Abstract
Postharvest diseases of fruits caused by phytopathogens cause losses up to 50% of global production. Phytopathogens control is performed with synthetic fungicides, but the application causes environmental contamination problems and human and animal health in addition to generating resistance. Yeasts are antagonist microorganisms that have been used in the last years as biocontrol agents and in sustainable postharvest disease management in fruits. Yeast application for biocontrol of phytopathogens has been an effective action worldwide. This review explores the sustainable use of yeasts in each continent, the main antagonistic mechanisms towards phytopathogens, their relationship with OMIC sciences, and patents at the world level that involve yeast-based-products for their biocontrol.
1. Introduction
Fruit is an important resource in human diet because of its contribution in vitamins, minerals, organic acids, fiber, among others [1]. Moreover, obesity, cardiovascular, cognitive, skin, eye, lung, and bone diseases could be prevented through regular fruit intake [2,3]. Nowadays, the consumer demands fruit with a high-quality standard, both in appearance and in nutritional content [4,5]. However, postharvest fruit quality is affected by various factors, especially fungal diseases [6], which decrease its organoleptic properties and cause significant losses during storage, affecting up to 25% of total production in industrialized countries and more than 50% in developing countries [7,8].
The main strategy to control fungal infections at the postharvest level in fruit is the application of synthetic fungicides [9]. Nevertheless, these products have negative effects on human, animal, and environmental health [10,11] and induce resistance in phytopathogens [12,13].
The rise of biotechnology in the last decade has made biocontrol one of the most studied sustainable alternatives in reducing postharvest diseases by using antagonistic microorganisms against phytopathogens [14,15], which is considered a viable alternative to synthetic fungicides [16]. Among the microorganisms, yeasts stand out for their antagonistic capacity, for example, they have certain characteristics, such as genetic stability, efficacy at low concentrations; control towards different phytopathogens [17]; simple nutritional requirements; survival under adverse environmental conditions; compatibility with other chemical and physical treatments; resistance to synthetic fungicides; and absence of pathogenicity towards the host [18,19]. Additionally, the yeasts do not produce metabolites potentially toxic to humans or animals and do not contaminate the environment [20,21,22].
In this review, we describe the use and applications of yeasts as biocontrol agents and its role in global sustainable postharvest disease management of fruits, including the characteristics of antagonist yeasts, their mechanisms of action, interaction with OMIC sciences, and future trends in their application.
2. Global Overview of the Use of Yeasts for Fruit Disease Biocontrol
Around the world, different yeast species have been evaluated for in vitro and in vivo control of postharvest fruit pathogens (Table 1). Although biocontrol commercial products for postharvest disease control have been developed, the search for new antagonists continues to allow the development of more effective biocontrol products that can be incorporated into crop sustainable management including fruits [23].

Table 1.
Yeast antagonists evaluated for the biocontrol of postharvest diseases in five continents of the earth.
3. Mechanisms of Action of Antagonistic Yeast towards Fruit Fungal Phytopathogens
3.1. Competition for Space and Nutrients
Competition for nutrients and space has been suggested to be the major mechanism of action by which yeasts exert their antagonistic action in inhibiting pathogenic fungi. Yeasts consume the necessary nutrients for their colonization and growth faster than the pathogens resulting in inhibiting spore germination, reducing its growth and infection level and, thus, decreasing infection and diseases development [50,51]. In addition, the synthesis of inhibitory compounds in yeasts is increased by the absorption of nutrients in situ or ex situ, improving their ability to biocontrol plant diseases [52].
The carbon sources that yeast consume include glucose, maltose, fructose, melezitose, and lactose, among others [53]. The determination of the nutritional needs and adaptation to the host of each yeast are important for their capacity as an antagonist [54].
3.2. Killer Toxin
Killer toxins are often glycosylated proteins produced by yeast of different species and can disrupt specific cell wall components (β-1,3-d-glucans, β-1,6-d-glucans, mannoproteins, and chitin), which result in fungal cell death (Table 2) [55,56]. Killer toxins attach to the cell membrane where they interact with a secondary receptor that result in changes in cell membrane permeabilization, DNA synthesis inhibition, cell cycle disruption, and RNA fragmentation [57,58].

Table 2.
Inhibition of phytopathogens cause of postharvest disease of fruits by yeasts producing killer toxins.
Genetic studies in Saccharomyces cerevisiae have shown that the ability to produce killer toxins is cytoplasmically inherited and related to the presence of double-stranded linear RNA (dsRNA) plasmids, which are then encapsulated, forming non-infectious virus-like particles (VLP) within the cell cytoplasm [66]. All killer toxins are produced under acidic conditions, and their activity decrease with the increase in pH and temperature of the medium in which they are found—an increase in these variables is sufficient for the yeasts to stop producing them [67,68].
3.3. Lytic Enzymes
One of the antagonistic mechanisms of yeasts against phytopathogens is the production of lytic enzymes, such as glucanases, chitinases, and proteases, which act on different sites of the fungal cell wall, causing cell lysis and death (Figure 1) [69,70].
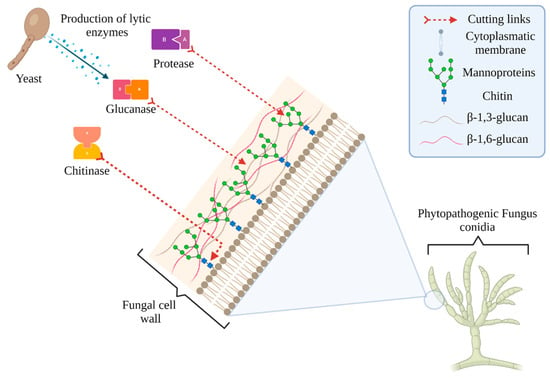
Figure 1.
Enzyme production by antagonistic yeasts and their lytic effect on phytopathogenic fungus cell wall.
