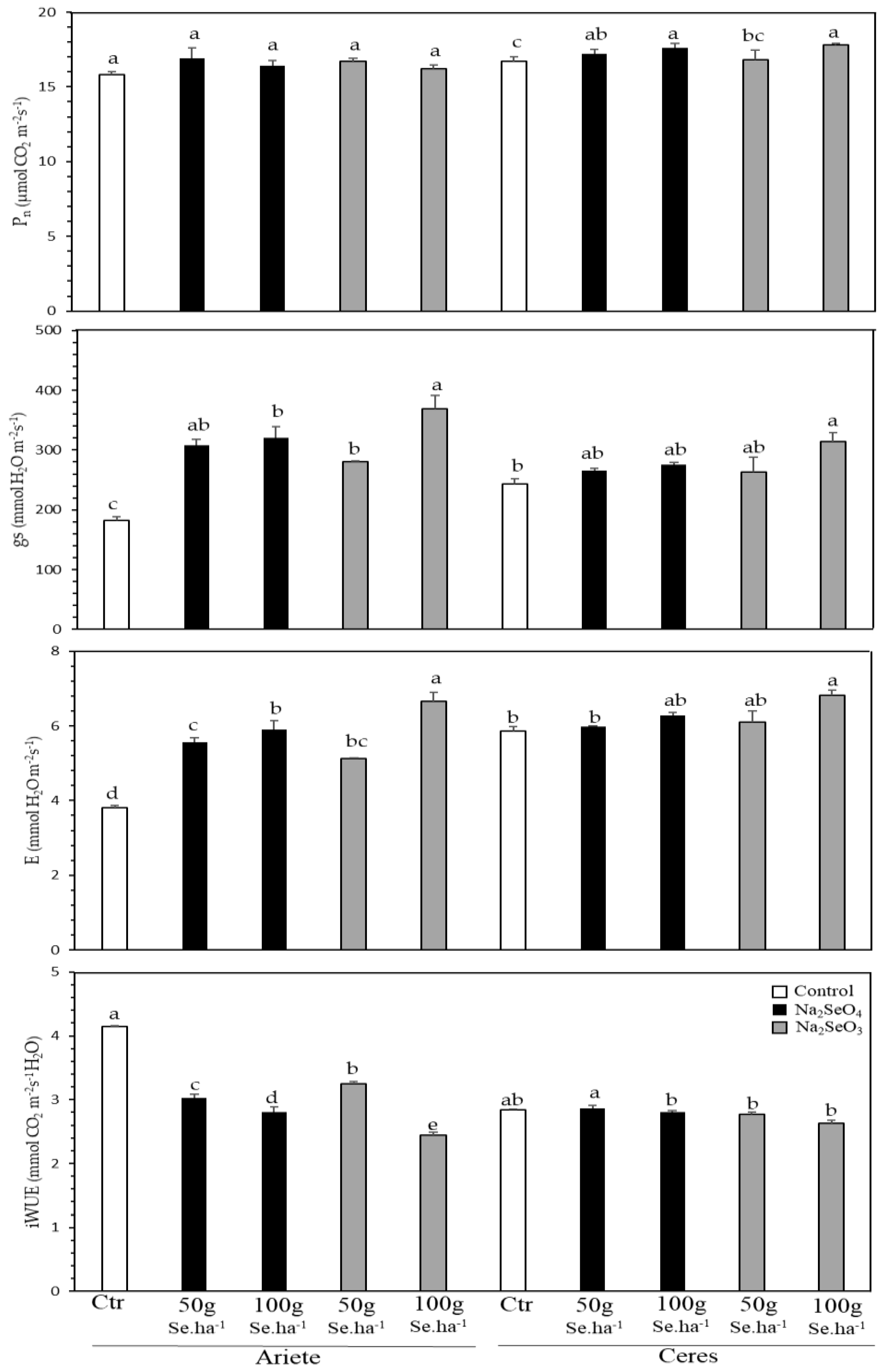
2.1. Physiological Monitoring during Biofortification
2.2. Accumulation of Selenium in Rice Grain
2.3. Macro and Micronutrient Quantification in Rice Grains
2.3.1. Paddy Rice
2.3.2. Brown Rice
2.3.3. White Rice
2.3.4. Comparison between Elemental Levels in Paddy and White Rice Grains
2.4. Colour Analysis
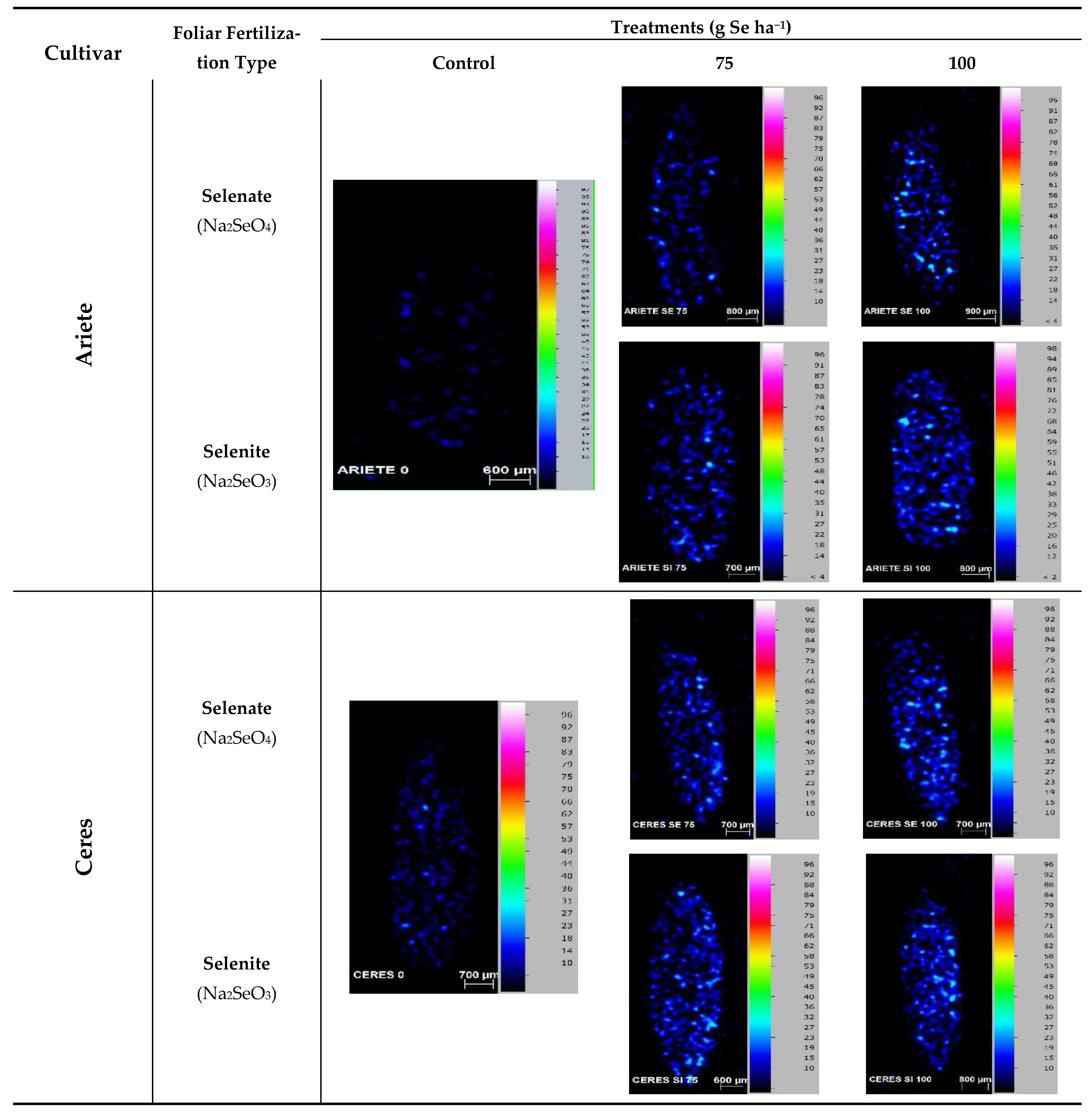
2.5. Location of Se in Grains
3. Discussion
4. Materials and Methods
4.1. Experimental Fields
4.2. Leaf Gas Exchange Measurements
4.3. Atomic Absorption Spectrometry
4.4. Analysis of Micronutrients and Se Content and Location in the Grain Tissues
4.5. Colorimetric Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Bañuelos, M.L.; Hermosillo-Cereceres, M.A.; Sanchez, E. The importance of selenium biofortification in food crops. Curr. Nutr. Food Sci. 2011, 7, 181–190. [Google Scholar] [CrossRef]
- Broadley, M.; Alcock, J.; Alford, J.; Cartwright, P.; Foot, I.; Fairweather-Tait, S.; Hart, D.; Hurst, R.; Knott, P.; MacGrath, S.; et al. Selenium biofortification of high-yielding winter wheat (Triticum aestivum L.) by liquid or granular Se fertilisation. Plant Soil. 2010, 332, 5–18. [Google Scholar] [CrossRef]
- Hart, D.; Fairweather-Tait, S.; Broadley, M.; Dickinson, S.; Foot, I.; Knott, P.; MacGrath, S.; Mowat, H.; Norman, K.; Scott, P.; et al. Selenium concentration and speciation in biofortified flour and bread: Retention of selenium during grain biofortification, processing and production of Se-enriched food. Food Chem. 2011, 126, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Longchamp, M.; Angeli, N.; Castrec-Rouelle, M. Effects on the accumulation of calcium, magnesium, iron, manganese, copper and zinc of adding the two inorganic forms of selenium to solution cultures of Zea mays. Plant Physiol. Biochem. 2016, 98, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Kápolna, E.; Hillestrom, P.R.; Laursen, K.H.; Husted, S.; Larsen, E.H. Effect of foliar application of selenium on its uptake and speciation in carrot. Food Chem. 2009, 115, 1357–1363. [Google Scholar] [CrossRef]
- White, P.J. Selenium accumulation by plants. Ann. Bot. 2016, 117, 217–235. [Google Scholar] [CrossRef]
- Alshall, T.; El-Ramady, H. Foliar application: From plant nutrition to biofortification. Environ. Biodivers. Soil Secur. 2017, 1, 71–83. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2017, 69, 172–180. [Google Scholar] [CrossRef]
- Chen, L.; Yang, F.; Xu, J.; Hu, Y.; Hu, Q.; Zhang, Y.; Pan, G. Determination of selenium concentration of rice in China and effect of fertilization of selenite and selenate on selenium content of rice. J. Agric. Food Chem. 2002, 50, 5128–5130. [Google Scholar] [CrossRef]
- Boldrin, P.F.; Faquin, V.; Ramos, S.J.; Boldrin, K.V.F.; Ávila, F.W.; Guilherme, L.R.G. Soil and foliar application of selenium in rice biofortification. J. Food Compos. Anal. 2013, 238–244. [Google Scholar] [CrossRef]
- Ros, G.; van Rotterdam, A.; Bussink, D.; Bindraban, P. Selenium fertilization strategies for bio-fortification of food: An agro-ecosystem approach. Plant Soil 2016, 404, 99–112. [Google Scholar] [CrossRef]
- Poblaciones, M.J.; Santamaría, O.; García-White, T.; Rodrigo, S.M. Selenium biofortification in bread-making wheat under Mediterranean conditions: Influence on grain yield and quality parameters. Crop Pasture Sci. 2014, 65, 362–369. [Google Scholar] [CrossRef]
