Toxicity of meta-Tyrosine
Abstract
:1. Introduction
2. Tyrosine Structural Isomers: meta-, ortho-, para-Tyr
3. Fescue as the Biological Source of m-Tyr
4. Mode of Action of m-Tyr
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vranova, V.; Rejsek, K.; Skene, K.R.; Formanek, P. Non-Protein Amino Acids: Plant, Soil and Ecosystem Interactions. Plant Soil 2011, 342, 31–48. [Google Scholar] [CrossRef]
- Grishin, D.V.; Zhdanov, D.D.; Pokrovskaya, M.V.; Sokolov, N.N. D-Amino Acids in Nature, Agriculture and Biomedicine. All Life 2020, 13, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araújo, W.L.; Braun, H.-P. Amino Acid Catabolism in Plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [Green Version]
- Schenck, C.A.; Maeda, H.A. Tyrosine Biosynthesis, Metabolism, and Catabolism in Plants. Phytochemistry 2018, 149, 82–102. [Google Scholar] [CrossRef] [PubMed]
- Abello, N.; Kerstjens, H.A.M.; Postma, D.S.; Bischoff, R. Protein Tyrosine Nitration: Selectivity, Physicochemical and Biological Consequences, Denitration, and Proteomics Methods for the Identification of Tyrosine-Nitrated Proteins. J. Proteome Res. 2009, 8, 3222–3238. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; Soto-Hernández, M., García-Mateos, R., Palma-Tenango, M., Eds.; IntechOpen: London, UK, 2019; pp. 35–50. [Google Scholar]
- Wang, M.; Toda, K.; Maeda, H.A. Biochemical Properties and Subcellular Localization of Tyrosine Aminotransferases in Arabidopsis thaliana. Phytochemistry 2016, 132, 16–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, J.; Serrani-Yarce, J.C.; Chen, F.; Baxter, D.; Venables, B.J.; Dixon, R.A. Role of Bifunctional Ammonia-Lyase in Grass Cell Wall Biosynthesis. Nat. Plants 2016, 2, 16050. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.A. Lignin Biosynthesis: Tyrosine Shortcut in Grasses. Nat. Plants 2016, 2, 16080. [Google Scholar] [CrossRef]
- Liu, M.; Lu, S. Plastoquinone and Ubiquinone in Plants: Biosynthesis, Physiological Function and Metabolic Engineering. Front. Plant Sci. 2016, 7, 1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lokvam, J.; Brenes-Arguedas, T.; Lee, J.S.; Coley, P.D.; Kursar, T.A. Allelochemic Function for a Primary Metabolite: The Case of L-Tyrosine Hyper-production in Inga umbellifera (Fabaceae). Am. J. Bot. 2006, 93, 1109–1115. [Google Scholar] [CrossRef] [Green Version]
- Bixenmann, R.J.; Coley, P.D.; Weinhold, A.; Kursar, T.A. High Herbivore Pressure Favors Constitutive over Induced Defense. Ecol. Evol. 2016, 6, 6037–6049. [Google Scholar] [CrossRef]