β-glucanases are enzymes that hydrolyze the β-glucosidic bond of β-glucans. There are two types of glucanases: those that randomly hydrolyze intra-chain bonds giving rise to oligosaccharides (endoglucanases) and those that release glucose molecules by hydrolyzing bonds at the non-reducing end of the chain (exoglucanase). There are also yeasts that can produce both types of enzymes [71,72]. Different mechanisms for glucanase synthesis and secretion have been suggested, but the most important one involves a synthesis regulated by repression in glucose when it is not found in sufficient quantities in the medium [73]. In relation to chitinases, these enzymes hydrolyze β-1,4 bonds of chitin N-acetyl-β-d-glycosamide, which is one of its main cellular fungus components, breaking it into oligomers and monomers of N-acetyl-β-d-glucosaminidase and causing cell death [74,75].
Five types of chitinases have been identified, of which the most common is type I with a molecular weight of around 30 kDa. In its sequence, it has an N-terminal domain similar to hevein and type II, which possess a lower molecular weight of 25 kDa and lack the N-terminal hevein domain [76,77]. Finally, proteases have a molecular weight of approximately 35 kDa, stability at a pH from 2 to 5, a low isoelectric point, are insensitive to metal and heavy metal chelators, and have a high capacity to hydrolyze a wide range of peptide bonds of the mannoproteins that make up the fungus cell wall [51,78].
3.4. Induction of Host Resistance
Yeasts can induce resistance in the host as an indirect mechanism to prevent infections caused by fungi [79]. At the initial stages of fungus invasion into the tissue, the fruit or plant cells begin with a hypersensitivity reaction (HR), which necrotizes the tissue invaded by the fungus to isolate the infection, to prevent, or to slow the advance towards healthy cells [80]. HR can be activated by many agents called inducers, such as synthetic products, phytopathogens, non-pathogenic microorganisms (such as yeasts, fungi, and bacteria), ultraviolet light, and insects, among others [81,82]. This reaction in the host can be systemic; due to this characteristic, induction is defined as systemic acquired resistance (SAR) [83].
In response to any inducer, the plant overexpresses genes and enzymes related to plant defense by increasing the production of substances. For example, these substances include proteins related to pathogenesis (PR-proteins, classified in 14 families) [84] and phytoalexins (characterized around 300, including coumarins, flavonoids, diterpenes, and benzofuran, among others) [85] and/or lytic enzymes (proteases, glucanases, and chitinases) [86] and reactive oxygen species (ROS) [87], among others, which have resulted in inhibition effects and/or cell lysis or disruption of the phytopathogenic fungus.
PR proteins are defined as proteins that are absent or detected at a low basal level in healthy tissues but significantly accumulate during pathological conditions in both compatible and incompatible host–pathogen interactions [88]. Research studies involved PR-proteins following yeast treatment of fruit, i.e., Pichia membranaefaciens induced PR-9 and PR-10 in peach fruit [89]. However, PR-protein responses are too variable in relation to specific host tissue as well as microbial stimuli. The gene expression of PR-5 and PR-8 was characterized in apple fruit after treatment with Candida oleophila as a biocontrol agent against Botrytis cinerea. As a result, PR-8 was significantly overexpressed in response to both microorganisms while neither B. cinerea nor C. oleophila treatment significantly overexpressed the PR-5 gene [39].
Phytoalexin and lytic enzyme production by yeast resistance induction was demonstrated by Nantawanit et al. [90], who concluded that resistance induction in chili fruit treated with Pichia guilliermondii significantly enhanced the activities of phenylalanine ammonia-lyase (PAL), chitinase, and β-1,3-glucanase, and capsidiol phytoalexin accumulation in chili tissue. PAL is a fundamental enzyme during the first steps of the phenylpropanoid pathway to synthetize lignin, phenols, phytoalexins, and other compounds related to the plant resistance process [91].
Moreover, biocontrol yeast agents can enhance antioxidant enzyme activity to alleviate the oxidative damage caused by ROS produced in response to pathogen infection [65]. After cherry treatment with P. membranaefaciens at 5 × 107 cells mL−1, peroxidase (POD) activity was enhanced, but catalase (CAT) and superoxide dismutase (SOD) decreased [92]. Many mechanisms related to resistance induction are simultaneously promoted by yeast antagonists. For example, Rodosporidium spp., Pichia spp., and Cryptococcus laurentii enhanced the activity of antioxidant enzymes and enzymes related to defense [93,94].
4. Antagonistic Yeasts and OMIC Sciences
Conventionally, the study of the mechanisms of action is related to the evaluation of the production of antibiotics, lytic enzymes, or other metabolites in vitro or in co-culture against the phytopathogen [95]. Information of the antagonistic mechanisms of antagonist yeasts is crucial for improving their efficiency against phytopathogens. Therefore, OMIC approaches, such as genomic, transcriptomic, and proteomic, are modern molecular technologies that help in their characterization [96]. Information on efficacy and consistency of an antagonist yeast helps to select the best antagonist against a specific phytopathogen [39]. The study of the microbial antagonist genome helps to understand the potential genes involved in biocontrol activity, characterizes groups of genes with unknown functions, compares the genome with other biological control agents, and, finally, helps in study gene transcription [97].
Proteomic approaches provide information on changes in metabolic/physiological functions within the cell. Additionally, any biotic or abiotic factors that induce changes during microbial growth can be studied by this molecular tool [98]. Proteomic analysis plays a key role in host–phytopathogen interactions, and this technique can help identify key proteins involved in antagonist–phytopathogen–host interaction [71].
Metabolomics analyses allow an understanding of cell physiology in real time. The production of secondary metabolites, antibiotics, and lytic enzymes is one of the main mechanisms of action for the control of phytopathogens [99]. The interaction of microbial antagonists with phytopathogens can change the proteome and transcriptome of plants or fruit, as well as their response to biotic stress through the induction of defense-related metabolic pathways [100].
Transcriptomic studies of biological control agents provide useful information on the genes involved in the production of secondary metabolites mostly studied in bacteria and yeasts [101]. In the case of fungal biocontrol agents, studies have focused on the genes involved in the influence of lytic enzymes, such as glucanases, proteases, and chitinases on fungal cell wall [102]. Transcriptomic analysis is not limited to the study of biological control agents; the study of phytopathogens also provides useful knowledge associated with its virulence [103].
