Tragia L. Genus: Ethnopharmacological Use, Phytochemical Composition and Biological Activity
Abstract
1. Introduction
2. Genus
3. Distribution and Localization
4. Methodology
5. Ethnopharmacological Usage
6. Biological Activity
6.1. In Vitro Activity
6.2. In Vivo Activity
7. Phytochemical Composition
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Solecki, R.S. Shanidar IV, a Neanderthal Flower Burial in Northern Iraq. Science 1975, 190, 880–881. [Google Scholar] [CrossRef]
- Ur Rehman, F.; Kalsoom, M.; Adnan, M.; Fazeli-Nasab, B.; Naz, N.; Ilahi, H.; Ali, M.F.; Ilyas, M.A.; Yousaf, G.; Toor, M.D.; et al. Importance of Medicinal Plants in Human and Plant Pathology: A Review. Int. J. Pharm. Biomed. Res. 2021, 8, 1–11. [Google Scholar] [CrossRef]
- Marrelli, M. Medicinal Plants. Plants 2021, 10, 1355. [Google Scholar] [CrossRef]
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal Plants: Past History and Future Perspective. J. Herbmed Pharmacol. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Katiyar, C.; Kanjilal, S.; Gupta, A.; Katiyar, S. Drug Discovery from Plant Sources: An Integrated Approach. AYU Int. Q. J. Res. Ayurveda 2012, 33, 10–19. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The Effect of Developmental and Environmental Factors on Secondary Metabolites in Medicinal Plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Coy Barrera, C.A.; Gómez, D.C.; Castiblanco, F.A. Importancia Medicinal Del Género Croton (Euphorbiaceae). Rev. Cuba. Plantas Med. 2016, 21, 234–247. [Google Scholar]
- Mwine, J.T.; Damme, P.V. Why Do Euphorbiaceae Tick as Medicinal Plants? A Review of Euphorbiaceae Family and Its Medicinal Features. J. Med. Plants Res. 2011, 5, 652–662. [Google Scholar]
- Gillespie, L.J.; Cardinal-McTeague, W.M.; Wurdack, K.J. Monadelpha (Euphorbiaceae, Plukenetieae), a New Genus of Tragiinae from the Amazon Rainforest of Venezuela and Brazil. PhytoKeys 2020, 169, 119–135. [Google Scholar] [CrossRef]
- POWO Tragia Plum. Ex L. | Plants of the World Online | Kew Science. Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:327688-2 (accessed on 25 October 2021).
- Urtecho, R. Tragia—FNA. Available online: http://dev.semanticfna.org/Tragia (accessed on 26 April 2021).
- Naudé, T.W.; Naidoo, V. Oxalates-containing plants. In Veterinary Toxicology; Elsevier: Amsterdam, The Netherlands, 2007; pp. 880–891. ISBN 978-0-12-370467-2. [Google Scholar]
- Prasad, R.; Shivay, Y.S. Oxalic Acid/Oxalates in Plants: From Self-Defence to Phytoremediation. Curr. Sci. 2017, 112, 1665–1667. [Google Scholar] [CrossRef]
- Ensikat, H.-J.; Wessely, H.; Engeser, M.; Weigend, M. Distribution, Ecology, Chemistry and Toxicology of Plant Stinging Hairs. Toxins 2021, 13, 141. [Google Scholar] [CrossRef]
- Freire de Sá Cordeiro, W.P.; Athiê-Souza, S.M.; Laurênio de Melo, A.; Ferreira de Sales, M. A New Endangered Species of Tragia (Euphorbiaceae) from the Brazilian Atlantic Forest. Syst. Bot. 2020, 45, 839–844. [Google Scholar] [CrossRef]
- Freire de Sá Cordeiro, W.P.; Athiê-Souza, S.M.; Buril, M.T.; de Melo, A.L.; de Sales, M.F. Chicomendes (Euphorbiaceae, Tragiinae): A New Amazonian Genus Segregated from Tragia. Plant Syst. Evol. 2021, 307, 46. [Google Scholar] [CrossRef]
- Govaerts, R. World Checklist and Bibliography of Euphorbiaceae (and Pandaceae); Royal Botanic Gardens: Kew, UK, 2000; Volume 1, ISBN 978-1-900347-83-9. [Google Scholar]
- Digital Science Dimensions [Software]. Available online: https://app.dimensions.ai/analytics/publication/overview/timeline (accessed on 18 June 2021).
- Kar, A.; Choudhary, B.K.; Bandyopadhyay, N.G. Comparative Evaluation of Hypoglycaemic Activity of Some Indian Medicinal Plants in Alloxan Diabetic Rats. J. Ethnopharmacol. 2003, 84, 105–108. [Google Scholar] [CrossRef]
- Pallie, M.S.; Perera, P.K.; Kumarasinghe, N.; Arawwawala, M.; Goonasekara, C.L. Ethnopharmacological Use and Biological Activities of Tragia involucrata L. Evid. Based Complement. Altern. Med. 2020, 2020, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Abdulhameed, S.; Pradeep, N.S.; Sugathan, S. (Eds.) . Bioresources and Bioprocess in Biotechnology, 1st ed.; Springer: Singapore, 2017; ISBN 978-981-10-3571-5. [Google Scholar]
- Jose, P.A.; Hussain, K.H.; Sreekumar, V.B. Developing an Information System for the Rare Endangered and Threatened (RET) Plants of Southern Western Ghats; Kerala Forest Research Institute: Peechi, India, 2014; p. 42. [Google Scholar]
- Reddy, B.S.; Rao, N.R.; Vijeepallam, K.; Pandy, V. Phytochemical, Pharmacological and Biological Profiles of Tragia Species (Family: Euphorbiaceae). Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 105–112. [Google Scholar] [CrossRef] [PubMed]
- WHO. Anatomical Therapeutic Chemical (ATC) Classification. Available online: https://www.who.int/tools/atc-ddd-toolkit/atc-classification (accessed on 8 June 2021).
- WFO. Tragia L. Available online: http://www.worldfloraonline.org/taxon/wfo-4000038765 (accessed on 7 November 2021).
- Trindade, M.T. Espécies úteis da família Euphorbiaceae no Brasil. Rev. Cuba. Plantas Med. 2015, 19. Available online: http://revplantasmedicinales.sld.cu/index.php/pla/article/view/113/105 (accessed on 8 June 2021).
