Vapour Application of Sage Essential Oil Maintain Tomato Fruit Quality in Breaker and Red Ripening Stages
Abstract
1. Introduction
2. Results
2.1. Fruit Decay
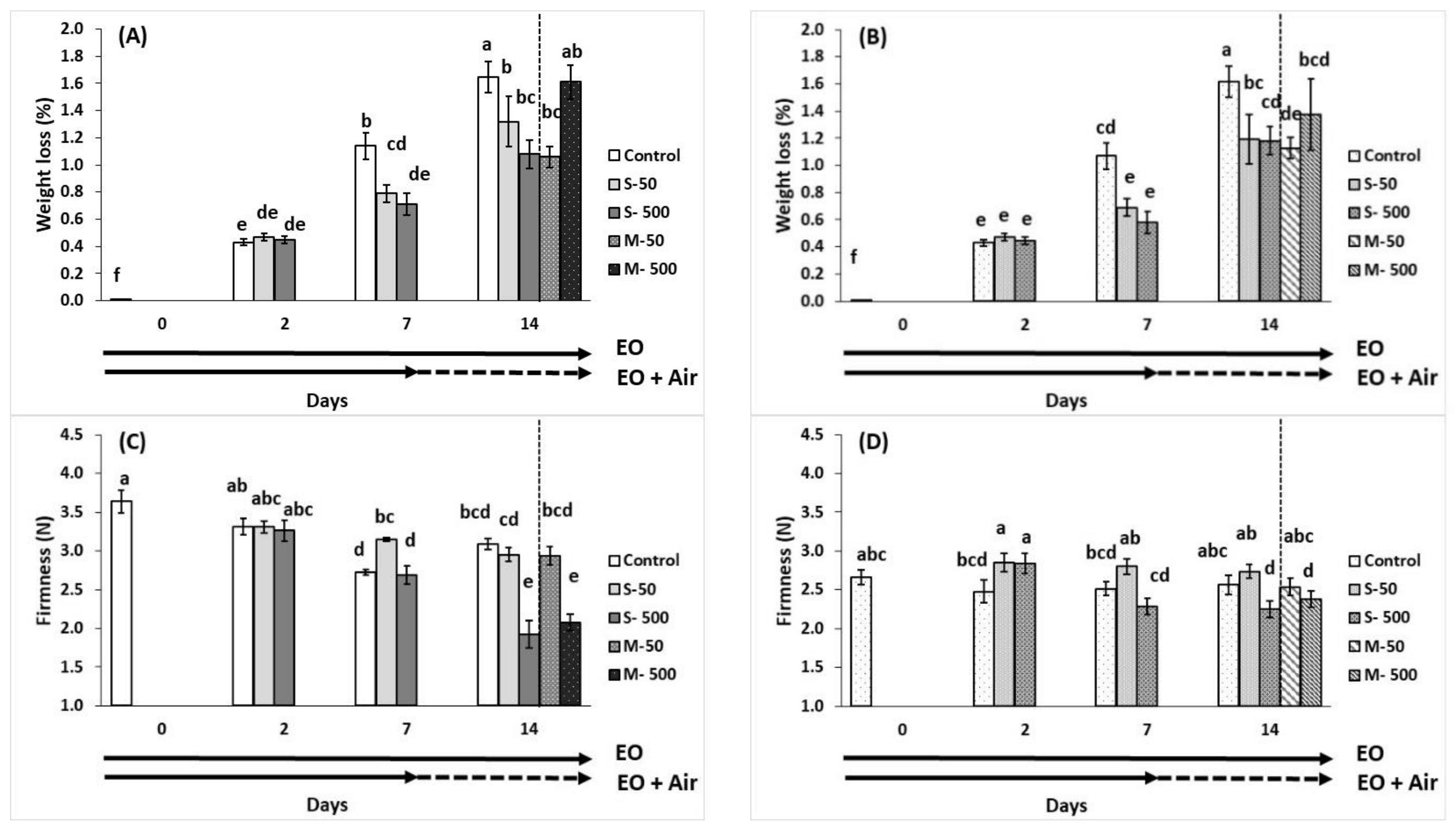
2.2. Fruit Weight Loss, Firmness and Colour
2.3. Soluble Solids, Organic Acid and Ripening Index
2.4. Respiration Rate and Ethylene Emission
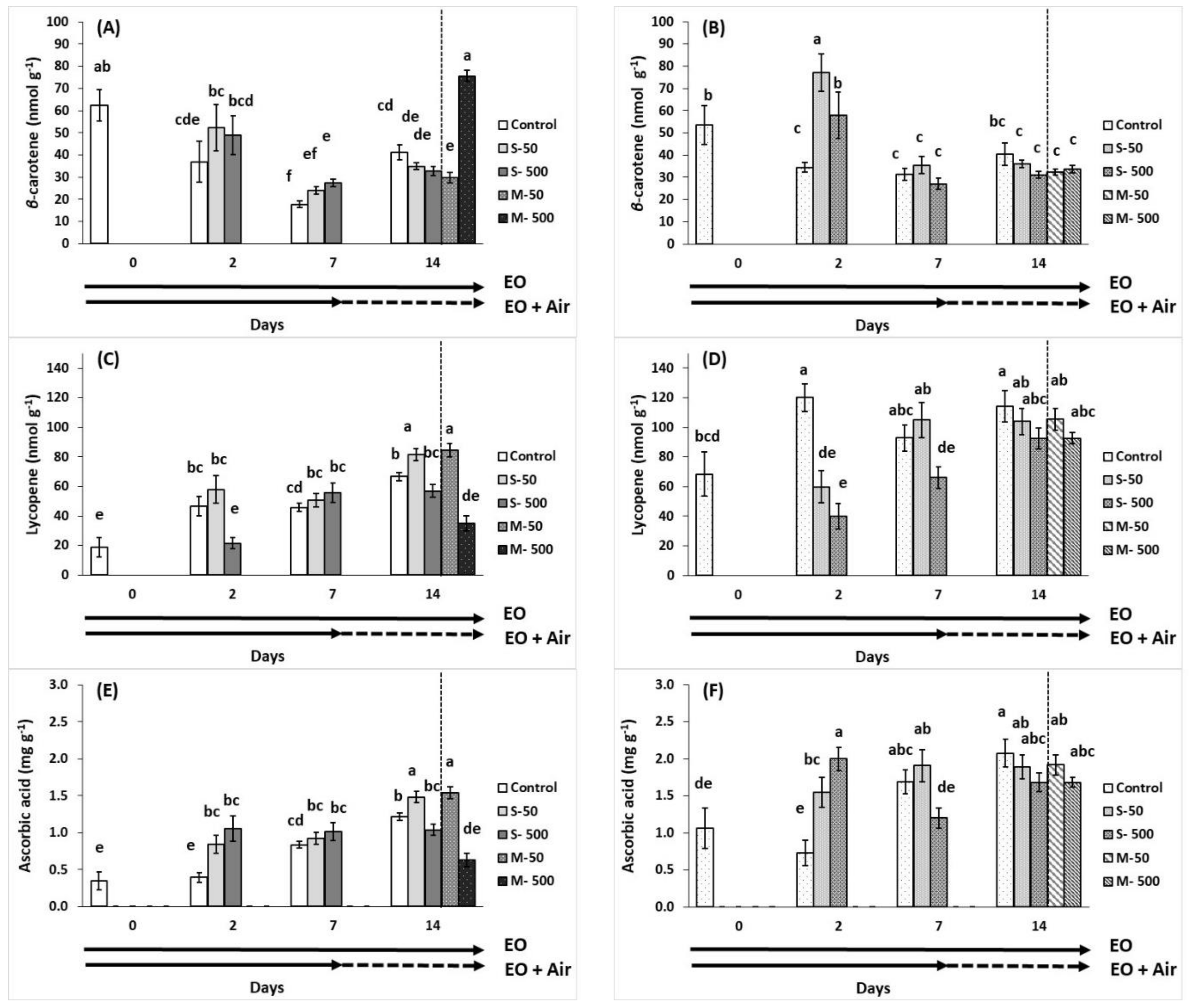
2.5. Carotenoid Composition and Ascorbic Acid
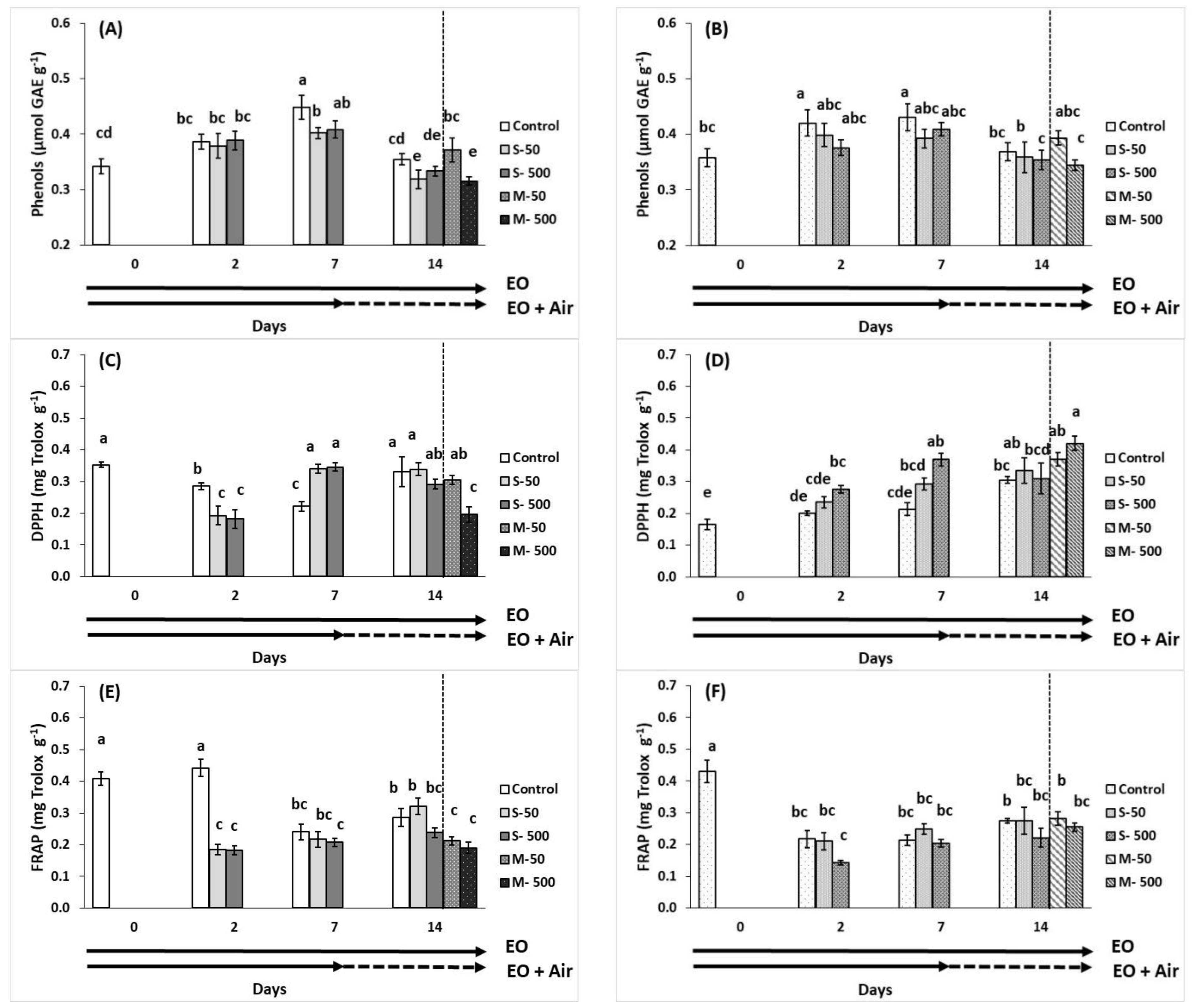
2.6. Total Phenols Content and Antioxidant Activity
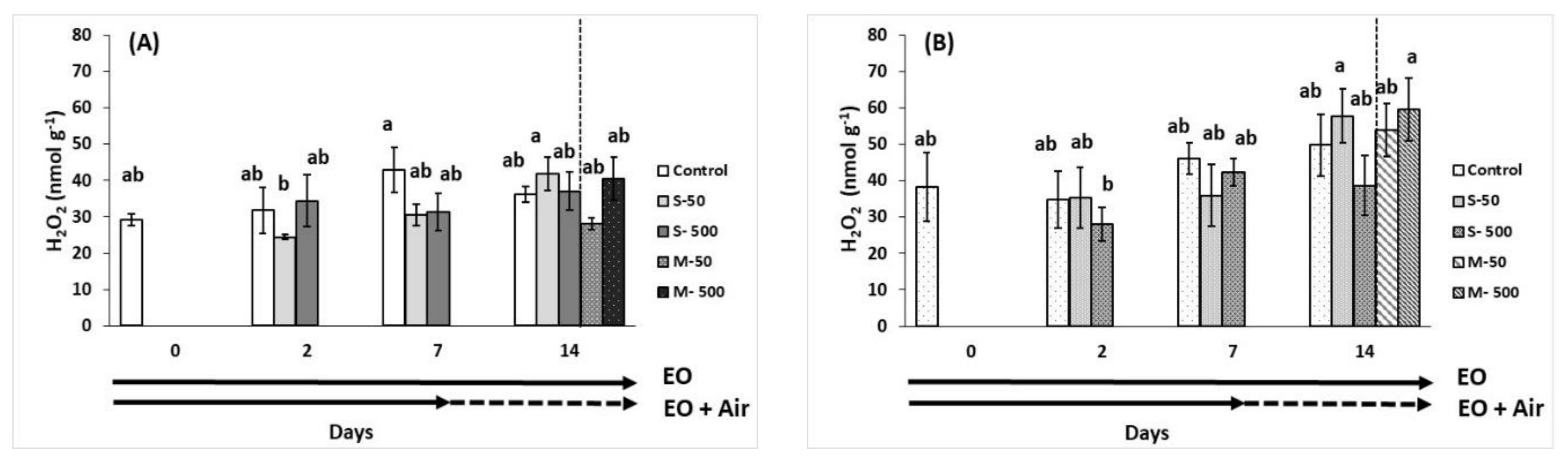
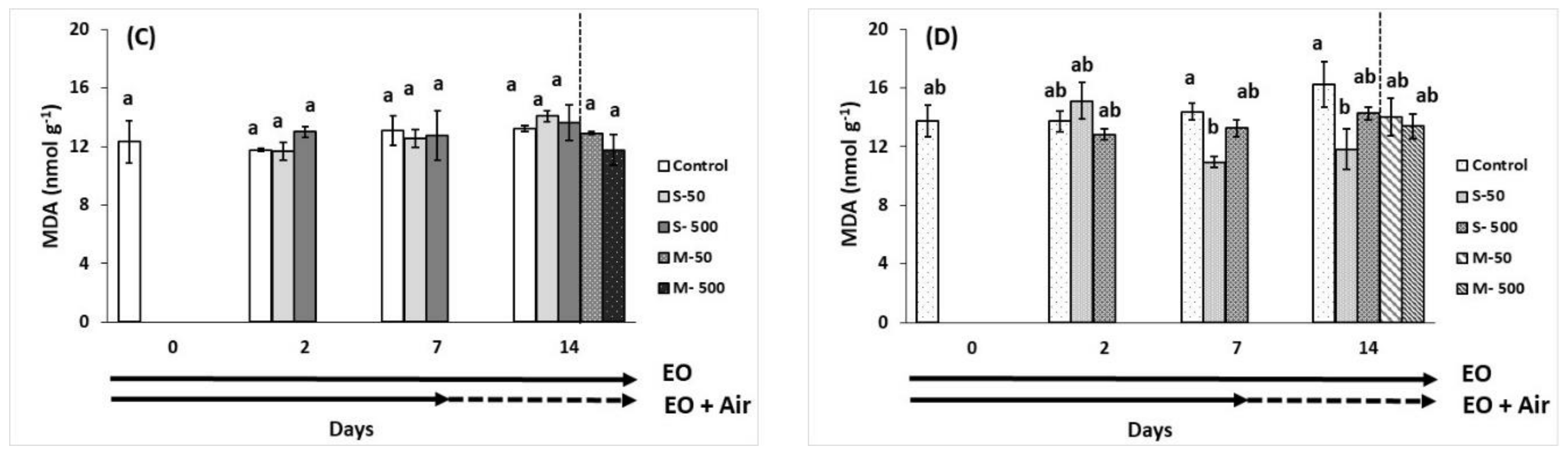
2.7. Plant Stress Indicators
2.8. Sensory Evaluation
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Design
4.2. Decay Evaluation
4.3. Respiration Rate and Ethylene Emission
4.4. Weight Loss, Colour and Fruit Firmness
4.5. Soluble Solids, Titratable Acidity, Ripening Index, Ascorbic Acid and Carotenoids
4.6. Total Phenols and Antioxidant Activity
4.7. Plant Stress Indicators
4.8. Sensory Evaluation
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Brummell, D.A.; Harpster, M.H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 2001, 47, 311–340. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Duan, Y.; Ju, Z. Plant oil emulsion modifies internal atmosphere, delays fruit ripening, and inhibits internal browning in Chinese pears. Postharvest Biol. Technol. 2000, 20, 243–250. [Google Scholar] [CrossRef]
