Molecular Authentication, Phytochemical Evaluation and Asexual Propagation of Wild-Growing Rosa canina L. (Rosaceae) Genotypes of Northern Greece for Sustainable Exploitation
Abstract
:1. Introduction
2. Results
2.1. Authentication Efficiency of ITS2
2.2. Phytochemical Analysis of Greek Native Rosa canina Rosehips
2.3. Preliminary Propagation Trials
2.4. Assessment of Greek Native Rosa canina Genotypes
2.5. Experimentation on Asexual Propagation of R. canina
3. Discussion
3.1. Molecular Authentication of Greek Native Genotypes of Rosa canina
3.2. Phytochemical Potential of Greek Native Genotypes of Rosa canina
3.3. Propagation Potential of Greek Native Genotypes
4. Materials and Methods
4.1. Plant Populations of Rosa canina Sampled
4.2. DNA Isolation
4.3. Polymerase Chain Reaction (PCR) Amplification
4.4. Sequence Analysis
4.5. Molecular Data Analysis
4.6. Phylogenetic Relationships
4.7. Phytochemical Analysis of Rosa canina Rosehips
4.8. Preliminary Propagation Trials and Mother Plants’ Growth Conditions
4.9. Propagation Experimental Design, Cutting Types, Hormone Applications and Rooting Conditions
4.10. Cuttings’ Performance and Growth Measurements
4.11. Statistical Analysis of Rooting Data and Phytochemical Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Young, M.A.; Schorr, P. Modern Roses, 12th ed.; Young, M.A., Ed.; American Rose Society: Shreveport, LA, USA, 2007; pp. 1–576. ISBN 1597250988. [Google Scholar]
- Suprun, I.I.; Plugatar, S.A.; Stepanov, I.V.; Naumenko, T.S. Analysis of genetic relationships of genotypes of the genus Rosa L. from the collection of Nikita Botanical Gardens using ISSR and IRAP DNA markers. Vavilovskii Zhurnal Genet. Sel. 2020, 24, 474–480. [Google Scholar] [CrossRef]
- Wylie, A.P. The history of garden roses, part 1. J. R. Hortic. Soc. 1954, 79, 555–571. [Google Scholar]
- Tomljenovic, N.; Pejić, I. Taxonomic review of the genus Rosa. Agric. Conspec. Sci. 2018, 83, 139–147. Available online: https://hrcak.srce.hr/203011 (accessed on 1 November 2021).
- Wissemann, V.; Ritz, C. Evolutionary patterns and processes in the genus Rosa (Rosaceae) and their implications for host-parasite co-evolution. Plant Syst. Evol. 2007, 266, 79–89. [Google Scholar] [CrossRef]
- Pang, X.H.; Song, J.Y.; Zhu, Y.J.; Xu, H.X.; Huang, L.F.; Chen, S.L. Applying plant DNA barcodes for Rosaceae species identification. Cladistics 2010, 27, 165–170. [Google Scholar] [CrossRef]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; De Waard, J.R. Biological identifications through DNA barcodes. Proc. Royal Soc. B 2003, 270, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Millan, T.; Osuna, F.; Cobos, S.; Torres, A.M.; Cubero, J.I. Using RAPDs to study phylogenetic relationships in Rosa. Theor. Appl. Genet. 1996, 92, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, A.; Starr, J.R.; Joly, S. Phylogenetic relationships in the genus Rosa: New evidence from chloroplast DNA sequences and an appraisal of current knowledge. Syst. Bot. 2007, 32, 366–378. [Google Scholar] [CrossRef]
- Oğraş, T.L.; Baştanlar, E.K.; Metin Karakaş, Ö.; Kandemir, I.; Özcelik, H. Assessment of genetic diversity of rose genotypes using ISSR markers. Turk. J. Bot. 2017, 41, 347–355. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, V.; Haq, S.U.; Jatav, P.K.; Kothari, S.L.; Kachhwaha, S. Assessment of genetic diversity in 29 rose germplasms using SCoT marker. J. King Saud Univ. Sci. 2019, 31, 780–788. [Google Scholar] [CrossRef]