- Oladosu, I.A.; Balogun, S.O.; Ademowo, G.O. Phytochemical Screening, Antimalarial and Histopathological Studies of Allophylus Africanus and Tragia benthamii. Chin. J. Nat. Med. 2013, 11, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Cho-Ngwa, F.; Monya, E.; Azantsa, B.K.; Manfo, F.P.T.; Babiaka, S.B.; Mbah, J.A.; Samje, M. Filaricidal Activities on Onchocerca Ochengi and Loa Loa, Toxicity and Phytochemical Screening of Extracts of Tragia Benthami and Piper Umbellatum. BMC Complement. Altern. Med. 2016, 16, 326. [Google Scholar] [CrossRef] [PubMed]
- Ajao, A.A.; Sibiya, N.P.; Moteetee, A.N. Sexual Prowess from Nature: A Systematic Review of Medicinal Plants Used as Aphrodisiacs and Sexual Dysfunction in Sub-Saharan Africa. S. Afr. J. Bot. 2019, 122, 342–359. [Google Scholar] [CrossRef]
- Raimi, I.O.; Kopaopa, B.G.; Mugivhisa, L.L.; Lewu, F.B.; Amoo, S.O.; Olowoyo, J.O. An Appraisal of Documented Medicinal Plants Used for the Treatment of Cancer in Africa over a Twenty-Year Period (1998–2018). J. Herb. Med. 2020, 23, 100371. [Google Scholar] [CrossRef]
- Ochwang’i, D.O.; Kimwele, C.N.; Oduma, J.A.; Gathumbi, P.K.; Kiama, S.G.; Efferth, T. Cytotoxic Activity of Medicinal Plants of the Kakamega County (Kenya) against Drug-Sensitive and Multidrug-Resistant Cancer Cells. J. Ethnopharmacol. 2018, 215, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Chekole, G. Ethnobotanical Study of Medicinal Plants Used against Human Ailments in Gubalafto District, Northern Ethiopia. J. Ethnobiol. Ethnomed. 2017, 13, 55. [Google Scholar] [CrossRef]
- Jeruto, P.; Too, E.; Mwamburi, L.; Omari, A. An Inventory of Medicinal Plants Used to Treat Gynaecological- Obstetric-Urino-Genital Disorders in South Nandi Sub County in Kenya. J. Nat. Sci. Res. 2015, 5, 136–152. [Google Scholar]
- Asmerom, D.; Kalay, T.H.; Araya, T.Y.; Desta, D.M.; Wondafrash, D.Z.; Tafere, G.G. Medicinal Plants Used for the Treatment of Erectile Dysfunction in Ethiopia: A Systematic Review. BioMed Res. Int. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Pascaline, J.; Charles, M.; George, O.; Lukhoba, C. Ethnobotanical Survey and Propagation of Some Endangered Medicinal Plants from South Nandi District of Kenya. J. Anim. Plant Sci. 2012, 8, 28. [Google Scholar]
- Aumeeruddy, M.Z.; Mahomoodally, M.F. Global Documentation of Traditionally Used Medicinal Plants in Cancer Management: A Systematic Review. S. Afr. J. Bot. 2021, 138, 424–494. [Google Scholar] [CrossRef]
- Amare, F.; Getachew, G. An Ethnobotanical Study of Medicinal Plants in Chiro District, West Hararghe, Ethiopia. Afr. J. Plant Sci. 2019, 13, 309–323. [Google Scholar] [CrossRef]
- Bekele, G.; Reddy, P.R. Ethnobotanical Study of Medicinal Plants Used to Treat Human Ailments by Guji Oromo Tribes in Abaya District, Borana, Oromia, Ethiopia. Univers. J. Plant Sci. 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Moges, A.; Moges, Y. Ethiopian Common Medicinal Plants: Their Parts and Uses in Traditional Medicine—Ecology and Quality Control. In Plant Science—Structure, Anatomy and Physiology in Plants Cultured in Vivo and in Vitro; Gonzalez, A., Rodriguez, M., Gören Sağlam, N., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-78984-746-8. [Google Scholar]
- Semenya, S.S.; Maroyi, A. Ethnobotanical Survey of Plants Used by Bapedi Traditional Healers to Treat Tuberculosis and Its Opportunistic Infections in the Limpopo Province, South Africa. S. Afr. J. Bot. 2019, 122, 401–421. [Google Scholar] [CrossRef]
- Tolossa, K.; Debela, E.; Athanasiadou, S.; Tolera, A.; Ganga, G.; Houdijk, J.G. Ethno-Medicinal Study of Plants Used for Treatment of Human and Livestock Ailments by Traditional Healers in South Omo, Southern Ethiopia. J. Ethnobiol. Ethnomed. 2013, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Ndhlovu, P.T.; Omotayo, A.O.; Otang-Mbeng, W.; Aremu, A.O. Ethnobotanical Review of Plants Used for the Management and Treatment of Childhood Diseases and Well-Being in South Africa. S. Afr. J. Bot. 2021, 137, 197–215. [Google Scholar] [CrossRef]
- Innocent, E.; Moshi, M.; Masimba, P.; Mbwambo, Z.; Kapingu, M.; Kamuhabwa, A. Screening of Traditionally Used Plants for in Vivo Antimalarial Activity in Mice. Afr. J. Tradit. Complement. Altern. Med. 2010, 6, 163–167. [Google Scholar] [CrossRef][Green Version]
- Moshi, M.J.; Otieno, D.F.; Mbabazi, P.K.; Weisheit, A. Ethnomedicine of the Kagera Region, North Western Tanzania. Part 2: The Medicinal Plants Used in Katoro Ward, Bukoba District. J. Ethnobiol. Ethnomed. 2010, 6, 19. [Google Scholar] [CrossRef]
- Tida, M.M.A.; Nanjarisoa, O.; Rabearivony, J.; Ranarijaona, H.L.T.; Fenoradosoa, T.A. Ethnobotanical Survey Of Plant Species Used In Traditional Medicine In Bekaraoka Region, Northeastern Madagascar. Int. J. Adv. Res. Publ. 2020, 4, 107–114. [Google Scholar]
- Barboza, G.E.; Cantero, J.J.; Núñez, C.; Arisa Espinar, L.; Pacciaroni, A. del V. Medicinal Plants: A General Review and a Phytochemical and Ethnopharmacological Screening of the Native Argentine Flora. Kurtziana 2009, 34, 7–365. [Google Scholar]
- Goleniowski, M.E.; Bongiovanni, G.A.; Palacio, L.; Nuñez, C.O.; Cantero, J.J. Medicinal Plants from the “Sierra de Comechingones”, Argentina. J. Ethnopharmacol. 2006, 107, 324–341. [Google Scholar] [CrossRef]
- Carlomagno, A.; Pardini, A.; Contino Esquijerosa, Y. Medicinal Plants in Ethnobotanical and Religious Traditions in Cuba: A First Review and Updating. 2015. Available online: https://www.researchgate.net/profile/Anna-Carlomagno/publication/276886636_Medicinal_plants_in_ethnobotanical_and_religious_traditions_in_Cuba_a_first_review_and_updating/links/555b088a08aeaaff3bfaefa7/Medicinal-plants-in-ethnobotanical-and-religious-traditions-in-Cuba-a-first-review-and-updating.pdf (accessed on 15 April 2021).