- Coley, P.D.; Endara, M.; Ghabash, G.; Kidner, C.A.; Nicholls, J.A.; Pennington, R.T.; Mills, A.G.; Soule, A.J.; Lemes, M.R.; Stone, G.N.; et al. Macroevolutionary Patterns in Overexpression of Tyrosine: An Anti-herbivore Defence in a Speciose Tropical Tree Genus. Inga (Fabaceae). J. Ecol. 2019, 107, 1620–1632. [Google Scholar] [CrossRef]
- Laursen, T.; Borch, J.; Knudsen, C.; Bavishi, K.; Torta, F.; Martens, H.J.; Silvestro, D.; Hatzakis, N.S.; Wenk, M.R.; Dafforn, T.R.; et al. Characterization of a Dynamic Metabolon Producing the Defense Compound Dhurrin in Sorghum. Science 2016, 354, 890–893. [Google Scholar] [CrossRef] [PubMed]
- De Prá, S.D.T.; Ferreira, G.K.; Carvalho-Silva, M.; Vieira, J.S.; Scaini, G.; Leffa, D.D.; Fagundes, G.E.; Bristot, B.N.; Borges, G.D.; Ferreira, G.C.; et al. L-Tyrosine Induces DNA Damage in Brain and Blood of Rats. Neurochem. Res. 2014, 39, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Galili, G. New Insights into the Shikimate and Aromatic Amino Acids Biosynthesis Pathways in Plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef] [PubMed]
- Widhalm, J.R.; Gutensohn, M.; Yoo, H.; Adebesin, F.; Qian, Y.; Guo, L.; Jaini, R.; Lynch, J.H.; McCoy, R.M.; Shreve, J.T.; et al. Identification of a Plastidial Phenylalanine Exporter That Influences Flux Distribution through the Phenylalanine Biosynthetic Network. Nat. Commun. 2015, 6, 8142. [Google Scholar] [CrossRef] [PubMed]
- Ipson, B.R.; Fisher, A.L. Roles of the Tyrosine Isomers Meta-Tyrosine and Ortho-Tyrosine in Oxidative Stress. Ageing Res. Rev. 2016, 27, 93–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parthasarathy, A.; Cross, P.J.; Dobson, R.C.J.; Adams, L.E.; Savka, M.A.; Hudson, A.O. A Three-Ring Circus: Metabolism of the Three Proteogenic Aromatic Amino Acids and Their Role in the Health of Plants and Animals. Front. Mol. Biosci. 2018, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Molnár, G.A.; Kun, S.; Sélley, E.; Kertész, M.; Szélig, L.; Csontos, C.; Böddi, K.; Bogár, L.; Miseta, A.; Wittmann, I. Role of Tyrosine Isomers in Acute and Chronic Diseases Leading to Oxidative Stress-A Review. Curr. Med. Chem. 2016, 23, 667–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. On the History of Oxidative Stress: Concept and Some Aspects of Current Development. Curr. Opin. Toxicol. 2018, 7, 122–126. [Google Scholar] [CrossRef]