- Spotts, R.A.; Peters, B.B. Chlorine and Chlorine Dioxide for Control of d’ Anjou Pear Decay. Plant Dis. 1980, 64, 1095. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Singleton, I.; Barnes, J. Deployment of low-level ozone-enrichment for the preservation of chilled fresh produce. Postharvest Biol. Technol. 2007, 43, 261–270. [Google Scholar] [CrossRef]
- Tzortzakis, N. Ozone: A powerful tool for the fresh produce preservation. In Postharvest Management Approaches for Maintaining Quality of Fresh Produce; Siddiqui, M., Zavala, J., Hang, C.-A., Eds.; Springer: Cham, Switzerland, 2016; pp. 175–208. [Google Scholar]
- Serrano, M.; Martínez-Romero, D.; Castillo, S.; Guillén, F.; Valero, D. The use of natural antifungal compounds improves the beneficial effect of MAP in sweet cherry storage. Innov. Food Sci. Emerg. Technol. 2005, 6, 115–123. [Google Scholar] [CrossRef]
- Tzortzakis, N. Essential oil: Innovative tool to improve the preservation of fresh produce—A review. Fresh Prod. 2009, 3, 87–97. [Google Scholar]
- Abdollahi, M.; Rezaei, M.; Farzi, G. Improvement of active chitosan film properties with rosemary essential oil for food packaging. Int. J. Food Sci. Technol. 2012, 47, 847–853. [Google Scholar] [CrossRef]
- Castillo, S.; Navarro, D.; Zapata, P.J.; Guillén, F.; Valero, D.; Serrano, M.; Martínez-Romero, D. Antifungal efficacy of Aloe vera in vitro and its use as a preharvest treatment to maintain postharvest table grape quality. Postharvest Biol. Technol. 2010, 57, 183–188. [Google Scholar] [CrossRef]
