Debaryomyces hansenii, Stenotrophomonas rhizophila, and Ulvan as Biocontrol Agents of Fruit Rot Disease in Muskmelon (Cucumis melo L.)
Abstract
:1. Introduction
2. Results
2.1. In Vivo Control Assay and Microscopic Visualization
2.2. Effect of Biocontrol Treatment Time on Their Biocontrol Efficacy
2.3. Efficacy of Biocontrol Treatments on Natural Fruit Rot Development and Fruit Quality Parameters
2.4. Antioxidant Enzymatic Activity on Muskmelon Fruit after Biocontrol Treatments
3. Discussion
4. Materials and Methods
4.1. Marine Microbial Antagonists Source and Concentration
4.2. Chemical Treatments Source and Concentration
4.3. Fusarium proliferatum Source and Concentration
4.4. Muskmelon Fruit Source and Pre-Treatment
4.5. In Vivo Biocontrol Assay and Microscopic Visualization
4.6. Effect of Biocontrol Treatment Time on the Control of Fruit Rot Disease
4.7. Efficacy of Biocontrol Treatments on Natural Fruit Rot Development and Fruit Quality Parameters
4.8. Antioxidant Enzymatic Activity on Muskmelon Fruit
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 3 September 2021).
- Wang, Y.; Xu, Z.; Zhu, P.; Liu, Y.; Zhang, Z.; Mastuda, Y.; Toyoda, H.; Xu, L. Postharvest biological control of melon pathogens using Bacillus subtilis EXWB1. J. Plant Pathol. 2010, 92, 645–652. [Google Scholar] [CrossRef]
- Wonglom, P.; Sunpapao, A. Fusarium incarnatum is associated with postharvest fruit rot of muskmelon (Cucumis melo). J. Phytopathol. 2020, 168, 204–210. [Google Scholar] [CrossRef]
- Agehara, S.; Crosby, K.; Holcroft, D.; Leskovar, D.I. Optimizing 1-methylcyclopropene concentration and immersion time to extend shelf life of muskmelon (Cucumis melo L. var. reticulatus) fruit. Sci. Hortic. 2018, 230, 117–125. [Google Scholar] [CrossRef]
- Abubakar, M.; Norida, M.; Rafii, M.; Nakasha, J. Effects of post-harvest hot water treatments on the fungi contamination, physiology and quality of rock melon fruit. Aust. J. Crop Sci. 2020, 14, 1081–1087. [Google Scholar] [CrossRef]
- Sui, Y.; Droby, S.; Zhang, D.; Wang, W.; Liu, Y. Reduction of Fusarium rot and maintenance of fruit quality in melon using eco-friendly hot water treatment. Environ. Sci. Pollut. Res. 2014, 21, 13956–13963. [Google Scholar] [CrossRef] [PubMed]
- Kaonga, C.C.; Chidya, R.C.G.; Kosamu, I.B.M.; Abdel-Dayem, S.M.; Mapoma, H.W.T.; Thole, B.; Mbewe, R.; Sakugawa, H. Trends in usage of selected fungicides in Japan between 1962 and 2014: A review. Int. J. Environ. Sci. Technol. 2017, 15, 1801–1814. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, R. Postharvest Diseases of Fruits and Vegetables and Their Management. In Postharvest Disinfection of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–52. [Google Scholar]