- Kaur, H.; Whiteman, M.; Halliwell, B. Peroxynitrite-Dependent Aromatic Hydroxylation and Nitration of Salicylate and Phenylalanine. Is Hydroxyl Radical Involved? Free Radic. Res. 1997, 26, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Ruemmele, B.A.; Wipff, J.; Brilman, L.; Hignight, K. Fine-Leaved Fescue Species. In Turfgrass Biology, Genetics and Breeding; Casler, M.D., Duncan, R.R., Eds.; John Wiley & Sons: New York, NY, USA, 2003; pp. 129–174. [Google Scholar]
- Bertin, C.; Paul, R.N.; Duake, S.O.; Weston, L.A. Laboratory Assessment of the Allelopathic Effects of Fine Leaf Fescues. J. Chem. Ecol. 2003, 29, 1919–1937. [Google Scholar] [CrossRef]
- Peters, E.J. Toxicity of Tall Fescue to Rape and Birdsfoot Trefoil Seeds and Seedlings. Crop Sci. 1968, 8, 650. [Google Scholar] [CrossRef]
- Stephenson, R.; Posler, G. The Influence of Tall Fescue on the Germination, Seedling Growth and Yield of Birdsfoot Trefoil. Grass Forage Sci. 1988, 43, 273–278. [Google Scholar] [CrossRef]
- Peters, E.J.; Mohammed Zam, A.H.B. Allelopathic Effects of Tall Fescue Genotypes. Agron. J. 1981, 73, 56. [Google Scholar] [CrossRef]
- Bertin, C.; Weston, L.A.; Huang, T.; Jander, G.; Owens, T.; Meinwald, J.; Schroeder, F.C. Grass Roots Chemistry: Meta-Tyrosine, an Herbicidal Nonprotein Amino Acid. Proc. Natl. Acad. Sci. USA 2007, 104, 16964–16969. [Google Scholar] [CrossRef] [Green Version]
- Duke, S.O. The Emergence of Grass Root Chemical Ecology. Proc. Natl. Acad. Sci. USA 2007, 104, 16729–16730. [Google Scholar] [CrossRef] [Green Version]
- Bertin, C.; Harmon, R.; Akaogi, M.; Weidenhamer, J.D.; Weston, L.A. Assessment of the Phytotoxic Potential of m-Tyrosine in Laboratory Soil Bioassays. J. Chem. Ecol. 2009, 35, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ames, B.D.; Walsh, C.T. Identification of Phenylalanine 3-Hydroxylase for Meta-Tyrosine Biosynthesis. Biochemistry 2011, 50, 5401–5403. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Rehak, L.; Jander, G. Meta-Tyrosine in Festuca rubra Ssp. commutata (Chewings Fescue) Is Synthesized by Hydroxylation of Phenylalanine. Phytochemistry 2012, 75, 60–66. [Google Scholar] [CrossRef]
- Gurer-Orhan, H.; Ercal, N.; Mare, S.; Pennathur, S.; Orhan, H.; Heinecke, J.W. Misincorporation of Free m-Tyrosine into Cellular Proteins: A Potential Cytotoxic Mechanism for Oxidized Amino Acids. Biochem. J. 2006, 395, 277–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bullwinkle, T.J.; Reynolds, N.M.; Raina, M.; Moghal, A.; Matsa, E.; Rajkovic, A.; Kayadibi, H.; Fazlollahi, F.; Ryan, C.; Howitz, N.; et al. Oxidation of Cellular Amino Acid Pools Leads to Cytotoxic Mistranslation of the Genetic Code. Elife 2014, 3, e02501. [Google Scholar] [CrossRef]