- Camele, I.; Elshafie, H.S.; Caputo, L.; Sakr, S.H.; De Feo, V. Bacillus mojavensis: Biofilm formation and biochemical investigation of its bioactive metabolites. J. Biol. Res. 2019, 92, 39–45. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sakr, S.; Bufo, S.A.; Camele, I. An attempt of biocontrol the tomato-wilt disease caused by Verticillium dahliae using Burkholderia gladioli pv. Agaricicola and its bioactive secondary metabolites. Int. J. Plant Biol. 2017, 8, 57–60. [Google Scholar]
- Stavropoulou, A.; Loulakakis, K.; Magan, N.; Tzortzakis, N. Origanum dictamnus Oil Vapour Suppresses the Development of Grey Mould in Eggplant Fruit in Vitro. Biomed Res. Int. 2014, 2014, 562679. [Google Scholar] [CrossRef]
- Tzortzakis, N.G.; Economakis, C.D. Antifungal activity of lemongrass (Cympopogon citratus L.) essential oil against key postharvest pathogens. Innov. Food Sci. Emerg. Technol. 2007, 8, 253–258. [Google Scholar] [CrossRef]
- Tzortzakis, N.G. Ethanol, vinegar and Origanum vulgare oil vapour suppress the development of anthracnose rot in tomato fruit. Int. J. Food Microbiol. 2010, 142, 14–18. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Botsaris, G.; Tzortzakis, N. Antioxidant and antibacterial activities, mineral and essential oil composition of spearmint (Mentha spicata L.) affected by the potassium levels. Ind. Crops Prod. 2017, 103, 202–212. [Google Scholar] [CrossRef]
- Basile, A.; Senatore, F.; Gargano, R.; Sorbo, S.; Del Pezzo, M.; Lavitola, A.; Ritieni, A.; Bruno, M.; Spatuzzi, D.; Rigano, D.; et al. Antibacterial and antioxidant activities in Sideritis italica (Miller) Greuter et Burdet essential oils. J. Ethnopharmacol. 2006, 107, 240–248. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crops Prod. 2013, 43, 587–595. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Saab, A.M.; Statti, G.A.; Menichini, F. Cytotoxic activity of essential oils from Labiatae and Lauraceae families against in vitro human tumor models. Anticancer Res. 2007, 27, 3293–3299. [Google Scholar] [PubMed]