Another important aspect is microbial interaction on the fruit’s surface with antagonistic microorganisms since they are an integral part of the host’s composition. The study of the microbiome is important to understand the key role of the microorganisms present and their role in fruit health and physiology, as well as their possible positive or negative interactions with artificially applied antagonists [104,105].
5. Patents on Yeast-Based Products for Plant and Fruit Disease Biocontrol
A patent, understood as the title that the state grants for the exclusive exploitation of an invention for a specified period [106,107], is a method used to protect intellectual property and, in many cases, is required to advance on the development of a biological product for the control of plant diseases. The first yeast-related patents date from 1842 in Finland [108] and 1873 in the United States by Luis Pasteur (US141972) [109].
Globally, from 2009 to 2021, 163 patents were reported in the Derwent Innovation database related to yeasts as biological control agents for plants or parts of them (Figure 2). Germany, USA, Australia, and China account for 53% of all patents with yeasts worldwide.
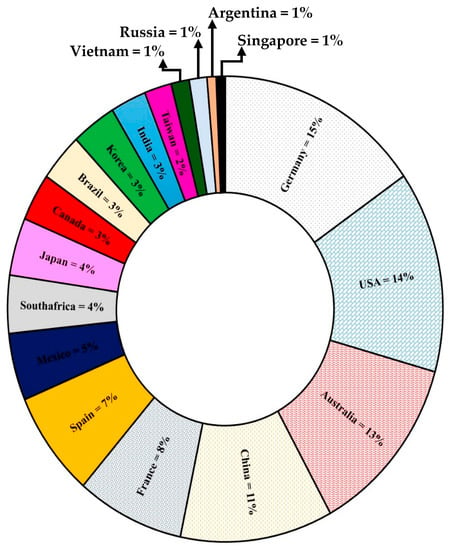
Figure 2.
Percentage of patents by country of biocontrol products containing yeasts used in plants or plant parts.
Of the 163 patents, 73.68% of the records have the name of the genus or genus and species of the yeast contained in the patented product, and 26.31% only indicated the word “yeast” among its components. Generally, the products contain yeasts and other microorganisms. Related to these products, 32.89% contained Metschnikowia fructicola; 11.18% contained Candida sp.; 11.18% contained a mixture of Candida oleophila, Metschnikovia fructicola, and Pichia anomala; 9.86% contained Pichia sp.; 7.89% contained Rhodotorula sp.; 5.92% contained Cryptococcus alone or mixed with Rhodotorula sp.; and 1.97% contained Debaryomyces sp.
Moreover, 84.21% of the patents belong to companies, where Bayer® is the predominant one. A low percentage is occupied by academic institutions (15.78%). This analysis reflects little participation of academics belonging to higher education institutions such as universities or public research centers in intellectual property registries. Much of the valuable information generated in universities has not been recorded, probably because the main objective is teaching and in addition to the lack of equipment to carry out mass formulations of the new product or an entity dedicated to marketing within these institutions. The development of biocontrol products containing yeasts for use in the post-harvest period is in high demand by entrepreneurs related to post-harvest and end consumer because it is a harmless product.
6. Conclusions
The use of yeasts as a post-harvest treatment to reduce decay caused by various phytopathogenic fungi in fruit of commercial interest is a sustainable and efficient alternative to the utilization of synthetic fungicides. The application of yeasts will be able to reduce the levels of fruit losses caused by phytopathogens, which will increase economic gains because of a greater volume of production for commercialization. Its implementation in postharvest will improve shelf life of the fruit and may lower crop costs by reducing the use of synthetic products. The acceptance of the consumer for product acquisition—not treated with any chemical—allowed opening new markets since it is a fruit not treated with synthetic fungicides.
Author Contributions
Conceptualization, L.G.H.-M., S.D. and G.D.Á.-Q.; original draft preparation; L.G.H.-M. and T.R.-G.; data curation and analyses, R.R.G.-E. and P.P.-R.; writing—original draft preparation, L.G.H.-M., R.R.G.-E. and G.D.Á.-Q.; writing—review and editing, L.G.H.-M., S.D. and P.P.-R.; visualization, P.G.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the grant project Problemas Nacionales 2015-01-352, Consejo Nacional de Ciencia y Tecnología (CONACYT, México).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Diana Fischer for English editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wallace, T.C.; Bailey, R.L.; Blumberg, J.B.; Burton-Freeman, B.; Chen, C.O.; Crowe-White, K.M.; Drewnowski, A.; Hooshmand, S.; Johnson, E.; Lewis, R.; et al. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit. Rev. Food Sci. Nutr. 2020, 60, 2174–2211. [Google Scholar] [CrossRef] [Green Version]
- Valcke, M.; Bourgault, M.H.; Rochette, L.; Normandin, L.; Samuel, O.; Belleville, D.; Blanchet, C.; Phaneuf, D. Human health risk assessment on the consumption of fruits and vegetables containing residual pesticides: A cancer and non-cancer risk/benefit perspective. Environ. Int. 2017, 108, 63–74. [Google Scholar] [CrossRef]
- Yahia, E.M.; García-Solís, P.; Celis, M.E.M. Contribution of fruits and vegetables to human nutrition and health. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Woodhead Publishing: Sawston, UK, 2019; pp. 19–45. [Google Scholar] [CrossRef]
- Brasil, I.M.; Siddiqui, M. Postharvest quality of fruits and vegetables: An overview. In Preharvest Modulation of Postharvest Fruit and Vegetable Quality: An Overview; Academic Press: Cambridge, MA, USA, 2018; pp. 1–40. [Google Scholar] [CrossRef]