- Zhang, L.H.; Byrne, D.H.; Ballard, R.E.; Rajapakse, S. Microsatellite marker development in rose and its application in tetraploid mapping. J. Am. Soc. Hortic. Sci. 2006, 131, 380–387. [Google Scholar] [CrossRef] [Green Version]
- Crespel, L.; Pernet, A.; Le Bris, M.; Gudin, S.; Saint-Oyant, L.H. Application of ISSRs for cultivar identification and assessment of genetic relationships in rose. Plant Breed. 2009, 12, 501–506. [Google Scholar] [CrossRef]
- Koopman, W.J.M.; Wissemann, V.; De Cock, K.; Van Huylenbroeck, J.; De Riek, J.; Sabatino, G.J.H.; Visser, D.; Vosman, B.; Ritz, C.M.; Maes, B.; et al. AFLP markers as a tool to reconstruct complex relationships: A case study in Rosa (Rosaceae). Am. J. Bot. 2008, 95, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Pang, X.; Song, J.; Shi, L.; Yao, H.; Han, J.; Leon, C. A renaissance in herbal medicine identification: From morphology to DNA. Biotechol. Adv. 2014, 32, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Hirakawa, H.; Sato, S.; Otagaki, S.; Matsumoto, S.; Tabata, S.; Tanaka, Y. Genome structure of Rosa multiflora, a wild ancestor of cultivated roses. DNA Res. 2018, 2, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saint-Oyant, L.H.; Ruttink, T.; Hamama, L.; Kirov, I.; Lakhwani, D.; Zhou, N.N.; Bourke, P.M.; Daccord, N.; Leus, L.; Schulz, D.; et al. A high-quality genome sequence of Rosa chinensis to elucidate ornamental traits. Nat. Plants 2018, 4, 473–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourke, P.M.; Arens, P.; Voorrips, R.E.; Esselink, G.D.; Koning-Boucoiran, C.F.; van’t Westende, W.P.; Santos Leonardo, T.; Wissink, P.; Zheng, C.; van Geest, G.; et al. Partial preferential chromosome pairing is genotype dependent in tetraploid rose. Plant J. 2017, 90, 330–343. [Google Scholar] [CrossRef] [Green Version]
- Raymond, O.; Gouzy, J.; Just, J.; Badouin, H.; Verdenaud, M.; Lemainque, A.; Vergne, P.; Moja, S.; Choisne, N.; Pont, C.; et al. The Rosa genome provides new insights into the domestication of modern roses. Nat. Genet. 2018, 50, 772–777. [Google Scholar] [CrossRef]
- Dubois, A.; Carrere, S.; Raymond, O.; Pouvreau, B.; Cottret, L.; Roccia, A.; Onesto, J.P.; Sakr, S.; Atanassova, R.; Baudino, S.; et al. Transcriptome database resource and gene expression atlas for the rose. BMC Genom. 2012, 13, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Cao, X.; Shi, S.; Zhao, N.; Li, D.; Fang, P.; Chen, X.; Qi, W.; Zhang, Z. Comparative RNA-Seq analysis reveals a critical role for brassinosteroids in rose (Rosa hybrida) petal defense against Botrytis cinerea infection. BMC Genet. 2018, 19, 62. [Google Scholar] [CrossRef]
- Neu, E.; Domes, H.S.; Menz, I.; Kaufmann, H.; Linde, M.; Debener, T. Interaction of roses with a biotrophic and a hemibiotrophic leaf pathogen leads to differences in defense transcriptome activation. Plant Mol. Biol. 2019, 99, 299–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pehlivan, M.; Mohammed, F.; Sevindik, M.; Akgul, H. Antioxidant and oxidant potential of Rosa canina. Eurasian J. Forest Sci. 2018, 6, 22–25. [Google Scholar] [CrossRef]
- Ayati, Z.; Amiri, M.S.; Ramezani, M.; Delshad, E.; Sahebkar, A.; Emami, S.A. Phytochemistry, traditional uses and pharmacological profile of rose hip: A review. Curr. Pharm. Des. 2018, 24, 4101–4124. [Google Scholar] [CrossRef] [PubMed]