- Borges, R.S.; Ortiz, B.L.S.; Pereira, A.C.M.; Keita, H.; Carvalho, J.C.T. Rosmarinus officinalis essential oil: A review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J. Ethnopharmacol. 2019, 229, 29–45. [Google Scholar] [CrossRef]
- Lopez-Reyes, J.G.; Spadaro, D.; Prelle, A.; Garibaldi, A.; Gullino, M.L. Efficacy of plant essential oils on postharvest control of rots caused by fungi on different stone fruits in vivo. J. Food Prot. 2013, 76, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Ben Farhat, M.; Jordán, M.J.; Chaouech-Hamada, R.; Landoulsi, A.; Sotomayor, J.A. Variations in essential oil, phenolic compounds, and antioxidant activity of tunisian cultivated Salvia officinalis L. J. Agric. Food Chem. 2009, 57, 10349–10356. [Google Scholar] [CrossRef]
- Tzortzakis, N.G. Maintaining postharvest quality of fresh produce with volatile compounds. Innov. Food Sci. Emerg. Technol. 2007, 8, 111–116. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Xylia, P.; Chrysargyris, A. Sage essential oil improves the effectiveness of Aloe vera gel on postharvest quality of tomato fruit. Agronomy 2019, 9, 635. [Google Scholar] [CrossRef]
- Xylia, P.; Clark, A.; Chrysargyris, A.; Romanazzi, G.; Tzortzakis, N. Quality and safety attributes on shredded carrots by using Origanum majorana and ascorbic acid. Postharvest Biol. Technol. 2019, 155, 120–129. [Google Scholar] [CrossRef]
