Inoculum Sources Modulate Mycorrhizal Inoculation Effect on Tamarix articulata Development and Its Associated Rhizosphere Microbiota
Abstract
:1. Introduction
2. Results
2.1. AMF T. articulata Colonization and Diversity
2.1.1. AMF T. articulata Colonization
2.1.2. AMF Spore Diversity
2.2. Effect of Mycorrhizal Inoculation on T. articulata Growth
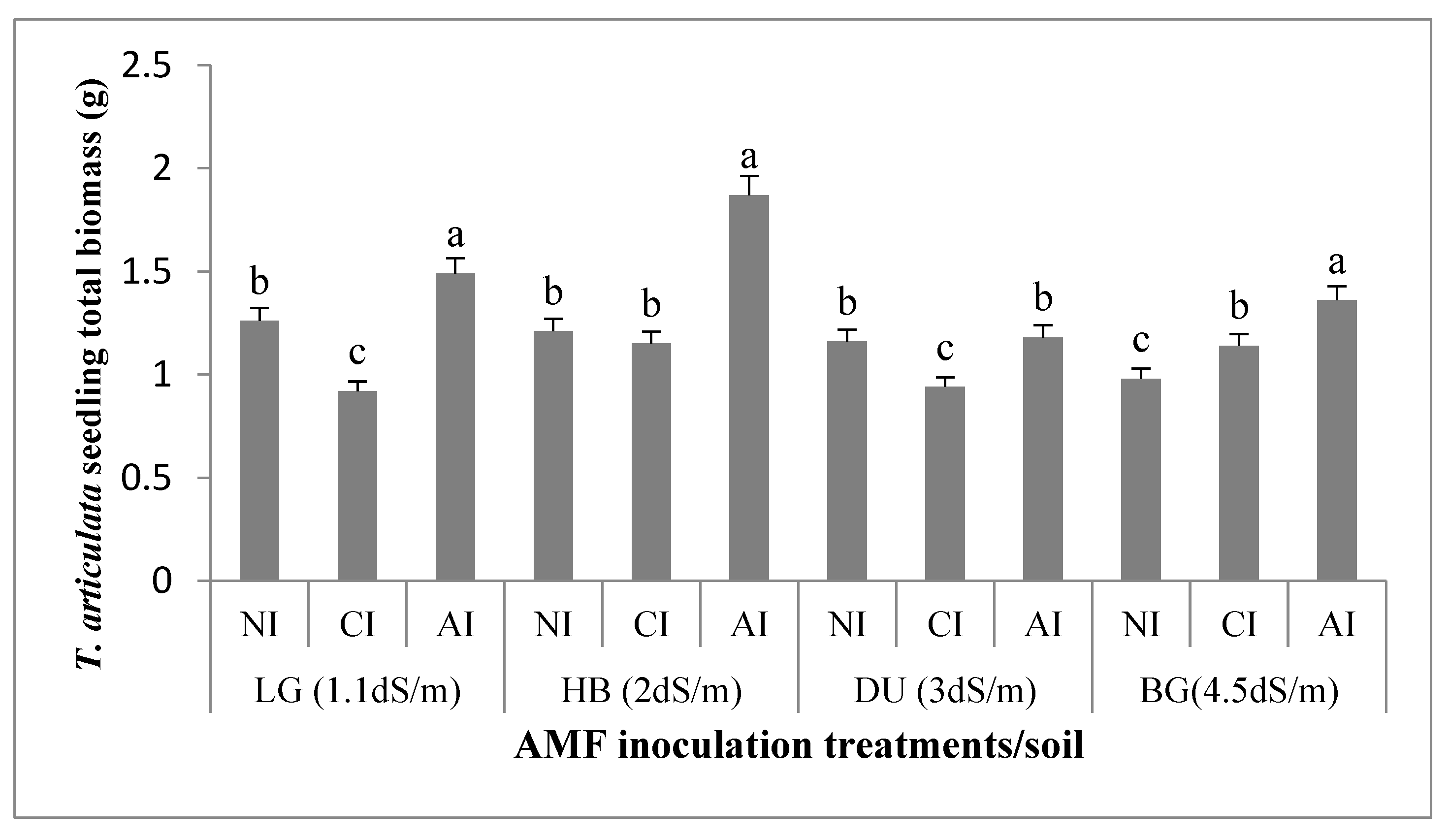
2.2.1. Seedling Biomasses
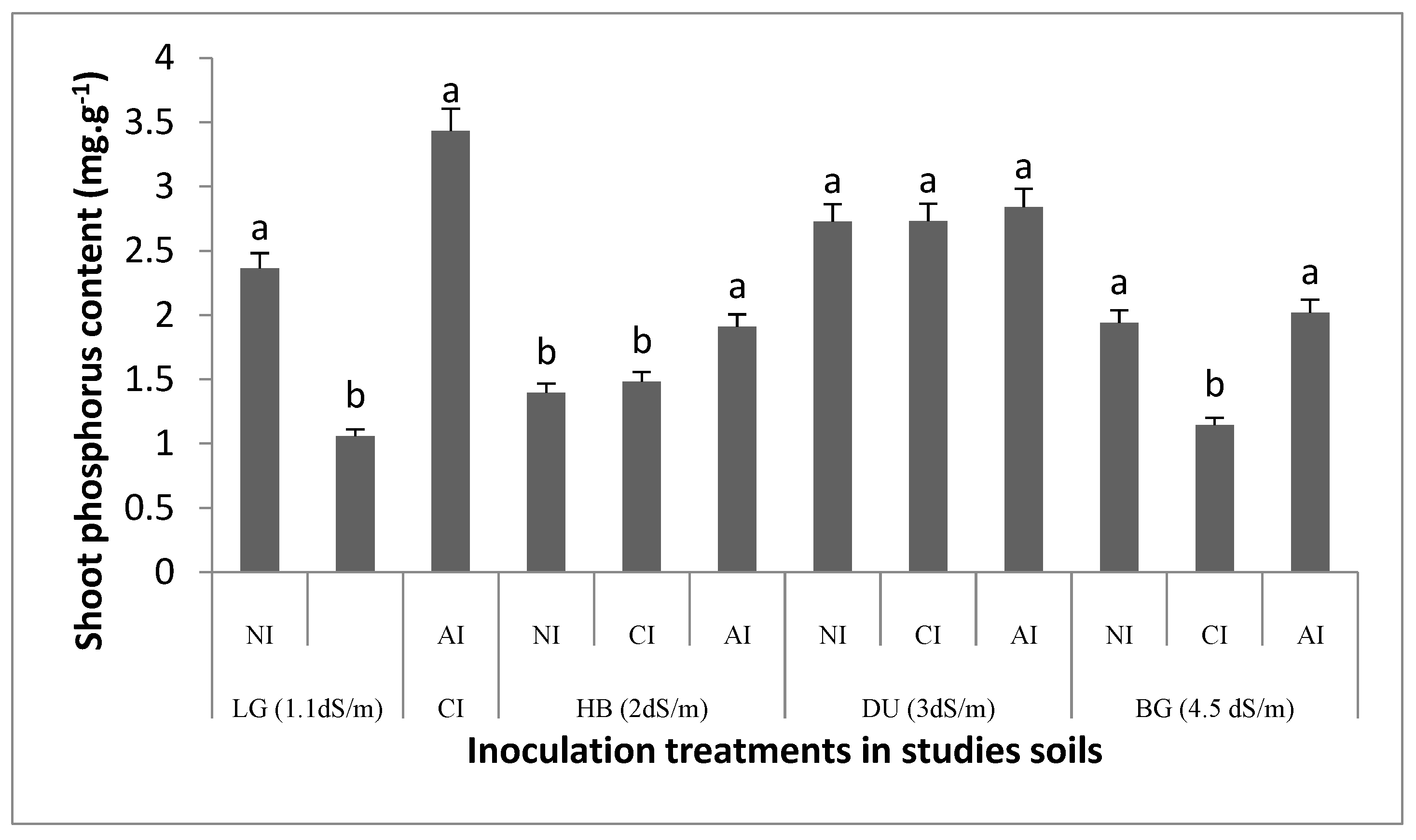
2.2.2. Phosphorus Contents in Soil and Shoots
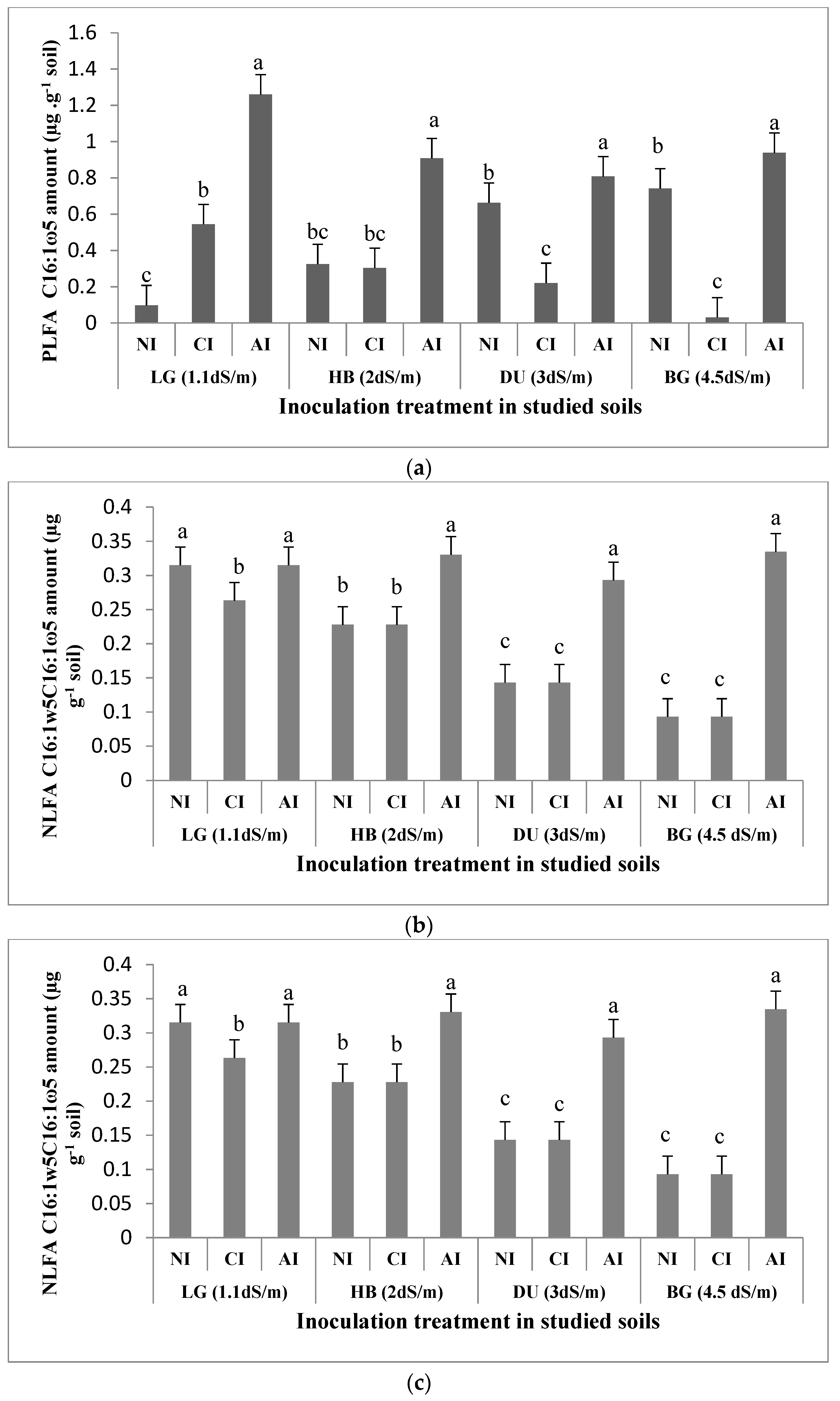
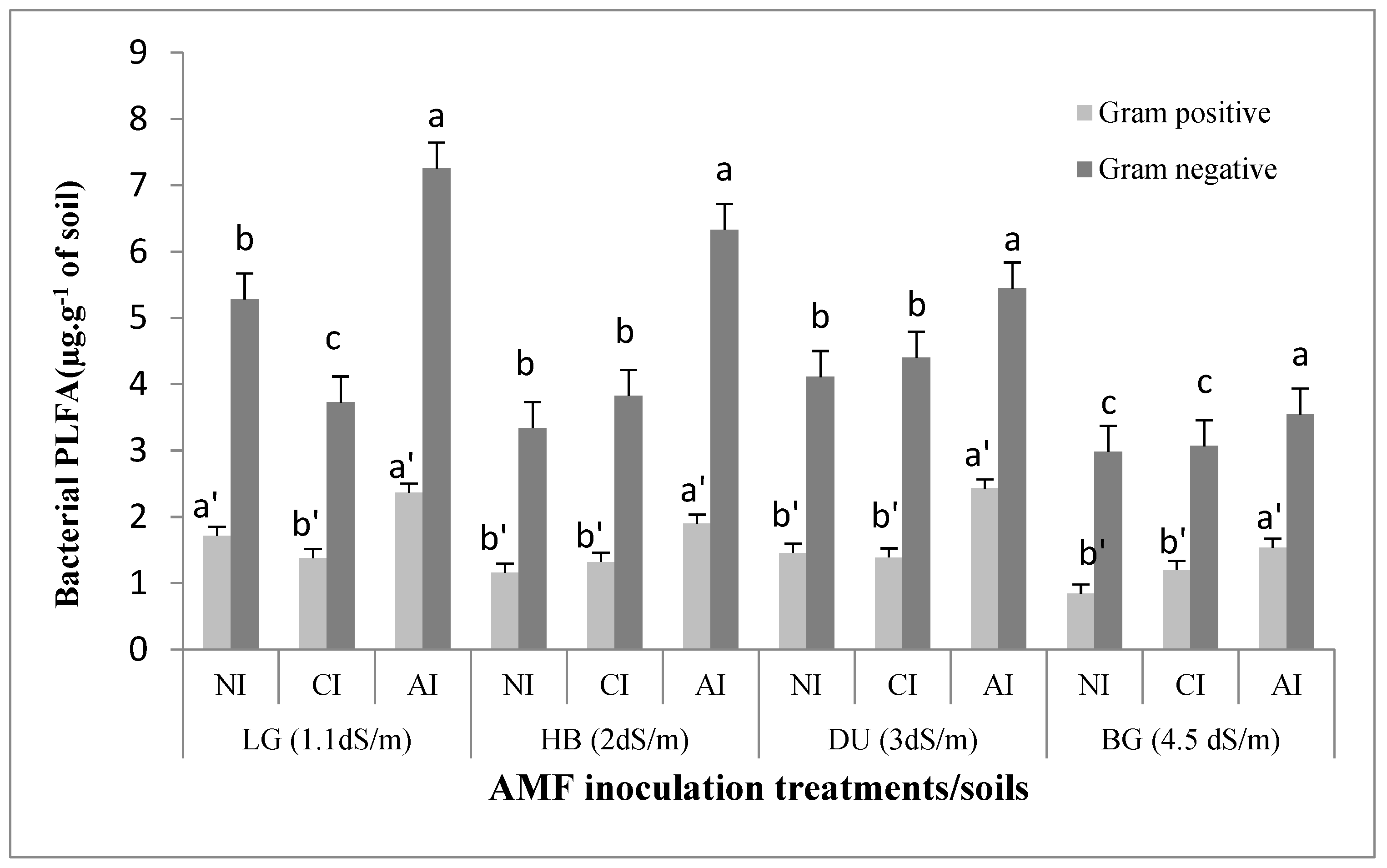
2.3. Influence of Mycorrhizal Inoculation on Soil Microbial Biomass
3. Discussion
4. Materials and Methods
4.1. Experimental Sites
4.2. Treatments and Experimental Design
4.3. Soil Microbial Analysis
4.3.1. Description of AMF Communities
4.3.2. Fatty Acid Analysis
4.3.3. Ergosterol Extraction
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manaut, N.; Sanguin, H.; Ouahman, L.; Bressan, M.; Thioulouse, J.; Baudin, E.; Galiana, A.; Hafidi, M.; Prin, Y.; Duponnois, R. Potentialities of ecological engineering strategy based on autochthonous arbuscular mycorrhizal community for improving afforestation programs with carob trees in degraded environments. Ecol. Eng. 2015, 79, 113–119. [Google Scholar] [CrossRef]
- Saifi, M.; Boulghobra, N.; Lakhdari, F. The Green Dam in Algeria as a Tool to Combat Desertification. In Proceedings of the Global Risk Forum: GRF Davos Planet@Risk, Volume 3, Number 1, Special Issue on the 5th IDRC Davos 2014, Davos, Switzerland, 24–28 August 2014. [Google Scholar]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traoré, S.; Ouattara, K.; Ilstedt, U.; Scmidt, M.; Thiombiano, A.; Malmer, A.; Nyberg, G. Effect of land degradation on carbon and nitrogen pools in two soil types of a semi-arid landscape in West Africa. Geoderma 2015, 241, 330–338. [Google Scholar] [CrossRef]