- Fan, H.; Zhang, Z.; Li, Y.; Zhang, X.; Duan, Y.; Wang, Q. Biocontrol of Bacterial Fruit Blotch by Bacillus subtilis 9407 via Surfactin-Mediated Antibacterial Activity and Colonization. Front. Microbiol. 2017, 8, 1973. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, P.; Xiao, Q.; Chen, J. Effect of phosphate-solubilizing bacteria on the gene expression and inhibition of bacterial fruit blotch in melon. Sci. Hortic. 2021, 282, 110018. [Google Scholar] [CrossRef]
- Collazo, C.; Abadias, M.; Aguiló-Aguayo, I.; Alegre, I.; Chenoll, E.; Viñas, I. Studies on the biocontrol mechanisms of Pseudomonas graminis strain CPA-7 against food-borne pathogens in vitro and on fresh-cut melon. LWT 2017, 85, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Intana, W.; Kheawleng, S.; Sunpapao, A. Trichoderma asperellum T76-14 Released Volatile Organic Compounds against Postharvest Fruit Rot in Muskmelons (Cucumis melo) Caused by Fusarium incarnatum. J. Fungi 2021, 7, 46. [Google Scholar] [CrossRef]
- Lv, X.; Ma, H.; Lin, Y.; Bai, F.; Ge, Y.; Zhang, D.; Li, J. Antifungal activity of Lactobacillus plantarum C10 against Trichothecium roseum and its application in promotion of defense responses in muskmelon (Cucumis melo L.) fruit. J. Food Sci. Technol. 2018, 55, 3703–3711. [Google Scholar] [CrossRef] [PubMed]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2018, 59, 1498–1513. [Google Scholar] [CrossRef]
- Vero, S.; Garmendia, G.; Gonzalez, M.; Bentancur, O.; Wisniewski, M. Evaluation of yeasts obtained from Antarctic soil samples as biocontrol agents for the management of postharvest diseases of apple (Malus × domestica). FEMS Yeast Res. 2012, 13, 189–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez-Montiel, L.; Gutierrez-Perez, E.D.; Murillo-Amador, B.; Vero, S.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G. Mechanisms employed by Debaryomyces hansenii in biological control of anthracnose disease on papaya fruit. Postharvest Biol. Technol. 2018, 139, 31–37. [Google Scholar] [CrossRef]
- Medina-Córdova, N.; Rosales-Mendoza, S.; Hernandez-Montiel, L.; Angulo, C. The potential use of Debaryomyces hansenii for the biological control of pathogenic fungi in food. Biol. Control 2018, 121, 216–222. [Google Scholar] [CrossRef]
- Reyes-Perez, J.J.; Hernandez-Montiel, L.G.; Vero, S.; Noa-Carrazana, J.C.; Quiñones-Aguilar, E.E.; Rincón-Enríquez, G. Postharvest biocontrol of Colletotrichum gloeosporioides on mango using the marine bacterium Stenotrophomonas rhizophila and its possible mechanisms of action. J. Food Sci. Technol. 2019, 56, 4992–4999. [Google Scholar] [CrossRef]
- Rivas-Garcia, T.; Murillo-Amador, B.; Nieto-Garibay, A.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G.; Hernandez-Montiel, L.G. Effect of Ulvan on the Biocontrol Activity of Debaryomyces hansenii and Stenotrophomonas rhizophila against Fruit Rot of Cucumis melo L. Agronomy 2018, 8, 273. [Google Scholar] [CrossRef] [Green Version]