- Longchamp, M.; Castrec-Rouelle, M.; Biron, P.; Bariac, T. Variations in the accumulation, localization and rate of metabolization of selenium in mature Zea mays plants supplied with selenite or selenate. Food Chem. 2015, 182, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Lidon, F.; Oliveira, K.; Galhano, C.; Guerra, M.; Ribeiro, M.; Pelica, J.; Pataco, I.; Ramalho, J.; Leitão, A.; Almeida, A.; et al. Selenium biofortification of rice through foliar application with selenite and selenate. Exp. Agric. 2018, 55, 528–542. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, G.; Chen, J.; Hu, Q. Uptake and transport of selenite and selenate by soybean seedlings of two genotypes. Plant Soil 2003, 253, 437–443. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B. Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Regul. 2013, 70, 149–157. [Google Scholar] [CrossRef]
- Oliveira, K.; Pataco, I.M.; Mourinho, M.P.; Santos, C.; Pelica, J.; Leitão, A.E.; Pais, I.P.; Campos, P.S.; Lidon, F.C.; Reboredo, F.H.; et al. Selenium biofortification in rice—A pragmatic perspective. Emir. J. Food Agric. 2015, 27, 231–241. [Google Scholar] [CrossRef]
- Mangueze, A.; Pessoa, M.F.; Silva, M.J.; Ndayiragije, A.; Magaia, H.; Cossa, V.; Reboredo, F.; Carvalho, M.L.; Santos, J.P.; Guerra, M.; et al. Simultaneous zinc and selenium biofortification in rice accumulation, localization and implications on the overall mineral content of the flour. J. Cereal Sci. 2018, 82, 34–41. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Roda, F.A.; Pessoa, M.F.G.; Reboredo, F.H.; Pais, I.P.; Ndayiragije, A.; Lidon, F.C.; Ribeiro-Barros, A.I. Selenium agronomic biofortification in rice: Improving crop quality against malnutrition. In The Future of Rice Demand: Quality beyond Productivity; de Oliveira, A.C., Pegoraro, C., Viana, V.E., Eds.; Springer: Cham, Switzerland, 2020; pp. 179–203. [Google Scholar] [CrossRef]
- Ramkissoon, C.; Degryse, F.; da Silva, R.C.; Baird, R.; Young, S.D.; Bailey, E.H.; McLaughlin, M.J. Improving the efficacy of selenium fertilizers for wheat biofortification. Sci. Rep. 2019, 9, 19520. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Zhang, X.; Zhang, W.; Huang, L.; Zhang, Z.; Yuan, L.; Liu, X. Effects of foliar application of selenate and selenite at different growth stages on selenium accumulation and speciation in potato (Solanum tuberosum L.). Food Chem. 2019, 286, 550–556. [Google Scholar] [CrossRef]
- Hartikainen, H. Biogeochemistry of selenium and its impact of food chain quality and human health. J. Trace Elem. Med. Biol. 2005, 18, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, B.; Llugany, M.; Palacios, O.; Valiente, M. Dual effects of different selenium species on wheat. Plant Physiol. Biochem. 2014, 83, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: London, UK, 1995. [Google Scholar]
- White, P.; Bowen, H.; Parmaguru, P.; Fritz, M.; Spracklen, W.; Spiby, R.E. Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J. Exp. Bot. 2004, 55, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Dong, T.; Ye, J.; Hou, Z. Selenium accumulation in wheat (Triticum aestivum L) as affected by coapplication of either selenite or selenate with phosphorus. Soil Sci. Plant Nutr. 2017, 63, 37–44. [Google Scholar] [CrossRef]
- Zembala, M.; Filek, M.; Walas, S.; Mrowiec, H.; Kornaś, A.; Miszalski, Z.; Hartikainen, H. Effect of selenium on macro- and microelement distribution and physiological parameters of rape and wheat seedlings exposed to cadmium stress. Plant Soil 2010, 329, 457–468. [Google Scholar] [CrossRef]
- Goufo, P.; Ferreira, L.M.M.; Trindade, H.; Rosa, E.A.S. Distribution of antioxidant compounds in the grain of the Mediterranean rice variety ‘Ariete’. CyTA J. Food 2015, 13, 140–150. [Google Scholar] [CrossRef][Green Version]
- Almeida, A.S.; Coutinho, J.; Brites, C.; Maçãs, B.; Marques, P.; Silva, C.; Jordão, A. Variedades Portuguesas de Arroz—Presente e Future. Dossier Técnico-Vida Rural. 2020; pp. 42–45. Available online: https://www.drapc.gov.pt/base/documentos/vr_variedades_portuguesas_%20arroz.pdf (accessed on 19 January 2021).