- Teerarak, M.; Laosinwattana, C. Essential oil from ginger as a novel agent in delaying senescence of cut fronds of the fern (Davallia solida (G. Forst.) Sw.). Postharvest Biol. Technol. 2019, 156, 110927. [Google Scholar] [CrossRef]
- Hassani, R.N.; Haghi, D.Z.; Pouya, Z. The effect of clove oil as preservative solution on vase life of cut rose flower. Int. J. Adv. Sci. Eng. Technol. 2017, 5, 28–30. [Google Scholar]
- Cindi, M.D.; Soundy, P.; Romanazzi, G.; Sivakumar, D. Different defense responses and brown rot control in two Prunus persica cultivars to essential oil vapours after storage. Postharvest Biol. Technol. 2016, 119, 9–17. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Caputo, L.; De Martino, L.; Gruľová, D.; Zheljazkov, V.Z.; De Feo, V.; Camele, I. Biological investigations of essential oils extracted from three Juniperus species and evaluation of their antimicrobial, antioxidant and cytotoxic activities. J. Appl. Microbiol. 2020, 129, 1261–1271. [Google Scholar] [CrossRef]
- Ben-Jabeur, M.; Ghabri, E.; Myriam, M.; Hamada, W. Thyme essential oil as a defense inducer of tomato against gray mold and Fusarium wilt. Plant Physiol. Biochem. 2015, 94, 35–40. [Google Scholar] [CrossRef]
- Nikkhah, M.; Hashemi, M. Boosting antifungal effect of essential oils using combination approach as an efficient strategy to control postharvest spoilage and preserving the jujube fruit quality. Postharvest Biol. Technol. 2020, 164, 111159. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). USDA, Agricultural Marketing Service; USDA: Washington, DC, USA, 1991.
