Monoterpenoids from the Fruits of Amomum tsao-ko Have Inhibitory Effects on Nitric Oxide Production
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Characterization
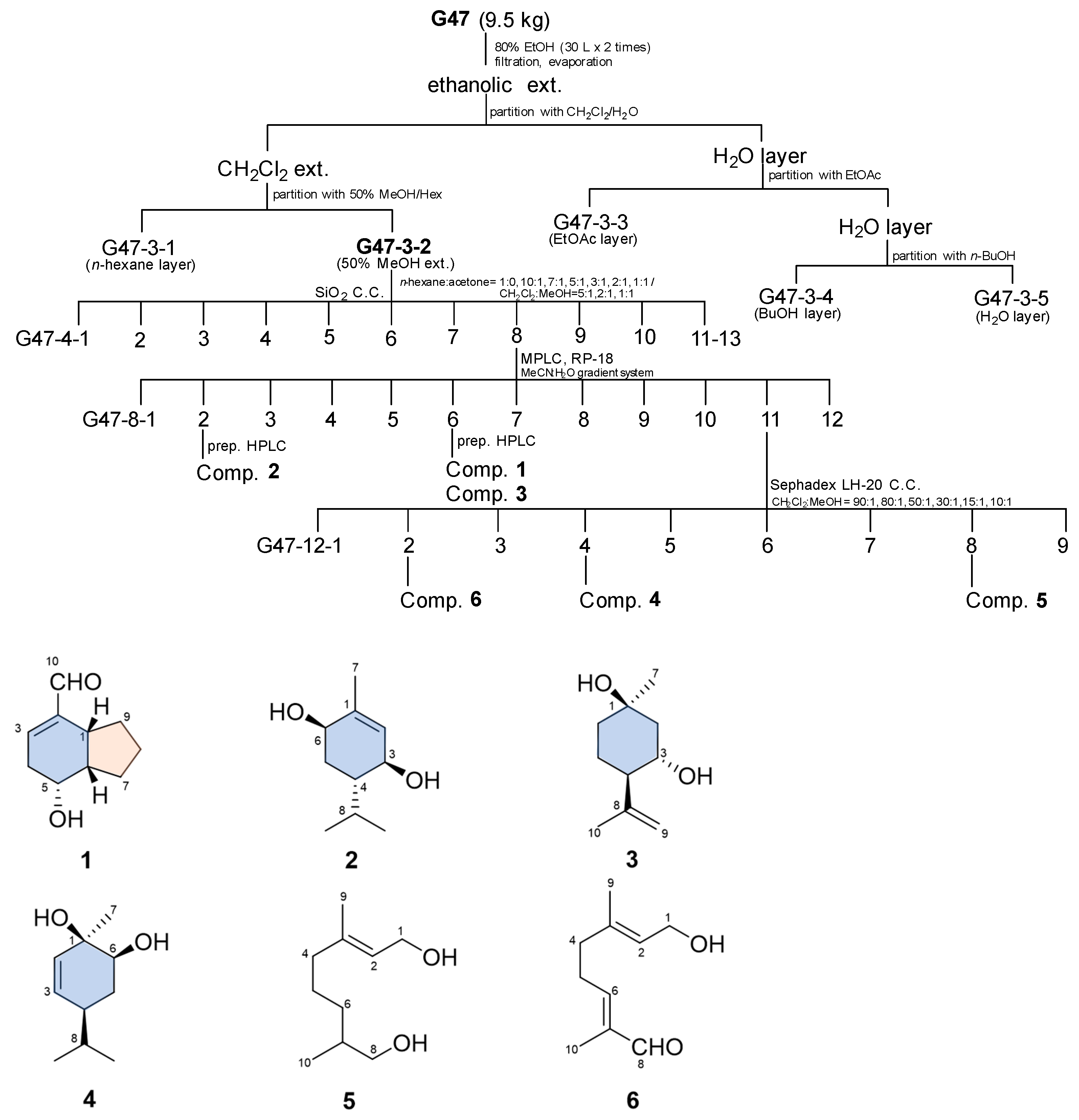
2.1.1. Isolation Compounds
2.1.2. Identification of Compound Structures
2.2. Nitric Oxide Inhibition Activity and Cell Viability
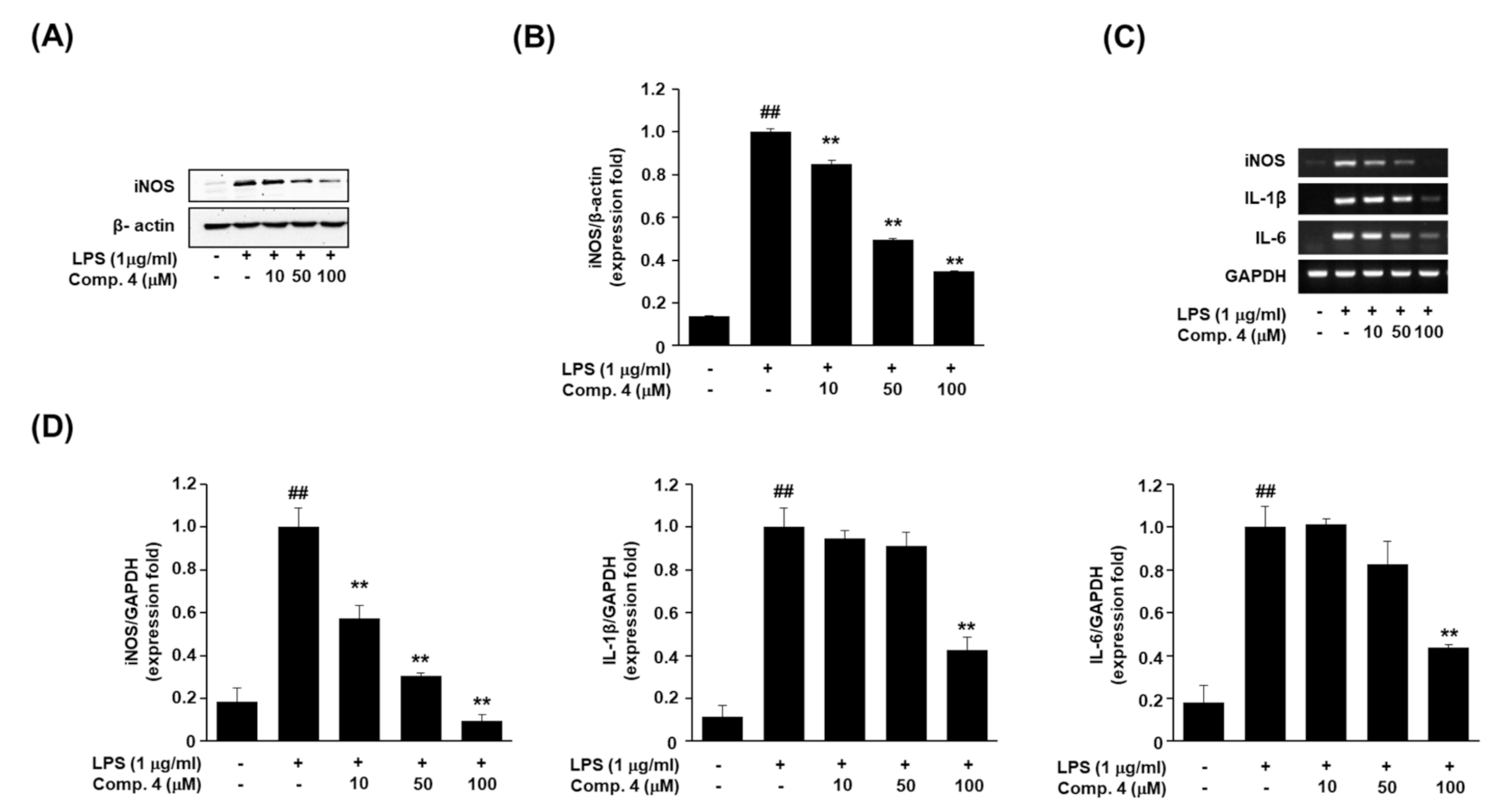
2.3. Evaluation of iNOS Protein Expression and Pro-Inflammatory Cytokine mRNA Expressions
3. Materials and Methods
3.1. General
3.2. Source of Plant Material
3.3. Extraction and Isolation of Compounds
3.3.1. Tsaokoin (1)
3.3.2. (3S,4S,6R)-3,6-Dihydroxy-1-Menthene (2)
3.3.3. (1R,3S,4R)-3-Hydroxy Isopulegol (3)
3.3.4. (1R,4S,6S)-1,6-Dihydroxy-2-Menthene (4)
3.3.5. 3,7-Dimethyl-2-Octene-1,8-Diol (5)
3.3.6. 8-Oxogeraniol (6)
3.4. Anti-Inflammatory Activities
3.4.1. Cell Culture Conditions
3.4.2. Measurement of LPS-Induced NO Production and MTT Assay for Cell Viability
3.4.3. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
3.4.4. Western Blot Analysis
3.4.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cheuka, P.M.; Mayoka, G.; Mutai, P.; Chibale, K. The role of natural products in drug discovery and development against neglected tropical diseases. Molecules 2017, 22, 58. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2002, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.M.; Kress, W.J.; Prince, L.M. Phylogenetic analyses of Amomum (Alpinioideae: Zingiberaceae) using ITS and matK DNA sequence data. Syst. Bot. 2004, 29, 334–344. [Google Scholar] [CrossRef]
- Kaewsri, W.; Paisooksantivatana, Y. Morphology and palynology of Amomum Roxb. in Thailand. Gard. Bull. 2007, 59, 105–112. [Google Scholar]
- Lee, S.; Lee, J.C.; Subedi, L.; Cho, K.H.; Kim, S.Y.; Park, H.J.; Kim, K.H. Bioactive compounds from the seeds of Amomum tsaoko Crevost et Lemaire, a Chinese spice as inhibitors of sphingosine kinases, SPHK1/2. RSC Adv. 2019, 9, 33957–33968. [Google Scholar] [CrossRef]
- Sim, S.; Tan, S.K.; Kohlenberg, B.; Braun, N.A. Amomum tsao-ko-Chinese black cardamom: Detailed oil composition and comparison with two other cardamom species. Nat. Prod. Commun. 2019, 14, 1934578X19857675. [Google Scholar] [CrossRef]
