Essential Role of Potassium in Apple and Its Implications for Management of Orchard Fertilization
Abstract
1. Introduction
2. The Coverage and the Focus of the Review
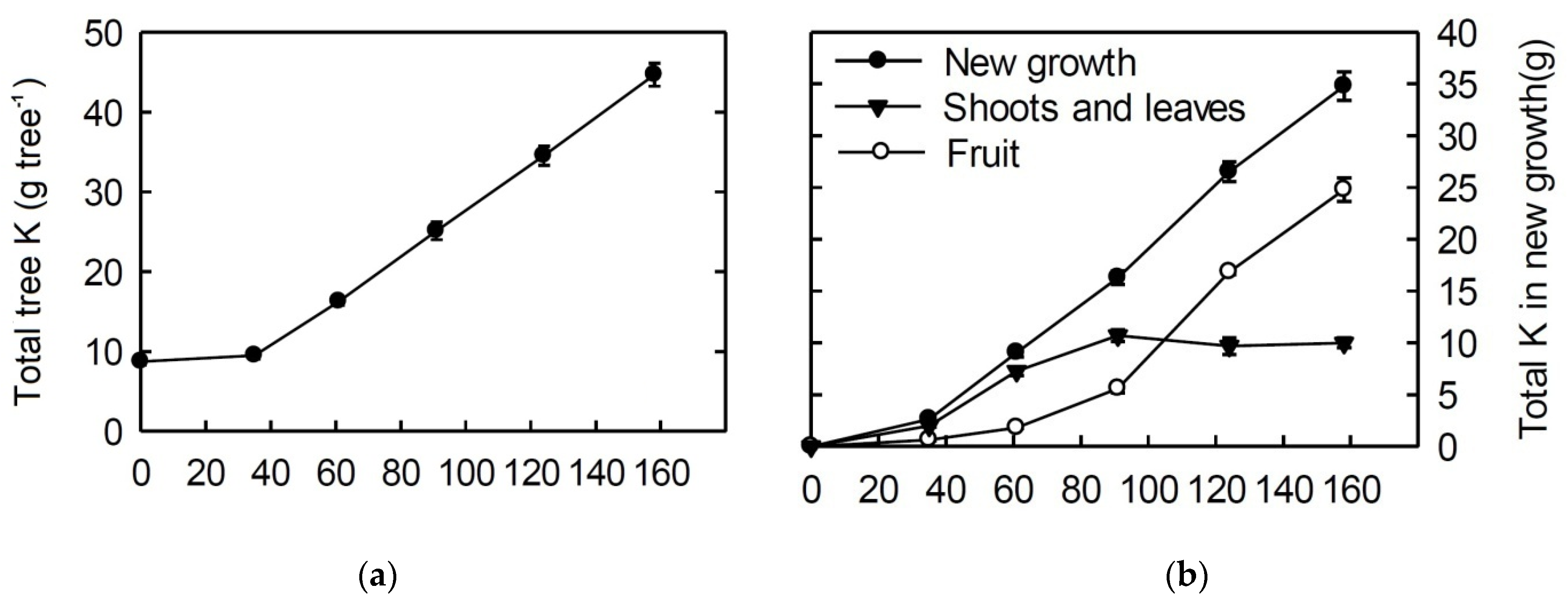
3. Seasonal and Developmental Variation of K Demand in Apple
3.1. Bud Break—Full Bloom (Phases 07–65 BBCH Scale)
3.2. Full Bloom—40-mm Fruit (65–74 BBCH Scale)
3.3. 40-mm Fruit—Picking Maturity (74–87 BBCH Scale)
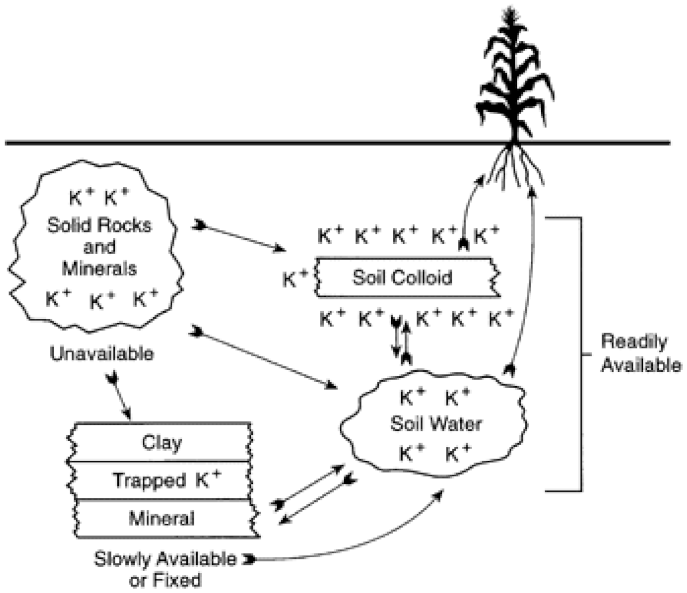
4. Uptake of K by Apple Trees
4.1. Influence of Soil Acidity (Soil pH)
4.2. Interactive Effects of Ions in the Soil on K Uptake
4.3. Soil Temperature and Humidity Effects on K Uptake
4.4. The Application of Microbial Cultures for Soil K Mobilization
5. Approaches to Automated Precision Adjustment of K Application Rate
6. Conclusions and Future Research Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gaerdt, H. Gartnerische Düngerlehre, 1st ed.; Translation from German by Prof. N.I. Kichunov; Typo-Litographia Gerold: Sankt-Petersburg, Russia, 1907; pp. 6–163. (In Russian) [Google Scholar]
- Sardanse, J.; Peñuelas, J. K Control of Plant Functions: Ecological and Agricultural Implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- Maathuis, F.J.M.; Amtmann, A. K+ Nutrition and Na+ Toxicity: The Basis of Cellular K+/Na+ Ratios. Ann. Bot. 1999, 84, 123–133. [Google Scholar] [CrossRef]
- Tränker, M.; Tavakol, A.; Jákli, B. Functioning of K and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Talbot, L.D.; Zeiger, E. Central Roles for K and Sucrose in Guard-Cell Osmoregulation. Plant Physiol. 1996, 111, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, T.T.; Cochrane, T.A. The vital role of K in the osmotic mechanism of stomata aperture modulation and its links with K deficiency. Plant Signal. Behav. 2009, 4, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Shin, R.; Schlachtmann, D.P. Ethylene Mediates Response and Tolerance to K Deprivation in Arabidopsis. Plant Cell 2009, 21, 607–621. [Google Scholar] [CrossRef]
- Araujo, L.; Bispo, W.S.; Rios, V.S.; Fernandes, S.A.; Rodrigues, F.A. Induction of the phenylpropanoid pathway by acibenzolar-S-methyl and K phosphite increases mango resistance to Ceratocystis fimbriata infection. Plant Dis. 2015, 99, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Zheng, C.; Sun, S.; Amjad, H.; Liang, K.; Lin, W. The role of plant cation/proton antiporter gene family in salt tolerance. Biol. Plant. 2018, 62, 617–629. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The Critical Role of K in Plant Stress Response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I. The role of K in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Perrenoud, S. K and Plant Health, 2nd ed.; International Potash Institute: Bern, Switzerland, 1990; pp. 8–10. [Google Scholar]