- Shiddamallayya, N.; Rao, R.; Doddamani, S.; Venkateshwarlu, G. A Glimpse on Forest Flora and Indian System of Medicine Plants of Chitradurga District, Karnataka. Int. J. Herb. Med. 2016, 4, 25–33. [Google Scholar]
- Hmhl, K.; Nwgnd, G. Medical Formulas for Krimidanta (Dental Caries) in Indigenous Medicine in Sri Lanka—A Literary Review. Int. J. Ayurveda Pharma Res. 2016, 4, 52–56. [Google Scholar]
- Velma, W.N.; Isabel, N.W.; Meshack, A.O.; Josphat, C.M. Isolation, Identification and Bioactivity of Fungal Endophytes from Selected Kenyan Medicinal Plants. Afr. J. Microbiol. Res. 2018, 12, 405–412. [Google Scholar] [CrossRef][Green Version]
- Pallie, M.; Perera, P.; Goonasekara, C.; Kumarasinghe, N.; Arawwawala, M. Efficacy and Safety of Freeze-Dried Form of Tragia involucrata L. Decoction in Treating Diabetes: A Randomized Controlled Clinical Trial. Clin. Trials Degener. Dis. 2020, 5, 31–36. [Google Scholar] [CrossRef]
- Panda, D.; Dash, S.K. Phytochemical Examination and Antimicrobial Activity of Various Solvent Extracts and the Selected Isolated Compounds from Roots of Tragia involucrata Linn. Int. J. Pharm. Sci. Drug Res. 2012, 4, 44–48. [Google Scholar]
- Hosahally, R.V.; Seru, G.; Sutar, P.S.; Joshi, V.G.; Sutar, K.P.; Karigar, A.A. Phytochemical and Pharmacological Evaluation of Tragia Cannabina for Anti-Inflammatory Activity. Int. Curr. Pharm. J. 2012, 1, 213–216. [Google Scholar] [CrossRef]
- Alimuzzaman, M.; Ahmed, M. Analgesic Activity of Tragia involucrata. Dhaka Univ. J. Pharm. Sci. 2007, 4, 35–38. [Google Scholar] [CrossRef]
- Pallie, M.S.; Perera, P.K.; Goonasekara, C.L.; Kumarasinghe, K.M.N.; Arawwawala, L.D.A.M. Evaluation of Diuretic Effect of the Hot Water Extract of Standardized Tragia involucrata Linn., in Rats. Int. J. Pharmacol. 2018, 13, 83–90. [Google Scholar] [CrossRef]
- Alanazi, A.; Anwar, M.J.; Ahmad, M.A. Hepatoprotective and Antioxidant Activity of Tragia involucrata Root Extracts against CCl4 Induced Hepatotoxicity in Rats. Pharm. Lett. 2015, 7, 146–152. [Google Scholar]
- Ediriweera, E.; Ratnasooriya, W. A Review on Herbs Used in Treatment of Diabetes Mellitus by Sri Lankan Ayurvedic and Traditional Physicians. Ayu 2009, 30, 373–391. [Google Scholar]
- Drewes, S.E.; Selepe, M.A.; Van Heerden, F.R.; Archer, R.H.; Mitchell, D. Unravelling the Names, Origins and Chemistry of “Muthis” Used for Male Sexual Disorders in KwaZulu-Natal, South Africa. S. Afr. J. Bot. 2013, 88, 310–316. [Google Scholar] [CrossRef][Green Version]
- Charlson, A.J. Antineoplastic Constituents of Some Southern African Plants. J. Ethnopharmacol. 1980, 2, 323–335. [Google Scholar] [CrossRef]
- Ngcobo, M.; Gqaleni, N.; Naidoo, V.; Cele, P. The Immune Effects of an African Traditional Energy Tonic in In Vitro and In Vivo Models. Evid. Based Complement. Altern. Med. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Yineger, H.; Yewhalaw, D.; Teketay, D. Ethnomedicinal Plant Knowledge and Practice of the Oromo Ethnic Group in Southwestern Ethiopia. J. Ethnobiol. Ethnomed. 2008, 4, 11. [Google Scholar] [CrossRef]
- Hassan-Abdallah, A.; Merito, A.; Hassan, S.; Aboubaker, D.; Djama, M.; Asfaw, Z.; Kelbessa, E. Medicinal Plants and Their Uses by the People in the Region of Randa, Djibouti. J. Ethnopharmacol. 2013, 148, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Abdela, G.; Sultan, M. Indigenous Knowledge, Major Threats and Conservation Practices of Medicinal Plants by Local Community in Heban Arsi District, Oromia, South Eastern Ethiopia. Adv. Life Sci. Technol. 2018, 68, 19. [Google Scholar]
- Cheikhyoussef, A.; Shapi, M.; Matengu, K.; Mu Ashekele, H. Ethnobotanical Study of Indigenous Knowledge on Medicinal Plant Use by Traditional Healers in Oshikoto Region, Namibia. J. Ethnobiol. Ethnomed. 2011, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Setshogo, M.P.; Mbereki, C.M. Floristic Diversity and Uses of Medicinal Plants Sold by Street Vendors in Gaborone, Botswana. Afr. J. Plant Sci. Biotechnol. 2011, 6, 69–74. [Google Scholar]
- Vergiat, A.M. Plantes magiques et médicinales des Féticheurs de l’Oubangui (Région de Bangui). J. Agric. Tradit. Bot. Appliquée 1969, 16, 335–367. [Google Scholar]
- Al-Fatimi, M. Ethnobotanical Survey of Medicinal Plants in Central Abyan Governorate, Yemen. J. Ethnopharmacol. 2019, 241, 111973. [Google Scholar] [CrossRef]
- Mothana, R.A.A.; Abdo, S.A.A.; Hasson, S.; Althawab, F.M.N.; Alaghbari, S.A.Z.; Lindequist, U. Antimicrobial, Antioxidant and Cytotoxic Activities and Phytochemical Screening of Some Yemeni Medicinal Plants. Evid. Based Complement. Altern. Med. 2010, 7, 323–330. [Google Scholar] [CrossRef]
- Desta, B. Ethiopian Traditional Herbal Drugs. Part III: Anti-Fertility Activity of 70 Medicinal Plants. J. Ethnopharmacol. 1994, 44, 199–209. [Google Scholar] [CrossRef]
- Suryawanshi, V. Extraction and Isolation of Clerodane as a Bioactive Molecule from Tragia Ramosa. J. Pharmacogn. Phytochem. 2019, 8, 1135–1138. [Google Scholar]
- Welcome, A.K.; Wyk, B.-E.V. An Inventory and Analysis of the Food Plants of Southern Africa. S. Afr. J. Bot. 2019, 122, 136–179. [Google Scholar] [CrossRef]
- Magwede, K.; van Wyk, B.-E.; van Wyk, A.E. An Inventory of Vhavenda Useful Plants. S. Afr. J. Bot. 2019, 122, 57–89. [Google Scholar] [CrossRef]
- Agbor, G.A.; Ndjib, R. Systematic Review of Plants Used Against Respiratory Diseases Related to COVID-19 in Africa. J. Drug Deliv. Ther. 2021, 11, 141–153. [Google Scholar] [CrossRef]