- Ribbeck-Busch, K.; Röder, A.; Hasse, D.; De Boer, W.; Martínez, J.L.; Hagemann, M.; Berg, G. A molecular biological protocol to distinguish potentially human pathogenic Stenotrophomonas maltophilia from plant-associated Stenotrophomonas rhizophila. Environ. Microbiol. 2005, 7, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, M.; Droby, S.; Norelli, J.; Liu, J.; Schena, L. Alternative management technologies for postharvest disease control: The journey from simplicity to complexity. Postharvest Biol. Technol. 2016, 122, 3–10. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- Spadaro, D.; Gullino, M.L. State of the art and future prospects of the biological control of postharvest fruit diseases. Int. J. Food Microbiol. 2004, 91, 185–194. [Google Scholar] [CrossRef]
- Teixidó, N.; Torres, R.; Viñas, I.; Abadias, M.; Usall, J. Biological Control of Postharvest Diseases in Fruit and Vegetables. In Protective Cultures, Antimicrobial Metabolites and Bacteriophages for Food and Beverage Biopreservation; Elsevier: Amsterdam, The Netherlands, 2011; pp. 364–402. [Google Scholar]
- Blackburn, D.; Shapiro-Ilan, D.I.; Adams, B.J. Biological control and nutrition: Food for thought. Biol. Control 2016, 97, 131–138. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Yin, J. Effects of hot air treatment in combination with Pichia guilliermondiion postharvest preservation of peach fruit. J. Sci. Food Agric. 2018, 99, 647–655. [Google Scholar] [CrossRef]
- He, F.; Zhao, L.; Zheng, X.; Abdelhai, M.H.; Boateng, N.S.; Zhang, X.; Zhang, H. Investigating the effect of methyl jasmonate on the biocontrol activity of Meyerozyma guilliermondii against blue mold decay of apples and the possible mechanisms involved. Physiol. Mol. Plant Pathol. 2019, 109, 101454. [Google Scholar] [CrossRef]
- Aguirre-Güitrón, L.; Calderón-Santoyo, M.; Bautista-Rosales, P.U.; Ragazzo-Sánchez, J.A. Application of powder formulation of Meyerozyma caribbica for postharvest control of Colletotrichum gloeosporioides in mango (Mangifera indica L.). LWT 2019, 113, 108271. [Google Scholar] [CrossRef]
- Restuccia, C.; Lombardo, M.; Scavo, A.; Mauromicale, G.; Cirvilleri, G. Combined application of antagonistic Wickerhamomyces anomalus BS91 strain and Cynara cardunculus L. leaf extracts for the control of postharvest decay of citrus fruit. Food Microbiol. 2020, 92, 103583. [Google Scholar] [CrossRef] [PubMed]
- Moretto, C.; Cervantes, A.L.L.; Filho, A.B.; Kupper, K.C. Integrated control of green mold to reduce chemical treatment in post-harvest citrus fruits. Sci. Hortic. 2014, 165, 433–438. [Google Scholar] [CrossRef]