- Alasalvar, C.; Salvadó, J.S.; Ros, E. Bioactives and health benefits of nuts and dried fruits. Food Chem. 2020, 314, 126192. [Google Scholar] [CrossRef]
- Kausar, R.; Iram, S.; Ahmad, K.S.; Jaffri, S.B. Molecular characterization of Fusarium solani and Fusarium oxysporum phyto-pathogens causing mango maturity malconformation. Arch. Phytopathol. Plant Prot. 2021, 54, 1372–1390. [Google Scholar] [CrossRef]
- Nunes, C.A. Biological control of postharvest diseases of fruit. Eur. J. Plant Pathol. 2012, 133, 181–196. [Google Scholar] [CrossRef]
- Gunny, A.A.N.; Fang, L.P.; Misnan, N.M.; Gopinath, S.C.; Salleh, N.H.M.; Hashim, R.H.R.; Mat, M.H.C. Microwave-assisted solvent-free extraction of essential oil from Coleus aromaticus: Anti-phytopathogenic potential for fruit post-harvesting. 3 Biotech 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Gandía, M.; Kakar, A.; Giner-Llorca, M.; Holzknecht, J.; Martínez-Culebras, P.; Galgóczy, L.; Marx, F.; Marcos, J.F.; Manzanares, P. Potential of antifungal proteins (AFPs) to control Penicillium postharvest fruit decay. J. Fungi 2021, 7, 449. [Google Scholar] [CrossRef]
- van der Ven, L.T.M.; Rorije, E.; Sprong, R.C.; Zink, D.; Derr, R.; Hendriks, G.; Loo, L.H.; Luijten, M. A case study with triazole fungicides to explore practical application of next-generation hazard assessment methods for human health. Chem. Res. Toxicol. 2020, 33, 834–848. [Google Scholar] [CrossRef]
- Ramos-Bell, S.; Hernandez-Montiel, L.G.; González-Estrada, R.R.; Gutiérrez-Martínez, P. Main diseases in postharvest blueberries, conventional and eco-friendly control methods: A review. LWT 2021, 149, 112046. [Google Scholar] [CrossRef]
- Agathokleous, E.; Calabrese, E.J. Fungicide-induced hormesis in phytopathogenic fungi: A critical determinant of successful agriculture and environmental sustainability. J. Agric. Food Chem. 2021, 69, 4561–4563. [Google Scholar] [CrossRef]
- Malakar, C.; Deka, S. Biosurfactants against drug-resistant human and plant pathogens: Recent advances. In Biosurfactants for a Sustainable Future: Production and Applications in the Environment and Biomedicine; Wiley: Hoboken, NJ, USA, 2021; pp. 353–372. [Google Scholar] [CrossRef]
- Wisniewski, M.; Droby, S. The postharvest microbiome: The other half of sustainability. Biol. Control 2019, 137, 104025. [Google Scholar] [CrossRef]
- Sare, A.R.; Jijakli, M.H.; Massart, S. Microbial ecology to support integrative efficacy improvement of biocontrol agents for postharvest diseases management. Postharvest Biol. Technol. 2021, 179, 111572. [Google Scholar] [CrossRef]
- González-Estrada, R.R.; Blancas-Benitez, F.J.; Aguirre-Güitrón, L.; Hernandez-Montiel, L.G.; Moreno-Hernández, C.; Cortés-Rivera, H.J.; Herrera-González, J.A.; Rayón-Díaz, E.; Velázquez-Estrada, R.M.; Santoyo-González, M.A.; et al. Alternative management technologies for postharvest disease control. In Food Losses, Sustainable Postharvest and Food Technologies; Elsevier: London, UK, 2021; pp. 153–190. [Google Scholar] [CrossRef]
- Spadaro, D.; Droby, S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Zhimo, V.Y.; Biasi, A.; Kumar, A.; Feygenberg, O.; Salim, S.; Vero, S.; Wisniewski, M.; Droby, S. Yeasts and bacterial consortia from kefir grains are effective biocontrol agents of postharvest diseases of fruits. Microorganisms 2020, 8, 428. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Zhang, R.; Xiong, B. Management of postharvest diseases of kiwifruit with a combination of the biocontrol yeast Candida oleophila and an oligogalacturonide. Biol. Control 2021, 156, 104549. [Google Scholar] [CrossRef]
- Hernandez-Montiel, L.G.; Gutierrez-Perez, E.D.; Murillo-Amador, B.; Vero, S.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G. Mechanisms employed by Debaryomyces hansenii in biological control of anthracnose disease on papaya fruit. Postharvest Biol. Technol. 2018, 139, 31–37. [Google Scholar] [CrossRef]
- Lutz, M.C.; Lopes, C.A.; Sosa, M.C.; Sangorrín, M.P. Semi-commercial testing of regional yeasts selected from North Patagonia Argentina for the biocontrol of pear postharvest decays. Biol. Control 2020, 150, 104246. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Hernández, A.; Galvan, A.I.; Córdoba, M.G.; Casquete, R.; Serradilla, M.J.; Martín, A. Selection and application of antifungal VOCs-producing yeasts as biocontrol agents of grey mould in fruits. Food Microbiol. 2020, 92, 103556. [Google Scholar] [CrossRef]
- Mukherjee, A.; Verma, J.P.; Gaurav, A.K.; Chouhan, G.K.; Patel, J.S.; Hesham, A.E.L. Yeast a potential bio-agent: Future for plant growth and postharvest disease management for sustainable agriculture. Appl. Microbiol. Biotechnol. 2020, 104, 1497–1510. [Google Scholar] [CrossRef]
- Sui, Y.; Sun, Z.; Zou, Y.; Li, W.; Jiang, M.; Luo, Y.; Liao, W.; Wang, Y.; Gao, X.; Liu, J.; et al. The Rlm1 transcription factor in Candida oleophila contributes to abiotic stress resistance and biocontrol efficacy against postharvest gray mold of kiwifruit. Postharvest Biol. Technol. 2020, 166, 111222. [Google Scholar] [CrossRef]