- Zapata, P.; Guillen, F.; Martinez-Romero, D.; Castillo, S.; Valero, D.; Serrano, M. Use of alginate or zein as edible coatings to delay postharvest ripening process and to maintain tomato (Solanum lycopersicon Mill) quality. J. Sci. Food Agric. 2008, 88, 1287–1293. [Google Scholar] [CrossRef]
- Cara, B.; Giovannoni, J.J. Molecular biology of ethylene during tomato fruit development and maturation. Plant Sci. 2008, 175, 106–113. [Google Scholar] [CrossRef]
- Tiecher, A.; de Paula, L.A.; Chaves, F.C.; Rombaldi, C.V. UV-C effect on ethylene, polyamines and the regulation of tomato fruit ripening. Postharvest Biol. Technol. 2013, 86, 230–239. [Google Scholar] [CrossRef]
- Chomchalow, S.; El Assi, N.M.; Sargent, S.A.; Brecht, J.K. Fruit maturity and timing of ethylene treatment affect storage performance of green tomatoes at chilling and nonchilling temperatures. Horttechnology 2002, 12, 104–114. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Chrysargyris, A.; Sivakumar, D.; Loulakakis, K. Vapour or dipping applications of methyl jasmonate, vinegar and sage oil for pepper fruit sanitation towards grey mould. Postharvest Biol. Technol. 2016, 118, 120–127. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nagata, Y. Postharvest ethanol vapor treatment of tomato fruit stimulates gene expression of ethylene biosynthetic enzymes and ripening related transcription factors, although it suppresses ripening. Postharvest Biol. Technol. 2019, 152, 118–126. [Google Scholar] [CrossRef]
- Wang, K.L.C.; Li, H.; Ecker, J.R. Ethylene biosynthesis and signaling networks. Plant Cell 2002, 14, 131–151. [Google Scholar] [CrossRef]
- Colombié, S.; Beauvoit, B.; Nazaret, C.; Bénard, C.; Vercambre, G.; Le Gall, S.; Biais, B.; Cabasson, C.; Maucourt, M.; Bernillon, S.; et al. Respiration climacteric in tomato fruits elucidated by constraint-based modelling. New Phytol. 2017, 213, 1726–1739. [Google Scholar] [CrossRef]
- de Jesús Salas-Méndez, E.; Vicente, A.; Pinheiro, A.C.; Ballesteros, L.F.; Silva, P.; Rodríguez-García, R.; Hernández-Castillo, F.D.; de Lourdes Virginia Díaz-Jiménez, M.; Flores-López, M.L.; Villarreal-Quintanilla, J.Á.; et al. Application of edible nanolaminate coatings with antimicrobial extract of Flourensia cernua to extend the shelf-life of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2019, 150, 19–27. [Google Scholar]
- Grierson, D.; Kader, A.A. Fruit ripening and quality. In The Tomato Crop; Chapman and Hall: London, UK, 1986; pp. 241–280. [Google Scholar]
- Yaman, Ö.; Bayoindirli, L. Effects of an edible coating and cold storage on shelf-life and quality of cherries. LWT-Food Sci. Technol. 2002, 35, 146–150. [Google Scholar] [CrossRef]
- Pan, J.C.; Bhowmilk, S.R. Shelf-life of mature green tomatoes stored in controlled atmosphere and high humidity. J. Food Sci. 1992, 57, 948–953. [Google Scholar]
- Aktas, H.; Bayindir, D.; Dilmaçünal, T.; Koyuncu, M.A. The effects of minerals, ascorbic acid, and salicylic acid on the bunch quality of tomatoes (Solanum lycopersicum) at high and low temperatures. HortScience 2012, 47, 1478–1483. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdeldaym, E.A.; Ali, M.R.; Mohamed, R.M.; Bob, R.I.; Abdelgawad, K.F. Effect of some citrus essential oils on post-harvest shelf life and physicochemical Quality of Strawberries during Cold Storage. Agronomy 2020, 10, 1466. [Google Scholar] [CrossRef]
- Martínez, K.; Ortiz, M.; Albis, A.; Castañeda, C.G.G.; Valencia, M.E.; Tovar, C.D.G. The effect of edible chitosan coatings incorporated with thymus capitatus essential oil on the shelf-life of strawberry (Fragaria x ananassa) during cold storage. Biomolecules 2018, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Dhital, R.; Mora, N.B.; Watson, D.G.; Kohli, P.; Choudhary, R. Efficacy of limonene nano coatings on post-harvest shelf life of strawberries. LWT 2018, 97, 124–134. [Google Scholar]