- Amusan, O.O. Some Ethnoremedies Used for HIV/AIDS and Related Diseases in Swaziland. Afr. J. Plant Sci. Biotechnol. 2009, 3, 20–26. [Google Scholar]
- Ogundare, A.O.; Olorunfemi, O.B. Antimicrobial Efficacy of the Leaves of Dioclea Reflexa, Mucuna Pruriens, Ficus Asperifolia and Tragia spathulata. Res. J. Microbiol. 2007, 2, 392–396. [Google Scholar] [CrossRef]
- Tabuti, J.R.S.; Kukunda, C.B.; Waako, P.J. Medicinal Plants Used by Traditional Medicine Practitioners in the Treatment of Tuberculosis and Related Ailments in Uganda. J. Ethnopharmacol. 2010, 127, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Silberbauer-Gottsberger, I. O Cerrado Como Potencial de Plantas Medicinais e Tóxicas. Oréades 1982, 8, 15–30. [Google Scholar]
- Ouöba, P.; Lykke, A.; Boussim, J.; Guinko, S. La flore médicinale de la Forêt Classée de Niangoloko (Burkina Faso). Etudes Flor Vég Burkina Faso 2006, 10, 5–16. [Google Scholar]
- Reko, B. La Hierba de Quetzalcoatl. Bot. Sci. 1946, 4, 13–14. [Google Scholar] [CrossRef][Green Version]
- Jiofack, T.; Fokunang, C.; Guedje, N.; Kemeuze, V.; Fongnzossie, E.; Nkongmeneck, B.A.; Mapongmetsem, P.M.; Tsabang, N. Ethnobotanical Uses of Medicinal Plants of Two Ethnoecological Regions of Cameroon. Int. J. Med. Med. Sci. 2010, 2, 60–79. [Google Scholar]
- Arnason, T.; Uck, F.; Lambert, J.; Hebda, R. Maya Medicinal Plants of San Jose Succotz, Belize. J. Ethnopharmacol. 1980, 2, 345–364. [Google Scholar] [CrossRef]
- Seukep, J.A.; Ngadjui, B.; Kuete, V. Antibacterial Activities of Fagara Macrophylla, Canarium Schweinfurthii, Myrianthus Arboreus, Dischistocalyx Grandifolius and Tragia benthamii against Multi-Drug Resistant Gram-Negative Bacteria. SpringerPlus 2015, 4, 567. [Google Scholar] [CrossRef] [PubMed]
- Anthoney, S.T.; Ngule, M.C.; Jackie, O.K. In Vitro Antibacterial Activity of Methanolic-Aqua Extract of Tragia Brevipes Leaves. Int. J. Pharm. Life Sci. 2014, 5, 3289–3294. [Google Scholar]
- Chepng’etich, J.; Ngule, C.; Jepkorir, M.; Mwangangi, R.; Njuguna, D.; Ndung’u, J.; Kiboi, D.; Mwitari, P. Total Phenolic Content and in Vitro Antiproliferative Activity of Tragia Brevipes (Pax) and Tetradenia Riparia (Hochst) Leaves Extract. Eur. J. Med. Plants 2018, 22, 1–10. [Google Scholar] [CrossRef]
- Sivajothi, V.; Dakappa, S.S. In Vitro and in Silico Antidiabetic Activity of Pyran Ester Derivative Isolated from Tragia Cannabina. Asian Pac. J. Trop. Biomed. 2014, 4, S455–S459. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.M.; Kumar, K.; Dwivedi, S.K.; Bala, M. Bioactive Potential of Indian Stinging Plants Leaf Extract against Pathogenic Fungi. J. Complement. Integr. Med. 2019, 16, 20170125. [Google Scholar] [CrossRef]
- Perumal Samy, R.; Manikandan, J.; Al Qahtani, M. Evaluation of Aromatic Plants and Compounds Used to Fight Multidrug Resistant Infections. Evid. Based Complement. Altern. Med. 2013, 2013, 1–17. [Google Scholar] [CrossRef]
- Thomas, R.; Megha, K.; Surya, P.; Rosalin, T.; Varghese, S.; Elyas, K. Investigation on the Biological Attributes of Tragia involucrata Linn. Using in Vitro Methods. J. Pharmacogn. Phytochem. 2021, 10, 398–404. [Google Scholar] [CrossRef]
- Velu, V.; Das, M.; Raj, N.A.N.; Dua, K.; Malipeddi, H. Evaluation of in Vitro and in Vivo Anti-Urolithiatic Activity of Silver Nanoparticles Containing Aqueous Leaf Extract of Tragia involucrata. Drug Deliv. Transl. Res. 2017, 7, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Nivya, M.T.; Patil, R.K.; Rao, G.M.A.; Khandagale, A.S.; Somashekarappa, H.; Ananda, D.; Manjunath, H.; Joshi, C.G. Cytotoxicity Based Screening for Radioprotective Properties of Methanolic Extract of Tragia involucrata L. on Cultured Human Peripheral Lymphocytes Exposed to Gamma Radiation. Indian J. Exp. Biol. 2019, 57, 469–477. [Google Scholar]
- Leonti, M.; Casu, L. Ethnopharmacology of Love. Front. Pharmacol. 2018, 9, 567. [Google Scholar] [CrossRef] [PubMed]
- Attah, A.F.; Omobola, A.I.; Moody, J.O.; Sonibare, M.A.; Adebukola, O.M.; Onasanwo, S.A. Detection of Cysteine-Rich Peptides in Tragia benthamii Baker (Euphorbiaceae) and in Vivo Antiinflammatory Effect in a Chick Model. Phys. Sci. Rev. 2021, 10151520200125. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Chandra, G. Phagodeterrence, Larvicidal and Oviposition Deterrence Activity of Tragia involucrata L. (Euphorbiaceae) Root Extractives against Vector of Lymphatic Filariasis Culex Quinquefasciatus (Diptera: Culicidae). Asian Pac. J. Trop. Dis. 2014, 4, S226–S232. [Google Scholar] [CrossRef]
- Subramani, P.; Sampathkumar, N.; Ravindiran, G.; Rajalingam, D.; Kumar, B. Evaluation of Nephroprotective and Antioxidant Potential of Tragia involucrata. Drug Invent. Today 2009, 1, 55–60. [Google Scholar]
- Joshi, C.G.; Gopal, M.; Kumari, N. Antitumor Activity of Hexane and Ethyl Acetate Extracts of Tragia involucrata. Int. J. Cancer Res. 2011, 7, 267–277. [Google Scholar] [CrossRef][Green Version]
- Islam, M.S.; Sana, S.; Haque, M.E.; Rahman, S.M.M.; Samad, A.; Noman, A.A.; Alam, R.; Rana, S.; Meem, R.I.; Mondol, D.; et al. Methanol, Ethyl Acetate and n-Hexane Extracts of Tragia involucrata L. Leaves Exhibit Anxiolytic, Sedative and Analgesic Activity in Swiss Albino Mice. Heliyon 2021, 7, e05814. [Google Scholar] [CrossRef]
- Samy, R.P.; Gopalakrishnakone, P.; Houghton, P.; Ignacimuthu, S. Purification of Antibacterial Agents from Tragia involucrata—A Popular Tribal Medicine for Wound Healing. J. Ethnopharmacol. 2006, 107, 99–106. [Google Scholar] [CrossRef]