- Peng, H.X.; Wei, X.Y.; Xiao, Y.X.; Sun, Y.; Biggs, A.R.; Gleason, M.L.; Shang, S.P.; Zhu, M.Q.; Guo, Y.Z.; Sun, G.Y. Management of valsa canker on apple with adjustments to K nutrition. Plant Dis. 2016, 100, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Shankar, A.; Chandran, A.K.N.; Sharma, M.; Jung, K.H.; Suprasanna, P.; Pandey, G.K. Emerging concepts of K homeostasis in plants. J. Exp. Bot. 2019, 71, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Gitea, M.A.; Gitea, D.; Tit, D.M.; Purza, L.; Samuel, A.D.; Bungău, S.; Badea, G.E.; Aleya, L. Orchard management under the effects of climate change: Implications for apple, plum, and almond growing. Environ. Sci. Pollut. Res. 2019, 26, 9908–9915. [Google Scholar] [CrossRef]
- Bai, Q.; Shen, Y.; Huang, Y. Advances in Mineral Nutrition Transport and Signal Transduction in Rosaceae Fruit Quality and Postharvest Storage. Front. Plant Sci. 2021, 12, 620018. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Wang, Y.; Zhang, N.; Guo, Y.; Ren, X.; Zhao, Z. K fertilization arrests malate accumulation and alters soluble sugar metabolism in apple fruit. Biol. Open 2018, 7, bio024745. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, C.; Dong, Q.; Huang, D.; Li, C.; Li, P.; Ma, F. Genome-Wide Identification and Analysis of Apple NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER Family (NPF) Genes Reveals MdNPF6.5 Confers High Capacity for Nitrogen Uptake under Low-Nitrogen Conditions. Int. J. Mol. Sci. 2018, 19, 2761. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Du, X.; Wang, F.; Sha, J.; Chen, Q.; Tian, G.; Zhu, Z.; Ge, S.; Jiang, Y. Effects of K levels on plant growth, accumulation and distribution of carbon, and nitrate metabolism in apple dwarf rootstock seedlings. Front. Plant Sci. 2020, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Zavalonni, C.; Marangoni, B.; Tagliavini, M.; Scudellari, D. Dynamics of Uptake of Calcium, K and Magnesium into Apple Fruit in a High Density Planting. Acta Hortic. 2001, 564, 113–121. [Google Scholar] [CrossRef]
- Brunetto, G.; de Melo, G.W.B.; Toselli, M.; Quartieri, M.; Tagliavini, M. The role of mineral nutrition on yields and fruit quality in grapevine, pear and apple. Rev. Bras. Frutic. 2015, 37, 1089–1104. [Google Scholar] [CrossRef]
- Kuzin, A.I.; Kashirskaya, N.Y.; Kochkina, A.M.; Kushner, A.V. Correction of K Fertigation Rate of Apple Tree (Malus domestica Borkh.) in Central Russia during the Growing Season. Plants 2020, 9, 1366. [Google Scholar] [CrossRef] [PubMed]
- Neilsen, D.; Roberts, T.L. Potassium Fertigation of High Density Apple Orchard. Better Crops 1996, 80, 12–13. Available online: http://www.ipni.net/publication/bettercrops.nsf/0/779E482A09FE907285257D2E004D0A32/$FILE/%20BC-1996-4%20p12.pdf (accessed on 23 November 2021).
- Magen, H. Potassium in Fertigation Systems; 5th Fertigation Training Course, Baoding, AUH; International Potash Institute: Basel, Switzerland, June 2004. Available online: https://silo.tips/queue/potassium-in-fertigation-systems?&queue_id=-1&v=1637631916&u=MTc4Ljc1LjEwNC4xMjk= (accessed on 23 November 2021).