- Hong, S.S.; Lee, J.H.; Choi, Y.H.; Jeong, W.; Ahn, E.K.; Lym, S.H.; Oh, J.S. Amotsaokonal A-C, benzaldehyde and cycloterpenal from Amomum tsao-ko. Tetrahedron Lett. 2015, 56, 6681–6684. [Google Scholar] [CrossRef]
- Liu, H.; Yan, Q.; Zou, D.; Bu, X.; Zhang, B.; Ma, X.; Leng, A.; Zhang, H.; Li, D.; Wang, C. Identification and bioactivity evaluation of ingredients from the fruits of Amomum tsaoko Crevost et Lemaire. Phytochem. Lett. 2018, 28, 111–115. [Google Scholar] [CrossRef]
- Zhang, T.T.; Lu, C.L.; Jiang, J.G. Neuroprotective and anti-inflammatory effects of diphenylheptanes from the fruits of Amomum tsaoko, a Chinese spice. Plant Foods Hum. Nutr. 2016, 71, 450–453. [Google Scholar] [CrossRef]
- Zhang, T.T.; Lu, C.L.; Jiang, J.G. Antioxidant and anti-tumour evaluation of compounds identified from fruit of Amomum tsaoko Crevost et Lemaire. J. Funct. Foods 2015, 18, 423–431. [Google Scholar] [CrossRef]
- Zhang, T.T.; Lu, C.L.; Jiang, J.G. Bioactivity evaluation of ingredients identified from the fruits of Amomum tsaoko Crevost et Lemaire, a Chinese spice. Food Funct. 2014, 5, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.S.; Lee, J.Y.; Cho, S.C. Isotsaokoin, an antifungal agent from Amomum tsao-ko. J. Nat. Prod. 2004, 67, 889–891. [Google Scholar] [CrossRef]
- He, X.F.; Wang, H.M.; Geng, C.A.; Hu, J.; Zhang, X.M.; Guo, Y.Q.; Chen, J.J. Amomutsaokols A-K, diarylheptanoids from Amomum tsao-ko and their α-glucosidase inhibitory activity. Phytochemistry 2020, 177, 112418. [Google Scholar] [CrossRef] [PubMed]
- He, X.F.; Zhang, X.K.; Geng, C.A.; Hu, J.; Zhang, X.M.; Guo, Y.Q.; Chen, J.J. Tsaokopyranols A-M, 2,6-epoxydiarylheptanoids from Amomum tsao-ko and their α-glucosidase inhibitory activity. Bioorg. Chem. 2020, 96, 103638. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Jang, H.; Le, T.P.L.; Hong, H.R.; Lee, M.K.; Hong, J.T.; Lee, D.; Hwang, B.Y. Pyranoflavanones and pyranochalcones from the fruits of Amomum tsao-ko. J. Nat. Prod. 2019, 82, 1886–1892. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [CrossRef]
