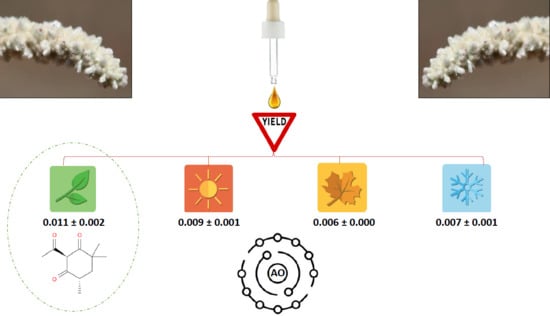
Yield and In Vitro Antioxidant Potential of Essential Oil from Aerva javanica (Burm. f.) Juss. ex Schul. Flower with Special Emphasis on Seasonal Changes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material Identification
2.2. Study Location and Plant Collection Schedules
2.3. Soil Physical and Chemical Analysis
2.4. Statistics and Experimental Design
2.5. EO Extraction by Hydrodistillation
2.5.1. Sample Preparation
2.5.2. Extraction Conditions and Yield Determination
2.6. Antioxidant Activity
2.6.1. Sample Preparation
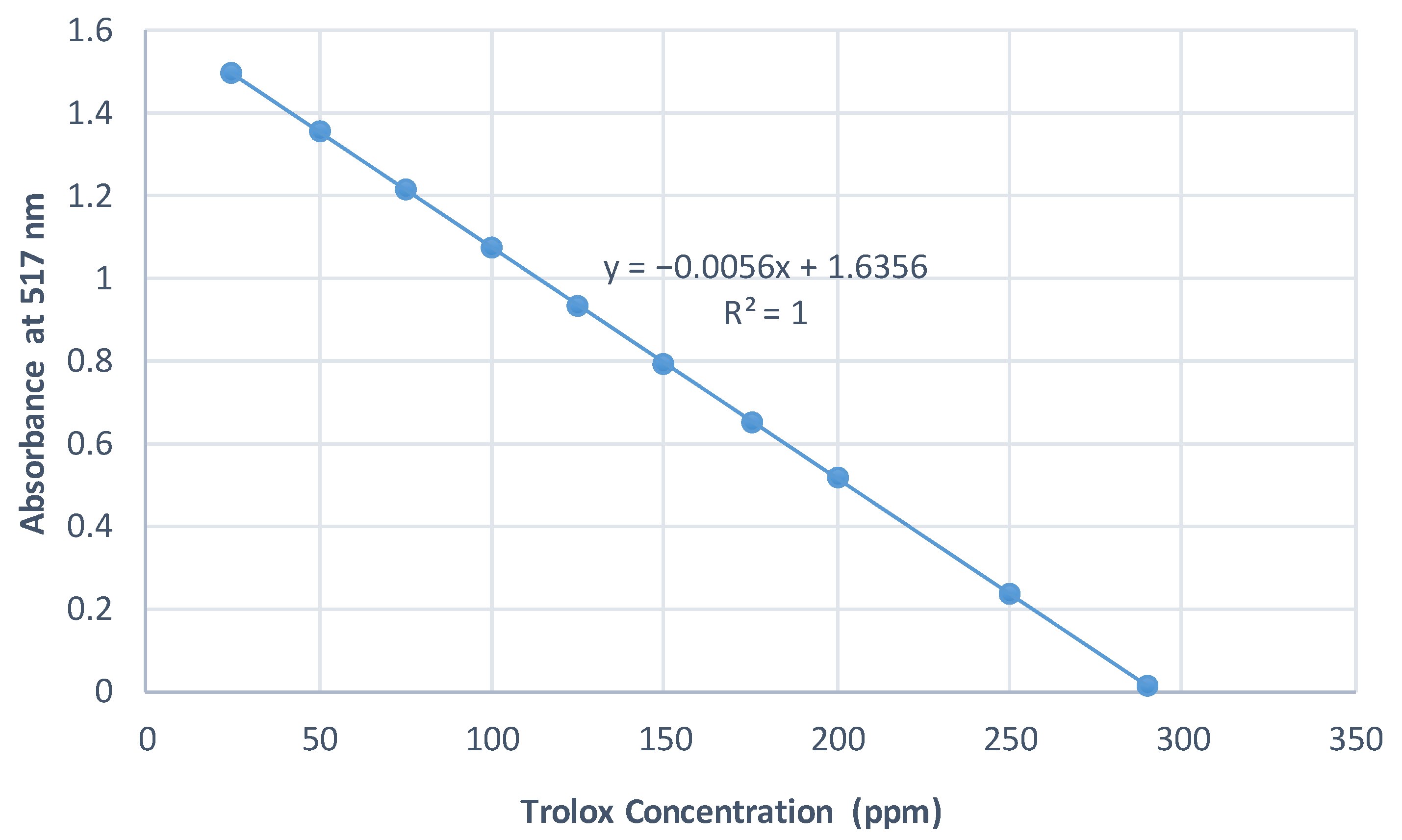
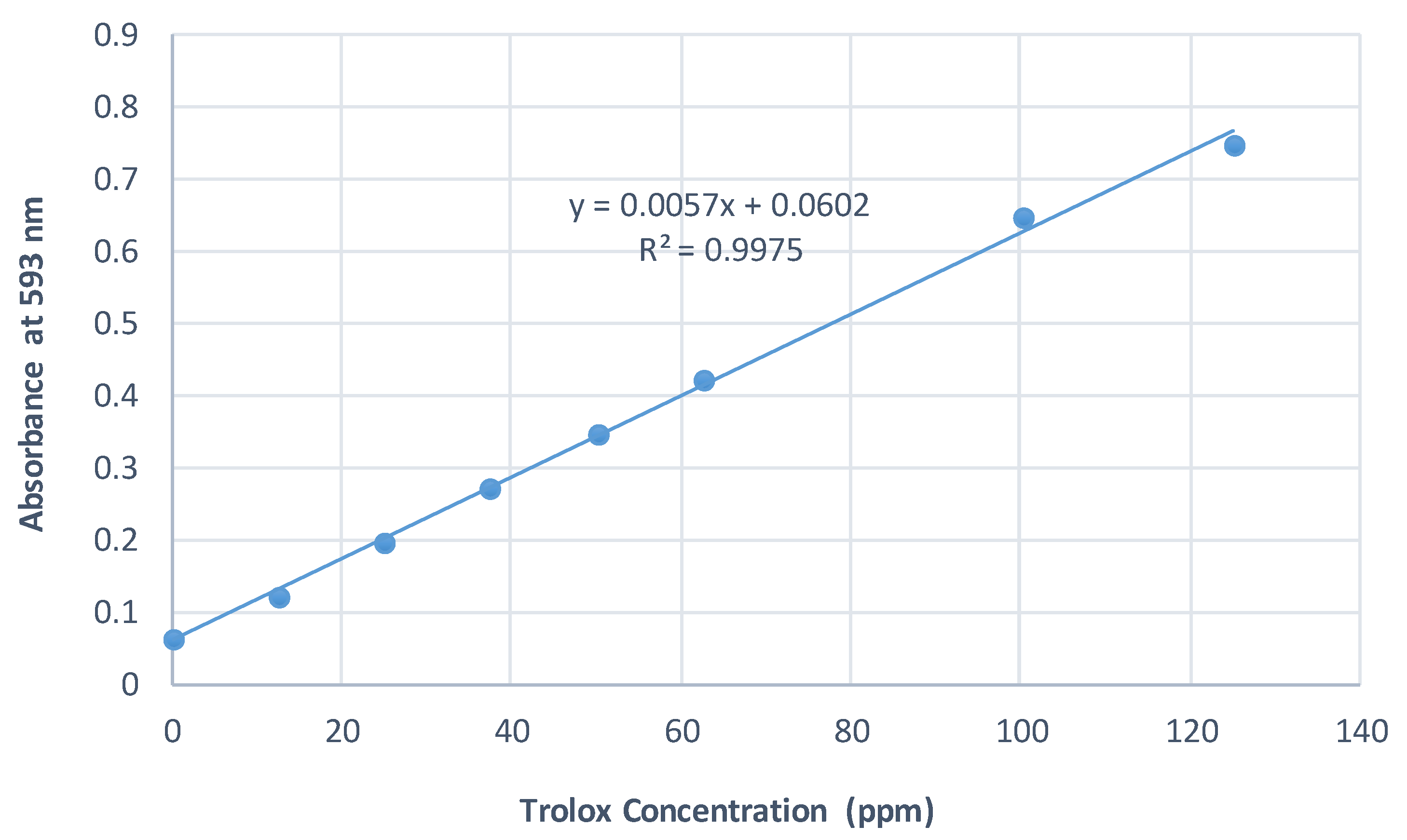
2.6.2. Standard Curves
2.7. Chromatographic Analysis
GC-MS Analysis
2.8. Plant Morphology by SEM Photos
2.8.1. Chemicals
2.8.2. Sample Preparation Protocol
3. Results and Discussion
3.1. EO Extraction by Hydrodistillation
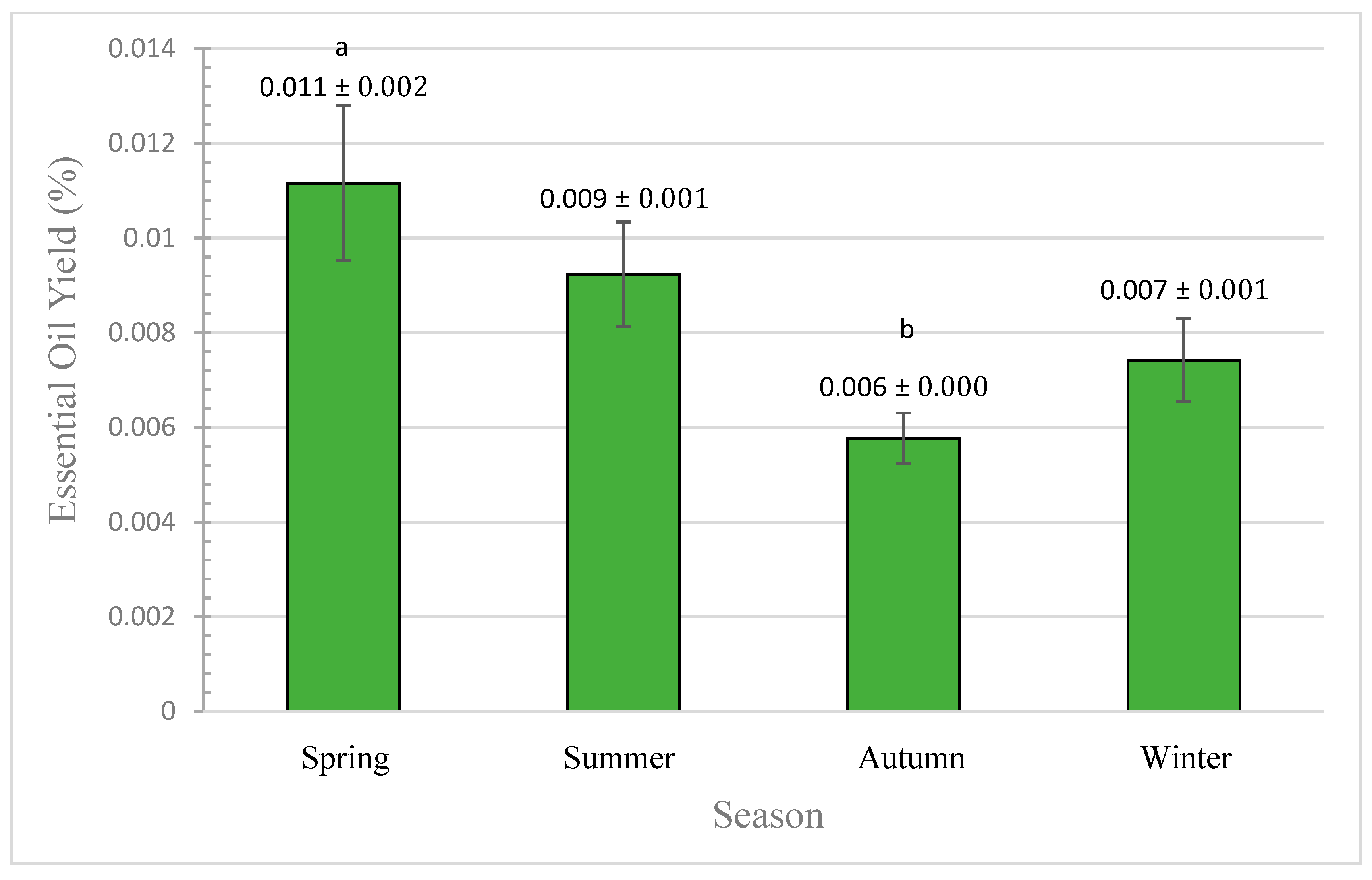
3.2. Effect of Seasonal Variation
3.2.1. Quantitative Analysis (by Yield)
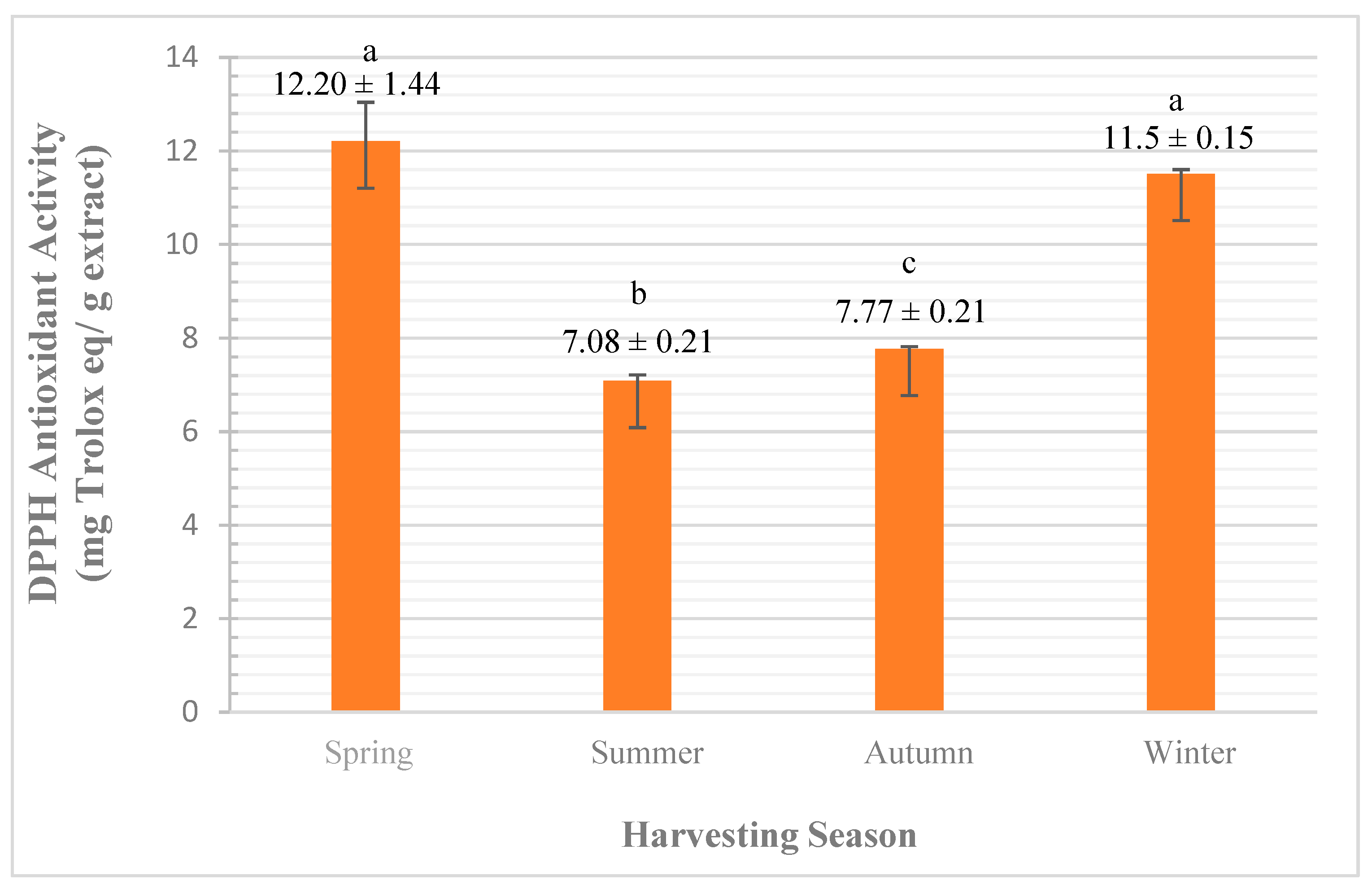
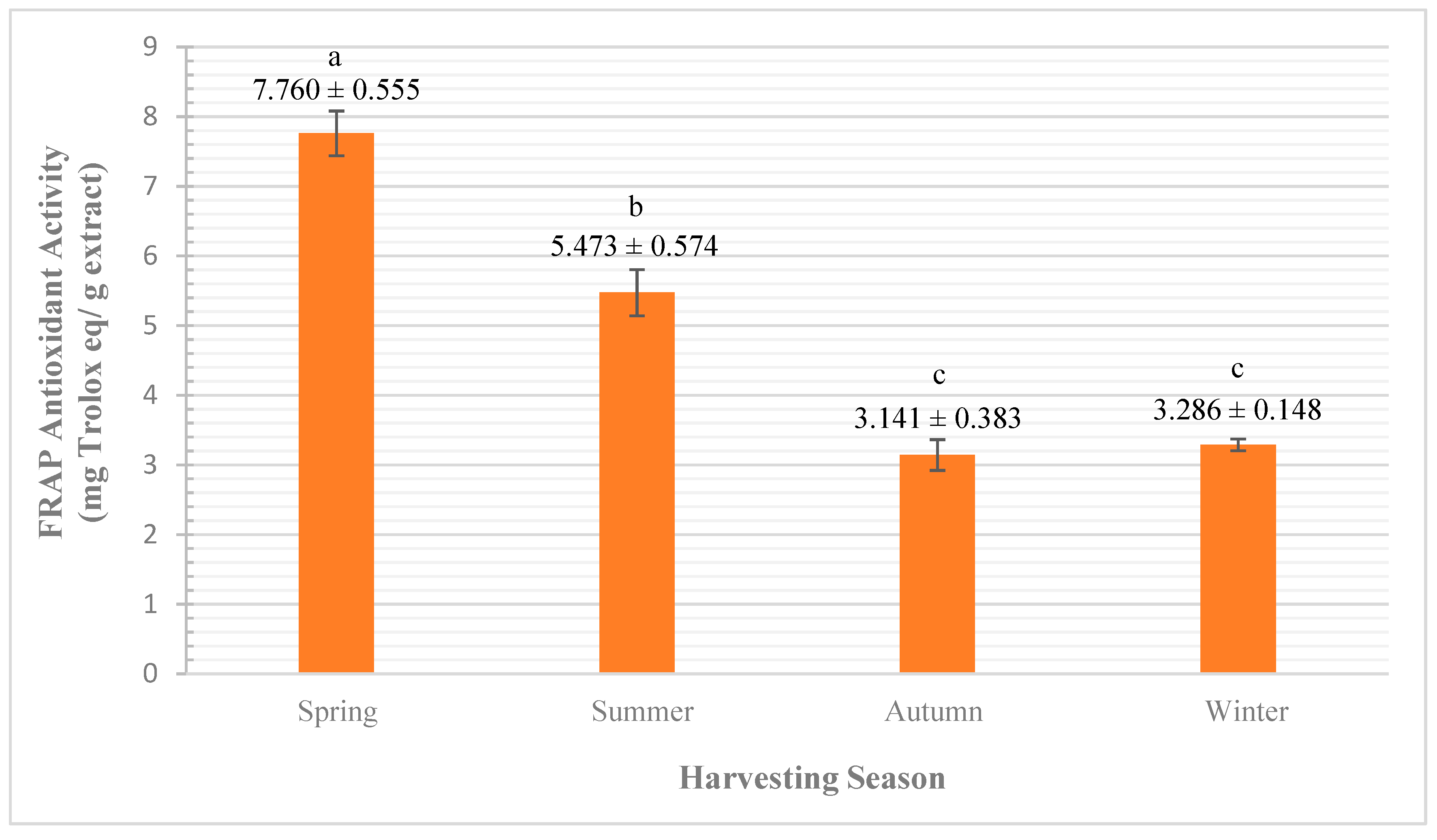
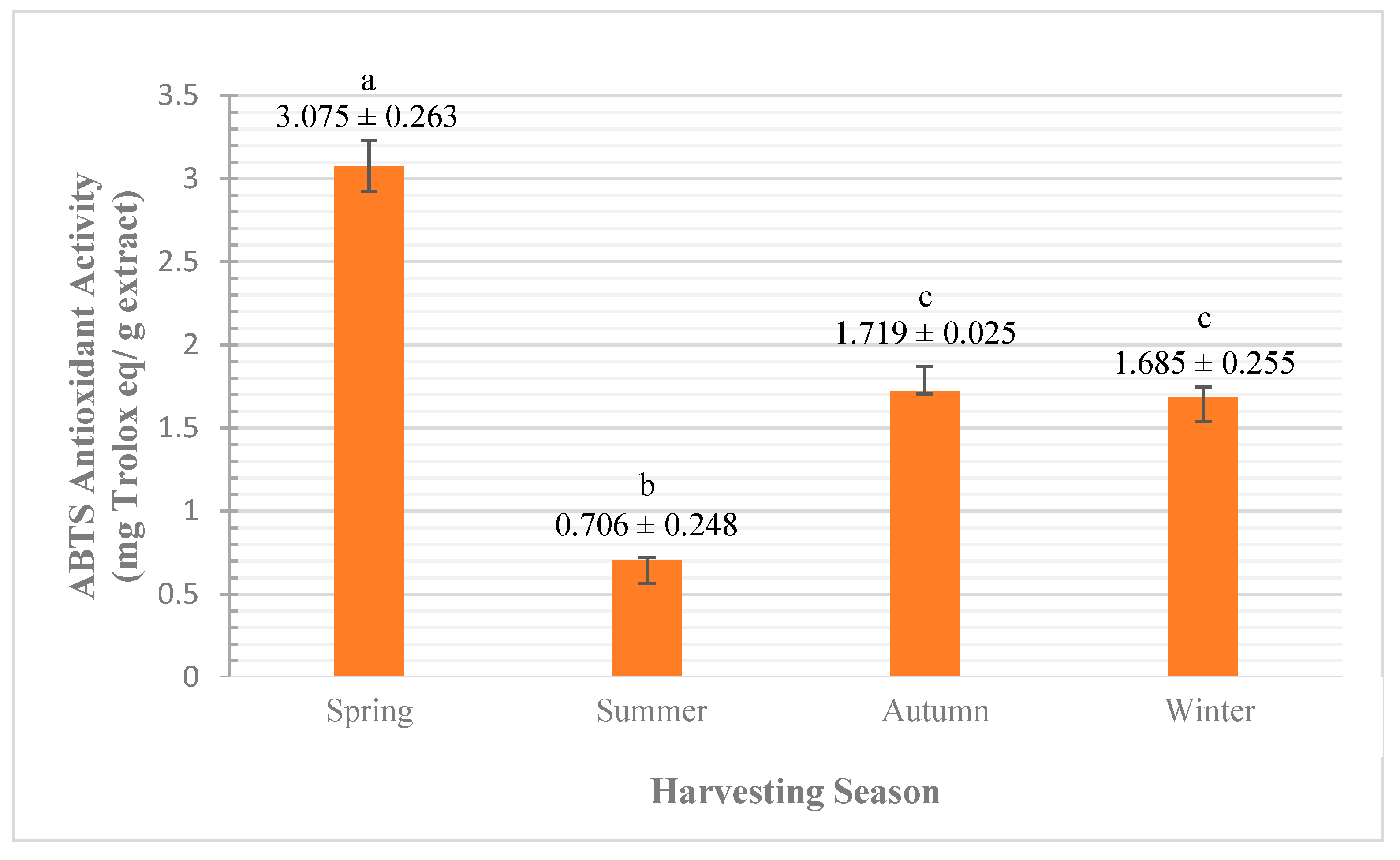
3.2.2. Qualitative Analysis (by Antioxidant Activity)
DPPH Assay
FRAP Assay