- Navarta, L.G.; Calvo, J.; Posetto, P.; Benuzzi, D.; Sanz, M.I. Freeze-drying of a mixture of bacterium and yeast for application in postharvest control of pathogenic fungi. SN Appl. Sci. 2020, 2, 1223. [Google Scholar] [CrossRef]
- Ebrahimi, L.; Etebarian, H.R.; Aminian, H.; Sahebani, N. Effect of Metschnikowia pulcherrima and methyl jasmonate on apple blue mold disease and the possible mechanisms involved. Phytoparasitica 2013, 41, 515–519. [Google Scholar] [CrossRef]
- Farahani, L.; Etebarian, H.R. Enhancement of the efficacy of two antagonistic yeasts with salicylic acid against Penicillium expansum. Arch. Phytopathol. Plant Prot. 2012, 45, 260–267. [Google Scholar] [CrossRef]
- Yu, T.; Zheng, X.D. Salicylic Acid Enhances Biocontrol Efficacy of the Antagonist Cryptococcus laurentii in Apple Fruit. J. Plant Growth Regul. 2006, 25, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Yu, T.; Chen, R.; Huang, B.; Wu, V.C.-H. Inhibiting Penicillium expansum infection on pear fruit by Cryptococcus laurentii and cytokinin. Postharvest Biol. Technol. 2007, 45, 221–227. [Google Scholar] [CrossRef]
- Nunes, C.A. Biological control of postharvest diseases of fruit. Eur. J. Plant Pathol. 2012, 133, 181–196. [Google Scholar] [CrossRef]
- Rivas-Garcia, T.; Murillo-Amador, B.; Nieto-Garibay, A.; Rincon-Enriquez, G.; Chiquito-Contreras, R.G.; Hernandez-Montiel, L.G. Enhanced biocontrol of fruit rot on muskmelon by combination treatment with marine Debaryomyces hansenii and Stenotrophomonas rhizophila and their potential modes of action. Postharvest Biol. Technol. 2019, 151, 61–67. [Google Scholar] [CrossRef]
- García, T.R.; Montiel, L.G.H.; Amador, B.M.; Garibay, A.N.; Contreras, R.G.C.; Enriquez, G.R. Identification and Characterization of Fusarium spp. from muskmelon in northwest Mexico. Biotecnia 2018, 20, 71–75. [Google Scholar] [CrossRef]
- Montiel, L.G.H.; Rodriguez, R.Z.; Angulo, C.; Puente, E.O.R.; Aguilar, E.E.Q.; Galicia, R. Marine yeasts and bacteria as biological control agents against anthracnose on mango. J. Phytopathol. 2017, 165, 833–840. [Google Scholar] [CrossRef]
- Chiquito-Contreras, R.G.; Murillo-Amador, B.; Carmona-Hernandez, S.; Chiquito-Contreras, C.J.; Hernandez-Montiel, L.G. Effect of Marine Bacteria and Ulvan on the Activity of Antioxidant Defense Enzymes and the Bio-Protection of Papaya Fruit against Colletotrichum gloeosporioides. Antioxidants 2019, 8, 580. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Ran, W.; Wang, H.; Li, X.; Shen, Q.; Shen, S.; Xu, Y. Biocontrol of Fusarium wilt disease in muskmelon with Bacillus subtilis Y-IVI. BioControl 2012, 58, 283–292. [Google Scholar] [CrossRef]
- Lima, J.; Gondim, D.; Oliveira, J.; Oliveira, F.; Goncalves, L.R.B.; Viana, F. Use of killer yeast in the management of postharvest papaya anthracnose. Postharvest Biol. Technol. 2013, 83, 58–64. [Google Scholar] [CrossRef]