- Zhang, M.; Tang, S.; Huang, X.; Zhang, F.; Pang, Y.; Huang, Q.; Yi, Q. Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fluorescence in rice (Oryza sativa L.). Environ. Exp. Bot. 2014, 107, 3945. [Google Scholar] [CrossRef]
- Reboredo, F.; Henriques, F. Some observations on the leaf ultrastructure of Halimione portulacoides (L.) Aellen grown in a medium containing copper. J. Plant Physiol. 1991, 137, 717–722. [Google Scholar] [CrossRef]
- Jiang, C.; Zu, C.; Lu, D.; Zheng, Q.; Shen, J.; Wang, H.; Li, D. Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci. Rep. 2017, 7, 42039. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, L.; Xu, J.; Zhang, Y.; Pan, G. Determination of selenium concentration in rice and the effect of foliar application of Se enriched fertilizer or sodium selenite on the selenium content of rice. J. Sci. Food Agric. 2002, 82, 869–872. [Google Scholar] [CrossRef]
- Wang, Y.D.; Wang, X.; Wong, Y.S. Generation of selenium-enriched rice with enhanced grain yield, selenium content and bioavailability through fertilisation with selenite. Food Chem. 2013, 141, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Chen, S.; Gao, D.; Liu, G.; Bai, H.; Li, A.; Peng, L.; Ren, Z. Selenium improves photosynthesis and protects photosystem II in pear (Pyrus bretschneideri), grape (Vitis vinifera), and peach (Prunus persica). Photosynthetica 2015, 53, 609–612. [Google Scholar] [CrossRef]
- González-Morales, S.; Pérez-Labrada, F.; García-Enciso, E.L.; Leija-Martínez, P.; Medrano-Macías, J.; Dávila-Rangel, I.E.; Benavides-Mendoza, A. Selenium and sulfur to produce allium functional crops. Molecules 2017, 22, 558. [Google Scholar] [CrossRef] [PubMed]
- Turakainen, M.; Hartikainen, H.; Seppanen, M.M. Effects of selenium treatments on potato (Solanum tuberosum L.) growth and concentrations of soluble sugars and starch. J. Agric. Food Chem. 2004, 52, 5378–5382. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, Z.; Bu, Y.; Ren, C.; Li, J.; Han, J.; Tao, C.; Zhang, K.; Wang, X.; Lu, G. Effects of selenium fertilizer on grain yield, Se uptake and distribution in common buckwheat (Fagopyrum esculentum Moench). Plant Soil Environ. 2015, 61, 371–377. [Google Scholar] [CrossRef]
- Thavarajah, P.; Vial, E.; Gebhardt, M.; Lacher, C.; Kumar, S.; Combs, G.F. Will selenium increase lentil (Lens culinaris Medik) yield and seed quality? Front. Plant Sci. 2015, 6, 356. [Google Scholar] [CrossRef]
- Da Silva, D.F.; Cipriano, P.E.; de Souza, R.R.; Júnior, M.S.; da Silva, R.F.; Faquin, V.; Silva, M.L.S.; Guilherme, L.R.G. Anatomical and physiological characteristics of Raphanus sativus L. submitted to different selenium sources and forms application. Sci. Hortic. 2020, 260, 108839. [Google Scholar] [CrossRef]
- Deng, X.; Liu, K.; Li, M.; Zhang, W.; Zhao, X.; Zhao, Z.; Liu, X. Difference of selenium uptake and distribution in the plant and selenium form in the grains of rice with foliar spray of selenite or selenate at different stages. Field Crops Res. 2017, 211, 165–171. [Google Scholar] [CrossRef]
- Marques, A.C.; Lidon, F.C.; Coelho, A.R.F.; Pessoa, C.C.; Luís, I.C.; Scotti-Campos, P.; Simões, M.; Almeida, A.S.; Legoinha, P.; Pessoa, M.F.; et al. Quantification and tissue localization of selenium in rice (Oryza sativa L. Poaceae) grains: A perspective of agronomic biofortification. Plants 2020, 9, 1670. [Google Scholar] [CrossRef]
- Lidon, F.C.; Ribeiro, A.; Leitão, A.; Pataco, I.; Ribeiro, M.M.; Reboredo, F.; Almeida, A.; Silva, M.M.; Pais, I.; Pelica, J.; et al. Selenium biofortification of rice grains and implications in the nutritional quality. J. Cereal Sci. 2018, 81, 22–29. [Google Scholar] [CrossRef]