- Varma, G.G.; Mathai, B.K.; Das, K.; Gowda, G.; Rammohan, S.; Einstein, J.W. Evaluation of Antiepileptic Activity of Methanolic Leaves Extract of Tragia involucrata Linn. in Mice. Int. Lett. Nat. Sci. 2014, 12, 167–179. [Google Scholar] [CrossRef]
- Thimmaiah, N.; Joshi, C.; Patil, R.; Khandagale, A.; Somashekarappa, H.; Ananda, D.; Manjunath, H. Mitigation of Radiation-Induced Oxidative Stress by Methanolic Extract of Tragia involucrata in Swiss Albino Mice. Pharmacogn. Res. 2019, 11, 236. [Google Scholar] [CrossRef]
- Dhara, A.K.; Suba, V.; Sen, T.; Pal, S.; Chaudhuri, A.K.N. Preliminary Studies on the Anti-Inflammatory and Analgesic Activity of the Methanolic Fraction of the Root Extract of Tragia involucrata Linn. J. Ethnopharmacol. 2000, 72, 265–268. [Google Scholar] [CrossRef]
- Samy, R.P.; Gopalakrishnakone, P.; Sarumathi, M.; Ignacimuthu, S. Wound Healing Potential of Tragia involucrata Extract in Rats. Fitoterapia 2006, 77, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Dhara, A.K.; Pal, S.; Nag Chaudhuri, A.K. Psychopharmacological Studies on Tragia involucrata Root Extract. Phytother. Res. 2002, 16, 326–330. [Google Scholar] [CrossRef]
- Samy, R.P.; Gopalakrishnakone, P.; Houghton, P.; Thwin, M.M.; Ignacimuthu, S. Effect of Aqueous Extract of Tragia involucrata Linn. on Acute and Subacute Inflammation. Phytother. Res. 2006, 20, 310–312. [Google Scholar] [CrossRef]
- Sama, V.; Rajini, T.; Afrooz, H.; Balaraju, P.; Reddy, B.M.; Mullangi, R. Antihyperglycemic Effects of Tragia Plukenetii Ethanolic Extract. Int. J. Pharm. Sci. Nanotechnol. 2014, 7, 2436–2440. [Google Scholar] [CrossRef]
- Kalaivanan, M.; Jesudass, L. Pharmacological Studies on Ethanol Extract of Tragia Plukenetii R. Smith. IOSR J. Pharm. 2012, 2, 1–7. [Google Scholar]
- Muthuraman, M.; Dorairaj, S.; Rangarajan, P.; Pemaiah, B. Antitumor and Antioxidant Potential of Tragia Plukenetii R. Smith on Ehrlich Ascites Carcinoma in Mice. Afr. J. Biotechnol. 2008, 7, 3527–3530. [Google Scholar]
- Manoharan, S.K. Evaluation of Anticonvulsant Activity of Tragia Plukenetii R. Smith Leaf Extracts against Chemoshock Induced by Pentylenetetrazole in Mice. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 750–753. [Google Scholar]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.; Lightfoot, D. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Thangiah, A.S.; Mutuku, N.; Ngule, E.; Francis, R. Qualitative Analysis of Phytoconstituents in Tragia Brevipes Plant. Int. J. Pharm. Res. Anal. 2018, 3, 93–98. [Google Scholar]
- Antony, C. Phytochemical and Spectral Study of the Medicinal Plant: Tragia Plukenetii. J. Pharm. Res. 2012, 5, 1701–1703. [Google Scholar]
- Olaoye, S.B.; Ibrahim, A.O.; Zhiqiang, L. Chemical Compositions and Radical Scavenging Potentials of Essential Oils from Tragia benthamii (BAKER) and Cissus Aralioides (WELW). J. Biol. Act. Prod. Nat. 2016, 6, 59–64. [Google Scholar] [CrossRef]
- Gobalakrishnan, R.; Kulandaivelu, M.; Bhuvaneswari, R.; Kandavel, D.; Kannan, L. Screening of Wild Plant Species for Antibacterial Activity and Phytochemical Analysis of Tragia involucrata L. J. Pharm. Anal. 2013, 3, 460–465. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Balogun, O.S.; Oladosu, I.A.; Liu, Z. Isolation of 2, 5-Dithia-3, 6-Diazabicyclo [2.2.1] Heptane and GC-MS Analysis of Silylated Extract from Tragia benthamii. Ife J. Sci. 2020, 22, 75–80. [Google Scholar] [CrossRef]
- Alagar Yadav, S.; Ramalingam, S.; Jabamalai Raj, A.; Subban, R. Antihistamine from Tragia involucrata L. Leaves. J. Complement. Integr. Med. 2015, 12, 217–226. [Google Scholar] [CrossRef] [PubMed]

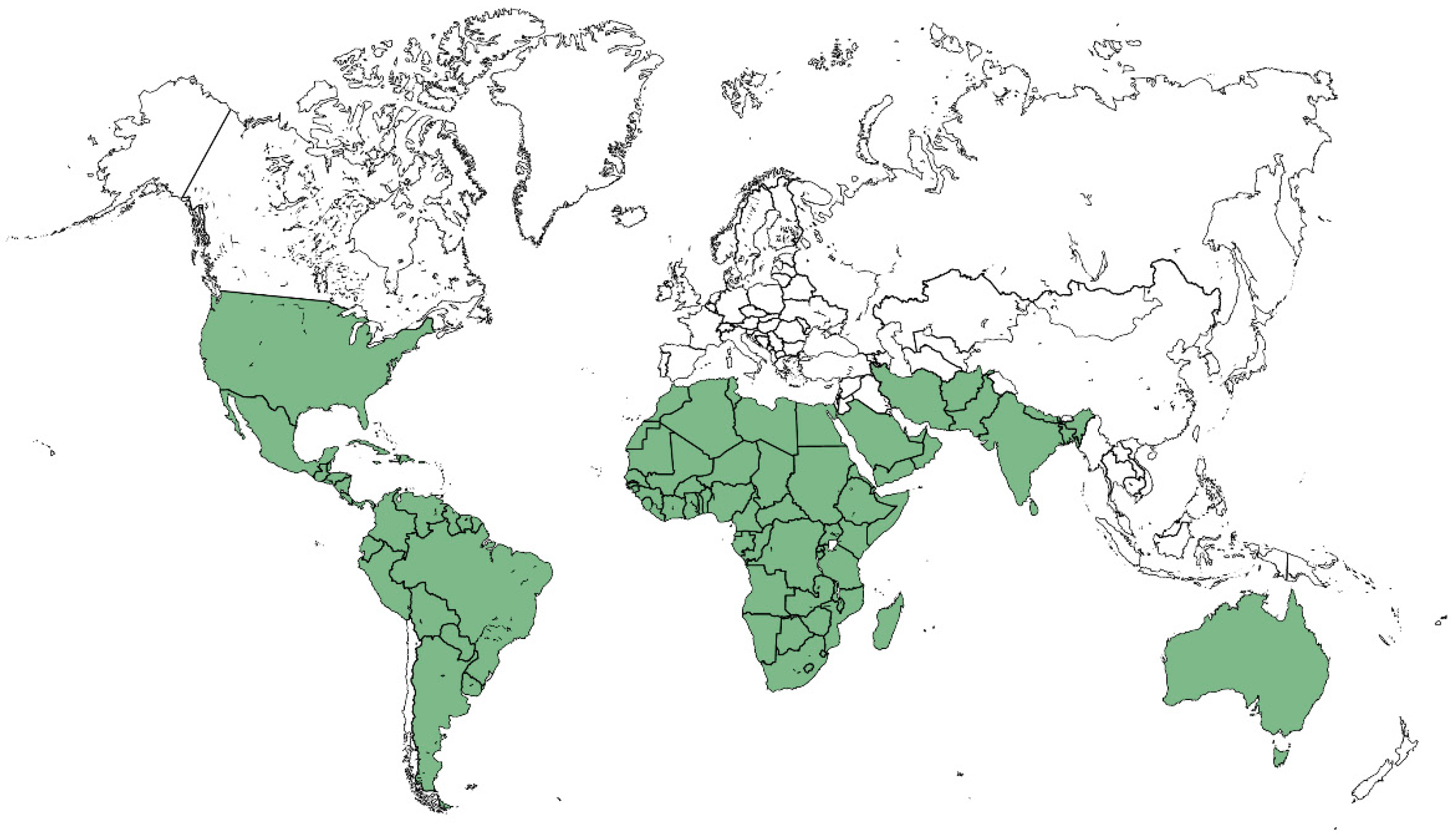
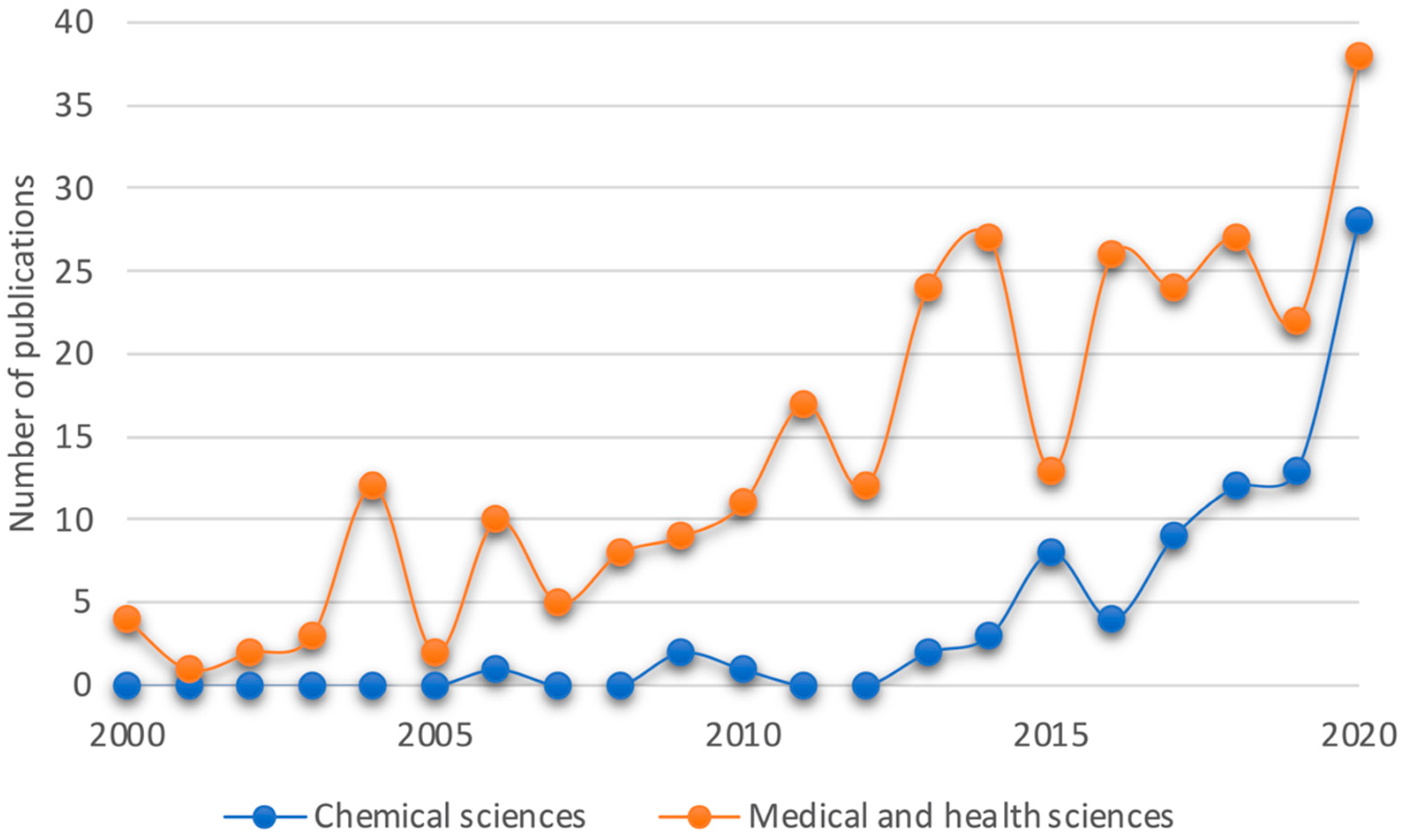

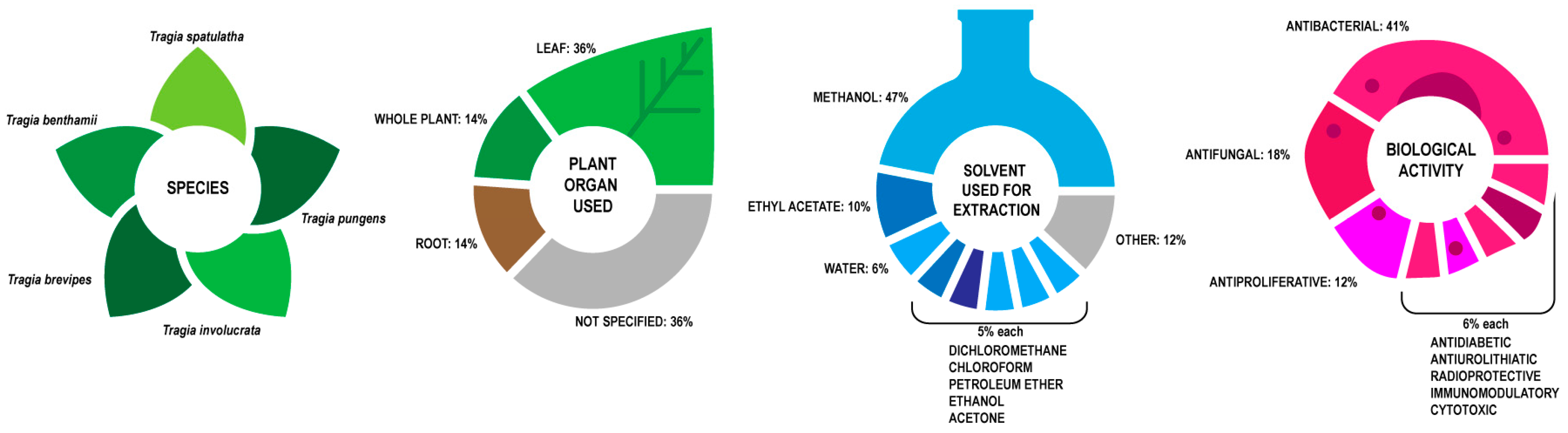

| Species | Region | Plant Organs Used | Use | Form of Usage | ATC Category | References |
|---|---|---|---|---|---|---|
| Tragia aliena Pax and K.Hoffm. | Brazil | NS | Medicinal (not specified) | NS | V | [26] |
| Tragia benthamii Baker | Nigeria, Cameroon | Whole plant Leaves, roots Whole plant | Abortifacient Antimalarial Filaricidal | Decoction NS | G P P | [27] [28] [23] |
| Tragia bicolor Miq. | India, Sri Lanka | NS | Medicinal | NS | V | [21] |
| Tragia brevipes Pax. | Rwanda, Kenya | Leaves | Anticancer Antigonorrhoeic Aphrodisiac Erectile dysfunction Obesity Uterotonic | Decoction Chewing Ash | L G G G A G | [29] [30] [31] [32] [33] [34] [35] [36] |
| Tragia cinerea (Pax) M.G.Gilbert and Radcl.-Sm. | Ethiopia | Leaves NS | Antigonorrhoeic Anti-inflammatory Aphrodisiac | Powdered plant, drunk mixed with butter/honey | G M G | [37] [38] |
| Tragia cordata Michx. | America, Ethiopia | Roots | Urinary tract and external parasites | Decoction Topical (powdered root) | G D | [39] |
| Tragia dioica Sond. | South Africa | Leaves | Fatigue Tuberculosis | NS | V J | [40] |
| Tragia doryodes M.G. Gilbert | Ethiopia | Leaves | Anthrax | Decoction | J | [41] |
| Tragia durbanensis Kuntze. | South Africa | NS | Skin rashes | NS | D | [42] |
| Tragia furialis Bojer | Tanzania, Madagascar | Roots | Abscess Analgesic Antimalarial Aphrodisiac Paralysis | Cold water maceration, drunk | J N P G N | [43] [44] [45] |
| Tragia geraniifolia Klotzsch ex Müll.Arg. | Argentina | Roots NS | Emollient Rubefacient Diuretic Antirheumatic | NS | D D G M | [46] [47] |
| Tragia gracilis Griseb. | Cuba | NS | Not specified | NS | V | [48] |
| Tragia hildebrandtii Müll.Arg. | India | NS | Not specified | NS | V | [49] |
| Tragia hispida Willd. | Sri Lanka | NS | Tooth decay | NS | A | [50] |
| Tragia insuavis Prain. | Kenya | Endophytes | Antibacterial | NS | J | [51] |