- Chanchaichaovivat, A.; Panijpan, B.; Ruenwongsa, P. Putative modes of action of Pichia guilliermondii strain R13 in controlling chilli anthracnose after harvest. Biol. Control 2008, 47, 207–215. [Google Scholar] [CrossRef]
- Jamalizadeh, M.; Etebarian, H.R.; Aminian, H.; Alizadeh, A. A review of mechanisms of action of biological control organisms against post-harvest fruit spoilage. EPPO Bull. 2011, 41, 65–71. [Google Scholar] [CrossRef]
- Zhao, Y.; Tu, K.; Shao, X.; Jing, W.; Su, Z. Effects of the yeast Pichia guilliermondii against Rhizopus nigricans on tomato fruit. Postharvest Biol. Technol. 2008, 49, 113–120. [Google Scholar] [CrossRef]
- Pech, J.; Bouzayen, M.; Latché, A. Climacteric fruit ripening: Ethylene-dependent and independent regulation of ripening pathways in melon fruit. Plant Sci. 2008, 175, 114–120. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Yang, Z.; Hu, Z.; Zheng, Y. The effects of the combination of Pichia membranefaciens and BTH on controlling of blue mould decay caused by Penicillium expansum in peach fruit. Food Chem. 2011, 124, 991–996. [Google Scholar] [CrossRef]
- Gupta, N.; Jain, S.K. Storage behavior of mango as affected by post harvest application of plant extracts and storage conditions. J. Food Sci. Technol. 2012, 51, 2499–2507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwel publishing: Ames, IA, USA, 2008; ISBN 0813819199. [Google Scholar]
- Zhang, J.; Bruton, B.D.; Biles, C.L. Fusarium solani endo-polygalacturonase from decayed muskmelon fruit: Purification and characterization. Physiol. Mol. Plant Pathol. 1999, 54, 171–186. [Google Scholar] [CrossRef]
- Burketova, L.; Trda, L.; Ott, P.G.; Valentova, O. Bio-based resistance inducers for sustainable plant protection against pathogens. Biotechnol. Adv. 2015, 33, 994–1004. [Google Scholar] [CrossRef]
- Arras, G.; Arru, S. Integrated control of postharvest citrus decay and induction of phytoalexins by Debaryomyces hansenii. Adv. Hortic. Sci. 1999, 13, 76–81. [Google Scholar]
- Romanazzi, G.; Sanzani, S.M.; Bi, Y.; Tian, S.; Martínez, P.G.; Alkan, N. Induced resistance to control postharvest decay of fruit and vegetables. Postharvest Biol. Technol. 2016, 122, 82–94. [Google Scholar] [CrossRef]
- Jaulneau, V.; Lafitte, C.; Jacquet, C.; Fournier, S.; Salamagne, S.; Briand, X.; Esquerré-Tugayé, M.-T.; Dumas, B. Ulvan, a Sulfated Polysaccharide from Green Algae, Activates Plant Immunity through the Jasmonic Acid Signaling Pathway. J. Biomed. Biotechnol. 2010, 2010, 525291. [Google Scholar] [CrossRef] [Green Version]