- Lamberts, L.; Bie, E.D.; Greet, E.; Vandeputte, W.S.; Veraverbeke, V.; Man, W.; Delcour, J.A. Effect of milling on colour and nutritional properties of rice. Food Chem. 2007, 100, 1496–1503. [Google Scholar] [CrossRef]
- Dillahunty, A.L.; Siebenmorgen, T.J.; Mauromoustakos, A. Effect of temperature, exposure duration, and moisture content on color and viscosity of rice. Cereal Chem. 2001, 78, 559–563. [Google Scholar] [CrossRef]
- Lu, L.; Tian, S.; Liao, H.; Zhang, J.; Yang, X.; Labavitch, J.; Chen, W. Analysis of metal element distributions in rice (Oryza sativa L.) seeds and relocation during germination based on X-Ray fluorescence imaging of Zn, Fe, K, Ca, and Mn. PLoS ONE 2013, 8, e57360. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.-M.; Lombi, E.; Donner, E.; de Jonge, M.D.; Punshon, T.; Jackson, B.P.; Guerinot, M.L.; Price, A.H.; Meharg, A.A. A review of recent developments in the speciation and location of arsenic and selenium in rice grain. Anal. Bioanal. Chem. 2012, 402, 3275–3286. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.N.; Lombi, E.; Sun, G.X.; Scheckel, K.; Zhu, Y.G.; Feng, X.; Zhu, J.; Carey, A.M.; Adomako, E.; Lawgali, Y.; et al. Selenium characterization in the global rice supply chain. Environ. Sci. Technol. 2009, 43, 6024–6030. [Google Scholar] [CrossRef]
- Sun, G.X.; Liu, X.; Williams, P.N.; Zhu, Y.G. Distribution and translocation of selenium from soil to grain and its speciation in paddy rice (Oryza sativa L.). Environ. Sci. Technol. 2010, 44, 6706–6711. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.H.; Lombi, E.; Fitzgerald, M.; Laursen, K.H.; Frydenvang, J.; Husted, S.; Boualaphanh, C.; Resurreccion, A.; Howard, D.L.; de Jonge, M.D.; et al. Losses of essential mineral nutrients by polishing of rice differ among genotypes due to contrasting grain hardness and mineral distribution. J. Cereal Sci. 2012, 56, 307–315. [Google Scholar] [CrossRef]
- Reddy, C.K.; Kimi, L.; Haripriya, S.; Kang, N. Effects of polishing on proximate composition, physico-chemical characteristics, mineral composition and antioxidant properties of pigmented rice. Rice Sci. 2017, 24, 241–252. [Google Scholar] [CrossRef]
- Doesthale, Y.G.; Devara, S.; Rao, S.; Belavady, B. Effect of milling on mineral and trace element composition of raw and parboiled rice. J. Sci. Food Agric. 1979, 30, 40–46. [Google Scholar] [CrossRef]
- Saleh, A.S.M.; Wang, P.; Wang, N.; Yang, L.; Xiao, Z. Brown rice versus white rice: Nutritional quality, potential health benefits, development of food products, and preservation technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1070–1096. [Google Scholar] [CrossRef]
- Ravichanthiran, K.; Ma, Z.F.; Zhang, H.; Cao, Y.; Wang, C.W.; Muhammad, S.; Aglago, E.K.; Zhang, Y.; Jin, Y.; Pan, B. Phytochemical profile of brown rice and its nutrigenomic implications. Antioxidants 2018, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, W.P.; Martins, M.Q.; Fortunato, A.S.; Rodrigues, A.P.; Semedo, J.N.; Simões-Costa, M.C.; Pais, I.P.; Leitão, A.E.; Colwell, F.; Goulao, L.; et al. Long-term elevated air [CO2] strengthens photosynthetic functioning and mitigates the impact of supra-optimal temperatures in tropical Coffea arabica and Coffea canephora species. Glob. Chang. Biol. 2016, 22, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Carrondo, M.; Reboredo, F.; Ganho, R.; Santos Oliveira, J.F. Heavy metal analysis of sediments in Tejo estuary, Portugal, using a rapid flameless atomic absorption procedure. Talanta 1984, 31, 561–564. [Google Scholar] [CrossRef]