ABTS Assay
Correlation between Antioxidant Assays
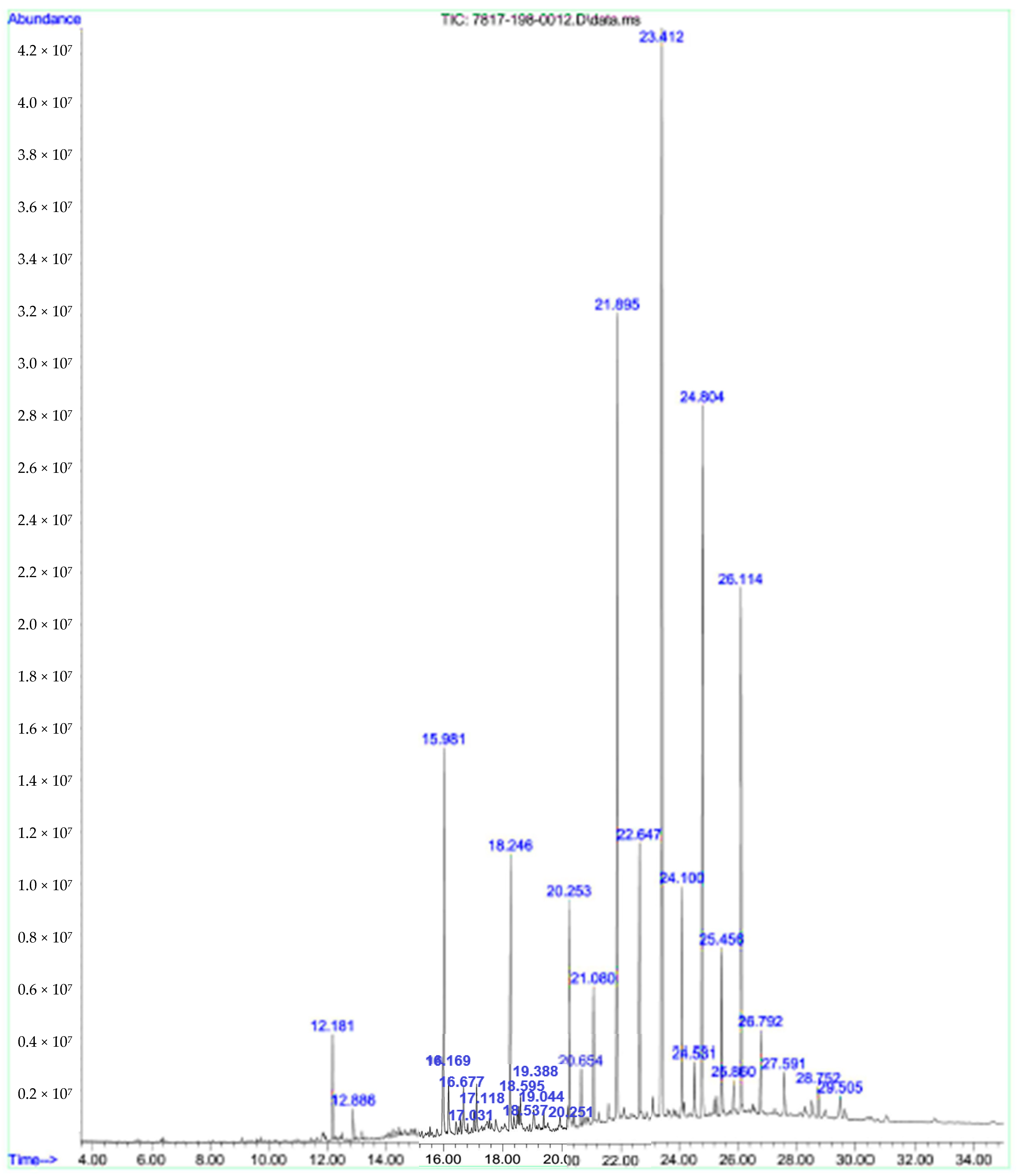
3.3. Chemical Composition (by GC-MS)
3.4. Morphology by SEM
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jongbloed, M.; Feulner, G.; Böer, B.; Western, A.R. The Comprehensive Guide to the Wild Flowers of the United Arab Emirates; Environmental Research and Wildlife Development Agency: Abu Dhabi, United Arab Emirates, 2003. [Google Scholar]
- Shahin, S. The United Arab Emirates Indigenous Essential Oil-bearing Plants: Essential oils of Aerva javanica and Cleome ambolyocarpa Grown in Sandy Soils under Arid Conditions. Ph.D. Thesis, United Arab Emirates University (UAEU), Al Ain, United Arab Emirates, 2018. [Google Scholar]
- Saleem, M.; Parveen, S.; Riaz, N.; Tahir, M.N.; Ashraf, M.; Afzal, I.; Jabbar, A. New bioactive natural products from Launaea nudicaulis. Phytochem. Lett. 2012, 5, 793–799. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Compounds by Gas Chromatography and Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2009. [Google Scholar]