- Paulert, R.; Ebbinghaus, D.; Urlass, C.; Moerschbacher, B.M. Priming of the oxidative burst in rice and wheat cell cultures by ulvan, a polysaccharide from green macroalgae, and enhanced resistance against powdery mildew in wheat and barley plants. Plant Pathol. 2010, 59, 634–642. [Google Scholar] [CrossRef]
- Cluzet, S.; Torregrosa, C.; Jacquet, C.; Lafitte, C.; Fournier, J.; Mercier, L.; Salamagne, S.; Briand, X.; Esquerre-Tugaye, M.-T.; Dumas, B. Gene expression profiling and protection of Medicago truncatula against a fungal infection in response to an elicitor from green algae Ulva spp. Plant Cell Environ. 2004, 27, 917–928. [Google Scholar] [CrossRef]
- Bi, Y.; Ge, Y.; Li, Y.; Wang, J.; Miao, X.; Li, X. Postharvest Acibenzolar-S-Methyl Treatment Suppresses Decay and Induces Resistance in Hami Melons. Acta Hortic. 2006, 712, 393–399. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Y.; Bi, Y.; Ge, Y.; Wang, Y.; Fan, C.; Li, D.; Deng, H. Postharvest BTH treatment induced disease resistance and enhanced reactive oxygen species metabolism in muskmelon (Cucumis melo L.) fruit. Eur. Food Res. Technol. 2012, 234, 963–971. [Google Scholar] [CrossRef]
- Zhang, Z.; Bi, Y.; Ge, Y.; Wang, J.; Deng, J.; Xie, D.; Wang, Y. Multiple pre-harvest treatments with acibenzolar-S-methyl reduce latent infection and induce resistance in muskmelon fruit. Sci. Hortic. 2011, 130, 126–132. [Google Scholar] [CrossRef]
- Tian, S.; Torres, R.; Ballester, A.-R.; Li, B.; Vilanova, L.; González-Candelas, L. Molecular aspects in pathogen-fruit interactions: Virulence and resistance. Postharvest Biol. Technol. 2016, 122, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Tian, S.; Qin, G.; Li, B.; Wang, Q.; Meng, X. Effects of salicylic acid on disease resistance and postharvest decay control of fruits. Stewart Postharvest Rev. 2007, 3, 1–7. [Google Scholar] [CrossRef]
- Lacan, D.; Baccou, J.-C. High levels of antioxidant enzymes correlate with delayed senescence in nonnetted muskmelon fruits. Planta 1998, 204, 377–382. [Google Scholar] [CrossRef]
- Dov, P.; Ludovica, G.M. Post-Harvest Pathology; Springer International Publishing: Cham, Switzerland, 2014. [Google Scholar]
- Jetiyanon, K. Defensive-related enzyme response in plants treated with a mixture of Bacillus strains (IN937a and IN937b) against different pathogens. Biol. Control 2007, 42, 178–185. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, J.; Zhou, Y.; Deng, L.; Yao, S.; Zeng, K. Inhibitory effect of Pichia membranaefaciens and Kloeckera apiculata against Monilinia fructicola and their biocontrol ability of brown rot in postharvest plum. Biol. Control 2017, 114, 51–58. [Google Scholar] [CrossRef]
- Levy, Y.; Benderly, M.; Cohen, Y.; Gisi, U.; Bassand, D. The joint action of fungicides in mixtures: Comparison of two methods for synergy calculation. EPPO Bull. 1986, 16, 651–657. [Google Scholar] [CrossRef]