| Tragia involucrata L. | Southern Asia (India, Sri Lanka, Bangladesh) | Whole plant, Leaves, Roots | Analgesic Antidiabetic Anti-inflammatory Antimicrobial Antinociceptive Antioxidant Antiparasitic Antitumor Diuretic Hepatoprotective | Decoction Juice Poultice | N A M J N – D L G N | [20,52] [53] [23] [54] [55] [56] [57] [58] |
| Tragia meyeriana Müll.Arg. | South Africa | NS Leaves, Stems NS (barks, stems and corms mentioned) | Aphrodisiac Antineoplastic Immune booster | Decoction | G L L | [59] [60] [61] |
| Tragia mitis Hochst. ex A.Rich. | Ethiopia | Root | Antidiarrheal | Crushed, mixed with water and sugar | A | [62] |
| Tragia mixta M.G.Gilbert | Djibouti | Leaves | Analgesic Stomach aches Tonsilitis | Heated Poultice | N A A | [63] [64] |
| Tragia okanyua Pax | Namibia | NS Root | Dizziness Snake bite Cardiovascular problems Sexually transmitted diseases (STD) | Powdered, drunk with water | N V B G | [65] [66] |
| Tragia plukenetii Radcl.-Sm. | East Africa, India | Leaves | Antihyperglycemic Antitumor | Decoction | A L | [23] |
| Tragia praetervisa Chakrab. & N.P.Balakr. | India, Sri Lanka | NS | Not specified | NS | V | [49] |
| Tragia preussii Pax | Central African Republic | Leaves | Rheumatism | NS | M | [67] |
| Tragia pungens (Forssk.) Müll.Arg. | Yemen | Whole plant | Allergy and skin diseases Antirheumatic Cytotoxic Anti-impotence | Paste | D M L G | [68] [69] [70] |
| Tragia ramosa Torr. | U.S.A., Mexico | Leaves | Not specified | NS | V | [71] |
| Tragia rupestris Sond. | South Africa | Whole plant | Medicine (not specified) | NS | V V | [72] [73] |
| Tragia senegalensis Müll. Arg | Benin | Leaves | Azoospermia | NS | G | [74] |
| Tragia sonderi Prain | Swaziland | Root | HIV/AIDS | Decoction Topical | L | [75] |
| Tragia spathulata Benth. | West Africa | Leaves | Antibacterial | NS | J | [23] [76] |
| Tragia subsessilis Pax | Uganda | Root | Tuberculosis | NS | J | [77] |
| Tragia uberabana Müll. Arg | Brazil | NS | Medicinal Toxic | NS | V V | [78] |
| Tragia vogelii Keay | Burkina Faso | Whole plant | Abortifacient | Decoction | G | [79] |
| Tragia volubilis L. | Mexico, Antilles, Brazil | Leaves, Stem, Root | Diuretic Medicinal STDs | Decoction | G V G | [80] [26] [46,81] |
| Tragia yucatanensis Millsp. | Belize, Guatemala, Mexico | Leaves | Burns Rheumatism | Topical | D M | [82] |
| Species | Extract | Plant Organs Used | Biological Activity | Biological Model | Effect | Methodology | Reference |
|---|---|---|---|---|---|---|---|
| T. benthamii | Methanol | Whole plant | Antibacterial | 28 strains (sensitive and MDR) of Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterobacter aerogenes, Escherichia coli, Providencia stuartii | Effective against 11/28 strains (39.3%) | 256–1024 μg/mL INT colorimetric assay | [83] |
| T. brevipes | Methanol: water 9:1 | Leaf | Antibacterial | Inhibition zones (mm) | 500 mg/mL extract—well diffusion assay | [84] | |
| Escherichia coli, | +2 | ||||||
| Salmonella spp., | +10 | ||||||
| Enterobacter aerogenes, | +9 | ||||||
| Bacillus cereus, | +24 | ||||||
| Serratia liquefaciens, | +5 | ||||||
| Proteus vulgaris | +8 | ||||||
| T. brevipes | Methanol:DCM 1:1 | Leaf | Antiproliferative | DU145 | +IC50: 30 μg/mL | Extract MTT | [85] |
| HCC | - | ||||||
| HELA | - | ||||||
| T. involucrata | Chloroform | Root | Antidiabetic | Fertile eggs of white leghorn chicken | + | 0.5, 1 mg/egg. Streptozotocin-induced diabetes | [86] |
| T. involucrata | Ethyl acetate | Root | Antibacterial Antifungal | Inhibition zones (mm) | 50–250 mg/mL. Disc diffusion | [53] | |
| Staphylococcus aureus | +18 | ||||||
| Bacillus subtilis | +14 | ||||||
| Bacillus brevis | +5.7 | ||||||
| Staphylococcus epidermidis | +0.6 | ||||||
| Escherichia coli | +17 | ||||||
| Shigella disenteriae | +3.7 | ||||||
| Pseudomonas aeruginosa | +9.4 | ||||||
| Vibrio cholera | +4.7 | ||||||
| Trichophyton rubrum | +3.7 | ||||||
| Malassezia furfur | +13.5 | ||||||
| T. involucrata | Methanol | Leaf | Antifungal | Inhibition zone | Agar disc diffusion | [87] | |
| Rhizopus stolonifer, | +16 ± 0.3 mm | ||||||
| Aspergillus niger, | +15 ± 0.2 mm | ||||||
| Alternaria solani, | +15 ± 0.6 mm | ||||||
| Mucor indicus, | - | ||||||
| Chaetomium globosum, | - | ||||||
| Tilletia indica | +10 ± 0.5 mm | ||||||
| T. involucrata | Isolated hydrocarbons and ethers | - | Antibacterial | Inhibition zone mm | Agar disc diffusion | [88] | |
| Burkholderia pseudomallei (TES21), | +23 | ||||||
| Burkholderia pseudomallei (KHW), | +25 | ||||||
| Klebsiella pneumoniae (ATCC15380) | - | ||||||
| Klebsiella pneumoniae | +20 | ||||||
| Pseudomonas aeruginosa (ATCC27853), | - | ||||||
| Vibrio damsela, | +19 | ||||||
| Salmonella typhi (ATCC51812) | +28 | ||||||
| T. involucrata | Methanol Ethyl acetate Chloroform Petroleum ether | Leaf | Antiproliferative | K562 cell lines |
| MTT | [89] |
| T. involucrata | Water +NP | Leaf | Antiurolithiatic | - | +Struvite crystal growth inhibitory effect | 2% extract; AgNPs (200 μg mL−1) | [90] |
| T. involucrata | Methanol | Whole plant | Radioprotective | Cultured human peripheral lymphocytes | +Pretreatment (10 μg mL−1) | 60Co gamma irradiation Comet assay | [91] |
| T. meyeriana and other plant species | Boiling water | Whole plant | Immunomodulatory | Isolated peripheral blood mononuclear cells | + | S. aureus stimulation. Inflammatory cytokine secretion in THP-1 monocytes | [61] |
| T. pungens | Methanol | NS | Antibacterial Cytotoxic | Staphylococcus aureus | +(8–14 mm) | Disk diffusion assay, Neutral red uptake assay | [69] |
| Bacillus subtilis | - | ||||||