- Hussain, A.I.; Anwar, F.; Sherazi, S.T.H.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Omer, E.A.; Abou Hussein, E.A.; Hendawy, S.F.; Ezz, E.D.; Azza, A.; El Gendy, A.G. Effect of soil type and seasonal variation on growth, yield, essential oil and artemisinin content of Artemisia annua L. Int. Res. J. Hortic. 2013, 1, 15–27. [Google Scholar] [CrossRef]
- Villa-Ruano, N.; Pacheco-Hernández, Y.; Cruz-Durán, R.; Lozoya-Gloria, E. Volatiles and seasonal variation of the essential oil composition from the leaves of Clinopodium macrostemum var. laevigatum and its biological activities. Ind. Crops Prod. 2015, 77, 741–747. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Laribi, B.; Bettaieb, I.; Kouki, K.; Sahli, A.; Mougou, A.; Marzouk, B. Water deficit effects on caraway (Carum carvi L.) growth, essential oil and fatty acid composition. Ind. Crops Prod. 2009, 30, 372–379. [Google Scholar] [CrossRef]
- Tátrai, Z.A.; Sanoubar, R.; Pluhár, Z.; Mancarella, S.; Orsini, F.; Gianquinto, G. Morphological and physiological plant responses to drought stress in Thymus citriodorus. Int. J. Agron. 2016, 2016, 4165750. [Google Scholar] [CrossRef] [Green Version]
- Shahin, S.M.; Kurup, S.; Jaleel, A.; Alyafei, M.A.M. Chemical composition of Cleome amblyocarpa Barr. & Murb. essential oil under different irrigation levels in sandy soils with antioxidant activity. J. Essen. Oil Bear. Plants 2018, 21, 1235–1256. [Google Scholar]