- Konsue, W.; Dethoup, T.; Limtong, S. Biological control of fruit rot and anthracnose of postharvest mango by antagonistic yeasts from economic crops leaves. Microorganisms 2020, 8, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhimo, V.Y.; Bhutia, D.D.; Saha, J. Biological control of postharvest fruit diseases using antagonistic yeasts in India. J. Plant Pathol. 2016, 98, 275–283. [Google Scholar]
- Hassan, H.; Mohamed, M.T.M.; Yusoff, S.F.; Hata, E.M.; Tajidin, N.E. Selecting antagonistic yeast for postharvest biocontrol of Colletotrichum gloeosporioides in papaya fruit and possible mechanisms involved. Agronomy 2021, 11, 760. [Google Scholar] [CrossRef]
- Droby, S.; Vinokur, V.; Weiss, B.; Cohen, L.; Daus, A.; Goldschmidt, E.E.; Porat, R. Induction of resistance to Penicillium digitatum in grapefruit by the yeast biocontrol agent Candida oleophila. Phytopathology 2002, 92, 393–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.H.; Chen, R.Y.; Chou, J.Y. Screening and evaluation of yeast antagonists for biological control of Botrytis cinerea on strawberry fruits. Mycobiology 2018, 46, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Hartati, S.; Wiyono, S.; Hidayat, S.H.; Sinaga, M.S. Mode of action of yeast-like fungus Aureobasidium pullulans in controlling anthracnose of postharvest chili. Int. J. Sci. Basic Appl. Res. 2015, 20, 253–263. [Google Scholar]
- Mohamed, H.; Saad, A. The biocontrol of postharvest disease (Botryodiplodia theobromae) of guava (Psidium guajava L.) by the application of yeast strains. Postharvest Biol. Technol. 2009, 53, 123–130. [Google Scholar] [CrossRef]
- Oro, L.; Feliziani, E.; Ciani, M.; Romanazzi, G.; Comitini, F. Volatile organic compounds from Wickerhamomyces anomalus, Metschnikowia pulcherrima and Saccharomyces cerevisiae inhibit growth of decay causing fungi and control postharvest diseases of strawberries. Int. J. Food Microbiol. 2018, 265, 18–22. [Google Scholar] [CrossRef]
- Settier-Ramírez, L.; López-Carballo, G.; Hernández-Muñoz, P.; Fontana, A.; Strub, C.; Schorr-Galindo, S. New isolated Metschnikowia pulcherrima strains from apples for postharvest biocontrol of Penicillium expansum and patulin accumulation. Toxins 2021, 13, 397. [Google Scholar] [CrossRef]
- Czarnecka, M.; Żarowska, B.; Połomska, X.; Restuccia, C.; Cirvilleri, G. Role of biocontrol yeasts Debaryomyces hansenii and Wickerhamomyces anomalus in plants defense mechanisms against Monilinia fructicola in apple fruits. Food Microbiol. 2019, 83, 1–8. [Google Scholar] [CrossRef]
- Zhu, R.; Yu, T.; Guo, S.; Hu, H.A.O.; Zheng, X.; Karlovsky, P. Effect of the yeast Rhodosporidium paludigenum on postharvest decay and patulin accumulation in apples and pears. J. Food Prot. 2015, 78, 157–163. [Google Scholar] [CrossRef]
- Manso, T.; Nunes, C. Metschnikowia andauensis as a new biocontrol agent of fruit postharvest diseases. Postharvest Biol. Technol. 2011, 61, 64–71. [Google Scholar] [CrossRef]
- Arrarte, E.; Garmendia, G.; Rossini, C.; Wisniewski, M.; Vero, S. Volatile organic compounds produced by Antarctic strains of Candida sake play a role in the control of postharvest pathogens of apples. Biol. Control 2017, 109, 14–20. [Google Scholar] [CrossRef]
- Vilaplana, R.; Cifuentes, C.; Vaca, L.; Cevallos-Cevallos, J.M.; Valencia-Chamorro, S. Curative activity of possible biocontrol agents in the postharvest of yellow pitahaya and organic banana. Postharvest Biol. Technol. 2020, 159, 111030. [Google Scholar] [CrossRef]
- Liu, J.; Wisniewski, M.; Artlip, T.; Sui, Y.; Droby, S.; Norelli, J. The potential role of PR-8 gene of apple fruit in the mode of action of the yeast antagonist, Candida oleophila, in postharvest biocontrol of Botrytis cinerea. Postharvest Biol. Technol. 2013, 85, 203–209. [Google Scholar] [CrossRef]
- Sperandio, E.M.; do Vale, H.M.M.; Moreira, G.A.M. Yeasts from native Brazilian Cerrado plants: Occurrence, diversity and use in the biocontrol of citrus green mould. Fungal Biol. 2015, 119, 984–993. [Google Scholar] [CrossRef]
- Reyes-Bravo, P.; Acuña-Fontecilla, A.; Rosales, I.M.; Godoy, L. Evaluation of native wine yeast as biocontrol agents against fungal pathogens related to postharvest diseases. Agric. Sci. Agron. 2019, 1–10. [Google Scholar] [CrossRef]
- Mewa-Ngongang, M.; Du Plessis, H.W.; Chidi, B.S.; Hutchinson, U.F.; Ntwampe, K.S.O.; Okudoh, V.I.; Jolly, N.P. Physiological and antagonistic properties of Pichia kluyveri for curative and preventive treatments against post-harvest fruit fungi. Pol. J. Food Nutr. Sci. 2021, 71, 245–253. [Google Scholar] [CrossRef]
- Aloui, H.; Licciardello, F.; Khwaldia, K.; Hamdi, M.; Restuccia, C. Physical properties and antifungal activity of bioactive films containing Wickerhamomyces anomalus killer yeast and their application for preservation of oranges and control of postharvest green mold caused by Penicillium digitatum. Int. J. Food Microbiol. 2015, 200, 22–30. [Google Scholar] [CrossRef]
- Abraham, A.O.; Laing, M.D.; Bower, J.P. Isolation and in vivo screening of yeast and Bacillus antagonists for the control of Penicillium digitatum of citrus fruit. Biol. Control 2010, 53, 32–38. [Google Scholar] [CrossRef]