- Garg, N.; Pandey, R. Effectiveness of autochthonous and exotic arbuscular mycorrhizal fungi on nutrient uptake and ion homeostasis in salt-stressed Cajanus cajan L. (Millsp.) genotypes. Mycorrhiza 2015, 25, 165–180. [Google Scholar] [CrossRef]
- ElHindi, K.M.; El-Din, A.S.; Elgorban, A.M. The impact of arbuscular mycorrhizal fungi in mitigating salt-induced adverse effects in sweet basil (Ocimum basilicum L.). Saudi J. Biol. Sci. 2017, 24, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Hashem, A.; Abd Allah, E.A.; Alqarawi, A.; Al-Huqail, A.A.; Wirth, S.; Egamberdieva, D. The Interaction between Arbuscular Mycorrhizal Fungi and Endophytic Bacteria Enhances Plant Growth of Acacia gerrardii under Salt Stress. Front. Microbiol. 2016, 7, 1089. [Google Scholar] [CrossRef] [Green Version]
- Richards, L.A. Diagnosis and Improvement of Saline Alkali Soils; Department of Agriculture: Washington, DC, USA, 1954.
- ERGR. Rapport d’Activité Sue les Projets de Plantation et de Reforeststion de l’Atlas Saharien; Ministère de l’Agriculture et du Développement Rurale: Abidjan, Cote D’Ivoire, 2012; p. 56.
- DGF. Direction Générale des Forêts. Rapport d’Activité de la Direction Générale des Forêts, Evaluation des Projets de Plantations Dans la Steppe Sud Algéroise; Direction Générale des Forêts: Ben Aknoun, Algeria, 2017; p. 48.
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Anthony, S.; World Database. Agro-Forest Tree Database: A Reference and Selection Guide Version 4.0. 2009. Available online: http://www.forestry.org (accessed on 22 June 2021).