- Zhimo, V.Y.; Dilip, D.; Sten, J.; Ravat, V.K.; Bhutia, D.D.; Panja, B.; Saha, J. Antagonistic Yeasts for Biocontrol of the Banana Postharvest Anthracnose Pathogen Colletotrichum musae. J. Phytopathol. 2016, 165, 35–43. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Johansson, L.H.; Borg, L.A.H. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- Cavalcanti, F.R.; Oliveira, J.T.A.; Martins-Miranda, A.S.; Viégas, R.A.; Silveira, J.A.G. Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt-stressed cowpea leaves. New Phytol. 2004, 163, 563–571. [Google Scholar] [CrossRef]
- Paoletti, F.; Aldinucci, D.; Mocali, A.; Caparrini, A. A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal. Biochem. 1986, 154, 536–541. [Google Scholar] [CrossRef]
- Uritani, I.; Tanaka, Y.; Kojima, M. Properties, development and cellular-localization of cinnamic acid 4-hydroxylase in cut-injured sweet potato1. Plant Cell Physiol. 1974, 15, 843–854. [Google Scholar] [CrossRef]
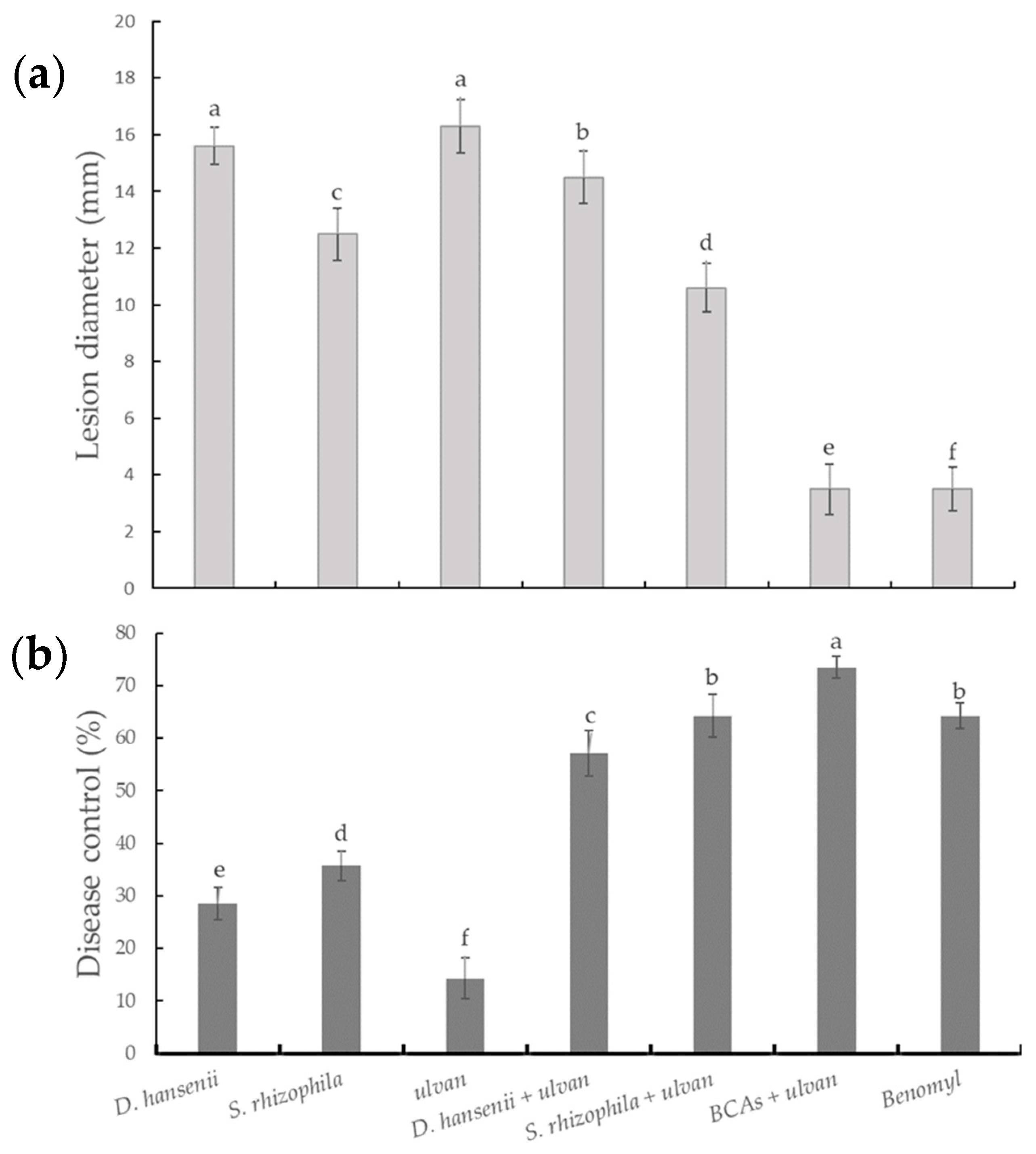
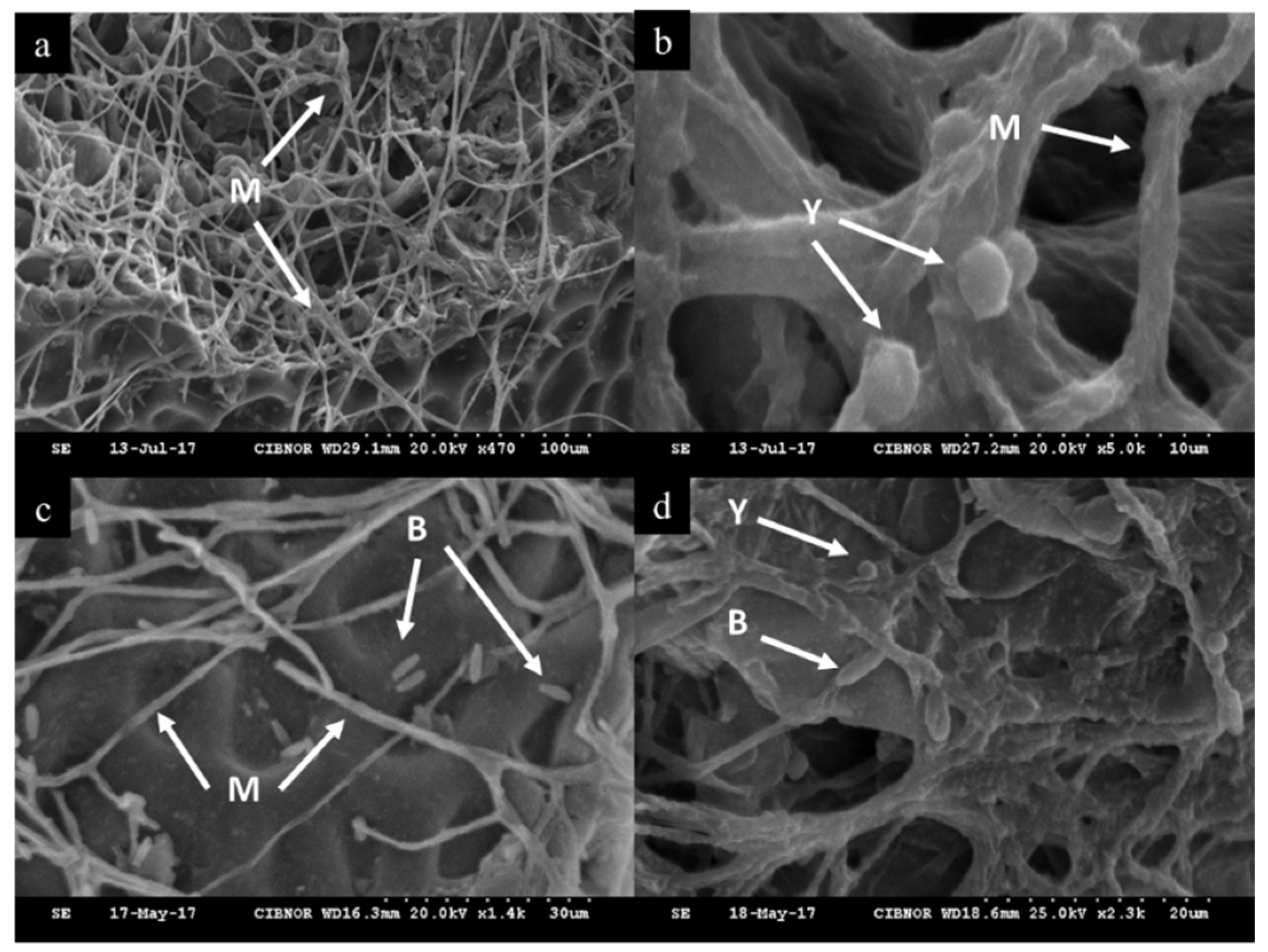

| Treatment | DCE * | SF |
|---|---|---|
| D. hansenii | - | - |
| S. rhizophila | - | - |
| Ulvan | - | - |
| D. hansenii + S. rhizophila | 49.2 | 1.7 |
| D. hansenii + ulvan | 38.4 | 1.5 |
| S. rhizophila + ulvan | 44.4 | 1.4 |
| BCAs + ulvan | 55.1 | 1.8 |
| Benomyl | - | - |
| Treatment | Before (h) | After (h) | |||
|---|---|---|---|---|---|
| 24 | 12 | 2 | 12 | 24 | |
| D. hansenii | 39.7 ± 2.3 g,* | 35.2 ± 2.3 g | 28.6 ± 3.1 f | 17.2 ± 1.4 g | 10.7 ± 1.8 g |
| S. rhizophila | 44.3 ± 3.1 f | 40.2 ± 3.3 f | 35.7 ± 2.8 e | 20.6 ± 2.3 f | 13.6 ± 3.3 e |
| Ulvan | 48.9 ± 2.1 e | 27.4 ± 1.4 h | 14.3 ± 3.9 g | 7.2 ± 2.1 h | 5.3 ± 1.7 h |
| BCAs | 75.8 ± 1.1 b | 72.5 ± 1.8 b | 68.2 ± 3.5 b | 39.2 ± 3.8 c | 18.3 ± 3.4 b |
| D. hansenii + ulvan | 70.2 ± 1.3 c | 60.3 ± 2.2 e | 57.1 ± 4.3 d | 34.3 ± 1.6 d | 11.1 ± 2.1 f |
| S. rhizophila + ulvan | 75.3 ± 2.4 b | 68.8 ± 4.1 c | 64.3 ± 4.1 c | 25.7 ± 2.0 e | 15.7 ± 1.1 d |
| BCAs + ulvan | 87.6 ± 2.3 a | 80.7 ± 3.4 a | 73.5 ± 2.1 a | 40.1 ± 1.2 b | 17.4 ± 1.3 c |
| Benomyl | 66.5 ± 2.2 d | 65.7 ± 3.2 d | 64.3 ± 2.4 c | 60.5 ± 2.4 a | 59.5 ± 1.2 a |
| Treatment | Before (h) | After (h) | |||
|---|---|---|---|---|---|
| 24 | 12 | 2 | 12 | 24 | |
| D. hansenii | 11.5 ± 0.5 b,* | 12.5 ± 0.7 b | 15.6 ± 0.7 c | 20.8 ± 0.9 c | 23.4 ± 0.7 d |
| S. rhizophila | 9.8 ± 0.6 d | 10.5 ± 0.8 c | 12.5 ± 0.9 e | 18.3 ± 0.5 e | 24.5 ± 1.3 c |
| Ulvan | 8.5 ± 0.3 e | 10.6 ± 0.6 c | 16.3 ± 0.9 b | 24.7 ± 1.1 b | 27.7 ± 0.8 b |
| BCAs | 7.8 ± 0.2 f | 8.7 ± 0.3 d | 10.6 ± 0.2 f | 15.3 ± 0.9 g | 17.1 ± 1.3 g |
| D. hansenii + ulvan | 10.2 ± 0.9 c | 12.4 ± 1.2 b | 14.5 ± 0.9 d | 19.4 ± 0.8 d | 20.6 ± 0.9 e |
| S. rhizophila + ulvan | 5.4 ± 0.2 h | 6.0 ± 0.3 e | 6.5 ± 0.9 g | 17.7 ± 1.1 f | 18.1 ± 1.2 f |