| Micrococcus flavus | - | ||||||
| Pseudomonas aeruginosa | - | ||||||
| Candida maltosa | - | ||||||
| FL cells | +Cytotoxicity. IC50: 70 μg/mL | ||||||
| T. spatulatha | Ethanol Methanol Acetone | Leaf | Antibacterial Antifungal | MIC (mg/mL) | Agar well diffusion | [76] | |
| Staphylococcus aureus, | +21 | ||||||
| Proteus mirabilis, | +21 | ||||||
| Klebsiella pneumoniae, | +25 | ||||||
| Salmonella typhi, | +25 | ||||||
| Streptococcus pneumoniae, | +25 | ||||||
| Escherichia coli, | - | ||||||
| Candida albicans, | - | ||||||
| Aspergillus flavus, | - | ||||||
| Fusarium solani | - |
| Species | Extract | Plant Organs Used | Animal Model | Activity | Results | Reference |
|---|---|---|---|---|---|---|
| T. benthamii | Ethanol | Whole plant | Swiss albino mice | Antimalarial | −Very poor activity against P. berghei (NK-65) at 50 mg·kg−1 bw. | [27] |
| T. benthamii | Water | NS | Chick | Anti-inflammatory | +Carrageenan-induced foot edema. Maximal inhibition 84.3% at 300 mg/kg bw. | [93] |
| T. furialis | Ethanol–water | NS | White albino mice | Antimalarial | +IC50: 639.3 mg·kg−1 bw against P. berghei. | [43] |
| T. involucrata | Root | Wistar rats | Hepatoprotective | +100–300 mg/kg bw. Hepatoprotective against CCl4 induced toxicity and antioxidant activity; Attenuation of biomarker alteration (SGOT, SGPT, ALP. TP). | [57] | |
| T. involucrata | Benzene: Ethyl acetate 1:1 | Root | Culex quinquefasciatus | Larvicidal | +0.1–0.4% w/v Oviposition and phagodeterrence, larvicidal. | [94] |
| T. involucrata | Ethanol | Leaf | Albino rats (male) | Nephroprotective | +250 and 500 mg/kg bw. Decrease in serum urea and creatinine in acetaminophen-induced toxicity. | [95] |
| T. involucrata | Hexane Ethyl acetate | Aerial parts | Swiss albino mice | Antitumor | +50–150 mg/kg bw. Ehrlich’s Ascites Carcinoma. DD antitumor activity and increased life span for both extracts. | [96] |
| T. involucrata | Hot water | NS | Wistar rats (male) | Diuretic | +1650, 2200 mg/kg bw. Loop diuretic action. | [56] |
| T. involucrata | Hot water—freeze dried | Whole plant | Clinical trial | Antidiabetic | 240 mL decoction/day. FPG decrease from 164.4 ± 20.4 to 130.9 ± 16.2 mg/dL. | [52] |
| T. involucrata | Methanol | Leaf | Swiss albino mice | Analgesic Anxiolytic Sedative | +200, 400 mg/kg bw. Acetic acid writhing and formalin-induced paw licking; behavioral tests; pentobarbital-induced sleep time. | [97] |
| T. involucrata | Methanol | Leaf | Wistar rats | Antibacterial | +100, 200 mg/kg bw. Wound healing in S. aureus infections. | [98] |
| T. involucrata | Methanol | Leaf | Swiss albino mice | Antiepileptic | +400, 800 mg/kg bw MES, PTZ, PTX induced convulsions DD. | [99] |
| T. involucrata | Methanol | NS | Swiss albino mice | Radioprotective | +100 mg/kg bw. DD survival increase | [100] |
| T. involucrata | Methanol | Root | Charles-Foster rats Swiss albino mice | Analgesic Anti-inflammatory | +Carrageenan paw edema, cotton pellet granulomata, acetic acid writhing. | [101] |
| T. involucrata | Methanol | Root | Wistar rats | Antibacterial | +100, 200 mg/kg bw. Wound healing in S. aureus infections | [102] |
| T. involucrata | Methanol | Root | Charles−Foster rats Swiss albino mice | CNS depressant | +100–300 mg/kg bw. Behavioral pattern, spontaneous motility, pentobarbitone-induced sleep, body temperature, aggressive behavior pattern and conditioned avoidance response (CAR). | [103] |
| T. involucrata | Methanol Chloroform | Whole plant | Albino rats | Anti-inflammatory | +100, 300 mg/kg bw. Both extracts. Carrageenan paw oedema. | [54] |
| T. involucrata | Methanol Ethyl acetate | Whole plant | Swiss albino mice | Analgesic | +500 mg/kg bw. Acetic acid model; tail flick model analgesic activity. | [55] |
| T. involucrata | Water | Leaf | Wistar rats Swiss mice (male) | Anti-inflammatory | +50–400 mg/kg bw in carrageenan-induced hindpaw edema and cotton pellet granuloma models. | [104] |
| T. involucrata | Water +NP | Leaf | Wistar rats (male) | Antiurolithiatic | +200 mg/kg bw. CaOx stone formation inhibition in ethylene glycol-induced urolithiasis. | [90] |
| T. plukenetii | Ethanol | Aerial parts | Wistar rats (male) | Antihyperglycemic | +At an oral dose of 150 and 300 mg/kg bw. Oral glucose tolerance test in alloxan induced diabetic rats. | [105] |
| T. plukenetii | Ethanol | Whole plant | Wistar rats Guinea pigs Rabbits | Antipyretic Diuretic Antiasthmatic Analgesic Antispasmodic | +100 mg/kg bw. +Antipyretic: Brewer’s yeast-induced hyperpyrexia method. +Diuretic: in vivo Lipschitz test method. +Antiasthmatic: Isolation of guinea pig ileum preparation; histamine-induced bronchoconstruction. +Analgesic: acetic acid writhing response. +Antispasmodic: studies on isolated rabbit jejunum. | [106] |
| T. plukenetii | Ethanol | Whole plant | Swiss albino mice (male) | Antitumor | +100–300mg/kg bw. Ehrlich ascites carcinoma survivability. Antioxidant parameters increased DD. | [107] |
| T. plukenetii | Methanol Benzene Chloroform | Leaf | Swiss albino mice | Anticonvulsant | +100 mg/kg bw. Methanol extract against PTZ-induced convulsions. | [108] |
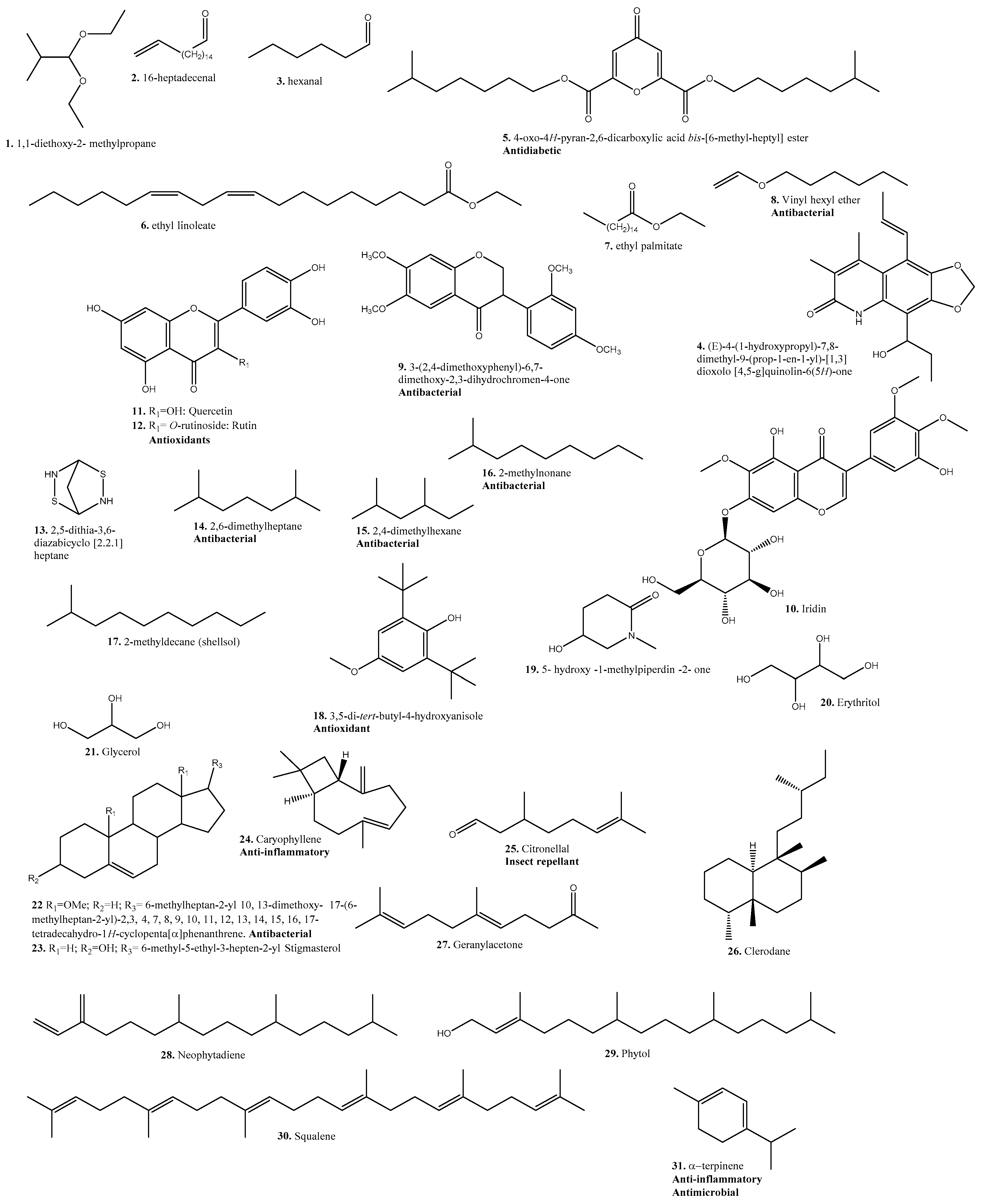
| No. | Compound | Identified | Isolated | Methodology Used | Species | Collection area | Plant Organ Used | Use | Effect | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Acetal | ||||||||||
| 1 | 1,1-diethoxy-2- methylpropane | X | Ethanol extract GC, MS | T. plukenetii | NS | Whole plant | NS | NS | [111] | |
| Aldehydes | ||||||||||
| 2 | 16-heptadecenal | X | Ethanol extract GC, MS | T. plukenetii | NS | Whole plant | NS | NS | [111] | |