- Camele, I.; Elshafie, H.S.; Caputo, L.; De Feo, V. Anti-quorum Sensing and Antimicrobial Effect of Mediterranean Plant Essential Oils Against Phytopathogenic Bacteria. Front. Microbiol. 2019, 10, 2619. [Google Scholar]
- Elshafie, H.S.; Camele, I. An overview of the biological effects of some mediterranean essential oils on human health. Biomed Res. Int. 2017, 2017, 9268468. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sakr, S.; Mang, S.M.; De Feo, V.; Camele, I. Antimicrobial activity and chemical composition of three essential oils extracted from Mediterranean aromatic plants. J. Med. Food. 2016, 19, 1096–1103. [Google Scholar] [CrossRef]
- Morcia, C.; Malnati, M.; Terzi, V. In vitro antifungal activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food Addit. Contam. Part A 2012, 29, 415–422. [Google Scholar]
- Xylia, P.; Chrysargyris, A.; Ahmed, Z.F.; Tzortzakis, Ν. Application of Rosemary and Eucalyptus Essential Oils and Their Main Component on the Preservation of Apple and Pear Fruits. Horticulture 2021, 7, 479. [Google Scholar] [CrossRef]
- Mokbel, A.A.; Alharbi, A.A. Antifungal effects of basil and camphor essential oils against Aspergillus flavus and A. parasiticus. Aust. J. Crop Sci. 2015, 9, 532–537. [Google Scholar]
- Da Silva, A.C.R.; Lopes, P.M.; de Azevedo, M.M.B.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological Activities of α-Pinene and β-Pinene Enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [PubMed]
- Jobling, J.; Pradhan, R.; Morris, S.C.; Mitchell, L.; Rath, A.C. The effect of ReTain plant growth regulator [aminoethoxyvinylglycine (AVG)] on the postharvest storage life of ‘Tegan Blue’ plums. Aust. J. Exp. Agric. 2003, 43, 515–518. [Google Scholar] [CrossRef]
- Onelli, E.; Ghiani, A.; Gentili, R.; Serra, S.; Musacchi, S.; Citterio, S. Specific changes of exocarp and mesocarp occurring during softening differently affect firmness in melting (MF) and non melting flesh (NMF) fruits. PLoS ONE 2015, 10, e0145341. [Google Scholar] [CrossRef][Green Version]
- Saladié, M.; Matas, A.J.; Isaacson, T.; Jenks, M.A.; Goodwin, S.M.; Niklas, K.J.; Xiaolin, R.; Labavitch, J.M.; Shackel, K.A.; Fernie, A.R.; et al. A reevaluation of the key factors that influence tomato fruit softening and integrity. Plant Physiol. 2007, 144, 1012–1028. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.R. Concise Reviews/Hypotheses in Food Science Effects of Production and Processing Factors on Major Fruit and Vegetable Antioxidants. J. Food Sci. 2005, 70, R11–R19. [Google Scholar] [CrossRef]
- Jagadeesh, S.L.; Charles, M.T.; Gariepy, Y.; Goyette, B.; Raghavan, G.S.V.; Vigneault, C. Influence of Postharvest UV-C Hormesis on the Bioactive Components of Tomato during Post-treatment Handling. Food Bioprocess Technol. 2011, 4, 1463–1472. [Google Scholar] [CrossRef]
- Toor, R.K.; Savage, G.P. Antioxidant activity in different fractions of tomatoes. Food Res. Int. 2005, 38, 487–494. [Google Scholar] [CrossRef]
- Andrews, P.K.; Fahy, D.A.; Foyer, C.H. Relationships between fruit exocarp antioxidants in the tomato (Lycopersicon esculentum) high pigment-1 mutant during development. Physiol. Plant. 2004, 120, 519–528. [Google Scholar] [CrossRef]
- Beecher, G. Nutrient content of tomatoes and tomato products. Proc. Soc. Exp. Biol. Med. 1998, 218, 98–100. [Google Scholar] [CrossRef]
- Scalfi, L.; Fogliano, V.; Pentangelo, A.; Graziani, G.; Giordano, I.; Ritieni, A. Antioxidant activity and general fruit characteristics in different ecotypes of Corbarini small tomatoes. J. Agric. Food Chem. 2000, 48, 1363–1366. [Google Scholar] [CrossRef]
- Arai, Y.; Watanabe, S.; Kimira, M.; Shimoi, K.; Mochizuki, R.; Kinae, N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr. 2000, 130, 2243–2250. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture (USDA). USDA Agricultural Handbook No. 18; U.S. Government Printing Office: Washington, DC, USA, 1951.