- Dinarello, C.A. Proinflammatory cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef]
- Song, Q.S.; Teng, R.W.; Liu, X.K.; Yang, C.R. Tsaokoin, a new bicyclic nonane from Amomum tsao-ko. Chin. Chem. Lett. 2001, 12, 227–230. [Google Scholar]
- Liu, J.F.; Li, H.J.; Wang, L.X.; Liu, M.Q.; Bi, Y.F.; Zhang, Y.B. Two new monoterpenes from the fruits of Illicium lanceolatum. Molecules 2013, 18, 11866–11872. [Google Scholar] [CrossRef]
- Pardo-Novoa, J.C.; Arreaga-González, H.M.; Gómez-Hurtado, M.A.; Rodríguez-García, G.; Cerda-García-Roja, C.M.; Joseph-Nathan, P.; del Río, R.E. Absolute configuration of menthene derivatives by vibrational circular dichroism. J. Nat. Prod. 2016, 79, 2570–2579. [Google Scholar] [CrossRef] [PubMed]
- Fkyerat, A.; Tabacchi, R. Enantioselective preparation of 1-hydroxy neoisopulegol and 1-hydroxy neoisomenthol. Tetrahedron: Asymmetry 1997, 8, 2231–2236. [Google Scholar] [CrossRef]
- De Pascual Teresa, J.; Torres, C.; Gonzalez, M.S.; Grande, M.; Bellido, I.S. Δ5-Dehydro-1-hydroxycarvomenthols from the essential oil of Chenopodium multifidum. Phytochemistry 1983, 22, 2749–2751. [Google Scholar] [CrossRef]
- Knapp, H.; Straubinger, M.; Fornari, S.; Oka, N.; Watanabe, N.; Winterhalter, P. (S)-3,7-Dimethyl-5-octene-1,7-diol and related oxygenated monoterpenoids from petals of Rosa damascena Mill. J. Agric. Food Chem. 1998, 46, 1966–1970. [Google Scholar] [CrossRef]
- Elsharif, S.A.; Buettner, A. Structure-odor relationship study on geraniol, nerol, and their synthesized oxygenated derivatives. J. Agric. Food Chem. 2018, 66, 2324–2333. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.W.; Shin, J.Y.; Seo, C.; Hong, S.S.; Ahn, E.K.; Jung, Y.H.; Oh, J.S. In vitro anti-inflammatory activity of the components of Amomum tsao-ko in murine macrophage raw 264.7 cells. Afr. J. Tradit. Complement. Altern. Med. 2018, 15, 26–34. [Google Scholar] [CrossRef]