| BCAs + ulvan | 1.7 ± 0.2 i | 2.3 ± 0.1 f | 3.5 ± 0.2 h | 14.5 ± 0.3 h | 18.5 ± 0.1 f |
| Benomyl | 6.2 ± 0.3 g | 6.4 ± 0.2 e | 6.5 ± 0.7 g | 6.9 ± 0.5 i | 6.7 ± 0.3 h |
| Control | 27.2 ± 0.7 a | 27.8 ± 1.3 a | 28.4 ± 1.2 a | 28.8 ± 0.9 a | 29.1 ± 1.2 a |
| Treatment | DI (%) | Weight Loss (g) | Firmness (N) | TSS (%) | pH |
|---|---|---|---|---|---|
| D. hansenii | 33.3 ± 1.2 b,* | 0.30 ± 0.02 c | 4.2 ± 0.5 c | 9.2 ± 0.08 a | 6.5 ± 0.1 a |
| S. rhizophila | 26.7 ± 1.6 c | 0.30 ± 0.01 c | 4.2 ± 0.4 c | 9.2 ± 0.09 a | 6.5 ± 0.1 a |
| Ulvan | 23.3 ± 0.8 d | 0.24 ± 0.03 e | 4.2 ± 0.4 c | 9.3 ± 0.06 a | 6.1 ± 0.1 b |
| BCAs | 17.2 ± 1.1 f | 0.26 ± 0.02 d | 4.3 ± 0.3 a | 9.3 ± 0.04 a | 6.2 ± 0.2 b |
| D. hansenii + ulvan | 20.0 ± 1.2 e | 0.21 ± 0.03 f | 4.3 ± 0.3 a | 9.3 ± 0.08 a | 6.2 ± 0.1 b |
| S. rhizophila + ulvan | 13.3 ± 0.7 g | 0.22 ± 0.02 f | 4.3 ± 0.3 a | 9.3 ± 0.06 a | 6.1 ± 0.1 b |
| BCAs + ulvan | 8.3 ± 0.8 i | 0.21 ± 0.02 f | 4.3 ± 0.2 a | 9.3 ± 0.05 a | 6.0 ± 0.3 c |
| Benomyl | 10.0 ± 0.4 h | 0.68 ± 0.05 b | 4.1 ± 0.8 b | 9.2 ± 0.08 a | 6.6 ± 0.1 a |
| Control | 70.0 ± 1.4 a | 1.06 ± 0.08 a | 4.0 ± 0.6 d | 9.2 ± 0.07 a | 6.6 ± 0.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivas-Garcia, T.; Murillo-Amador, B.; Reyes-Pérez, J.J.; Chiquito-Contreras, R.G.; Preciado-Rangel, P.; Ávila-Quezada, G.D.; Lara-Capistran, L.; Hernandez-Montiel, L.G. Debaryomyces hansenii, Stenotrophomonas rhizophila, and Ulvan as Biocontrol Agents of Fruit Rot Disease in Muskmelon (Cucumis melo L.). Plants 2022, 11, 184. https://doi.org/10.3390/plants11020184
Rivas-Garcia T, Murillo-Amador B, Reyes-Pérez JJ, Chiquito-Contreras RG, Preciado-Rangel P, Ávila-Quezada GD, Lara-Capistran L, Hernandez-Montiel LG. Debaryomyces hansenii, Stenotrophomonas rhizophila, and Ulvan as Biocontrol Agents of Fruit Rot Disease in Muskmelon (Cucumis melo L.). Plants. 2022; 11(2):184. https://doi.org/10.3390/plants11020184
Chicago/Turabian StyleRivas-Garcia, Tomas, Bernardo Murillo-Amador, Juan J. Reyes-Pérez, Roberto G. Chiquito-Contreras, Pablo Preciado-Rangel, Graciela D. Ávila-Quezada, Liliana Lara-Capistran, and Luis G. Hernandez-Montiel. 2022. "Debaryomyces hansenii, Stenotrophomonas rhizophila, and Ulvan as Biocontrol Agents of Fruit Rot Disease in Muskmelon (Cucumis melo L.)" Plants 11, no. 2: 184. https://doi.org/10.3390/plants11020184
APA StyleRivas-Garcia, T., Murillo-Amador, B., Reyes-Pérez, J. J., Chiquito-Contreras, R. G., Preciado-Rangel, P., Ávila-Quezada, G. D., Lara-Capistran, L., & Hernandez-Montiel, L. G. (2022). Debaryomyces hansenii, Stenotrophomonas rhizophila, and Ulvan as Biocontrol Agents of Fruit Rot Disease in Muskmelon (Cucumis melo L.). Plants, 11(2), 184. https://doi.org/10.3390/plants11020184