- Chaudhry, M.S.; Saeed, M.; Nasim, F.U.H. Soil chemical heterogeneity may affect the diversity of arbuscular mycorrhizal fungi in the rhizosphere of Tamarix aphylla under arid climate. An. Stiint. Univ. Alexandru Ioan Cuza Iasi Sect. II A Biol. Veg. 2013, 59, 53–63. [Google Scholar]
- Meinhard, A.K.; Gerhing, C.A. Tamarix and soil ecology. In Tamarix a Case Study of Ecological Change in American West; Sher, A.Q., Ed.; Oxford University Press: Oxford, UK, 2013; pp. 225–239. [Google Scholar]
- Alqarawi, A.A.; Abd Allah, E.F.; Hashem, A. Alleviation of salt-induced adverse impact via mycorrhizal fungi in Ephedra aphylla Forssk. J. Plant Interact. 2014, 9, 802–810. [Google Scholar] [CrossRef] [Green Version]
- Garg, N.; Pandey, R. High effectiveness of exotic arbuscular mycorrhizal fungi is reflected in improved rhizobial symbiosis and trehalose turnover in Cajanus cajan genotypes grown under salinity stress. Fungal Ecol. 2016, 21, 57–67. [Google Scholar] [CrossRef]
- Schüβler, A.; Schwarzott, D.; Walker, C. A new fungal phylum, the Glomeromycota: Phylogeny and evolution. Mycol. Res. 2001, 105, 1413–1421. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Plouznikoff, K.; Declerck, S.; Calonne-Salmon, M. Mitigating Abiotic Stresses in Crop Plants by Arbuscular Mycorrhizal Fungi. In Belowground Defense Strategies in Plants, Signaling and Communication in Plants; Vos, C.M.F., Kazan, K., Eds.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wu, Q.S. (Ed.) Arbuscular Mycorrhizas and Stress Tolerance of Plant; Springer: New York, NY, USA, 2017. [Google Scholar]
- Bencherif, K.; Dalpé, Y.; Hadj-Sahraoui, A. Arbuscular Mycorrhizal Fungi Alleviate Soil Salinity Stress in Arid and Semi-Arid Areas. Soil Biol. 2019, 56, 375–400. [Google Scholar]