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture—Status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: Upraited guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Nachtigall, G.R.; Dechen, A.R. Seasonality of nutrients in leaves and fruits of apple trees. Sci. Agric. 2006, 63, 493–501. [Google Scholar] [CrossRef]
- Cheng, L.; Raba, R. Accumulation of Macro- and Micronutrients and Nitrogen Demand-supply Relationship of ‘Gala’/’Malling 26′ Apple Tree Grown in Sand Culture. J. Amer. Soc. Hort. Sci. 2009, 134, 3–13. [Google Scholar] [CrossRef]
- Kuzin, A.I.; Kashirskaya, N.Y.; Solovhenko, A.E.; Kushner, A.V.; Kochkina, A.M.; Stepantsova, L.V. Interplay of foliar K and other macro- and micronutrient levels in the leaves of ‘Ligol,’ ‘Venjaminovskoye,’ and ‘Bogatyr’ apple cv. grafted on ‘Budagovski 396’ rootstock. Horticulurae SI “Precision management of fruit trees”. 2022. manuscript in preparation. [Google Scholar]
- Roeva, T.A.; Leonicheva, E.V.; Leontieva, L.I. The impact of foliar fertilization on the K and phosphorus content in apple shoots. Pomic. Small Fruits Cult. Russ. 2018, 53, 183–188. [Google Scholar] [CrossRef]
- Greer, D.Y.; Wünsche, J.N.; Norling, C.L.; Wiggins, H.N. Root-zone temperatures affect phenology of bud break, flower cluster development, shoot extension growth and gas exchange of ‘Braeburn’ (Malus domestica) apple trees. Tree Physiol. 2006, 26, 105–111. [Google Scholar] [CrossRef]
- Tagliavini, M.; Hogue, E.J.; Neilsen, G.H. Influence of phosphorus nutrition and root zone temperature on growth and mineral uptake of peach seedlings. J. Plant Nutr. 1991, 14, 1267–1276. [Google Scholar] [CrossRef]
- Engels, C.; Marschner, H. Root to shoot translocation in relation to shoot demand in maize (Zea mays L.) grown at different root zone temperatures. J. Plant Nutr. Soil Sci. 1991, 155, 121–128. [Google Scholar] [CrossRef]
- McMichael, B.L.; Burke, J.J. Soil Temperature and Root Growth. HortScience 1998, 33, 947–951. [Google Scholar] [CrossRef]
- Dong, S.; Scagel, C.F.; Cheng, L.; Fuchigami, L.H.; Rygiewicz, P.T. Soil temperature and plant growth stage influence nitrogen uptake and amino acid concentration of apple during early spring growth. Tree Physiol. 2001, 21, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L. Optimizing Nitrogen and K Management to Foster Apple Tree Growth and Cropping Without Getting ‘Burned’. NYFQ 2013, 21, 21–24. Available online: https://nyshs.org/wp-content/uploads/2016/10/4.Optimizing-Nitrogen-and-K-Management-to-Foster-Apple-Tree-Growth-and-Cropping-Without-Getting-Burned.pdf (accessed on 2 May 2020).
- Hoying, S.; Fargione, M.; Iungerman, K. Diagnosing apple tree nutritional status: Leaf analysis interpretation and defiency symptoms. NYFQ 2004, 12, 6–19. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.655.7994&rep=rep1&type=pdf (accessed on 13 October 2021).
- Uçgun, K.; Gezgin, S. Interpretation of Leaf Analysis Performed in Early Vegetation in Apple Orchards. Commun. Soil Sci. Plant Anal. 2017, 48, 1719–1725. [Google Scholar] [CrossRef]
- Rather, G.H.; Bansal, S.K.; Bashir, O.; Weida, U. Impact of K Nutrition on Fruit Yield and Physicochemical Characteristics of Apple Cultivar Red Delicious. Indian J. Fertil. 2019, 15, 790–797. Available online: https://www.ipipotash.org/uploads/abstracts/pdf/impact-K-nutrition-on-fruit-yield-and-characteristics-of-apple-ijf2019.pdf (accessed on 11 April 2020).
- Yousuf, S.; Ahmad Sheikh, M.; Chand, S.; Anjum, S. Effect of different sources of K on yield and quality of apple (cv. Red Delicious in temperate conditions. J. Appl. Nat. Sci. 2018, 10, 1332–1340. [Google Scholar] [CrossRef]
- Neilsen, G.H.; Neilsen, D.; Herbert, L.C.; Hogue, E.J. Response of Apple to Fertigation of N and K under Conditions Susceptible of K Deficiency. J. Am. Soc. Hort. Sci. 2004, 129, 26–31. [Google Scholar] [CrossRef]
- Neilsen, G.H.; Parchomchuk, P.; Meheriuk, M.; Neilsen, D. Development and correction of K-deficiency in drip irrigated apple. Hortscience 1998, 33, 258–261. [Google Scholar] [CrossRef]
- Daugaard, H.; Grauslund, J. Fruit colour and correlations with orchard factors and post-harvest characteristics in apple cv. Mutsu. J. Hortic. Sci. Biotechnol. 1999, 74, 283–287. [Google Scholar] [CrossRef]
- Neilsen, G.H.; Parchomchuk, P.; Neilsen, D.; Zebarth, B.J. Drip-fertirrigation of apples trees affects root distribution and the development of K deficiency. Can. J. Soil Sci. 2000, 80, 353–361. [Google Scholar] [CrossRef]
- Leonel, S.; dos Reis, L.L. K fertilization on Fruits Orchards: Study Case from Brazil. In Soil Fertility; Issaka, R.N., Ed.; Intechopen: London, UK, 2012. [Google Scholar] [CrossRef]
- Anjum, R.; Kirmani, N.A.; Nageena, N.; Sameere, S. Quality of apple cv. Red delicious as influenced by K. AJSS 2008, 3, 227–229. Available online: https://www.cabdirect.org/cabdirect/abstract/20093068848 (accessed on 13 October 2021).