- Taqarort, N.; Echairi, A.; Chaussod, R.; Nouaim, R.; Boubaker, H.; Benaoumar, A.A.; Boudyach, E. Screening and identification of epiphytic yeasts with potential for biological control of green mold of citrus fruits. World J. Microbiol. Biotechnol. 2008, 24, 3031–3038. [Google Scholar] [CrossRef]
- Zhao, Y.; Tu, K.; Su, J.; Tu, S.; Hou, Y.; Liu, F.; Zou, X. Heat treatment in combination with antagonistic yeast reduces diseases and elicits the active defense responses in harvested cherry tomato fruit. J. Agric. Food Chem. 2009, 57, 7565–7570. [Google Scholar] [CrossRef]
- Liu, F.; Tu, K.; Shao, X.; Zhao, Y.; Tu, S.; Su, J.; Hou, Y.; Zou, X. Effect of hot air treatment in combination with Pichia guilliermondii on postharvest anthracnose rot of loquat fruit. Postharvest Biol. Technol. 2010, 58, 65–71. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, M.; Wu, H.; Tu, S.; Pan, L.; Tu, K. Defense response of cherry tomato at different maturity stages to combined treatment of hot air and Cryptococcus laurentii. Postharvest Biol. Technol. 2016, 117, 177–186. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, L.; Turner, M.; Xu, H.; Dong, Y.; Jiang, S. Methyl jasmonate enhances biocontrol efficacy of Rhodotorula glutinis to postharvest blue mold decay of pears. Food Chem. 2009, 117, 621–626. [Google Scholar] [CrossRef]
- Hernandez-Montiel, L.G.; Ochoa, J.L.; Troyo-Diéguez, E.; Larralde-Corona, C.P. Biocontrol of postharvest blue mold (Penicillium italicum Wehmer) on Mexican lime by marine and citrus Debaryomyces hansenii isolates. Postharvest Biol. Technol. 2010, 56, 181–187. [Google Scholar] [CrossRef]
- Rivas-Garcia, T.; Murillo-Amador, B.; Nieto-Garibay, A.; Rincon-Enriquez, G.; Chiquito-Contreras, R.G.; Hernandez-Montiel, L.G. Enhanced biocontrol of fruit rot on muskmelon by combination treatment with marine Debaryomyces hansenii and Stenotrophomonas rhizophila and their potential modes of action. Postharvest Biol. Technol. 2019, 151, 61–67. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, S.; Sun, D.; Yang, Y.; Wei, Z.; Wang, C.; Lu, L. Cultivation of Rhodosporidium paludigenum in gluconic acid enhances effectiveness against Penicillium digitatum in citrus fruit. Postharvest Biol. Technol. 2021, 172, 111374. [Google Scholar] [CrossRef]
- Lima, G.; Arru, S.; De Curtis, F.; Arras, G. Influence of antagonist, host fruit and pathogen on the biological control of postharvest fungal diseases by yeasts. J. Ind. Microbiol. Biotechnol. 1999, 23, 223–229. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Liu, J.; Tian, X.; Zhang, D.; Wang, Q. Management of blue mold (Penicillium italicum) on mandarin fruit with a combination of the yeast, Meyerozyma guilliermondii and an alginate oligosaccharide. Biol. Control 2021, 152, 104451. [Google Scholar] [CrossRef]
- Mannazzu, I.; Domizio, P.; Carboni, G.; Zara, S.; Zara, G.; Comitini, F.; Budroni, M.; Ciani, M. Yeast killer toxins: From ecological significance to application. Crit. Rev. Biotechnol. 2019, 39, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Villalba, M.L.; Mazzucco, M.B.; Lopes, C.A.; Ganga, M.A.; Sangorrín, M.P. Purification and characterization of Saccharomyces eubayanus killer toxin: Biocontrol effectiveness against wine spoilage yeasts. Int. J. Food Microbiol. 2020, 331, 108714. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Alonso, A.; Marquina, D.; Santos, A. The biology of Pichia membranifaciens killer toxins. Toxins 2017, 9, 112. [Google Scholar] [CrossRef] [Green Version]
- Crabtree, A.M.; Kizer, E.A.; Hunter, S.S.; Van Leuven, J.T.; New, D.D.; Fagnan, M.W.; Rowley, P.A. A rapid method for sequencing double-stranded RNAs purified from yeasts and the identification of a potent K1 killer toxin isolated from Saccharomyces cerevisiae. Viruses 2019, 11, 70. [Google Scholar] [CrossRef] [Green Version]
- Çorbacı, C.; Uçar, F.B. Purification, characterization, and in vivo biocontrol efficiency of killer toxins from Debaryomyces hansenii strains. Int. J. Biol. Macromol. 2018, 119, 1077–1082. [Google Scholar] [CrossRef]
- de Lima, J.R.; Gonçalves, L.R.B.; Brandão, L.R.; Rosa, C.A.; Viana, F.M.P. Isolation, identification, and activity in vitro of killer yeasts against Colletotrichum gloeosporioides isolated from tropical fruits. J. Basic Microbiol. 2013, 53, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk, M.; Żarowska, B.; Restuccia, C.; Cirvilleri, G. Postharvest biocontrol ability of killer yeasts against Monilinia fructigena and Monilinia fructicola on stone fruit. Food Microbiol. 2017, 61, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Çorbacı, C.; Uçar, F.B. Production and optimization of killer toxin in Debaryomyces hansenii strains. Braz. Arch. Biol. Technol. 2017, 60, 1–11. [Google Scholar] [CrossRef]
- Perez, M.F.; Contreras, L.; Garnica, N.M.; Fernández-Zenoff, M.V.; Farías, M.E.; Sepulveda, M.; Ramallo, J.; Dib, J.R. Native killer yeasts as biocontrol agents of postharvest fungal diseases in lemons. PLoS ONE 2016, 11, e0165590. [Google Scholar] [CrossRef] [Green Version]
- Lima, J.R.; Gondim, D.M.; Oliveira, J.T.A.; Oliveira, F.S.; Gonçalves, L.R.; Viana, F.M. Use of killer yeast in the management of postharvest papaya anthracnose. Postharvest Biol. Technol. 2013, 83, 58–64. [Google Scholar] [CrossRef]