- Samejo, M.Q.; Memon, S.; Bhanger, M.I.; Khan, K.M. Chemical compositions of the essential oil of Aerva javanica leaves and stems. Pak. J. Anal. Env. Chem. 2012, 13, 48–52. [Google Scholar]
- Samejo, M.Q.; Memon, S.; Bhanger, M.I.; Khan, K.M. Comparison of chemical composition of Aerva javanica seed essential oils obtained by different extraction methods. Pak. J. Pharm. Sci. 2013, 26, 757–760. [Google Scholar]
- Hemmati, C.; Nikooei, M.; Bertaccini, A. Identification and transmission of phytoplasmas and their impact on essential oil composition in Aerva javanica. 3 Biotech 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Al-Shehri, M.; Moustafa, M. Anticancer, antibacterial, and phytochemicals derived from extract of Aerva javanica (Burm. f.) Juss. ex Schult. grown naturally in Saudi Arabia. Trop. Conserv. Sci. 2019, 12, 1940082919864262. [Google Scholar] [CrossRef] [Green Version]
- Robertshawe, P. Medicinal plants in Australia Volume 1: Bush Pharmacy. J. Aust. Trad. Med. Soc. 2011, 17, 173–174. [Google Scholar]
- Selvaraj, M.; Kandaswamy, M.; Park, D.W.; Ha, C.S. Highly efficient and clean synthesis of verbenone over well ordered two-dimensional mesoporous chromium silicate catalysts. Catal. Today 2010, 158, 286–295. [Google Scholar] [CrossRef]
- Huber, D.P.W.; Borden, J.H. Protection of lodgepole pines from mass attack by mountain pine beetle, Dendroctonus ponderosae, with nonhost angiosperm volatiles and verbenone. Entomol. Exp. Appl. 2001, 99, 131–141. [Google Scholar] [CrossRef]
- Akpuaka, A.; Ekwenchi, M.M.; Dashak, D.A.; Dildar, A. Biological activities of characterized isolates of n-hexane extract of Azadirachta indica A. Juss (Neem) leaves. N. Y. Sci. J. 2013, 6, 119–124. [Google Scholar]
- Gonzales, W.L.; Negritto, M.A.; Suarez, L.H.; Gianoli, E. Induction of glandular and non-glandular trichomes by damage in leaves of Madia sativa under contrasting water regimes. Acta Oecol. 2008, 33, 128–132. [Google Scholar] [CrossRef]



| Plant Part | Drying Methods | Particle Size | Harvesting Season |
|---|---|---|---|
| Flowers | Air-Drying: Shaded at 25 °C | 2 mm | Spring (Mid-April) Summer (First of June) Autumn (End of October) Winter (Mid-January) |
| DPPH | FRAP | ABTS | |
|---|---|---|---|