- Bencherif, K.; Boutekrabt, A.; Fontaine, J.; Laruelle, F.; Dalpé, Y.; Lounès-Haj Sahraoui, A. Impact of soil salinity on arbuscular mycorrhizal fungi biodiversity and microflora biomass associated with Tamarix articulata Vahll rhizosphere in arid and semi-arid Algerian areas. Sci. Total Environ. 2015, 533, 488–494. [Google Scholar] [CrossRef]
- Fall, D.; Bakhoum, N.; Fall, F.; Diouf, F.; Ndigue, M.; Ndiaye, C.; Hocher, V.; Diouf, D. Improvement of tree growth in salt-affected soils under greenhouse conditions using a combination of peanut shells and microbial inoculation. J. Agric. Biotechnol. Sustain. Dev. 2017, 9, 36–44. [Google Scholar]
- Porras-Soriano, A.; Soriano-Martin, M.L.; Porras-Piedra, A. Arbuscular mycorrhizal fungi increased growth, nutrient uptake and tolerance to salinity in olive trees under nursery conditions. J. Plant Physiol. 2009, 166, 1350–1359. [Google Scholar] [CrossRef]
- Hart, M.M.; Antunes, P.M.; Chaudhry, V.B.; Abbott, L. Fungal inoculants in the field: Is the reward greater than the risk. Funct. Ecol. 2018, 32, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Saxena, B.; Shukla, K.; Giri, B. Arbuscular Mycorrhizal Fungi and Tolerance of salt stress in plant. In Arbuscular Mycorrhizas and Stress Tolerance in Plants; Wu, Q.S., Ed.; Springer: New York, NY, USA, 2017; pp. 67–98. [Google Scholar]
- Smith, S.E.; Jakobsen, I.; Gronlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Guo, X.; Feng, G.; Maimaitiaili, B.; Fan, J.; He, X. Indigenous arbuscular mycorrhizal fungi can alleviate salt stress and promote growth of cotton and maize in saline fields. Plant Soil 2016, 398, 195–206. [Google Scholar] [CrossRef]