- Reboredo, F.H.S.; Ribeiro, C.A.G. Vertical distribution of Al, Cu, Fe and Zn in soil salt marshes of the Sado estuary, Portugal. Int. J. Environ. Stud. 1984, 23, 249–253. [Google Scholar] [CrossRef]
- Cardoso, P.; Mateus, T.; Velu, G.; Singh, R.P.; Santos, J.P.; Carvalho, M.L.; Lourenço, V.M.; Lidon, F.; Reboredo, F.; Guerra, M. Localization and distribution of Zn and Fe in grains of biofortified bread wheat lines through micro and triaxial-X-ray spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2018, 141, 70–79. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Pais, I.P.; Leitão, A.E.; Guerra, M.; Reboredo, F.H.; Máguas, C.M.; Carvalho, M.L.; Scotti-Campos, P.; Ribeiro-Barros, A.I.; Lidon, F.J.C.; et al. Can elevated air [CO2] conditions mitigate the predicted warming impact on the quality of coffee bean? Front. Plant Sci. 2018, 9, 287. [Google Scholar] [CrossRef] [PubMed]
| Treatments (g Se ha−1) | Se | Zn | Fe | Cu | Ca | Mg | K | ||
|---|---|---|---|---|---|---|---|---|---|
| Ariete | Na2SeO4 | Control | 1.69 ± 0.20 c | 47.0 ± 11.4 a | 34.9 ± 2.4 a | 5.13 ± 0.87 a | 43.9 ± 7.07 a | 443 ± 3.49 a | 6300 ± 1000 b |
| 25 | 2.66 ± 0.23 c | 36.0 ± 2.76 a | 35.1 ± 3.02 a | 4.85 ± 0.05 a | 42.4 ± 7.89 a | 448 ± 2.58 a | 6300 ± 200 b | ||
| 50 | 4.06 ± 1.38 b | 26.8 ± 4.14 b | 38.5 ± 5.49 a | 5.35 ± 0.93 a | 21.9 ± 5.89 a | 445 ± 0.40 a | 8900 ± 1600 a | ||
| 75 | 6.06 ± 0.42 ab | 30.3 ± 4.44 a | 35.0 ± 2.36 a | 5.19 ± 0.51 a | 27.1 ± 6.22 a | 418 ± 6.57 b | 8200 ± 2100 a | ||
| 100 | 8.10 ± 2.21 a | 30.7 ± 6.34 a | 36.8 ± 5.90 a | 5.94 ± 1.05 a | 30.9 ± 8.13 a | 415 ± 9.10 b | 8700 ± 1400 a | ||
| Na2SeO3 | Control | 1.69 ± 0.20 c | 47.0 ± 11.4 a | 34.9 ± 2.40 a | 5.13 ± 0.87 a | 43.9 ± 7.07 a | 443 ± 3.49 a | 6300 ± 1000 b | |
| 25 | 5.92 ± 2.38 ab | 28.3 ± 2.42 b | 31.0 ± 6.52 a | 5.34 ± 0.63 a | 40.0 ± 5.09 a | 440 ± 2.77 a | 9400 ± 900 a | ||
| 50 | 7.55 ± 1.79 ab | 33.0 ± 6.77 a | 39.6 ± 3.12 a | 5.30 ± 0.76 a | 44.7 ± 8.83 a | 449 ± 9.00 a | 8700 ± 2300 a | ||
| 75 | 9.88 ± 2.83 a | 31.6 ± 3.28 a | 35.8 ± 5.71 a | 5.79 ± 0.17 a | 45.3 ± 6.11 a | 441 ± 4.45 a | 9000 ± 1400 a | ||
| 100 | 13.0 ± 2.52 a | 29.6 ± 3.12 b | 39.2 ± 12.7 a | 5.02 ± 0.84 a | 39.8 ± 8.98 a | 456 ± 5.88 a | 6900 ± 300 b | ||
| Ceres | Na2SeO4 | Control | 2.01 ± 0.32 c | 69.7 ± 1.74 a | 42.7 ± 4.59 a | 7.43 ± 0.81 a | 76.1 ± 2.99 a | 424 ± 11.2 ab | 6700 ± 600 ab |
| 25 | 4.11 ± 0.76 b | 36.5 ± 3.90 b | 37.8 ± 8.46 a | 7.74 ± 0.98 a | 59.1 ± 5.29 b | 408 ± 5.90 b | 8700 ± 700 a | ||
| 50 | 5.67 ± 1.63 a | 33.6 ± 9.27 b | 40.1 ± 5.59 a | 6.50 ± 2.21 a | 51.1 ± 0.81 b | 453 ± 20.3 a | 6100 ± 700 b | ||
| 75 | 8.66 ± 3.25 a | 31.4 ± 1.94 b | 43.7 ± 8.92 a | 6.53 ± 1.19 a | 57.3 ± 2.19 b | 418 ± 12.9 b | 5500 ± 500 b | ||
| 100 | 11.6 ± 3.94 a | 49.3 ± 16.0 ab | 50.0 ± 2.05 a | 7.10 ± 1.07 a | 67.8 ± 2.31 ab | 419 ± 8.92 b | 7100 ± 500 ab | ||
| Na2SeO3 | Control | 2.01 ± 0.32 c | 69.7 ± 1.74 a | 42.7 ± 4.59 a | 7.43 ± 0.81 a | 76.1 ± 2.99 a | 424 ± 11.2 b | 6700 ± 600 a | |
| 25 | 6.30 ± 1.22 b | 39.2 ± 5.22 a | 51.0 ± 9.84 a | 7.26 ± 1.40 a | 40.6 ± 6.58 b | 437 ± 8.57 b | 8200 ± 900 a | ||
| 50 | 11.8 ± 3.85 a | 36.7 ± 7.97 a | 40.2 ± 4.28 a | 6.11 ± 1.12 a | 56.7 ± 5.78 b | 457 ± 4.38 a | 6300 ± 600 a | ||
| 75 | 10.3 ± 2.01 a | 33.4 ± 2.82 a | 50.0 ± 8.97 a | 5.75 ± 0.71 a | 57.8 ± 2.37 b | 429 ± 3.88 b | 6000 ± 1100 a | ||
| 100 | 12.7 ± 2.91 a | 38.0 ± 1.99 a | 45.2 ± 8.25 a | 5.99 ± 0.43 a | 58.4 ± 7.53 b | 444 ± 1.54 b | 6800 ± 1500 a | ||
| Treatments (g Se ha−1) | Se | Zn | Fe | Cu | Ca | Mg | K | ||
|---|---|---|---|---|---|---|---|---|---|
| Ariete | Na2SeO4 | Control | 1.75 ± 0.28 c | 29.5 ± 6.08 a | 24.3 ± 1.79 a | 5.48 ± 0.65 a | 25.9 ± 1.74 a | 448 ± 18.6 a | 5300 ± 100 b |