- Klipcan, L.; Moor, N.; Kessler, N.; Safro, M.G. Eukaryotic Cytosolic and Mitochondrial Phenylalanyl-tRNA Synthetases Catalyze the Charging of tRNA with the Meta-Tyrosine. Proc. Natl. Acad. Sci. USA 2009, 106, 11045–11048. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, K.J.; Samardzic, K.; Main, B.J. Toxic Nonprotein Amino Acids. In Plant Toxins. Toxicology; Gopalakrishnakone, P., Regina Carlini, C., Ligabue-Braun, R., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 263–285. [Google Scholar]
- Zer, H.; Mizrahi, H.; Malchenko, N.; Avin-Wittenberg, T.; Klipcan, L.; Ostersetzer-Biran, O. The Phytotoxicity of Meta-Tyrosine Is Associated with Altered Phenylalanine Metabolism and Misincorporation of This Non-Proteinogenic Phe-Analog to the Plant’s Proteome. Front. Plant Sci. 2020, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Andrzejczak, O.; Krasuska, U.; Olechowicz, J.; Staszek, P.; Ciacka, K.; Bogatek, R.; Hebelstrup, K.; Gniazdowska, A. Destabilization of ROS Metabolism in Tomato Roots as a Phytotoxic Effect of Meta-Tyrosine. Plant Physiol. Biochem. 2018, 123, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Tohge, T.; Lytovchenko, A.; Fernie, A.R.; Jander, G. Pleiotropic Physiological Consequences of Feedback-Insensitive Phenylalanine Biosynthesis in Arabidopsis thaliana. Plant J. 2010, 63, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Staszek, P.; Gniazdowska, A. Peroxynitrite Induced Signaling Pathways in Plant Response to Non-Proteinogenic Amino Acids. Planta 2020, 252, 5. [Google Scholar] [CrossRef] [PubMed]
- Ciacka, K.; Tymiński, M.; Gniazdowska, A.; Krasuska, U. Carbonylation of Proteins—An Element of Plant Ageing. Planta 2020, 252, 12. [Google Scholar] [CrossRef] [PubMed]
- Staszek, P.; Weston, L.A.; Ciacka, K.; Krasuska, U.; Gniazdowska, A. L-Canavanine: How Does a Simple Non-Protein Amino Acid Inhibit Cellular Function in a Diverse Living System? Phytochem. Rev. 2017, 16, 1269–1282. [Google Scholar] [CrossRef]
- Rosenthal, G.A. L-Canavanine: A Higher Plant Insecticidal Allelochemical. Amino Acids 2001, 21, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Krasuska, U.; Andrzejczak, O.; Staszek, P.; Bogatek, R.; Gniazdowska, A. Canavanine Alters ROS/RNS Level and Leads to Post-Translational Modification of Proteins in Roots of Tomato Seedlings. Front. Plant Sci. 2016, 7, 840. [Google Scholar] [CrossRef] [Green Version]