- Nava, G.; Dechen, A.R.; Nachtigall, G.R. Nitrogen and K Fertilization Affect Apple Fruit Quality in Southern Brazil. Commun. Soil Sci. Plant Anal. 2007, 39, 96–107. [Google Scholar] [CrossRef]
- Neilsen, G.H.; Neilsen, D. The effect of k-fertilization on apple fruit ca concentration and quality. Acta Hortic. 2006, 721, 177–184. [Google Scholar] [CrossRef]
- Dilmaghani, M.R.; Malakouti, M.J.; Neilsen, J.H.; Fallahi, E. Interactive Effects of K and Calcium on K/Ca Ration and its consequences on Apple Fruit Quality in Calcareous Soils of Iran. J. Plant Nutr. 2005, 27, 1149–1162. [Google Scholar] [CrossRef]
- Marchuk, S. The Dynamics of Potassium in Some Australian Soils. Ph.D. Thesis, The University of Adelaide, Adelaide, Australia, November 2015. [Google Scholar] [CrossRef]
- Wegner, L.H. Interplay of Water and Nutrient Transport: A Whole-Plant Perspective. In Progress in Botany (Genetics–Physiolog–Systematic–Ecology); Lüttge, U., Beyschlag, W., Eds.; Springer: Darmstadt, Germany, 2015. [Google Scholar] [CrossRef]
- Pardo, J.M.; Rubio, F. Na+ and K+ transporters in plant signaling. In Transporters and Pumps in Plant Signaling; Geisler, M., Venima, K., Eds.; Springer: Berlin/Hedelberg, Germany, 2011; pp. 65–98. [Google Scholar]
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K+ Nutrition in Plants. Front. Plant Sci. 2019, 10, 281. [Google Scholar] [CrossRef]
- Epstein, E.; Rains, D.W.; Elzam, O.E. Resolution of dual mechanisms of K absorption by barley roots. Proc. Natl. Acad. Sci. USA 1963, 49, 684–692. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. Cellular mechanism of K transport in plants. Physiol. Plant. 2008, 133, 637–650. [Google Scholar] [CrossRef]
- Gierth, M.; Maser, P. K transporters in plants-involvement in K+ acquisition, redistribution and homeostasis. FEBS Lett. 2007, 581, 2348–2356. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhaler, U. Drought and salinity: A comparison of their effect on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Fazio, G.; Kviklis, D.; Grusak, M.A.; Robinson, T. Soil pH, Soil Type and Replant Disease Affect Growth and Nutrient Absorption of Apple Rootstock. NYFQ 2012, 20, 22–29. Available online: https://nyshs.org/wp-content/uploads/2016/10/5.Soil-pH-Soil-Type-and-Replant-Disease-Affect-Growth-and-Nutrient-Absorption-of-Apple-Rootstocks.pdf (accessed on 18 October 2021).
- Valverdi, N.A.; Cheng, L.; Kalcsits, L. Apple Scion and Rootstock Contribute to Nutrient Uptake and Partitioning under Different Belowground Environments. Agronomy 2019, 9, 415. [Google Scholar] [CrossRef]
- Neilsen, G.H.; Parchomchuk, P.; Hogue, E.J.; Wolk, W.D.; Lau, O.L. Response of apple trees to fertigation-induced soil acidification. Can. J. Plant Sci. 1994, 74, 347–351. [Google Scholar] [CrossRef]
- Zhang, Y.; de Vries, W.; Thomas, B.W.; Hao, X.; Shi, X. Impacts of long-term nitrogen fertilization on acid buffering rates and mechanisms of a slightly calcareous clay soil. Geoderma 2017, 305, 92–99. [Google Scholar] [CrossRef]
- Raese, J.T. Effect of low soil pH from different fertilisers on performance of apple and pear trees. In Plant-Soil Interactions at Low pH: Principles and Management. Developments in Plant and Soil Sciences; Date, R.A., Grundon, N.J., Rayment, G.E., Probert, M.E., Eds.; Springer: Dordrecht, Germany, 1995; Volume 64, pp. 803–807. [Google Scholar] [CrossRef]
- Reid, J.B.; Trolove, S.N.; Tan, Y.; Johnstone, P.R. Nitrogen and K preconditioning affects uptake of both nitrate and K in young wheat. Ann. Appl. Biol. 2016, 168, 66–80. [Google Scholar] [CrossRef]
- Zhang, F.; Niu, J.; Zhang, W.; Chen, X.; Li, C.; Yuan, L.; Xie, J. K nutrition of crops under varied regimes of nitrogen supply. Plant Soil. 2010, 335, 21–34. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, R.; Xia, S.; Wang, L.; Liu, C.; Zhang, R.; Fan, Z.; Chen, F.; Liu, Y. Interactions between N, P, and K fertilizers affect the environment and the yield and quality of satsumas. Glob. Ecol. Conserv. 2019, 19, e00663. [Google Scholar] [CrossRef]
- Bouma, D.; Mcentyre, G.A. A Factorial Field Experiment with Citrus. J. Hortic. Sci. 1963, 38, 175–198. [Google Scholar] [CrossRef]