- Paunović, D.; Kalušević, A.; Petrović, T.; Urošević, T.; Djinović, D.; Nedović, V.; Popović-Djordjević, J. Assessment of chemical and antioxidant properties of fresh and dried rosehip (Rosa canina L.). Not. Bot. Hort Agrobot. 2018, 47, 108–113. [Google Scholar] [CrossRef] [Green Version]
- Gruenwald, J.; Uebelhack, R.; Moré, M.I. Rosa canina—Rose hip pharmacological ingredients and molecular mechanics counteracting osteoarthritis—A systematic review. Phytomedicine 2019, 60, 152958. [Google Scholar] [CrossRef]
- Fattahi, S.; Jamei, R.; Hosseini Sarghein, S. Antioxidant and antiradical activities of Rosa canina and Rosa pimpinellifolia fruits from West Azerbaijan. Iran. J. Plant Physiol. 2012, 2, 523–529. [Google Scholar]
- Kerasioti, E.; Apostolou, A.; Kafantaris, I.; Chronis, K.; Kokka, E.; Dimitriadou, C.; Tzanetou, E.N.; Priftis, A.; Koulocheri, S.D.; Haroutounian, S.A.; et al. Polyphenolic composition of Rosa canina, Rosa sempervivens and Pyrocantha coccinea extracts and assessment of their antioxidant activity in human endothelial cells. Antioxidants 2019, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Roman, I.; Stanila, A.; Stanila, S. Bioactive compounds and antioxidant activity of Rosa canina L. biotypes from spontaneous flora of Transylvania. Chem. Cent. J. 2013, 7, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães, R.; Barros, L.; Calhelha, R.C.; Carvalho, A.M.; Queiroz, M.J.R.; Ferreira, I.C. Bioactivity of different enriched phenolic extracts of wild fruits from northeastern Portugal: A comparative study. Plant Foods Hum. Nutr. 2014, 69, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Tumbas, V.T.; Canadanovic-Brunet, J.M.; Cetojevic-Simin, D.D.; Cetkovic, G.S.; Ethilas, S.M.; Gille, L. Effect of rosehip (Rosa canina L.) phytochemicals on stable free radicals and human cancer cells. J. Sci. Food Agric. 2012, 92, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Klastersky, I. Rosa L. In Flora Europaea; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1968; Volume 2, pp. 25–31. ISBN 9780521066624. [Google Scholar]
- Zieliński, J. Mountain Flora of Greece; Strid, A., Ed.; Cambridge University Press: Cambridge, UK, 1986; Volume 1, pp. 387–399. ISBN 9780748602070. [Google Scholar]
- Blythe, E.K.; Sibley, J.L.; Tilt, K.M.; Ruter, J.M. Methods of auxin application in cutting propagation: A review of 70 years of scientific discovery and commercial practice. J. Environ. Hortic. 2007, 25, 166–185. [Google Scholar] [CrossRef]
- Hoşafçi, H.; Arslan, N.; Sarihan, E.O. Propagation of dogrose (Rosa canina L.) plants by softwood cuttings. Acta Hortic. 2005, 690, 139–142. [Google Scholar] [CrossRef]
- Kazankaya, A.; Yörük, E.; Doğan, A. Effect of IBA on rooting of Rosa canina hardwood cuttings from lake Van region, Turkey. Acta Hortic. 2005, 690, 153–158. [Google Scholar] [CrossRef]
- Izadi, Z.; Zarei, H.; Alizadeh, M. Studies on vegetative propagation of Rosa canina. Indian J. Hort. 2012, 69, 598–601. [Google Scholar]
- Maloupa, E.; Krigas, N.; Grigoriadou, K.; Lazari, D.; Tsoktouridis, G. Conservation strategies for native plant species and their sustainable exploitation: Case of the Balkan Botanic Garden of Kroussia, N. Greece. In Floriculture Ornamental Plant Biotechnology, 1st ed.; Teixeira da Silva, J.A., Ed.; Global Science Books: Middlesex, UK, 2008; Volume V, pp. 37–56. ISBN 9784903313122. [Google Scholar]