- Costa, E.D.; Lemos, V.S.; Rezende, B.A.; Cortes, S.F. Neuronal nitric oxide synthase in vascular physiology and diseases. Front. Physiol. 2016, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.S.; Lee, S.A.; Han, X.H.; Hwang, J.S.; Lee, C.; Lee, D.; Hong, J.T.; Kim, Y.; Lee, H.; Hwang, B.Y. ent-Kaurane diterpenoids from Isodon japonicas. J. Nat. Prod. 2008, 71, 1055–1058. [Google Scholar] [CrossRef]
- Shin, J.Y.; Kang, J.S.; Byun, H.Y.; Ahn, E.K. Regulatory effects and molecular mechanism of Trigonostemon reidioides on lipopolysaccharide‑induced inflammatory responses in RAW264.7 cells. Mol. Med. Rep. 2017, 16, 5137–5142. [Google Scholar] [CrossRef]
- Sur, B.; Kim, M.; Villa, T.; Oh, S. Benzylideneacetophenone derivative alleviates arthritic symptoms via modulation of the MAPK signaling pathway. Molecules 2020, 25, 3319. [Google Scholar] [CrossRef]
- Mastinu, A.; Bonini, S.A.; Rungratanawanich, W.; Aria, F.; Marziano, M.; Maccarinelli, G.; Abate, G.; Premoli, M.; Memo, M.; Uberti, D. Gamma-oryzanol prevents LPS-induced brain inflammation and cognitive impairment in adult mice. Nutrients 2019, 11, 728. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Ryu, S.; Jang, D.S.; Cho, Y.W.; Chung, E.K.; Lee, K.T. Amomum tsao-ko fruit extract suppresses lipopolysaccharide-induced inducible nitric oxide synthase by inducing heme oxygenase-1 in macrophages and in septic mice. Int. J. Exp. Pathol. 2016, 96, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Ahn, E.K.; Hong, S.S.; Oh, J.S. 2,8-Decadiene-1,10-diol inhibits lipopolysaccharide-induced inflammatory responses through inactivation of mitogen-activated protein kinase and nuclear factor-κB signaling pathway. Inflammation 2016, 39, 583–591. [Google Scholar] [CrossRef] [PubMed]
| Position | 1 | 2 b | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1 | 37.2 d c | 137.4 s | 71.4 s | 69.1 s | 59.4 t | 59.3 t |
| 2 | 144.8 s | 131.0 d | 46.1 t | 132.1 d | 123.4 d | 124.5 d |
| 3 | 146.7 d | 69.9 d | 67.3 d | 134.1 d | 139.9 s | 137.9 s |
| 4 | 31.2 t | 42.8 d | 54.0 d | 42.6 d | 39.7 t | 37.8 t |
| 5 | 68.4 d | 30.9 t | 25.3 t | 30.1 t | 24.9 t | 27.1 t |
| 6 | 42.8 d | 68.4 d | 38.1 t | 73.6 d | 32.6 t | 153.7 d |
| 7 | 24.7 t | 20.7 q | 31.7 q | 25.9 q | 35.6 d | 139.6 s |