- Krasuska, U.; Andrzejczak, O.; Staszek, P.; Borucki, W.; Gniazdowska, A. Meta-Tyrosine Induces Modification of Reactive Nitrogen Species Level, Protein Nitration and Nitrosoglutathione Reductase in Tomato Roots. Nitric Oxide 2017, 68, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Bartesaghi, S.; Radi, R. Fundamentals on the Biochemistry of Peroxynitrite and Protein Tyrosine Nitration. Redox Biol. 2018, 14, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Staszek, P.; Krasuska, U.; Otulak-Kozieł, K.; Fettke, J.; Gniazdowska, A. Canavanine Induced Decrease in NO Synthesis Alters Activity of Antioxidant System but Does Not Impact GSNO Catabolism in Tomato Roots. Front. Plant Sci. 2019, 10, 1077. [Google Scholar] [CrossRef]
- Staszek, P.; Krasuska, U.; Ciacka, K.; Gniazdowska, A. ROS Metabolism Perturbation as an Element of Mode of Action of Allelochemicals. Antioxidants 2021, 10, 1648. [Google Scholar] [CrossRef] [PubMed]
- Howitz, N.; Su, T.; Lazazzera, B.A. Meta-Tyrosine Induces Cytotoxic Misregulation of Metabolism in Escherichia coli. J. Mol. Biol. 2020, 432, 166716. [Google Scholar] [CrossRef] [PubMed]
- Chiarella, P.; Bruzzo, J.; Meiss, R.P.; Ruggiero, R.A. Concomitant Tumor Resistance. Cancer Lett. 2012, 324, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, R.A.; Bruzzo, J.; Chiarella, P.; di Gianni, P.; Isturiz, M.A.; Linskens, S.; Speziale, N.; Meiss, R.P.; Bustuoabad, O.D.; Pasqualini, C.D. Tyrosine Isomers Mediate the Classical Phenomenon of Concomitant Tumor Resistance. Cancer Res. 2011, 71, 7113–7124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggiero, R.A.; Bruzzo, J.; Chiarella, P.; Bustuoabad, O.D.; Meiss, R.P.; Pasqualini, C.D. Concomitant Tumor Resistance: The Role of Tyrosine Isomers in the Mechanisms of Metastases Control. Cancer Res. 2012, 72, 1043–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gueron, G.; Anselmino, N.; Chiarella, P.; Ortiz, E.G.; Lage Vickers, S.; Paez, A.V.; Giudice, J.; Contin, M.D.; Leonardi, D.; Jaworski, F.; et al. Game-Changing Restraint of ROS-Damaged Phenylalanine, upon Tumor Metastasis. Cell Death Dis. 2018, 9, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fell, V.; Greenway, A.M.; Hoskins, J.A. The Metabolism of L-m-Tyrosine in Man. Biochem. Med. 1979, 22, 246–255. [Google Scholar] [CrossRef]
- Ipson, B.R.; Green, R.A.; Wilson, J.T.; Watson, J.N.; Faull, K.F.; Fisher, A.L. Tyrosine Aminotransferase Is Involved in the Oxidative Stress Response by Metabolizing Meta-Tyrosine in Caenorhabditis elegans. J. Biol. Chem. 2019, 294, 9536–9554. [Google Scholar] [CrossRef] [PubMed]