- Grigoriadou, K.; Sarropoulou, V.; Krigas, N.; Maloupa, E.; Tsoktouridis, G. GIS-facilitated effective propagation protocols of the Endangered local endemic of Crete Carlina diae (Rech. f.) Meusel and A. Kástner (Asteraceae): Serving ex situ conservation needs and its future sustainable utilization as an ornamental. Plants 2020, 9, 1465. [Google Scholar] [CrossRef]
- Krigas, N.; Mouflis, G.; Grigoriadou, K.; Maloupa, E. Conservation of important plants from the Ionian Islands at the Balkan Botanic Garden of Kroussia, N Greece: Using GIS to link the in situ collection data with plant propagation and ex situ cultivation. Biodiver. Conserv. 2010, 19, 3583–3603. [Google Scholar] [CrossRef]
- Krigas, N.; Tsoktouridis, G.; Anestis, I.; Khabbach, A.; Libiad, M.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Lamchouri, F.; Tsiripidis, I.; Tsiafouli, M.A.; et al. Exploring the potential of neglected local endemic plants of three Mediterranean regions in the ornamental sector: Value chain feasibility and readiness timescale for their sustainable exploitation. Sustainability 2021, 13, 2539. [Google Scholar] [CrossRef]
- Libiad, M.; Khabbach, A.; El Haissoufi, M.; Anestis, I.; Lamchouri, F.; Bourgou, S.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Greveniotis, V.; Tsiripidis, I.; et al. Agro-alimentary potential of the neglected and underutilized local endemic plants of Crete (Greece), Rif-Mediterranean coast of Morocco and Tunisia: Perspectives and challenges. Plants 2021, 10, 1770. [Google Scholar] [CrossRef] [PubMed]
- Bourgou, S.; Jilani, I.B.H.; Karous, O.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Khabbach, A.; Libiad, M.; El Haissoufi, M.; Lamchouri, F.; Greveniotis, V.; et al. Medicinal-cosmetic potential of the local endemic plants from of Crete (Greece), Rif-Mediterranean Coast of Morocco and Tunisia: Priorities for sustainable exploitation. Biology 2021. under review. [Google Scholar]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasri, F.; Fadakar, A.; Saba, M.K.; Yousefi, B. Study of indole butyric acid IBA effects on cutting rooting improving some of wild genotypes of damask roses Rosa damascena Mill. J. Agric. Sci. 2015, 60, 263–275. [Google Scholar] [CrossRef]
- Ercişli, S.; Güleryüz, M. A study of the propagation of the hardwood cuttings of some rose hips. Turk. J. Agric. For. 1999, 23, 305–310. Available online: https://atif.sobiad.com/index.jsp?modul=makale-goruntule&id=R3mSOnsBYbO9RkQmEnlm (accessed on 1 November 2021).
- Tawfik, A.A.; Ibrahim, O.H.; Abdul-Hafeez, E.; Ismail, S.A. Effect of cutting type, indol-3-butyric acid and the growing season on rooting of stem cuttings of Rosa hybrida cv Eiffel Tower. J. Plant Prod. 2018, 9, 537–542. [Google Scholar] [CrossRef] [Green Version]
- Abbas, H.; Jaskani, M.J.; Hussain, Z.; Asif, M. Response of rose cuttings against root promoting hormones during spring and autumn. Int. J. Biol. Biotech. 2006, 3, 201–204. Available online: https://www.researchgate.net/publication/259465811_Response_of_rose_cuttings_against_root_promoting_hormones_during_spring_and_autumn (accessed on 28 October 2021).
- Kashefi, M.; Zerei, H.; Bahadori, F. The regulating effect of the growth of indole butyric acid and the time of stem cutting preparation on propagation of damask rose ornamental shrub. J. Ornam. Plants 2014, 4, 49–55. Available online: https://www.sid.ir/en/journal/ViewPaper.aspx?id=438004 (accessed on 28 October 2021).