| DPPH | NA | +0.933 | +0.994 |
| FRAP | +0.933 | NA | +0.968 |
| ABTS | +0.994 | +0.968 | NA |
| Antioxidant Assays | DPPH | FRAP | ABTS |
|---|---|---|---|
| DPPH | NA | +1 * | +1 * |
| FRAP | +1 * | NA | +1 * |
| ABTS | +1 * | +1 * | NA |
| Antioxidant Assays | DPPH | FRAP | ABTS |
|---|---|---|---|
| DPPH | NA | +1 * | +1 * |
| FRAP | +1 * | NA | +1 * |
| ABTS | +1 * | +1 * | NA |
| Antioxidant Assays | DPPH | FRAP | ABTS |
|---|---|---|---|
| DPPH | NA | +1 * | +1 * |
| FRAP | +1 * | NA | +1 * |
| ABTS | +1 * | +1 * | NA |
| No. | Compound | RT | RI | Percent Composition (%) Spring |
|---|---|---|---|---|
| 1 | 2E,4E-Octadienol | 12.184 | 1113 | 1.35 |
| 2 | cis-Limonene oxide | 12.883 | 1132 | 0.42 |
| 3 | Verbenone | 15.973 | 1204 | 6.06 |
| 4 | (2E)-Octenol acetate | 16.171 | 1208 | 1.14 |
| 5 | endo-Fenchyl acetate | 16.573 | 1218 | ----- |
| 6 | Methyl-(2E)-nonenoate | 16.678 | 1221 | 1.20 |
| 7 | nor-Davanone | 17.033 | 1228 | 0.39 |
| 8 | Thymol methyl ether | 17.115 | 1132 | 0.84 |
| 9 | tetrahydro-Linalool acetate | 17.121 | 1231 | ----- |
| 10 | Pulegone | 17.261 | 1233 | ----- |
| 11 | Carvotanacetone | 17.768 | 1244 | ----- |
| 12 | (4Z)-Decen-1-ol | 18.246 | 1255 | 4.07 |