- Alva, A.K.; Mattos, D., Jr.; Paramasivam, S.; Patil, B.; Dou, H.; Sajwan, K.S. K management for optimizing citrus production and quality. Int. J. Fruit Sci. 2006, 6, 3–43. [Google Scholar] [CrossRef]
- Agbenin, J.O. Phosphate-induced zinc retention in a tropical semiarid soil. Eur. J. Soil Sci. 1998, 49, 693–700. [Google Scholar] [CrossRef]
- Malvi, U. Interaction of micronutrients with major nutrients with special reference to K. Karnataka J. Agric. Sci. 2011, 24, 106–109. [Google Scholar]
- Neilsen, G.H.; Neilsen, D. Consequences of K, magnesium sulphate fertilization of high density Fuji apple orchards. Can. J. Soil Sci. 2011, 91, 1013–1027. [Google Scholar] [CrossRef]
- Tang, W.; Wang, W.; Chen, D.; Cui, N.; Yang, H.; Hu, X. Evaluation of the Regional-Scale Optimal K Rate Based on Sustainable Apple Yield and High-Efficiency K Use in Loess Plateau and Bohai Bay of China: A Meta-Analysis. Agronomy 2021, 11, 1368. [Google Scholar] [CrossRef]
- Jungk, A.; Claasen, N. Ion Diffusion in the Soil-Root System. Adv. Agron. 1996, 61, 53–110. [Google Scholar]
- White, P.J. Ion Uptake Mechanism of Individual Cells and Roots. In Marschners Mineral Nutrition of Higher Plants, 3rd ed.; Marscner, P., Ed.; Academic Press: Amsterdam, The Netherlands, 2012; pp. 7–47. [Google Scholar] [CrossRef]
- Onwuka, B.M. Effects of soil temperature on some soil properties and plant growth. Adv. Plants Agric. Res. 2018, 8, 34–37. [Google Scholar] [CrossRef]
- Grossnickle, S. Ecophysiology of Northern Spruce Species in the Performance of Planted Seedlings; NRC–CNRC, NRC; Research Press: Ottawa, ON, Canada, 2000; pp. 325–407. [Google Scholar] [CrossRef]
- Lahti, M.; Aphalo, P.J.; Finér, L.; Lehto, T.; Leinonen, I.; Mannerkoski, H.; Ryyppö, A. Soil temperature, gas exchange and nitrogen status of 5-year-old Norway spruce seedlings. Tree Physiol. 2002, 22, 1311–1316. [Google Scholar] [CrossRef]
- Lösch, R.; Schulze, E.D. Internal Coordination of Plant Responses to Drought and Evaporational Demand. In Ecophysiology of Photosynthesis; Schulze, E.D., Caldwell, M.M., Eds.; Springer Study Edition; Springer: Berlin/Heidelberg, Germany, 1995; Volume 100, pp. 185–204. [Google Scholar] [CrossRef]
- Fuchs, E.E.; Livingston, N.J. Hydraulic control of stomatal conductance in Douglas fir [Pseudotsuga menziesii (Mirb.) Franco] and alder [Alnus rubra (Bong)] seedlings. Plant Cell Environ. 1996, 19, 1091–1098. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; King, J.S.; Burton, A.J.; Brown, E.S. Responses of tree fine roots to temperature. New Phytol. 2000, 147, 105–115. [Google Scholar] [CrossRef]
- Sparks, D.L. K Dynamics in Soils. In Advances in Soil Science; Stewart, B.A., Ed.; Springer: New York, NY, USA, 1987; Volume 6, pp. 1–63. [Google Scholar] [CrossRef]
- Sparks, D.L.; Huang, P.M. Physical chemistry of soil K. In K in Agriculture; Munson, R.D., Ed.; Soil Science Society of America: Madinson, WI, USA, 1985; pp. 201–276. [Google Scholar] [CrossRef]
- Simonson, M.; Hillier, S.; Öborn, I. Changes in clay minerals and K fixation capacity as a result of release and fixation of K in long-term field experiments. Geoderma 2009, 151, 109–120. [Google Scholar] [CrossRef]
- Bader, B.R.; Taban, S.K.; Fahmi, S.A.; Abood, M.A.; Hamdi, G.J. K availability in soil amended with organic matter and phosphorus fertilizer under water stress during maize (Zea mays L.) growth. J. Saudi Soc. Agric. Sci. 2021, 20, 390–394. [Google Scholar] [CrossRef]
- Kuchenbuch, R.; Claassen, N.; Jungk, A. K availability in relation to soil moisture. Plant Soil 1986, 95, 221–231. [Google Scholar] [CrossRef]
- Sardi, K.; Fulop, P. Relationship between soil K level and K uptake of corn affected by soil moisture. Commun. Soil Sci. Plant Anal. 1994, 25, 1735–1746. [Google Scholar] [CrossRef]
- Zeng, Q.; Brown, P.H. Soil K mobility and uptake by corn under differential soil moisture regimes. Plant Soil 2000, 221, 121–134. [Google Scholar] [CrossRef]
- Samuel, A.D.; Bungau, S.; Tit, D.M.; Melinte (Frunzilica), C.E.; Purza, L.; Badea, G. E Effects of Long term Application of Organic and Mineral fertilizers on Soil Enzyms. Rev. Chim. 2018, 69, 2608–2612. [Google Scholar] [CrossRef]