- Otiende, M.A.; Nyabundi, J.O.; Ngamau, K.; Opala, P. Effects of cutting position of rose rootstock cultivars on rooting and its relationship with mineral nutrient content and endogenous carbohydrates. Sci. Hortic. 2017, 225, 204–212. [Google Scholar] [CrossRef]
- Kinik, E.; Çelikel, F.G. Effects of plant growth promoting bacteria and auxin on cutting propagation of Rosa canina L. Turkish J. Agric. Food Sci. Techn. 2017, 5, 1714–1719. [Google Scholar] [CrossRef] [Green Version]
- Kinik, E.; Çelikel, F.G. Cutting propagation of Rosa canina by mycorrhiza and auxin. Int. J. Agric. Wildlife Sci. 2020, 6, 1–7. [Google Scholar] [CrossRef]
- Izadi, Z.; Zarei, H.; Alizadeh, M. Role of grafting technique on the success of stenting propagation of two rose (Rosa sp.) varieties. Amer. J. Plant Sci. 2013, 4, 41–44. [Google Scholar] [CrossRef] [Green Version]
- Agbaria, H.; Bruria, H.; Zieslin, N. Effects of grafting on transpiration, CO2 fixation and growth of rose plants (Rosa x hybrid cvs Liseta and Mercedes). J. Hortic. Sci. 1995, 70, 651–656. [Google Scholar] [CrossRef]
- Nazari, F.; Khosh-Khui, M.; Salehi, H. Growth and flower quality of four Rosa hybrida L. cultivars in response to propagation by stenting or cutting in soilless culture. Sci. Hortic. 2009, 119, 302–305. [Google Scholar] [CrossRef]
- Chen, S.; Yao, H.; Han, J.; Liu, C.; Song, J.; Shi, L.; Zhu, Y.; Ma, X.; Gao, T.; Pang, X.; et al. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS ONE 2010, 5, e8613. [Google Scholar] [CrossRef] [PubMed]
- Madesis, P.; Ganopoulos, I.; Ralli, P.; Tsaftaris, A. Barcoding the major Mediterranean leguminous crops by combining universal chloroplast and nuclear DNA sequence targets. Genet. Mol. Res. 2012, 11, 2548–2558. [Google Scholar] [CrossRef] [PubMed]
- Ross, H.A.; Murugan, S.; Sibon, L.W.L. Testing the reliability of genetic methods of species identification via simulation. Syst. Biol. 2008, 57, 216–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Vavoura, M.V.; Badeka, A.V.; Kontakos, S.; Kontominas, M.G. Characterization of four popular sweet cherry cultivars grown in Greece by volatile compound and physicochemical data analysis and sensory evaluation. Molecules 2015, 20, 1922–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.S.; Coates, G.A. Vitamin C in frozen, fresh squeezed, unpasteurized, polyethylene-bottled orange juice: A storage study. Food Chem. 1999, 65, 165–168. [Google Scholar] [CrossRef]