- Mechri, B.; Manga, A.G.B.; Tekaya, M.; Attia, F.; Cheheb, H.; BenMeriem, F.; Braham, M.; Boujnah, D.; Hammami, M. Changes in microbial communities and carbohydrate profiles induced by the mycorrhizal fungus (Glomus intraradices) in rhizospheres of olive trees (Olea europaea L.). Appl. Soil Ecol. 2014, 75, 124–133. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O. Biofertilizers and sustainable agriculture: Exploring arbuscular mycorrhizal fungi. Appl. Microbiol. Biotechnol. 2017, 101, 4871–4881. [Google Scholar] [CrossRef]
- Diagne, N.; Baudin, E.; Svistoonoff, S.; Ouattara, C.; Diouf, D.; Kan, A.; Ndiaye, C.; Noba, K.; Bogusz, D.; Franche, C. Effect of native and allochthones arbuscular mycorrhizal fungi on Casuarina equisetifolia growth and its root bacterial community. Arid Land Res. Manag. 2018, 32, 212–228. [Google Scholar] [CrossRef]
- Molineux, C.J.; Connop, S.P.; Gange, A.C. Manipulating soil microbial communities in extensive green roof substrates. Sci. Total Environ. 2014, 493, 632–638. [Google Scholar] [CrossRef]
- Changey, F.; Meglouli, H.; Fontaine, J.; Magnin-Robert, M.; Tisserant, B.; Lerch, T.Z.; Lounès-Hadj Sahraoui, A. Initial microbial status modulates mycorrhizal inoculation effect on rhizosphere microbial communities. Mycorrhiza 2019, 29, 475–487. [Google Scholar] [CrossRef]
- John, M.K. Colorimetric determination of phosphorus in soil and plant materials with ascorbic acid. Soil Sci. 1970, 109, 214–220. [Google Scholar] [CrossRef]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Blaszkowski, J. Glomeromycota; Polish Academy of Sciences: Warsaw, Poland, 2012; p. 303. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for cleaning roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–160. [Google Scholar] [CrossRef]