- Bungau, S.; Behl, T.; Aleya, L.; Bourgeade, P.; Aloi-Sosse, B.; Purza, A.L.; Abid, A.; Samuel, A.D. Expatiating the impact of anthropogenic aspects and climatic factors on long-term soil monitoring and management. Environ. Sci. Pollut. Res. 2021, 28, 30528–30550. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, P.; Mocek-Płóciniak, A.; Skowrońska, M.; Bednarek, W.; Kuśmierz, S.; Zawierucha, E. The Mineral Fertilizer-Dependent Chemical Parameters of Soil Acidification under Field Conditions. Sustainability 2020, 12, 7165. [Google Scholar] [CrossRef]
- Goulding, K.W.T. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Prakash, N.B. Effect of different biochars on acid soil and growth parameters of rice plants under aluminium toxicity. Sci. Rep. 2020, 10, 12249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Kong, F. Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Appl. Soil Ecol. 2014, 82, 18–25. [Google Scholar] [CrossRef]
- Somers, E.; Vanderleyden, J.; Srinivasan, M. Rhizosphere Bacterial Signalling: A Love Parade beneath Our Feet. Crit. Rev. Microbiol. 2004, 309, 205–240. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, B.S.; Babalola, O.O.; Glick, B. Plant growth promoting root-colonizing bacterial entophytes. Rhizosphere 2021, 20, 100433. [Google Scholar] [CrossRef]
- Yadav, A.N.; Kumar, V.; Dhaliwal, H.S.; Prasad, R.; Saxena, A.K. Microbiome in Crops: Diversity, Distribution, and Potential Role in Crop Improvement. In New and Future Developments in Microbial Biotechnology and Bioengineering: Crop Improvement through Microbial Biotechnology; Prasad, R., Gill, S.G., Tuteja, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 305–332. [Google Scholar] [CrossRef]
- Basak, B.B.; Biswas, D.R. Influence of K solubilizing microorganism (Bacillus mucilaginosus) and waste mica on K uptake dynamics by sudan grass (Sorghum vulgare Pers.) grown under two Alfisols. Plant Soil 2009, 317, 235–255. [Google Scholar] [CrossRef]
- Sheng, X.-F.; Xia, J.-J.; Jiang, C.-Y.; He, L.-Y.; Qian, M. Characterization of heavy metal-resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape. Environ. Pollut. 2008, 156, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Meena, V.S.; Maurya, B.R.; Verma, J.P. Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol. Res. 2014, 163, 337–347. [Google Scholar] [CrossRef]
- Hun, S.H.S.; Jung, J.S.; Lee, K.D. Rock phosphate-K and rock-solubilising bacteria as alternative, sustainable fertilisers. Agron. Sustain. Dev. 2006, 26, 233–240. [Google Scholar] [CrossRef]
- Mosa, W.; Paszt, L.; Frąc, M.; Trzciński, P. The Role of Biofertilization in Improving Apple Productivity―A Review. Adv. Microbiol. 2015, 5, 21–27. [Google Scholar] [CrossRef][Green Version]
- Kuzin, A.; Solovchenko, A.; Stepantsova, L.; Pugachev, G. Soil fertility management in apple orchard with microbial biofertilizers. E3S Web. Conf. 2020, 222, 03020. [Google Scholar] [CrossRef]
- Kuzin, A.; Akimov, M.; Pugachev, G. Stepantsova Evaluating the efficacy of bacterial phosphorus fertilizers in an apple or-chard on chernozem soil. In Proceedings of the 4th International Symposium on Horticulture in Europe, Stuttgart, Germany, 8–11 March 2021; ISHS: Leuven, Belgium, 2022. in press. [Google Scholar]
- Holb, I.J.; Gonda, I.; Vágó, I.; Nagy, P.T. Seasonal Dynamics of Nitrogen, Phosphorus, and K Contents of Leaf and Soil in Environmental Friendly Apple Orchards. Commun. Soil Sci. Plant Anal. 2009, 40, 694–705. [Google Scholar] [CrossRef]
- Saxena, A.K.; Kaushik, R.; Yadav, A.N.; Gulati, S.; Sharma, D. Role of Archaea in sustenance of plants in extreme saline environments. In Proceedings of the 56th Annual Conference of Association of Microbiologists of India and International Symposium on “Emerging Discoveries in Microbiology”, New Delhi, India, 7–10 December 2015. [Google Scholar] [CrossRef]
- Msimbira, L.A.; Smith, D.L. The Roles of Plant Growth Promoting Microbes in Enhancing Plant Tolerance to Acidity and Alkalinity Stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Sathya, A.; Vijayabharathi, R.; Varshney, R.K.; Gowda, C.L.; Krishnamurthy, L. Plant growth promoting rhizobia: Challenges and opportunities. 3 Biotech 2015, 5, 355–377. [Google Scholar] [CrossRef]