| 25 | 2.86 ± 0.37 c | 27.9 ± 3.41 a | 19.4 ± 3.50 a | 5.34 ± 0.40 a | 20.9 ± 3.88 a | 438 ± 6.87 a | 8500 ± 1600 a | ||
| 50 | 3.74 ± 0.39 ab | 28.5 ± 6.31 a | 18.5 ± 5.47 a | 5.05 ± 1.35 a | 11.3 ± 3.62 b | 453 ± 4.22 a | 7000 ± 2000 a | ||
| 75 | 5.51 ± 1.50 ab | 24.9 ± 1.18 a | 14.6 ± 2.60 a | 4.41 ± 0.57 a | 18.4 ± 4.13 a | 447 ± 6.74 a | 6000 ± 1400 a | ||
| 100 | 7.17 ± 1.84 a | 28.9 ± 4.74 a | 15.9 ± 2.44 a | 4.96 ± 0.12 a | 13.1 ± 2.49 b | 425 ± 11.8 a | 6600 ± 800 a | ||
| Na2SeO3 | Control | 1.75 ± 0.28 c | 29.5 ± 6.08 a | 24.3 ± 1.79 a | 5.48 ± 0.65 a | 25.9 ± 1.74 b | 448 ± 18.6 a | 5300 ± 100 b | |
| 25 | 2.15 ± 0.14 c | 27.6 ± 6.06 a | 19.7 ± 1.69 a | 4.20 ± 0.48 a | 26.8 ± 1.34 b | 443 ± 5.46 a | 5700 ± 1500 a | ||
| 50 | 6.76 ± 1.32 b | 28.3 ± 5.73 a | 17.5 ± 0.96 a | 7.67 ± 5.18 a | 45.1 ± 11.0 a | 454 ± 7.04 a | 4800 ± 700 a | ||
| 75 | 12.1 ± 4.13 ab | 27.6 ± 4.21 a | 19.8 ± 1.00 a | 4.96 ± 0.71 a | 33.0 ± 2.78 a | 454 ± 2.84 a | 7700 ± 600 a | ||
| 100 | 16.7 ± 4.30 a | 26.1 ± 6.07 a | 14.1 ± 1.15 a | 4.92 ± 0.62 a | 29.4 ± 5.92 ab | 446 ± 2.14 a | 7300 ± 2800 a | ||
| Ceres | Na2SeO4 | Control | 2.11 ± 0.25 c | 44.6 ± 7.53 a | 22.4 ± 1.56 a | 5.42 ± 0.62 a | 49.0 ± 7.51 a | 447 ± 8.03 a | 7800 ± 0300 a |
| 25 | 3.79 ± 0.29 b | 28.2 ± 5.90 b | 16.4 ± 3.06 a | 4.96 ± 0.29 a | 40.4 ± 4.37 a | 442 ± 6.28 a | 6300 ± 1200 a | ||
| 50 | 3.78 ± 0.83 b | 28.7 ± 4.45 b | 18.7 ± 1.96 a | 585 ± 1.52 a | 38.7 ± 2.80 a | 452 ± 5.53 a | 7400 ± 1000 a | ||
| 75 | 6.69 ± 2.31 ab | 30.8 ± 2.47 b | 21.6 ± 1.21 a | 5.13 ± 0.66 a | 34.6 ± 3.04 a | 446 ± 11.5 a | 7700 ± 2000 a | ||
| 100 | 9.44 ± 2.67 a | 39.0 ± 6.26 a | 21.5 ± 1.32 a | 5.78 ± 0.75 a | 35.6 ± 6.76 a | 454 ± 3.17 a | 9000 ± 0700 a | ||
| Na2SeO3 | Control | 2.11 ± 0.25 c | 44.6 ± 7.53 a | 22.4 ± 1.56 a | 5.42 ± 0.62 a | 49.0 ± 7.51 a | 447 ± 8.03 a | 7800 ± 300 ab | |
| 25 | 4.00 ± 1.50 b | 34.5 ± 6.99 a | 19.5 ± 1.45 a | 5.77 ± 0.31 a | 35.1 ± 0.13 a | 459 ± 6.06 a | 6600 ± 1100 b | ||
| 50 | 9.01 ± 1.52 ab | 37.6 ± 5.05 a | 20.4 ± 0.46 a | 5.98 ± 0.82 a | 29.9 ± 3.23 a | 452 ± 8.31 a | 9800 ± 500 a | ||
| 75 | 14.2 ± 5.65 a | 32.2 ± 2.79 a | 17.7 ± 2.41 a | 5.70 ± 0.55 a | 32.0 ± 3.34 a | 454 ± 4.45 a | 8300 ± 1600 ab | ||
| 100 | 17.7 ± 0.71 a | 28.5 ± 0.92 b | 19.2 ± 2.15 a | 5.24 ± 0.34 a | 35.0 ± 4.02 a | 457 ± 5.34 a | 7300 ± 200 ab | ||
| Treatments (g Se ha−1) | Se | Zn | Fe | Cu | Ca | Mg | K | ||
|---|---|---|---|---|---|---|---|---|---|
| Ariete | Na2SeO4 | Control | 2.06 ± 0.33 c (+ 21.9%) | 32.5 ± 8.77 a (30.8%) | 9.01 ± 2.47 a (74.2%) | 3.84 ± 0.49 a (25.1%) | 14.1 ± 1.34 a (67.9%) | 329 ± 27.4 a (25.7%) | 1700 ± 100 a (73.0%) |
| 25 | 3.08 ± 0.73 bc (+ 15.8%) | 24.4 ± 4.01 a (32.2%) | 7.85 ± 2.06 a (77.6%) | 4.19 ± 0.22 a (13.6%) | 23.0 ± 2.64 a (45.8%) | 309 ± 8.43 a (31.0%) | 1900 ± 300 a (70.3%) | ||
| 50 | 4.90 ± 2.14 ab (20.7%) | 21.6 ± 3.40 a (19.4%) | 8.66 ± 2.07 a (77.5%) | 4.05 ± 0.54 a (24.3%) | 21.3 ± 5.04 a (32.8%) | 238 ± 27.1 a (46.5%) | 1600 ± 300 a (81.9%) | ||
| 75 | 5.59 ± 0.75 ab (7.76%) | 18.3 ± 1.23 a (39.6%) | 8.63 ± 1.74 a (75.3%) | 4.02 ± 0.08 a (22.5%) | 15.4 ± 1.47 a (43.2%) | 295 ± 26.9 a (29.4%) | 2200 ± 200 a (73.7%) | ||
| 100 | 8.22 ± 3.07 a (+ 1.5%) | 22.3 ± 5.16 a (27.4%) | 8.58 ± 2.33 a (76.7%) | 4.29 ± 0.16 a (27.8%) | 10.7 ± 0.46 a (65.4%) | 286 ± 32.6 b (31.1%) | 2000 ± 500 a (76.6%) | ||
| Na2SeO3 | Control | 2.06 ± 0.33 c (+ 21.9%) | 32.5 ± 8.77 a (30.8%) | 9.01 ± 2.47 a (74.2%) | 3.84 ± 0.49 a (25.1%) | 14.1 ± 1.34 a (67.9%) | 329 ± 27.4 a (25.7%) | 1700 ± 100 a (73.0%) | |
| 25 | 4.07 ± 0.50 ab (31.2%) | 22.4 ± 3.79 a (13.8%) | 11.0 ± 1.63 a (64.5%) | 4.00 ± 0.03 a (25.1%) | 10.5 ± 2.80 a (73.8%) | 254 ± 18.2 b (42.3%) | 1900 ± 600 a (80.2%) | ||