| Population Sample | TPC (mg GAE/100 g) | AA (%RSA) | TF (mg CE/100 g) | Vitamin C (mg AAE/100 g) |
|---|---|---|---|---|
| GR-1-BBGK-19,191 | 83.56 ± 0.20 d | 95.82 ± 0.50 a | 1.44 ± 0.20 c | 426.12 ± 0.52 b |
| GR-1-BBGK-03,2229 | 62.98 ± 0.01 f | 95.36 ± 0.40 a | 0.87 ± 0.01 d | 350.38 ± 0.14 e |
| GR-1-BBGK-19,568 | 78.15 ± 0.02 e | 88.41 ± 0.46 b | 1.91 ± 0.02 b | 500.22 ± 0.15 a |
| GR-1-BBGK-19,674 | 90.88 ± 0.02 c | 95.31 ± 0.14 a | 2.46 ± 0.02 a | 398.32 ± 0.58 c |
| GR-1-BBGK-19,640 | 83.88 ± 0.03 d | 95.37 ± 0.80 a | 1.85 ± 0.03 b | 390.30 ± 0.24 d |
| GR-1-BBGK-19,635 | 97.03 ± 0.30 b | 95.71 ± 0.36 a | 2.09 ± 0.30 ab | 344.34 ± 0.55 f |
| GR-1-BBGK-19,504 | 215.46 ± 0.00 a | 95.71 ± 0.00 a | 2.00 ± 0.08 b | 71.85 ± 0.28 g |
| Average | 101.71 ± 48.65 | 94.53 ± 2.59 | 1.80 ± 0.50 | 354.50 ± 128.21 |
| Population Sample (ACN) | Hormone Treatment (ppm IBA) | Mother Plant Development Stage ** | Season of Year | Cutting Type | Rooting (%) |
|---|---|---|---|---|---|
| GR-1-BBGK-19,191 | 10,000 | Dormancy | Winter | Hardwood cuttings | 44.05 |
| GR-1-BBGK-19,191 | 2500 * | Early growth (bud break) | Spring | Softwood cuttings | 75.70 |
| GR-1-BBGK-19,193 | 4000 | Dormancy | Winter | Hardwood cuttings | 10.71 |
| GR-1-BBGK-19,193 | 2500 * | Early growth | Spring | Softwood cuttings | 77.80 |
| GR-1-BBGK-19,674 | 4000 | Late growth | Autumn | Semi-hardwood cuttings | 25.00 |
| GR-1-BBGK-19,568 | 4000 | Advanced growth | Summer | Softwood cuttings | 1.30 |
| GR-1-BBGK-19,579 | 2000 | Advanced growth | Summer | Softwood cuttings | 12.22 |
| GR-1-BBGK-19,635 | 4000 | Advanced growth | Summer | Softwood cuttings | 28.00 |
| IPEN Accession Number | DNA Barcoding | Success of Propagation Trials | Comparative Vitamin C Content | Comparative Total Phenolic Content |
|---|---|---|---|---|
| GR-1-BBGK-19,74 | Effective | - | - | - |
| GR-1-BBGK-19,504 | Effective | - | Low | Very high |
| GR-1-BBGK-19,560 | Effective | - | - | - |
| GR-1-BBGK-19,568 | Effective | Low | Very high | Low |
| GR-1-BBGK-19,635 | - | Low | High | Very high |
| GR-1-BBGK-19,640 | - | - | High | High |
| GR-1-BBGK-19,674 | - | Low | High | Very high |
| GR-1-BBGK-03,2229 | Effective | High | High | Low |
| GR-1-BBGK-19,186 | Effective | - | - | - |
| GR-1-BBGK-19,191 | Effective | High | Very high | High |
| GR-1-BBGK-19,193 | Effective | High | - | - |
| GR-1-BBGK-19,579 | Effective | Low | - | - |