| 13 | (2E)-Decenal | 18.485 | 1260 | 0.44 |
| 14 | cis-Chrysanthenyl acetate | 18.537 | 1261 | 0.43 |
| 15 | Geranial | 18.595 | 1264 | 0.97 |
| 16 | trans-Carvone oxide | 19.044 | 1273 | 0.71 |
| 17 | (2Z)-Hexenyl valerate | 19.388 | 1282 | 0.39 |
| 18 | n-Tridecane | 20.245 | 1300 | 3.79 |
| 19 | Isoamyl bemzyl ether | 20.653 | 1310 | 0.95 |
| 20 | 2E,4E-Decadienol | 21.078 | 1319 | 2.08 |
| 21 | 3-oxo-ρ-Menth-1-en-7-al | 21.580 | 1330 | ----- |
| 22 | Evodone | 21.877 | 1137 | 14.77 |
| 23 | 4-Hydroxybenzaldehyde | 22.641 | 1355 | 4.56 |
| 24 | Angustione | 23.392 | 1372 | 20.72 |
| 25 | (E)-Methyl cinnamate | 23.556 | 1376 | ----- |
| 26 | 1-Tetradecene | 24.092 | 1388 | 3.98 |
| 27 | α-Chamipinene | 24.448 | 1396 | ----- |
| 28 | Cyperene | 24.529 | 1398 | 1.16 |
| 29 | methyl-Cresol acetate | 24.791 | 1403 | 12.49 |
| 30 | (2E,4E)-Undecadienal | 25.281 | 1415 | ----- |
| 31 | (E)-Trimenal | 25.450 | 1419 | 2.77 |
| 32 | cis-Thujopsene | 25.858 | 1429 | 0.80 |
| 33 | γ-Elemene | 26.103 | 1434 | 9.15 |
| 34 | α-Humulene | 26.791 | 1452 | 1.65 |
| 35 | (2E)-Dodecen-1-ol | 27.589 | 1469 | 1.05 |
| 36 | α-Selinene | 28.755 | 1498 | 0.88 |