| 3 | Hexanal | X | Hydrodistillation GC/GC-MS | T. benthamii | Ibadan, Nigeria | Leaves | NS | NS | [112] | |
| Alkaloid | ||||||||||
| 4 | (E)-4-(1-hydroxypropyl)-7,8-dimethyl-9-(prop-1-en-1-yl)-[1,3] dioxolo [4,5-g]quinolin-6(5H)-one | X | X | Acidified ethanol extract GC, MS, LC | T. plukenetii | NS | Whole plant | NS | NS | [111] |
| Esters | ||||||||||
| 5 | 4-oxo-4H-pyran-2,6-dicarboxylic acid bis-[6-methyl-heptyl] ester | X | X | Ethanol extract IR 1H, 13C NMR, MS | T. involucrata | Salem, India | Roots | Antidiabetic | Blood glucose reduction | [86] |
| 6 | Ethyl linoleate | X | X | Ethanol extract GC, MS | T. plukenetii | NS | Whole plant | NS | NS | [111] |
| 7 | Ethyl palmitate | X | X | Ethanol extract GC, MS | T. plukenetii | NS | Whole plant | NS | NS | [111] |
| Ether | ||||||||||
| 8 | Vinyl hexyl ether | X | X | Aqueous extract GC, MS | T. involucrata | Tamil Nadu, India | Leaf | Antibacterial Escherichia coli Proteus vulgaris Staphylococcus aureus | MBC 12.25 μg/mL | [98,113] |
| Flavonoids | ||||||||||
| 9 | 3-(2,4-dimethoxyphenyl)-6,7-dimethoxy-2,3-dihydrochromen-4-one | X | X | Ethyl acetate extract FTIR, MS, 1H NMR | T. involucrata | Odisha, India | Root | Antibacterial Fungicidal | MIC 1.25-12.5 μg/mL | [53] |
| 10 | Iridin | X | X | Ethyl acetate extract FTIR, MS, 1H NMR | T. involucrata | Odisha, India | Root | Toxic | [53] | |
| 11 | Quercetin | X | X | Ethyl acetate extract FTIR, MS, 1H NMR | T. involucrata | Odisha, India | Root | Antioxidant | [53] | |
| 12 | Rutin | X | X | Ethyl acetate extract FTIR, MS, 1H NMR | T. involucrata | Odisha, India | Root | Antioxidant | [53] | |
| Heterocycle | ||||||||||
| 13 | 2,5-dithia-3,6-diazabicyclo[2.2.1]heptane | X | X | 95% aqueous ethanol extraction 1H, 13C NMR | T. benthamii | Ibadan, Nigeria | Whole plant | NS | [114] | |
| Hydrocarbons | ||||||||||
| 14 | 2,6-dimethylheptane | X | X | Aqueous extract GC, MS | T. involucrata | Tamil Nadu, India | Leaf | Antibacterial Proteus vulgaris | MBC 10 μg/mL | [98] |
| 15 | 2,4-dimethylhexane | X | X | Aqueous extract GC, MS | T. involucrata | Tamil Nadu, India | Leaf | Antibacterial Staphylococcus aureus | MBC 12.25 μg/mL | [98] |
| 16 | 2-methylnonane | X | X | Aqueous extract GC, MS | T. involucrata | Tamil Nadu, India | Leaf | Antibacterial Escherichia coli Proteus vulgaris Staphylococcus aureus | MIC 5.0 μg/mL | [98] |
| 17 | Shellsol (2-methyldecane) | X | X | Aqueous extract GC, MS | T. involucrata | Tamil Nadu, India | Leaf | Antibacterial Proteus vulgaris Staphylococcus aureus | MBC 25.0 μg/mL | [98] |
| 18 | 3,5-di-tert-butyl-4-hydroxyanisole | X | X | 95% aqueous ethanol extraction 1H, 13C NMR | T. benthamii | Ibadan, Nigeria | Whole plant | Antioxidant | [114] | |
| 19 | 5-hydroxy-1-methylpiperdin-2-one | X | X | Methanol extract IR, 1H, 13C RMN, LC | T. involucrata | Kerala, India | Leaf | Antihistamine | Muscle relaxant, bronchodilating and anti-allergic effects | [115] |
| Polyols | ||||||||||
| 20 | Erythritol | X | X | 95% aqueous ethanol extraction 1H, 13C NMR | T. benthamii | Ibadan, Nigeria | Whole plant | NS | NS | [114] |
| 21 | Glycerol | X | X | 95% aqueous ethanol extraction 1H, 13C NMR | T. benthamii | Ibadan, Nigeria | Whole plant | NS | NS | [114] |
| Terpenoids | ||||||||||
| 22 | 10,13-dimethoxy-17-(6-methylheptan-2-yl)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[α]phenanthrene. | X | X | Ethyl acetate extract FTIR, MS, 1H NMR | T. involucrata | Odisha, India | Root | NS | NS | [53] |
| 23 | Stigmasterol | X | Aqueous extract GC, MS | T. involucrata | Leaf | NS | NS | [98] | ||
| 24 | Caryophyllene | X | Hydrodistillation GC/GC-MS | T. benthamii | Ibadan, Nigeria | Leaves | Anti inflammatory | [112] | ||
| 25 | Citronellal | X | X | Ethanol extract IR, 1H RMN, LC | T. ramosa | Maharashtra, India | Leaves | Antibacterial | [71] | |
| 26 | Clerodane | X | X | Ethanol extract IR, 1H RMN, LC | T. ramosa | Maharashtra, India | Leaves | NS | NS | [71] |
| 27 | Geranylacetone | X | Hydrodistillation GC/GC-MS | T. benthamii | Ibadan, Nigeria | Leaves | NS | NS | [112] | |
| 28 | Neophytadiene (2-(4,8,12-Trimethyltridecyl) buta-1,3-diene) | X | X | Ethanol extract GC, MS | T. plukenetii | NS | Whole plant | NS | NS | [111] |
| 29 | Phytol | X | X | 95% aqueous ethanol extraction 1H, 13C NMR | T. benthamii | Ibadan, Nigeria | Whole plant | NS | NS | [114] |
| 30 | Squalene (all trans) | X | X | Ethanol extract GC, MS | T. plukenetii | NS | Whole plant | NS | NS | [111] |
| 31 | α-terpinene | X | X | Ethanol extract IR, 1H RMN, LC | T. ramosa | Maharashtra, India | Leaves | Antiinflammatory, Antimicrobial | NS | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte-Casar, R.; Romero-Benavides, J.C. Tragia L. Genus: Ethnopharmacological Use, Phytochemical Composition and Biological Activity. Plants 2021, 10, 2717. https://doi.org/10.3390/plants10122717
Duarte-Casar R, Romero-Benavides JC. Tragia L. Genus: Ethnopharmacological Use, Phytochemical Composition and Biological Activity. Plants. 2021; 10(12):2717. https://doi.org/10.3390/plants10122717
Chicago/Turabian StyleDuarte-Casar, Rodrigo, and Juan Carlos Romero-Benavides. 2021. "Tragia L. Genus: Ethnopharmacological Use, Phytochemical Composition and Biological Activity" Plants 10, no. 12: 2717. https://doi.org/10.3390/plants10122717
APA StyleDuarte-Casar, R., & Romero-Benavides, J. C. (2021). Tragia L. Genus: Ethnopharmacological Use, Phytochemical Composition and Biological Activity. Plants, 10(12), 2717. https://doi.org/10.3390/plants10122717