| Substrate Type | Cutting Type | Hormone Treatment (ppm IBA) | Rooting (%) | Root Number | Root Length (mm) |
|---|---|---|---|---|---|
| 3:1 | Apical Cuttings | Control | 0 | 0 | 0 |
| 1000 | 12.5 | 4.00 (±0.00) * | 28.50 (±0.00) | ||
| 2000 | 12.5 | 7.00 (±0.00) | 43.57 (±0.00) | ||
| 4000 | 0 | 0 | 0 | ||
| 6000 | 0 | 0 | 0 | ||
| Sub-apical Cuttings | Control | 0 | 0 | 0 | |
| 1000 | 0 | 0 | 0 | ||
| 2000 | 37.5 † | 5.00 (±1.52) a | 33.57 (±9.93) a | ||
| 4000 | 25.0 | 5.00 (±2.00) a | 23.07(±9.07) a | ||
| 6000 | 0 | 0 | 0 | ||
| 1:1 | Apical Cuttings | Control | 0 | 0 | 0 |
| 1000 | 0 | 0 | 0 | ||
| 2000 | 0 | 0 | 0 | ||
| 4000 | 37.5 † | 6.33 (±2.60) a | 51.79 (±9.31) a | ||
| 6000 | 0 | 0 | 0 | ||
| Sub-apical Cuttings | Control | 0 | 0 | 0 | |
| 1000 | 12.5 | 1.00 (±0.00) | 13.00 (±0.00) | ||
| 2000 | 25.0 | 3.50 (±2.50) a | 40.91 (±13.08) a | ||
| 4000 | 0 | 0 | 0 | ||
| 6000 | 12.5 | 22.00 (±0.00) | 18.59 (±0.00) |
| Substrate Type | Treatment | Rooting (%) | Root Number | Root Length (mm) |
|---|---|---|---|---|
| A | ||||
| 3:1 | Control | 6.25 | 1.00 (±0.00) ** | 30.00 (±00.00) |
| 1000 | 0 | 0 | 0 | |
| 2000 | 12.25 | 5.50 (±1.50) a | 26.17 (±13.67) a | |
| 4000 | 6.25 | 6.00 (±0.00) | 21.33 (±00.00) | |
| 2500 * | 25.00 | 3.00 (±0.91) a | 19.55 (±5.15) a | |
| 1:1 | Control | 6.25 | 3.00 (±0.00) | 56.00 (±00.00) |
| 1000 | 12.25 | 6.00 (±1.00) a | 43.14 (±12.85) a | |
| 2000 | 12.25 | 7.00 (±4.00) a | 80.08 (±27.25) a | |
| 4000 | 25 | 8.25 (±1.18) a | 52.54 (±8.88) a | |
| 2500 * | 12.25 | 7.00 (±3.00) a | 42.72 (±9.47) a | |
| B | ||||
| 3:1 | Control | 0 | 0 | 0 |
| 2000 | 16.7 | 1.00 (±0.16) A | 6.00 (±4.83) A | |
| 4000 | 66.7 † | 3.00 (±1.71) A | 14.75 (±6.65) A |
| Mother Plant Fertilization Status | Hormone Treatment (ppm IBA) | Rooting (%) | Root Number | Root Length (mm) |
|---|---|---|---|---|
| No fertilization | Control | 33.3 | 2.50 (±0.50) a | 20.41 (±2.91) a |
| 2000 | 16.7 | 2.00 (±0.00) * | 5.00 (±0.00) | |
| Conventional | Control | 33.3 | 2.00 (±1.00) a | 67.50 (±42.52) a |
| 2000 | 50.0 † | 3.33 (±0.33) a | 87.77 (±11.39) a | |
| Organic | Control | 16.7 | 3.00 (±0.00) | 86.67 (±0.00) |
| 2000 | 16.7 | 2.00 (±0.00) | 92.50 (±0.00) |
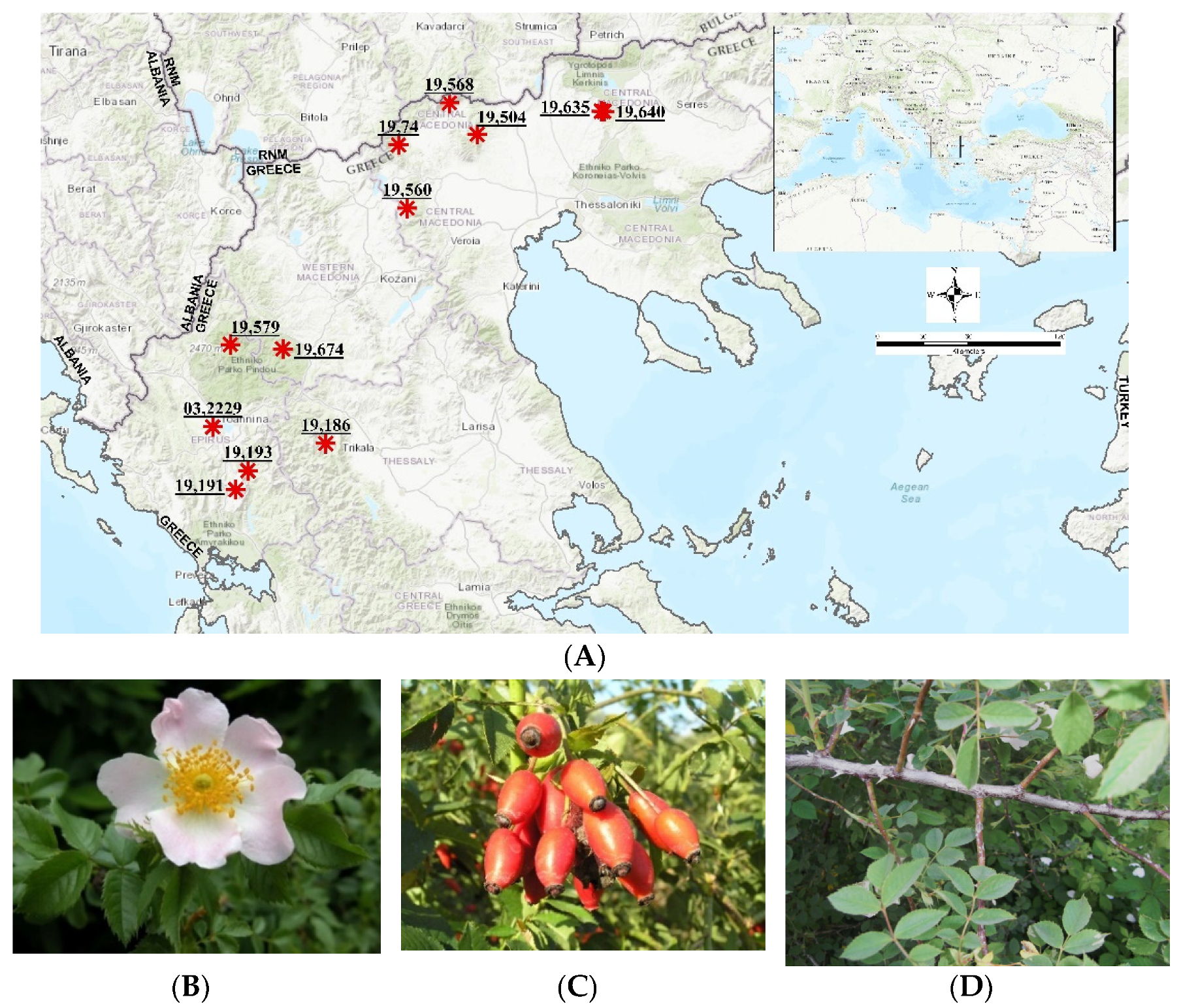
| IPEN Accession Number | Greek Prefecture | Area | Altitude (m) | Sampling |
|---|---|---|---|---|
| GR-1-BBGK-19,74 | Central Macedonia | Mt Voras | 862 | LS |