- Molnár, G.A.; Nemes, V.; Biró, Z.; Ludány, A.; Wagner, Z.; Wittmann, I. Accumulation of the Hydroxyl Free Radical Markers Meta-, Ortho-Tyrosine and DOPA in Cataractous Lenses Is Accompanied by a Lower Protein and Phenylalanine Content of the Water-Soluble Phase. Free Radic. Res. 2005, 39, 1359–1366. [Google Scholar] [CrossRef]
- Ogihara, T.; Hirano, K.; Ogihara, H.; Misaki, K.; Hiroi, M.; Morinobu, T.; Kim, H.-S.; Ogawa, S.; Ban, R.; Hasegawa, M.; et al. Non-Protein-Bound Transition Metals and Hydroxyl Radical Generation in Cerebrospinal Fluid of Newborn Infants with Hypoxic Ischemic Encephalopathy. Pediatr. Res. 2003, 53, 594–599. [Google Scholar] [CrossRef] [Green Version]
- Kaur, H.; Edmonds, S.E.; Blake, D.R.; Halliwell, B. Hydroxyl Radical Generation by Rheumatoid Blood and Knee Joint Synovial Fluid. Ann. Rheum. Dis. 1996, 55, 915–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molnár, G.A. Tyrosine Isomers and Hormonal Signaling: A Possible Role for the Hydroxyl Free Radical in Insulin Resistance. World J. Diabetes 2015, 6, 500. [Google Scholar] [CrossRef] [PubMed]
- Mikolás, E.; Kun, S.; Laczy, B.; Molnár, G.A.; Sélley, E.; Koszegi, T.; Wittmann, I. Incorporation of Ortho- and Meta-Tyrosine into Cellular Proteins Leads to Erythropoietin-Resistance in an Erythroid Cell Line. Kidney Blood Press. Res. 2013, 38, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Voll, L.M.; Allaire, E.E.; Fiene, G.; Weber, A.P.M. The Arabidopsis Phenylalanine Insensitive Growth Mutant Exhibits a Deregulated Amino Acid Metabolism. Plant Physiol. 2004, 136, 3058–3069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufman, S. A Model of Human Phenylalanine Metabolism in Normal Subjects and in Phenylketonuric Patients. Proc. Natl. Acad. Sci. USA 1999, 96, 3160–3164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
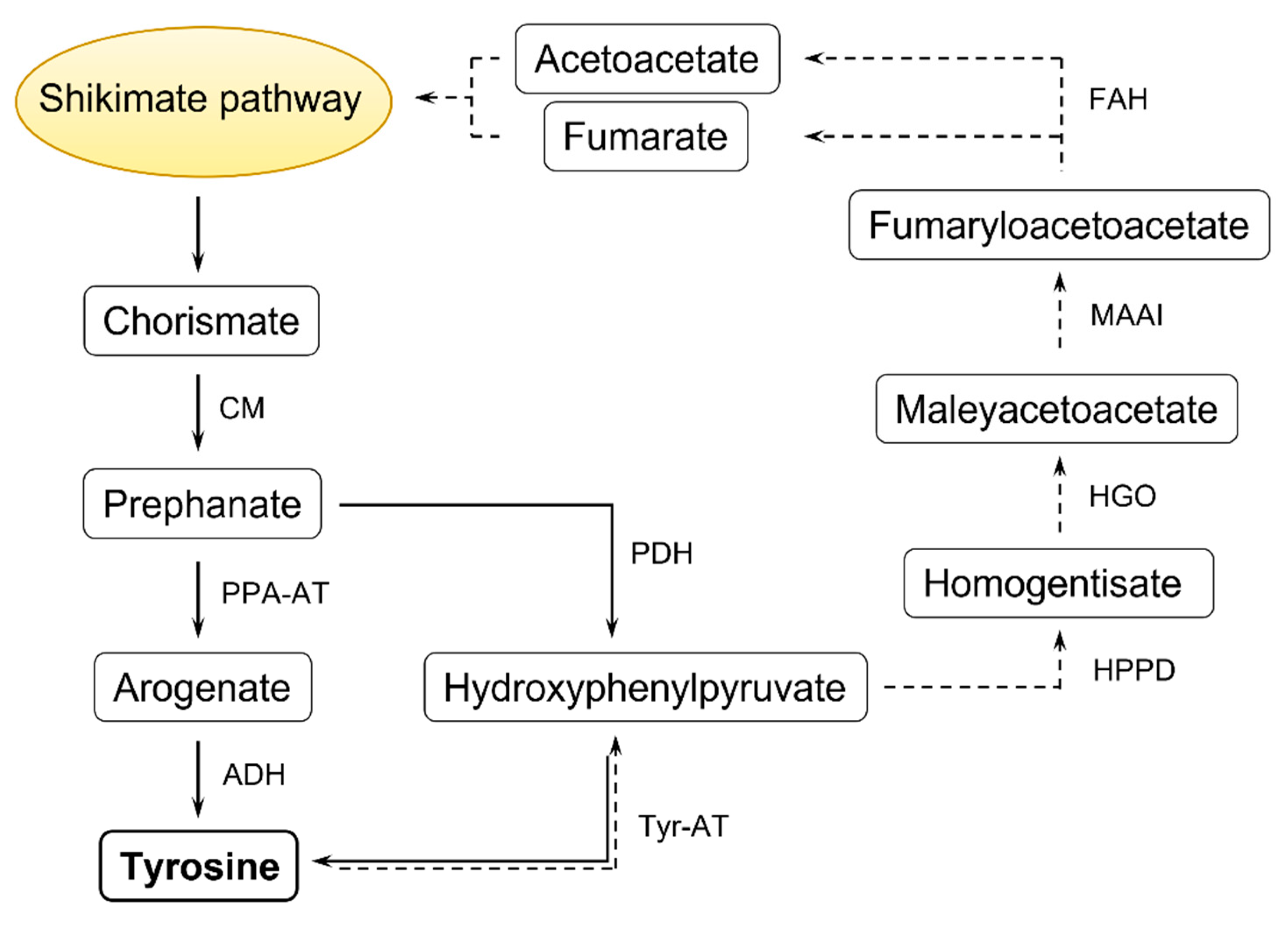
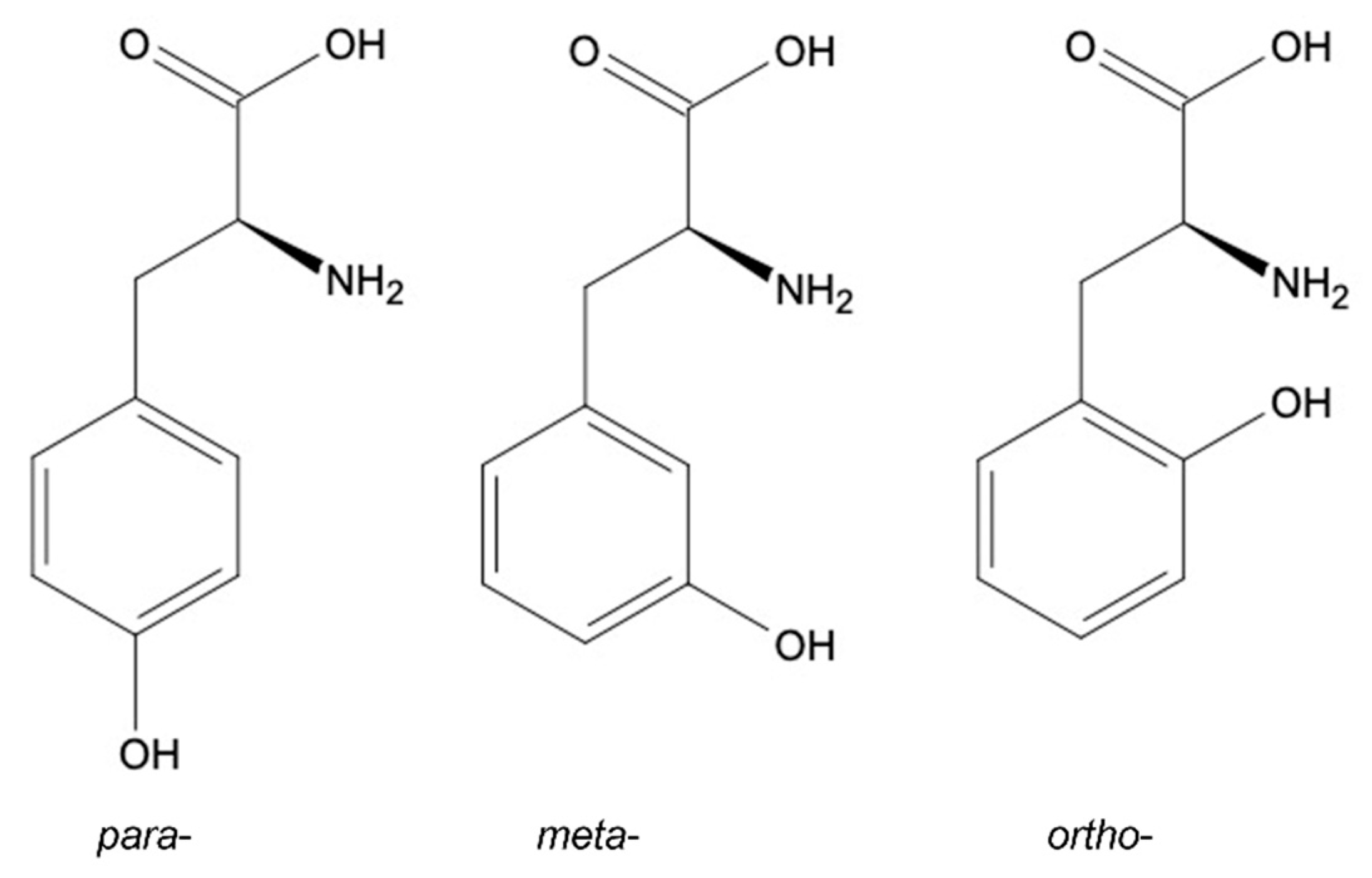
| Plant Material, m-Tyr Concentration | Effect of m-Tyr Application | References |
|---|---|---|
| Lettuce (Lactuca sativa L.) (DL-m-Tyr: 10–320 µM) | Inhibition of root growth (filter paper bioassays, soil bioassays) | [29,31] |
| Tomato (Solanum lycopersicum L.) (DL-m-Tyr: 50–250 µM) | Inhibition of root growth (filter paper bioassays) Accumulation of ROS (spectrophotometry) Increased emission of NO (fluorimetry) Modification of enzymatic and nonenzymatic cellular antioxidant system (spectrophotometry) Increased protein carbonylation level and protein nitration level (ELISA) | [39,46] |