- Platania, C.; Restuccia, C.; Muccilli, S.; Cirvilleri, G. Efficacy of killer yeasts in the biological control of Penicillium digitatum on Tarocco orange fruits (Citrus sinensis). Food Microbiol. 2012, 30, 219–225. [Google Scholar] [CrossRef]
- Nally, M.C.; Pesce, V.M.; Maturano, Y.P.; Assaf, L.R.; Toro, M.E.; De Figueroa, L.C.; Vazquez, F. Antifungal modes of action of Saccharomyces and other biocontrol yeasts against fungi isolated from sour and grey rots. Int. J. Food Microbiol. 2015, 204, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Abu-Mejdad, N.M.J.A.; Al-Saadoon, A.H.; Al-Badran, A.I.; Minati, M.H. Optimum conditions of killer toxins produced by Torulaspora delbrueckii and Wickerhamomyces anomalus and their action as antifungal agents. Bull. Natl. Res. Cent. 2020, 44, 1–9. [Google Scholar] [CrossRef]
- Moura, V.S.; Pollettini, F.L.; Ferraz, L.P.; Mazzi, M.V.; Kupper, K.C. Purification of a killer toxin from Aureobasidium pullulans for the biocontrol of phytopathogens. J. Basic Microbiol. 2021, 61, 77–87. [Google Scholar] [CrossRef]
- Rodrigues, P.L.; da Silva, J.L.; de Alfaia, J.P.; de Souza, J.C.; de Macedo, L.P. Biocontrol potential of yeasts in citrus postharvest by production of β-1,3-glucanase enzyme and killer activity: A review. Citrus Res. Technol. 2020, 41, 1–18. [Google Scholar] [CrossRef]
- Oztekin, S.; Karbancioglu-Guler, F. Bioprospection of Metschnikowia sp. isolates as biocontrol agents against postharvest fungal decays on lemons with their potential modes of action. Postharvest Biol. Technol. 2021, 181, 111634. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, J.; Zhou, Y.; Deng, L.; Yao, S.; Zeng, K. Inhibitory effect of Pichia membranaefaciens and Kloeckera apiculata against Monilinia fructicola and their biocontrol ability of brown rot in postharvest plum. Biol. Control 2017, 114, 51–58. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Wang, Y.; Li, B.; Gu, X.; Zhang, X.; Serwah, N.A.; Zhang, H. Effect of β-glucan on the biocontrol efficacy of Cryptococcus podzolicus against postharvest decay of pears and the possible mechanisms involved. Postharvest Biol. Technol. 2020, 160, 111057. [Google Scholar] [CrossRef]
- Santos, T.; Villanueva, J.R.; Nombela, C. Production and catabolite repression of Penicillium italicum beta-glucanases. J. Bacteriol. 1977, 129, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Brar, A.; Yadav, M.; Chawade, A.; Vivekanand, V.; Pareek, N. Chitinases-potential candidates for enhanced plant resistance towards fungal pathogens. Agriculture 2018, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Le, B.; Yang, S.H. Microbial chitinases: Properties, current state and biotechnological applications. World J. Microbiol. Biotechnol. 2019, 35, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Banani, H.; Spadaro, D.; Zhang, D.; Matic, S.; Garibaldi, A.; Gullino, M.L. Postharvest application of a novel chitinase cloned from Metschnikowia fructicola and overexpressed in Pichia pastoris to control brown rot of peaches. Int. J. Food Microbiol. 2015, 199, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.V.; Sambyal, K.; Negi, A.; Sonwani, S.; Mahajan, R. Chitinases production: A robust enzyme and its industrial applications. Biocatal. Biotransform. 2021, 39, 161–189. [Google Scholar] [CrossRef]
- Lario, L.D.; Chaud, L.; das Graças Almeida, M.; Converti, A.; Sette, L.D.; Pessoa, A. Production, purification, and characterization of an extracellular acid protease from the marine Antarctic yeast Rhodotorula mucilaginosa L7. Fungal Biol. 2015, 119, 1129–1136. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, F.; Gu, N.; Yan, X.; Wang, K.; Dhanasekaran, S.; Gu, X.; Zhao, L.; Zhang, H. Postharvest biological control of Rhizopus rot and the mechanisms involved in induced disease resistance of peaches by Pichia membranefaciens. Postharvest Biol. Technol. 2020, 163, 111146. [Google Scholar] [CrossRef]
- Noman, A.; Aqeel, M.; Qari, S.H.; Al Surhanee, A.A.; Yasin, G.; Alamri, S.; Hashem, M.; Al-Saadi, A.M. Plant hypersensitive response vs pathogen ingression: Death of few gives life to others. Microb. Pathog. 2020, 145, 104224. [Google Scholar] [CrossRef]
- Kamle, M.; Borah, R.; Bora, H.; Jaiswal, A.K.; Singh, R.K.; Kumar, P. Systemic acquired resistance (SAR) and induced systemic resistance (ISR): Role and mechanism of action against phytopathogens. In Fungal Biotechnology and Bioengineering; Springer: Cham, Switzerland, 2020; pp. 457–470. [Google Scholar] [CrossRef]
- Musheer, N.; Ashraf, S.; Choudhary, A.; Kumar, M.; Saeed, S. Role of microbiotic factors against the soil-borne phytopathogens. In Phytobiomes: Current Insights and Future Vistas; Springer: Singapore, 2020; pp. 251–280. [Google Scholar] [CrossRef]
- Romanazzi, G.; Sanzani, S.M.; Bi, Y.; Tian, S.; Martínez, P.G.; Alkan, N. Induced resistance to control postharvest decay of fruit and vegetables. Postharvest Biol. Technol. 2016, 122, 82–94. [Google Scholar] [CrossRef]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Tajamul, S.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212, 29–37. [Google Scholar] [CrossRef]