| 37 | Menthyl isovalerate | 29.507 | 1516 | 0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahin, S.M.; Jaleel, A.; Alyafei, M.A.M. Yield and In Vitro Antioxidant Potential of Essential Oil from Aerva javanica (Burm. f.) Juss. ex Schul. Flower with Special Emphasis on Seasonal Changes. Plants 2021, 10, 2618. https://doi.org/10.3390/plants10122618
Shahin SM, Jaleel A, Alyafei MAM. Yield and In Vitro Antioxidant Potential of Essential Oil from Aerva javanica (Burm. f.) Juss. ex Schul. Flower with Special Emphasis on Seasonal Changes. Plants. 2021; 10(12):2618. https://doi.org/10.3390/plants10122618
Chicago/Turabian StyleShahin, Suzan Marwan, Abdul Jaleel, and Mohammed Abdul Muhsen Alyafei. 2021. "Yield and In Vitro Antioxidant Potential of Essential Oil from Aerva javanica (Burm. f.) Juss. ex Schul. Flower with Special Emphasis on Seasonal Changes" Plants 10, no. 12: 2618. https://doi.org/10.3390/plants10122618
APA StyleShahin, S. M., Jaleel, A., & Alyafei, M. A. M. (2021). Yield and In Vitro Antioxidant Potential of Essential Oil from Aerva javanica (Burm. f.) Juss. ex Schul. Flower with Special Emphasis on Seasonal Changes. Plants, 10(12), 2618. https://doi.org/10.3390/plants10122618