- Dalpé, Y.; Seguin, S.M. Microwave-assisted technology for the clearing and staining of arbuscular mycorrhizal fungi in roots. Mycorrhiza 2013, 23, 333–340. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Microbial biomass measured as total lipid phosphate in soils of different organic content. J. Microbiol. Methods 1991, 14, 151–163. [Google Scholar] [CrossRef]
- Frostegård, A.; Tunlid, A.; Baath, E. Changes in microbial community structure during long-term incubation in two soils experimentally contaminated with metals. Soil Biol. Biochem. 1996, 28, 55–63. [Google Scholar] [CrossRef]

| Mycorrhizal Rate/Studied Soils | LG (1.1 dS·m−1) | HB (2.1 dS·m−1) | DU (3.1 dS·m−1) | BG (4.5 dS·m−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NI | CI | AI | NI | CI | AI | NI | CI | AI | NI | CI | AI | |
| Total mycorrhizal rate (%) | 15.4 c | 15.1 c | 21.9 a | 14.7 c | 10.9 c | 26.7 a | 12.2 c | 11.8 b | 29.1 a | 9.4 b | 15.4 b,c | 33.2 a |
| Total arbuscules (%) | 8.1 c’ | 11.6 b’ | 16.6 a’ | 9.3 c’ | 5.6 c’ | 12.2 a’ | 8.6 c’ | 8.8 c’ | 13.6 a’ | 4.2 c’ | 6.6 c’ | 16.6 a’ |
| Total vesicles (%) | 11.2 c,† | 14.8 b,† | 25.7 a,† | 13.2 c,† | 9.2 b,† | 16.6 a,† | 10.3 b,c,† | 10.6 c,† | 22.1 a,† | 8.3 c,† | 11.6 c,† | 28.3 a,† |
| NI Treatment | CI Treatment | AI Treatment | ||||
|---|---|---|---|---|---|---|
| MCR | SDW | MCR | SDW | MCR | SDW | |
| SDW | 0.5 * | – | 0.7 * | – | 0.9 ** | – |
| RDW | 0.6 * | – | –0.2 | 0.9 *** | 0.8 ** | 0.9 *** |
| Shoot Phosphorus | 0.6 * | 0.6 * | 0.7 * | 0.5 * | 0.9 ** | 0.7 * |
| Soil phosphorus | –0.3 | 0.9 *** | 0.3 | 0.7 ** | –0.8 ** | 0.2 |
| Shannon index | 0.3 | 0.1 | 0.3 | 0.2 | 0.9 ** | 0.4 ** |