- Wang, C.; Cui, Y.; Qu, X. Mechanisms and improvement of acid resistance in lactic acid bacteria. Arch. Microbiol. 2018, 200, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Zerrouk, I.Z.; Benchabane, M.; Khelifi, L.; Yokawa, K.; Ludwig-Müller, J.; Baluska, F. A Pseudomonas strain isolated from date-palm rhizospheres improves root growth and promotes root formation in maize exposed to salt and aluminum stress. J. Plant Physiol. 2016, 191, 111–119. [Google Scholar] [CrossRef]
- Wargo, J.M.; Mervin, I.A.; Watkins, C.B. Fruit Size, Yield, Market Value of ‘GoldRush’ Apple are Affected by Amount, Timing and Method of Nitrogen Fertilization. HortTecnology 2003, 13, 153–161. [Google Scholar] [CrossRef]
- Nava, G.; Dechen, A.R. Long-term annual fertilization with nitrogen and potassium affect yield and mineral composition of ‘Fuji’ apple. Sci. Agric. 2009, 66, 377–385. [Google Scholar] [CrossRef][Green Version]
- Kuzin, A.I.; Trunov, Y.V. Distribution of available K in the soil root zone under the influence of drip irrigation and fertigation in the intensive apple orchard. Pomic. Small Fruits Cult. Russ. 2015, 43, 119–128. (In Russian) [Google Scholar]
- Fomenko, T.G.; Popova, V.P.; Kuzin, A.I. The impact of apple orchard fertigation on seasonal dynamics of the soil nutrients. In Proceedings of the IX International Symposium on Mineral Nutrition of Fruit Crops, Tel-Aviv, Israel, 28–30 June 2021; ISHS: Leuven, Belgium, 2022. in press. [Google Scholar]
- Butler, O. On the Case of Alternate Bearing in the Apple. Bul. Torrey Bot. Club 1917, 44, 85–96. Available online: https://www.jstor.org/stable/pdf/2479535.pdf (accessed on 21 October 2021). [CrossRef]
- Monselise, S.P.; Goldschmidt, E.E. Alternate bearing in fruit trees. Hort. Rev. 1982, 4, 128–173. [Google Scholar] [CrossRef]
- Fioravanço, J.C.; Czermainski, A.B.C. Biennial bearing in apple cultivars. Rev. Ceres Viçosa 2018, 65, 144–149. [Google Scholar] [CrossRef]
- Grigorian, V.; Sharemi, S.B. Study on Effective Methods for Reducing the Alternate Bearing in Golden Delicious Apple Cultivar. J. Agric. Sci. Technol. 2003, 5, 31–37. Available online: https://jast.modares.ac.ir/article-23-12213-en.html (accessed on 21 October 2021).
- Meland, M. Effects of different crop loads and thinning times on yield, fruit quality, and return bloom in Malus × domestica Borkh. ‘Elstar’. J. Hortic. Sci. Biotechnol. 2009, 84, 117–121. [Google Scholar] [CrossRef]
- Kumar, A.; Bhuj, B.D.; Singh, C.P. Alternate Bearing in Fruits Trees: A Review. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 1218–1235. [Google Scholar] [CrossRef]
- Shalom, L.; Samuels, S.; Zur, N.; Shlizerman, L.; Zemach, H.; Weissberg, M.; Ophir, R.; Blumwald, E.; Sadka, A. Alternate Bearing in Citrus: Changes in the Expression of Flowering Control Genes and in Global Gene Expression in ON- versus OFF-Crop Trees. PLoS ONE 2012, 7, e46930. [Google Scholar] [CrossRef]
- Hanke, M.V.; Flachowsky, H.; Peil, A.; Hattasch, C. No flower no fruit—Genetic potentials to trigger flowering in fruit trees. In Genes Genomes and Genomics; Global Science Books: Ikenode, Japan, 2007; Volume 1, pp. 1–20. Available online: http://www.globalsciencebooks.info/Online/GSBOnline/images/0706/GGG_1(1)/GGG_1(1)1-20o.pdf (accessed on 21 October 2021).
- Hansen, P. Crop load and nutrient translocation. In Mineral Nutrition of Fruit Trees; Atkinson, D., Jackson, J.E., Sharples, R.O., Waller, W.M., Eds.; Butterworths: London, UK, 1980; pp. 201–212. [Google Scholar] [CrossRef]
- Szewczuk, A.; Komosa, A.; Gudarowska, E. Effect of soil K levels and different K fertilizer forms on yield and storability of ‘Golden delicious’ apples. Acta Sci. Pol. Hortorum Cult. 2008, 7, 53–59. Available online: http://www.hortorumcultus.actapol.net/pub/7_2_53.pdf (accessed on 21 October 2021).
- Stiles, W.C.; Reid, W.S. Orchard nutrition management. In Information Bulletin 219; Cornell Cooperative Extension: Ithaka, NY, USA, 1991; pp. 5–14. Available online: https://ecommons.cornell.edu/handle/1813/3305 (accessed on 21 October 2021).