- Nandakumar, M.; Malathi, P.; Sundar, A.R.; Viswanathan, R. Host-pathogen interaction in sugarcane and red rot pathogen: Exploring expression of phytoalexin biosynthesis pathway genes. Indian Phytopathol. 2021, 74, 529–535. [Google Scholar] [CrossRef]
- Das, J.; Yadav, S.K.; Ghosh, S.; Tyagi, K.; Magotra, A.; Krishnan, A.; Jha, G. Enzymatic and non-enzymatic functional attributes of plant microbiome. Curr. Opin. Biotechnol. 2021, 69, 162–171. [Google Scholar] [CrossRef]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Qin, G.; Tian, S. Effect of microbial biocontrol agents on alleviating oxidative damage of peach fruit subjected to fungal pathogen. Int. J. Food Microbiol. 2008, 126, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Nantawanit, N.; Chanchaichaovivat, A.; Panijpan, B.; Ruenwongsa, P. Induction of defense response against Colletotrichum capsici in chili fruit by the yeast Pichia guilliermondii strain R13. Biol. Control 2010, 52, 145–152. [Google Scholar] [CrossRef]
- MacDonald, M.J.; D’Cunha, G.B. A modern view of phenylalanine ammonia lyase. Biochem. Cell Biol. 2007, 85, 273–282. [Google Scholar] [CrossRef]
- Chan, Z.; Tian, S. Induction of H2O2-metabolizing enzymes and total protein synthesis by antagonistic yeast and salicylic acid in harvested sweet cherry fruit. Postharvest Biol. Technol. 2006, 39, 314–320. [Google Scholar] [CrossRef]
- Zhao, Y.; Yin, J. Effects of Pichia guilliermondii and hot air treatment on the postharvest preservation of red fuji apple quality attributes. J. Food Prot. 2018, 81, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Cao, X.; Yu, T.; Wang, Q.; Zhang, Y.; Zheng, X.; Lu, H. Effect of Cryptococcus laurentii on inducing disease resistance in cherry tomato fruit with focus on the expression of defense-related genes. Food Chem. 2018, 254, 208–216. [Google Scholar] [CrossRef]
- di Francesco, A.; Martini, C.; Mari, M. Biological control of postharvest diseases by microbial antagonists: How many mechanisms of action? Eur. J. Plant Pathol. 2016, 145, 711–717. [Google Scholar] [CrossRef]
- Sarethy, I.P.; Saharan, A. Genomics, proteomics and transcriptomics in the biological control of plant pathogens: A review. Indian Phytopathol. 2021, 74, 3–12. [Google Scholar] [CrossRef]
- Sui, Y.; Wisniewski, M.; Droby, S.; Piombo, E.; Wu, X.; Yue, J. Genome sequence, assembly, and characterization of the antagonistic yeast Candida oleophila used as a biocontrol agent against post-harvest diseases. Front. Microbiol. 2020, 11, 295. [Google Scholar] [CrossRef] [Green Version]
- Gu, N.; Zhang, X.; Gu, X.; Zhao, L.; Dhanasekaran, S.; Qian, X.; Zhang, H. Proteomic analysis reveals the mechanisms involved in the enhanced biocontrol efficacy of Rhodotorula mucilaginosa induced by chitosan. Biol. Control 2020, 149, 104325. [Google Scholar] [CrossRef]
- Belinato, J.R.; Kupper, K.C.; Augusto, F. In vivo investigation of the volatile metabolome of antiphytopathogenic yeast strains active against Penicillium digitatum using comprehensive two-dimensional gas chromatography and multivariate data analysis. Microchem. J. 2018, 141, 362–368. [Google Scholar] [CrossRef]
- Alawiye, T.T.; Babalola, O.O. Metabolomics: Current application and prospects in crop production. Biologia 2021, 76, 227–239. [Google Scholar] [CrossRef]
- Yan, Y.; Zheng, X.; Apaliya, M.T.; Yang, H.; Zhang, H. Transcriptome characterization and expression profile of defense-related genes in pear induced by Meyerozyma guilliermondii. Postharvest Biol. Technol. 2018, 141, 63–70. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, M.; Li, B.; Dhanasekaran, S.; Wang, K.; Gu, X.; Zhang, X.; Zhang, H. Investigating proteome and transcriptome defense response of table grapes induced by Yarrowia lipolytica. Sci. Hortic. 2021, 276, 109742. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Z.; Leng, J.; Wang, Q.; Liu, J. Heat stress alters the transcriptome of Debaryomyces hansenii and reduces its biocontrol activity against postharvest gray mold on kiwifruit. Postharvest Biol. Technol. 2021, 178, 111541. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M. The fruit microbiome: A new frontier for postharvest biocontrol and postharvest biology. Postharvest Biol. Technol. 2018, 140, 107–112. [Google Scholar] [CrossRef]
- Zhimo, V.Y.; Kumar, A.; Biasi, A.; Salim, S.; Feygenberg, O.; Toamy, M.A.; Abdelfattaah, A.; Medina, S.; Freilich, S.; Wisniewski, M.; et al. Compositional shifts in the strawberry fruit microbiome in response to near-harvest application of Metschnikowia fructicola, a yeast biocontrol agent. Postharvest Biol. Technol. 2021, 175, 111469. [Google Scholar] [CrossRef]
- Sampat, B.N. Patenting and US academic research in the 20th century: The world before and after Bayh-Dole. Res. Policy 2006, 35, 772–789. [Google Scholar] [CrossRef]
- Rathje, J.M.; Katila, R. Enabling technologies and the role of private firms: A machine learning matching analysis. Strategy Sci. 2021, 6, 5–21. [Google Scholar] [CrossRef]
- Bernardo-Álvarez, M.Á. Patentes fúngicas: De Pasteur a nuestros días. Rev. Iberoam. Micol. 2012, 29, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, M. Intellectual Property and Biotechnology: Biological Inventions; Edward Elgar Publishing: Northampton, MA, USA, 2008; p. 377. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).