| AMF species richness | 0.5 | 0.3 | −0.4 | –0.5 | 0.9 ** | 0.2 ** |
| NLFA C16:1ω5 | 0.6 * | 0.4 * | −0.1 | –0.5 | 0.7 * | 0.5 * |
| PLFA C16:1ω5 | –0.00 | 0.6 | −0.00 | 0.5 | 0.6 * | 0.2 * |
| NLFA/PLFA C16:1ω5 | 0.6 * | 0.3 ** | −0.1 | −0.3 | 0.6 * | 0.00 * |
| Ergosterol | 0.4 | 0.6 | 0.0 | 0.3 | 0.6 * | 0.2 * |
| Gram positive bacteria | 0.5 | 0.0 | 0.2 | 0.1 | 0.5 * | 0.2 * |
| Gram negative bacteria | 0.4 | 0.3 | 0.00 | 0.1 | 0.6 * | 0.2 ** |
| Sources of Variation/Parameters | Mycorrhizal Rate | Shoot Phosphorus | Total Dry Weight | NLFA | PLFA | Ergosterol | Gram + | Gram − | Soil Phosphorus | AMF Spore Biodiversity | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil salinity effect | R² | –0.461 | 0.135 | 0.313 | 0.564 | 0.304 | 0.161 | 0.329 | 0.409 | 0.139 | 0.78 | |
| F | 2.381 | 0.434 | 1.267 | 3.595 | 1.212 | 0.532 | 1.361 | 1.926 | 0.447 | 27.668 | ||
| Pr > F | 0.042 * | 0.904 | 0.302 | 0.005 ** | 0.331 | 0.838 | 0.257 | 0.095 * | 0.896 | 0.001 * | ||
| Intergroup Inoculum sources | NI | R² | 0.580 | 0.53 | 0.567 | 0.521 | 0.478 | 0.53 | 0.43 | 0.12 | 0.78 | 0.618 |
| F | 15.5 | 3.56 | 31.23 | 2.15 | 1.99 | 1.52 | 2.89 | –2.26 | 3.87 | 23.5 | ||
| Pr > F | 0.05 * | p < 0.02 * | 0.05 * | 0.02 * | 0.05 * | 0.05 * | 0.02 * | 0.45 ns | 0.02 * | 0.05 * | ||
| IC | R² | 0.406 | 0.135 | 0.479 | 0.46 | 0.347 | 0.19 | 0.32 | 0.24 | 0.24 | 0.56 | |
| 3.17 | 1.9 | 32.15 | 1.091 | 1.12 | 0.51 | 1.36 | –1.8 | 2.5 | 21.03 | |||
| FPr > F | 0.009 ** | 0.8 | 0.05 * | 0.1 | 0.12 | 0.8 | 0.1 | 0.19 | 0.4 | 0.05 * | ||
| AI | R² | 0.593 | 0.624 | 0.618 | 0.65 | 0.74 | 0.65 | 0.67 | 0.62 | 0.798 | 0.65 | |
| F | 14.9 | 4.34 | 7.62 | 4.12 | 2.39 | 2.11 | 2.06 | 6.19 | 21.34 | 2.758 | ||