| GR-1-BBGK-19,504 | Central Macedonia | Kastaneri | 780 | RR, LS |
| GR-1-BBGK-19,560 | Central Macedonia | Mt Vermio | 1615 | LS |
| GR-1-BBGK-19,568 | Central Macedonia | Mt Tzena | 1086 | SWSC, RR, LS |
| GR-1-BBGK-19,635 | Central Macedonia | Mt Kroussia | 650 | SWSC, RR |
| GR-1-BBGK-19,640 | Central Macedonia | Mt Kroussia | 700 | RR |
| GR-1-BBGK-19,674 | Western Macedonia | Ziaka | 900 | SWSC, RR |
| GR-1-BBGK-03,2229 | Epirus | Ioannina | 650 | SWSC, RR, LS |
| GR-1-BBGK-19,186 | Epirus | Mt Lakmos | 1370 | LS |
| GR-1-BBGK-19,191 | Epirus | Anogeia | 1081 | SWSC, RR, LS |
| GR-1-BBGK-19,193 | Epirus | Mt Xirovouni | 1070 | SWSC, LS |
| GR-1-BBGK-19,579 | Epirus | Pades | 1180 | SWSC, LS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maloupa, E.; Karapatzak, E.; Ganopoulos, I.; Karydas, A.; Papanastasi, K.; Kyrkas, D.; Yfanti, P.; Nikisianis, N.; Zahariadis, A.; Kosma, I.S.; et al. Molecular Authentication, Phytochemical Evaluation and Asexual Propagation of Wild-Growing Rosa canina L. (Rosaceae) Genotypes of Northern Greece for Sustainable Exploitation. Plants 2021, 10, 2634. https://doi.org/10.3390/plants10122634
Maloupa E, Karapatzak E, Ganopoulos I, Karydas A, Papanastasi K, Kyrkas D, Yfanti P, Nikisianis N, Zahariadis A, Kosma IS, et al. Molecular Authentication, Phytochemical Evaluation and Asexual Propagation of Wild-Growing Rosa canina L. (Rosaceae) Genotypes of Northern Greece for Sustainable Exploitation. Plants. 2021; 10(12):2634. https://doi.org/10.3390/plants10122634
Chicago/Turabian StyleMaloupa, Eleni, Eleftherios Karapatzak, Ioannis Ganopoulos, Antonis Karydas, Katerina Papanastasi, Dimitris Kyrkas, Paraskevi Yfanti, Nikos Nikisianis, Anthimos Zahariadis, Ioanna S. Kosma, and et al. 2021. "Molecular Authentication, Phytochemical Evaluation and Asexual Propagation of Wild-Growing Rosa canina L. (Rosaceae) Genotypes of Northern Greece for Sustainable Exploitation" Plants 10, no. 12: 2634. https://doi.org/10.3390/plants10122634
APA StyleMaloupa, E., Karapatzak, E., Ganopoulos, I., Karydas, A., Papanastasi, K., Kyrkas, D., Yfanti, P., Nikisianis, N., Zahariadis, A., Kosma, I. S., Badeka, A. V., Patakioutas, G., Fotakis, D., & Krigas, N. (2021). Molecular Authentication, Phytochemical Evaluation and Asexual Propagation of Wild-Growing Rosa canina L. (Rosaceae) Genotypes of Northern Greece for Sustainable Exploitation. Plants, 10(12), 2634. https://doi.org/10.3390/plants10122634