| 50 | 5.36 ± 2.37 ab (29.0%) | 18.9 ± 1.13 a (42.7%) | 8.71 ± 1.66 a (78.0%) | 4.24 ± 0.29 a (20.0%) | 15.4 ± 2.33 a (70.9%) | 283 ± 13.1 ab (37.0%) | 1900 ± 100 a (78.5%) | ||
| 75 | 10.4 ± 3.29 a (+ 5.3%) | 20.4 ± 2.28 a (35.4%) | 11.6 ± 4.60 a (67.6%) | 4.60 ± 0.46 a (20.6%) | 9.60 ± 1.78 a (78.8%) | 266 ± 18.6 ab (39.7%) | 1900 ± 200 a (78.6%) | ||
| 100 | 8.79 ± 1.33 a (32.4%) | 19.4 ± 1.21 a (34.4%) | 8.17 ± 1.02 a (79.1%) | 3.98 ± 0.65 a (20.7%) | 10.3 ± 0.89 a (74.1%) | 258 ± 24.6 b (43.4%) | 1700 ± 400 a (74.8%) | ||
| Ceres | Na2SeO4 | Control | 2.34 ± 0.17 c (+ 16.4%) | 45.8 ± 1.63 a (34.3%) | 8.02 ± 1.79 a (81.2%) | 4.51 ± 0.46 a (39.3%) | 18.9 ± 2.85 a (75.2%) | 293 ± 8.86 a (30.9%) | 2100 ± 100 a (69.0%) |
| 25 | 2.62 ± 0.38 c (36.2%) | 29.2 ± 3.51 a (20.0%) | 7.52 ± 1.14 a (80.1%) | 4.48 ± 0.30 a (42.1%) | 13.1 ± 1.31 a (77.8%) | 304 ± 9.59 a (25.5%) | 2100 ± 100 a (75.9%) | ||
| 50 | 3.72 ± 0.41 a (34.4%) | 25.5 ± 1.76 a (24.1%) | 7.93 ± 1.63 a (80.2%) | 4.48 ± 0.12 a (31.1%) | 17.1 ± 2.71 a (66.5%) | 308 ± 8.49 a (32.0%) | 1800 ± 400 a (70.2%) | ||
| 75 | 3.76 ± 1.14 a (56.6%) | 23.1 ± 3.46 a (26.4%) | 10.7 ± 2.07 a (75.5%) | 4.01 ± 0.84 a (38.6%) | 15.9 ± 1.68 a (72.2%) | 301 ± 11.7 a (28.0%) | 2000 ± 600 a (63.8%) | ||
| 100 | 4.30 ± 1.00 a (62.9%) | 29.5 ± 2.71 a (40.2%) | 8.45 ± 1.36 a (83.1%) | 4.19 ± 0.40 a (41.0%) | 15.2 ± 3.02 a (77.6%) | 264 ± 10.1 b (37.0%) | 1900 ± 200 a (72.7%) | ||
| Na2SeO3 | Control | 2.34 ± 0.17 c (+ 16.4%) | 45.8 ± 1.63 a (34.3%) | 8.02 ± 1.79 a (81.2%) | 4.51 ± 0.46 a (39.3%) | 18.9 ± 2.85 a (75.2%) | 293 ± 8.86 a (30.9%) | 2100 ± 1 ab (69.0%) | |
| 25 | 3.88 ± 1.13 b (38.4%) | 21.9 ± 2.22 a (44.1%) | 8.31 ± 2.07 a (83.7%) | 4.03 ± 0.10 a (45.8%) | 18.7 ± 2.45 a (53.9%) | 242 ± 11.3 b (44.6%) | 1600 ± 200 b (81.1%) | ||
| 50 | 6.09 ± 1.55 ab (48.4%) | 21.9 ± 1.36 a (40.3%) | 6.45 ± 0.84 a (84.0%) | 4.13 ± 0.36 a (32.4%) | 15.4 ± 2.43 a (72.8%) | 267 ± 8.94 a (41.6%) | 1800 ± 400 b (71.5%) | ||
| 75 | 9.47 ± 2.68 a (8.05%) | 24.2 ± 3.12 a (27.5%) | 5.93 ± 1.12 a (88.1%) | 4.75 ± 0.76 a (17.4%) | 23.9 ± 2.66 a (58.6%) | 276 ± 10.3 a (35.7%) | 2800 ± 300 a (54.0%) | ||
| 100 | 11.0 ± 1.51 a (13.4%) | 26.2 ± 5.85 a (31.0%) | 6.15 ± 2.43 a (86.4%) | 4.62 ± 0.37 a (22.9%) | 11.8 ± 0.14 a (79.8%) | 224 ± 12.2 c (49.5%) | 1700 ± 100 b (75.0%) | ||
| Treatments (g Se ha−1) | Paddy Rice | Brown Rice | White Rice | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L | a* | b* | L | a* | b* | L | a* | b* | ||
| Na2SeO4 | ||||||||||
| Ariete | Control | 59.8 ± 2.23 a | 5.57 ± 1.44 a | 27.7 ± 3.35 a | 63.5 ± 2.35 a | 3.48 ± 0.44 a | 23.1 ± 0.18 a | 73.6 ± 1.11 a | −0.80 ± 0.24 a | 9.52 ± 0.54 a |
| 25 | 58.6 ± 0.81 a | 5.97 ± 1.20 a | 27.9 ± 3.19 a | 63.4 ± 1.52 a | 2.81 ± 0.22 a | 22.1 ± 0.95 a | 74.2 ± 0.32 a | −0.86 ± 0.13 a | 9.25 ± 0.70 a | |
| 50 | 59.3 ± 2.14 a | 5.51 ± 1.52 a | 26.6 ± 3.31 a | 62.6 ± 3.05 a | 3.50 ± 0.96 a | 23.6 ± 1.51 a | 73.5 ± 1.40 a | −0.78 ± 0.12 a | 9.73 ± 1.07 a | |
| 75 | 58.9 ± 1.81 a | 5.78 ± 1.56 a | 27.6 ± 3.97 a | 62.5 ± 2.75 a | 3.20 ± 0.43 a | 22.8 ± 0.60 a | 74.0 ± 1.52 a | −0.80 ± 0.18 a | 9.51 ± 0.38 a | |
| 100 | 59.6 ± 3.33 a | 6.07 ± 0.98 a | 28.1 ± 2.48 a | 63.0 ± 2.00 a | 3.16 ± 0.48 a | 23.3 ± 0.58 a | 72.1 ± 1.66 a | −0.66 ± 0.10 a | 10.1 ± 1.01 a | |
| Na2SeO3 | ||||||||||
| Control | 59.0 ± 2.17 a | 5.78 ± 1.35 a | 27.3 ± 2.87 a | 63.6 ± 2.17 a | 3.45 ± 0.35 a | 23.5 ± 0.91 a | 74.3 ± 1.12 a | −0.88 ± 0.15 a | 8.78 ± 0.60 a | |
| 25 | 59.9 ± 2.15 a | 5.31 ± 1.71 a | 27.4 ± 3.64 a | 62.3 ± 1.08 a | 3.63 ± 1.12 a | 23.1 ± 0.87 a | 73.9 ± 2.35 a | −0.60 ± 0.20 a | 9.24 ± 0.65 a | |