- Tzortzakis, N.; Singleton, I.; Barnes, J. Impact of low-level atmospheric ozone-enrichment on black spot and anthracnose rot of tomato fruit. Postharvest Biol. Technol. 2008, 47, 1–9. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G.W.; AOAC International. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. Nippon Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Nikou, A.; Tzortzakis, N. Effectiveness of Aloe vera gel coating for maintaining tomato fruit quality. N. Z. J. Crop Hortic. Sci. 2016, 44, 203–217. [Google Scholar] [CrossRef]
- Tzortzakis, N.G.; Tzanakaki, K.; Economakis, C.D. Effect of origanum oil and vinegar on the maintenance of postharvest quality of tomato. Food Nutr. Sci. 2011, 2, 974–982. [Google Scholar] [CrossRef][Green Version]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- De Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; De Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
 ), EO exposure (→). Tomato exposed to air
), EO exposure (→). Tomato exposed to air  , tomato exposed to EOs
, tomato exposed to EOs  .
.
 ), EO exposure (→). Tomato exposed to air
), EO exposure (→). Tomato exposed to air  , tomato exposed to EOs
, tomato exposed to EOs  .
.


| Fruit Decay | |||||||
|---|---|---|---|---|---|---|---|
| Treatments | Breaker Stage | Red Stage | |||||
| 0 Days | 7 Days | 14 Days | 0 Days | 7 Days | 14 Days | ||
| S/M | Control | 1.00 ± 0.00 * | 1.17 ± 0.12 a | 2.05 ± 0.15 a | 1.00 ± 0.00 * | 1.16 ± 0.10 a | 2.75 ± 0.22 a |
| S | EO-50 μL L−1 | 1.03 ± 0.04 a | 1.10 ± 0.15 b | 1.06 ± 0.07 a | 1.25 ± 0.10 b | ||
| S | EO-500 μL L−1 | 1.00 ± 0.00 a | 1.00 ± 0.00 b | 1.00 ± 0.00 a | 1.00 ± 0.00 b | ||
| M | EO-50 μL L−1 | 1.15 ± 0.10 b | 1.40 ± 0.20 b | ||||
| M | EO-500 μL L−1 | 1.30 ± 0.25 b | 1.95 ± 0.35 ab | ||||
| Breaker Tomatoes | Red Tomatoes | |||||
|---|---|---|---|---|---|---|
| Control | EO-50 μL L−1 | EO-500 μL L−1 | Control | EO-50 μL L−1 | EO-500 μL L−1 | |
| Appearance | 61.8 ± 4.6 a | 64.4 ± 4.9 a | 40.0 ± 4.2 b | 71.8 ± 3.4 a | 78.8 ± 3.9 a | 38.5 ± 4.4 b |
| Color | 70.1 ± 5.8 a | 52.8 ± 5.4 b | 50.1 ± 5.8 b | 74.2 ± 4.9 a | 79.5 ± 3.3 a | 51.4 ± 5.0 b |
| Aroma | 70.0 ± 5.7 a | 57.0 ± 4.5 b | 24.2 ± 2.2 c | 68.5 ± 4.1 a | 67.1 ± 4.5 a | 27.1 ± 2.6 b |
| Texture | 65.8 ± 5.7 a | 72.7 ± 6.4 a | 34.2 ± 3.8 b | 65.2 ± 3.8 a | 74.0 ± 3.5 a | 30.0 ± 3.4 b |
| Sweetness | 45.7 ± 3.8 a | 38.5 ± 3.9 ab | 30.0 ± 4.1 b | 67.1 ± 5.7 a | 59.2 ± 3.8 a | 32.8 ± 4.5 b |
| Satisfaction | 61.4 ± 3.9 a | 49.7 ± 5.3 b | 22.8 ± 1.9 c | 68.5 ± 4.0 a | 55.7 ± 5.2 b | 21.4 ± 1.4 c |
| Marketability | 67.1 ± 5.7 a | 54.7 ± 5.8 b | 21.4 ± 1.4 c | 80.0 ± 5.5 a | 64.2 ± 7.6 b | 20.0 ± 0.0 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrysargyris, A.; Rousos, C.; Xylia, P.; Tzortzakis, N. Vapour Application of Sage Essential Oil Maintain Tomato Fruit Quality in Breaker and Red Ripening Stages. Plants 2021, 10, 2645. https://doi.org/10.3390/plants10122645
Chrysargyris A, Rousos C, Xylia P, Tzortzakis N. Vapour Application of Sage Essential Oil Maintain Tomato Fruit Quality in Breaker and Red Ripening Stages. Plants. 2021; 10(12):2645. https://doi.org/10.3390/plants10122645
Chicago/Turabian StyleChrysargyris, Antonios, Charalampos Rousos, Panayiota Xylia, and Nikolaos Tzortzakis. 2021. "Vapour Application of Sage Essential Oil Maintain Tomato Fruit Quality in Breaker and Red Ripening Stages" Plants 10, no. 12: 2645. https://doi.org/10.3390/plants10122645
APA StyleChrysargyris, A., Rousos, C., Xylia, P., & Tzortzakis, N. (2021). Vapour Application of Sage Essential Oil Maintain Tomato Fruit Quality in Breaker and Red Ripening Stages. Plants, 10(12), 2645. https://doi.org/10.3390/plants10122645