| Pr > F | 0.001 ** | p < 0.05 * | 0.002 ** | 0.002 ** | 0.05 * | 0.05 * | 0.01 ** | 0.02* | 0.05* | 0.001 ** | ||
| Contrast | NI vs. IC | p < 0.04 * | p < 0.02 * | p < 0.05 * | p < 0.01 ** | p < 0.03 * | p < 0.05 * | p < 0.98 | p < 0.32 | p < 0.311 | 0.02 * | |
| NI vs. AI | p < 0.02 * | p < 0.3 ns | p < 0.04 * | p < 0.03 * | p < 0.02 * | p < 0.812 | p < 0.9 | p < 0.19 | p < 0.261 ns | p < 0.03 * | ||
| IC vs. AI | p < 0.05 * | p < 0.05 * | p < 0.02 * | p < 0.01 ** | p < 0.04 * | p < 0.05 * | p < 0.5 | p < 0.78 ns | p < 0.385 ns | p < 0.04 * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bencherif, K.; Laruelle, F.; Dalpé, Y.; Lounès-Hadj Sahraoui, A. Inoculum Sources Modulate Mycorrhizal Inoculation Effect on Tamarix articulata Development and Its Associated Rhizosphere Microbiota. Plants 2021, 10, 2716. https://doi.org/10.3390/plants10122716
Bencherif K, Laruelle F, Dalpé Y, Lounès-Hadj Sahraoui A. Inoculum Sources Modulate Mycorrhizal Inoculation Effect on Tamarix articulata Development and Its Associated Rhizosphere Microbiota. Plants. 2021; 10(12):2716. https://doi.org/10.3390/plants10122716
Chicago/Turabian StyleBencherif, Karima, Frédéric Laruelle, Yolande Dalpé, and Anissa Lounès-Hadj Sahraoui. 2021. "Inoculum Sources Modulate Mycorrhizal Inoculation Effect on Tamarix articulata Development and Its Associated Rhizosphere Microbiota" Plants 10, no. 12: 2716. https://doi.org/10.3390/plants10122716
APA StyleBencherif, K., Laruelle, F., Dalpé, Y., & Lounès-Hadj Sahraoui, A. (2021). Inoculum Sources Modulate Mycorrhizal Inoculation Effect on Tamarix articulata Development and Its Associated Rhizosphere Microbiota. Plants, 10(12), 2716. https://doi.org/10.3390/plants10122716