| Arabidopsis (Arabidopsis thaliana ecotype Columbia (Col-0)) (DL-m-Tyr: 2.5–320 µM, according to personal information) | Inhibition of root growth and decreased leaves number (agar plate bioassays) Decreased total chlorophyll content (spectrophotometry), aberration in chloroplast ultrastructure (TEM), lowered photosynthesis rate (Clark-type electrode) Modification of mitochondria structure (TEM), lowered respiration rate (Clark-type electrode) | [38] |
| Tissue/Biological Material | Level of m-Tyr | Physiological Effect Linked to m-Tyr and Accompanied Biochemical Changes | References |
|---|---|---|---|
| Human lenses | 20.3 nmol g−1 protein | Cataract related with the lower content of soluble proteins (spectrophotometry) and Phe (HPLC) | [57] |
| Cerebrospinal fluid of newborn infants | 20.5 nM | Hypoxic ischemic encephalopathy related with the increased content of ascorbic acid (HPLC), the higher ortho-Tyr/Phe and m-Tyr/Phe ratio (GC/MS), and the detection of non-protein-bound iron (spectrophotometry) | [58] |
| Human synovial fluid | 0.5–3.5 µM | Rheumatoid arthritis. Determination of hydroxyradical attack on Phe (HPLC) | [59] |
| Human tumors (prostate, murine mammary carcinomas) in mice | * Injection of m-Tyr (67 mg kg−1 day−1) | Inhibition of the implantation of metastases (all treated and control mice were killed and metastases were counted) | [54] |
| T-lymphoma in BALB/c mice | * Injection of m-Tyr (0.2 mL of 500 μg mL−1 m-Tyr, daily for 5 days) | Decrease of tumor volume (histopathologic studies), the low expression of proliferation marker Ki-67 protein (immunostaining) | [52] |
| TF-1 erythroblast cell culture | * Treatment with m-Tyr (20 mg L−1) | Inhibition of cells proliferation (Bürker cell counting chamber) Induction of erythropoietin resistance (erythropoietin-response, cell counting) | [60,61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyminski, M.; Ciacka, K.; Staszek, P.; Gniazdowska, A.; Krasuska, U. Toxicity of meta-Tyrosine. Plants 2021, 10, 2800. https://doi.org/10.3390/plants10122800
Tyminski M, Ciacka K, Staszek P, Gniazdowska A, Krasuska U. Toxicity of meta-Tyrosine. Plants. 2021; 10(12):2800. https://doi.org/10.3390/plants10122800
Chicago/Turabian StyleTyminski, Marcin, Katarzyna Ciacka, Pawel Staszek, Agnieszka Gniazdowska, and Urszula Krasuska. 2021. "Toxicity of meta-Tyrosine" Plants 10, no. 12: 2800. https://doi.org/10.3390/plants10122800
APA StyleTyminski, M., Ciacka, K., Staszek, P., Gniazdowska, A., & Krasuska, U. (2021). Toxicity of meta-Tyrosine. Plants, 10(12), 2800. https://doi.org/10.3390/plants10122800