| 50 | 58.0 ± 2.98 a | 5.73 ± 1.89 a | 27.0 ± 3.10 a | 63.4 ± 2.22 a | 3.10 ± 0.43 a | 23.8 ± 1.04 a | 74.5 ± 0.24 a | −0.82 ± 0.11 a | 9.08 ± 0.48 a | |
| 75 | 59.1 ± 2.10 a | 5.76 ± 1.53 a | 27.5 ± 2.86 a | 64.2 ± 2.79 a | 3.13 ± 0.78 a | 23.5 ± 0.75 a | 74.4 ± 1.23 a | −0.88 ± 0.11 a | 9.12 ± 0.34 a | |
| 100 | 58.4 ± 2.38 a | 5.73 ± 1.90 a | 27.2 ± 3.90 a | 64.0 ± 1.80 a | 3.27 ± 0.56 a | 23.8 ± 0.43 a | 73.8 ± 1.92 a | −0.88 ± 0.04 a | 9.22 ± 0.40 a | |
| Na2SeO4 | ||||||||||
| Ceres | Control | 58.6 ± 1.39 a | 6.56 ± 0.32 a | 32.8 ± 0.88 ab | 62.0 ± 2.03 a | 3.57 ± 0.83 a | 22.8 ± 0.59 a | 72.1 ± 1.51 a | −1.02 ± 0.06 a | 8.42 ± 0.47 a |
| 25 | 57.5 ± 0.88 a | 6.98 ± 0.76 a | 33.2 ± 1.10 a | 63.1 ± 1.82 a | 2.85 ± 1.03 a | 22.1 ± 0.65 a | 74.0 ± 1.19 a | −1.12 ± 0.11 a | 7.59 ± 0.86 a | |
| 50 | 58.3 ± 0.68 a | 6.82 ± 0.14 a | 33.4 ± 0.42 a | 61.7 ± 2.32 a | 2.91 ± 0.47 a | 23.0 ± 0.50 a | 72.4 ± 0.99 a | −0.94 ± 0.20 a | 7.61 ± 1.15 a | |
| 75 | 58.7 ± 0.62 a | 6.60 ± 0.27 a | 32.0 ± 0.52 ab | 62.9 ± 1.76 a | 3.03 ± 0.12 a | 22.6 ± 0.49 a | 74.3 ± 0.60 a | −1.01 ± 0.15 a | 7.92 ± 1.02 a | |
| 100 | 56.5 ± 1.20 a | 6.36 ± 0.28 a | 31.2 ± 0.43b | 62.4 ± 2.88 a | 3.26 ± 0.57 a | 22.7 ± 0.44 a | 73.0 ± 1.66 a | −1.03 ± 0.08 a | 8.18 ± 0.50 a | |
| Na2SeO3 | ||||||||||
| Control | 57.5 ± 1.28 a | 6.09 ± 0.62 a | 30.4 ± 1.00 a | 61.8 ± 0.86 a | 3.50 ± 0.44 a | 23.1 ± 1.53 a | 72.0 ± 1.42 a | −1.03 ± 0.08 a | 8.17 ± 1.09 a | |
| 25 | 57.5 ± 1.12 a | 6.36 ± 0.27 a | 31.8 ± 0.83 a | 63.8 ± 2.85 a | 2.93 ± 0.28 a | 22.7 ± 0.77 a | 72.5 ± 1.67 a | −0.81 ± 0.38 a | 8.13 ± 1.58 a | |
| 50 | 57.8 ± 0.40 a | 6.29 ± 0.34 a | 31.7 ± 0.46 a | 62.9 ± 0.97 a | 3.20 ± 1.01 a | 23.6 ± 1.35 a | 74.7 ± 0.75 a | −0.98 ± 0.11 a | 8.26 ± 1.30 a | |
| 75 | 57.3 ± 1.47 a | 6.98 ± 0.14 a | 31.5 ± 0.70 a | 63.0 ± 1.81 a | 3.18 ± 0.85 a | 22.8 ± 2.06 a | 73.0 ± 1.40 a | −1.07 ± 0.08 a | 7.96 ± 0.83 a | |
| 100 | 56.9 ± 0.78 a | 6.51 ± 0.35 a | 31.4 ± 1.07 a | 63.1 ± 0.38 a | 2.90 ± 0.83 a | 22.4 ± 0.50 a | 74.2 ± 0.47 a | −1.01 ± 0.14 a | 8.31 ± 0.60 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, A.C.; Lidon, F.C.; Coelho, A.R.F.; Pessoa, C.C.; Luís, I.C.; Campos, P.S.; Simões, M.; Almeida, A.S.; Pessoa, M.F.; Galhano, C.; et al. Effect of Rice Grain (Oryza sativa L.) Enrichment with Selenium on Foliar Leaf Gas Exchanges and Accumulation of Nutrients. Plants 2021, 10, 288. https://doi.org/10.3390/plants10020288
Marques AC, Lidon FC, Coelho ARF, Pessoa CC, Luís IC, Campos PS, Simões M, Almeida AS, Pessoa MF, Galhano C, et al. Effect of Rice Grain (Oryza sativa L.) Enrichment with Selenium on Foliar Leaf Gas Exchanges and Accumulation of Nutrients. Plants. 2021; 10(2):288. https://doi.org/10.3390/plants10020288
Chicago/Turabian StyleMarques, Ana Coelho, Fernando C. Lidon, Ana Rita F. Coelho, Cláudia Campos Pessoa, Inês Carmo Luís, Paula Scotti Campos, Manuela Simões, Ana Sofia Almeida, Maria F. Pessoa, Carlos Galhano, and et al. 2021. "Effect of Rice Grain (Oryza sativa L.) Enrichment with Selenium on Foliar Leaf Gas Exchanges and Accumulation of Nutrients" Plants 10, no. 2: 288. https://doi.org/10.3390/plants10020288
APA StyleMarques, A. C., Lidon, F. C., Coelho, A. R. F., Pessoa, C. C., Luís, I. C., Campos, P. S., Simões, M., Almeida, A. S., Pessoa, M. F., Galhano, C., Guerra, M., Leitão, R. G., Legoinha, P., Ramalho, J. C., Semedo, J. N., Rodrigues, A. P., Marques, P., Silva, C., Ribeiro-Barros, A., ... Reboredo, F. H. (2021). Effect of Rice Grain (Oryza sativa L.) Enrichment with Selenium on Foliar Leaf Gas Exchanges and Accumulation of Nutrients. Plants, 10(2), 288. https://doi.org/10.3390/plants10020288

