| 8 | 24.9 t | 27.0 d | 146.3 s | 31.5 d | 68.3 t | 195.3 d |
| 9 | 32.2 t | 21.4 q | 113.1 t | 19.6 q | 16.2 q | 16.3 q |
| 10 | 194.0 d | 17.0 q | 19.3 q | 19.0 q | 16.5 q | 9.3 q |
| Compound | IC50 (μM) a | MTT (μM) |
|---|---|---|
| tsaokoin (1) | >100 | >100 |
| (3S,4S,6R)-3,6-dihydroxy-1-menthene (2) | >100 | >100 |
| (1R,3S,4R)-3-hydroxy isopulegol (3) | >100 | >100 |
| (1R,4S,6S)-1,6-dihydroxy-2-menthene (4) | 82.5 | >100 |
| 3,7-dimethyl-2-octene-1,8-diol (5) | >100 | >100 |
| 8-oxogeraniol (6) | >100 | >100 |
| NG-methyl-L-arginine acetate b | 34.2 | >100 |
| Gene | Primer Sequences | |
|---|---|---|
| iNOS | forward reverse | 5′-GAGTTCGAGACTTCTGTGA-3′ 5′-GGCGATCTGGTAGTAGTAG-3′ |
| IL-1β | forward reverse | 5′-CTTTGAAGAAGAGCCCATCC-3′ 5′ TTTGTCGTTGCTTGGTTCTC 3′ |
| IL-6 | forward reverse | 5′-CACTTCACAAGTCGGAGGCTT-3′ 5′-GCAAGTGCATCATCGTTGTTC-3′ |
| GAPDH | forward reverse | 5′-CAGGTAAACTCAGGAGAGTG-3′ 5′-GTAGACTCCACGACATACTC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.S.; Lee, J.E.; Jung, Y.W.; Park, J.-H.; Lee, J.A.; Jeong, W.; Ahn, E.-K.; Choi, C.W.; Oh, J.S. Monoterpenoids from the Fruits of Amomum tsao-ko Have Inhibitory Effects on Nitric Oxide Production. Plants 2021, 10, 257. https://doi.org/10.3390/plants10020257
Hong SS, Lee JE, Jung YW, Park J-H, Lee JA, Jeong W, Ahn E-K, Choi CW, Oh JS. Monoterpenoids from the Fruits of Amomum tsao-ko Have Inhibitory Effects on Nitric Oxide Production. Plants. 2021; 10(2):257. https://doi.org/10.3390/plants10020257
Chicago/Turabian StyleHong, Seong Su, Ji Eun Lee, Yeon Woo Jung, Ju-Hyoung Park, Jung A. Lee, Wonsik Jeong, Eun-Kyung Ahn, Chun Whan Choi, and Joa Sub Oh. 2021. "Monoterpenoids from the Fruits of Amomum tsao-ko Have Inhibitory Effects on Nitric Oxide Production" Plants 10, no. 2: 257. https://doi.org/10.3390/plants10020257
APA StyleHong, S. S., Lee, J. E., Jung, Y. W., Park, J.-H., Lee, J. A., Jeong, W., Ahn, E.-K., Choi, C. W., & Oh, J. S. (2021). Monoterpenoids from the Fruits of Amomum tsao-ko Have Inhibitory Effects on Nitric Oxide Production. Plants, 10(2), 257. https://doi.org/10.3390/plants10020257