- Vandermaesen, J.; Akkermans, W.; Delalieux, S.; Bal, J.; Smedts, Y.; Bylemans, D.; Remy, S. Evaluation and demonstration of precision management practices in pear orchards. Acta Hortic. 2021, 1314, 297–306. [Google Scholar] [CrossRef]
- Ali, A.; Imran, M. Remotely sensed real-time quantification of biophysical and biochemical traits of Citrus (Citrus sinensis L.) fruit orchards—A review. Sci. Hortic. 2021, 282, 11024. [Google Scholar] [CrossRef]
- Biegert, K.; McCormick, R.J.; Zoth, M.; Braun, P. A precision management approach to monitor apple fruit growth and quality. Acta Hortic. 2021, 1314, 447–454. [Google Scholar] [CrossRef]
- O’Connell, M.G.; Scalisi, A. Sensing fruit and tree performance under deficit irrigation in ‘September Bright’ nectarine. Acta Hortic. 2021, 1314, 9–16. [Google Scholar] [CrossRef]
- Solovchenko, A.; Lukyanov, A.; Nikolenko, A.; Shurygin, B.; Akimov, M.; Gitelson, A. Physiological foundations of spectral imaging-based monitoring of apple fruit ripening. Acta Hortic. 2021, 1314, 419–428. [Google Scholar] [CrossRef]

| K Supply (mM K+) | Shoot Dry Weight (g plant −1) | Root Dry Weight (g) | Root/Shoot Ratio |
|---|---|---|---|
| 0 | 3.93 ± 0.14 d | 1.45 ± 0.08 d | 0.37 ± 0.03 c |
| 3 | 4.30 ± 0.16 c | 1.77 ± 0.09 c | 0.41 ± 0.03 ab |
| 6 | 5.37 ± 0.15 a | 2.30 ± 0.13 a | 0.43 ± 0.01 a |
| 9 | 4.84 ± 0.15 b | 1.94 ± 0.07 b | 0.40 ± 0.00 ab |
| 12 | 4.34 ± 0.27 c | 1.66 ± 0.10 c | 0.38 ± 0.01 bc |
| K2O Rate oz. tree−1 year | 3-Year Old Trees | 4-Year Old Trees | ||
|---|---|---|---|---|
| Yield, lb tree−1 | Mean Fruit Weight, oz. | Yield, lb tree−1 | Mean Fruit Weight, oz. | |
| 0 | 7.5 | 7.4 | 9.9 | 6.3 |
| 0.6 | 8.4 | 7.8 | 11.9 | 6.7 |
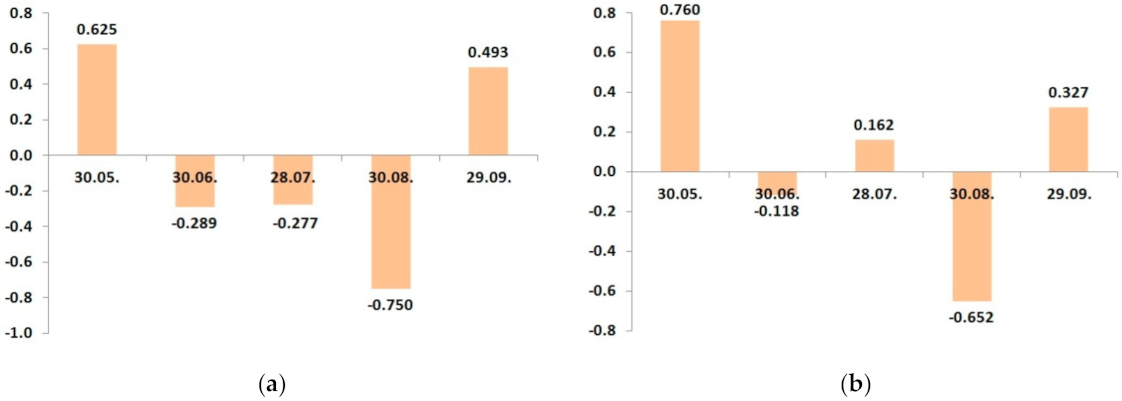
| Date | Control N20P15 | N20P15K25 | N20P15K35 | N20P15K45 | N20P15K20 | N20P15K25 | N20P15K30 | LSD05 |
|---|---|---|---|---|---|---|---|---|
| ‘Zhigulevskoye’ | ||||||||
| 30.05 | 1.81 | 1.89 | 1.74 | 1.97 | 1.76 | 1.92 | 1.73 | 0.10 |
| 30.06 | 1.61 | 1.21 | 1.29 | 1.56 | 1.28 | 1.21 | 1.26 | 0.08 |
| 28.07 | 1.36 | 1.09 | 1.32 | 1.33 | 1.33 | 1.29 | 1.24 | 0.08 |
| 30.08 | 1.22 | 1.05 | 1.00 | 0.98 | 0.94 | 0.88 | 0.98 | 0.06 |
| 29.09 | 1.30 | 1.27 | 1.31 | 1.52 | 1.12 | 1.37 | 1.44 | 0.08 |
| ‘Lobo’ | ||||||||
| 30.05 | 1.67 | 1.77 | 1.78 | 1.95 | 1.71 | 1.73 | 1.68 | 0.11 |
| 30.06 | 1.54 | 1.65 | 1.77 | 1.67 | 1.63 | 1.68 | 1.61 | 0.10 |
| 28.07 | 1.12 | 1.34 | 1.42 | 1.24 | 1.42 | 1.51 | 1.30 | 0.08 |
| 30.08 | 1.04 | 1.07 | 1.03 | 1.13 | 0.85 | 0.88 | 0.79 | 0.05 |
| 29.09 | 1.15 | 0.91 | 1.09 | 1.03 | 0.85 | 0.87 | 0.96 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzin, A.; Solovchenko, A. Essential Role of Potassium in Apple and Its Implications for Management of Orchard Fertilization. Plants 2021, 10, 2624. https://doi.org/10.3390/plants10122624
Kuzin A, Solovchenko A. Essential Role of Potassium in Apple and Its Implications for Management of Orchard Fertilization. Plants. 2021; 10(12):2624. https://doi.org/10.3390/plants10122624
Chicago/Turabian StyleKuzin, Andrei, and Alexei Solovchenko. 2021. "Essential Role of Potassium in Apple and Its Implications for Management of Orchard Fertilization" Plants 10, no. 12: 2624. https://doi.org/10.3390/plants10122624
APA StyleKuzin, A., & Solovchenko, A. (2021). Essential Role of Potassium in Apple and Its Implications for Management of Orchard Fertilization. Plants, 10(12), 2624. https://doi.org/10.3390/plants10122624






