Anatomical and Chemical Characterization of Ulmus Species from South Korea
Abstract
1. Introduction
2. Results and Discussion
2.1. Anatomical Characteristics of the Leaf Surface
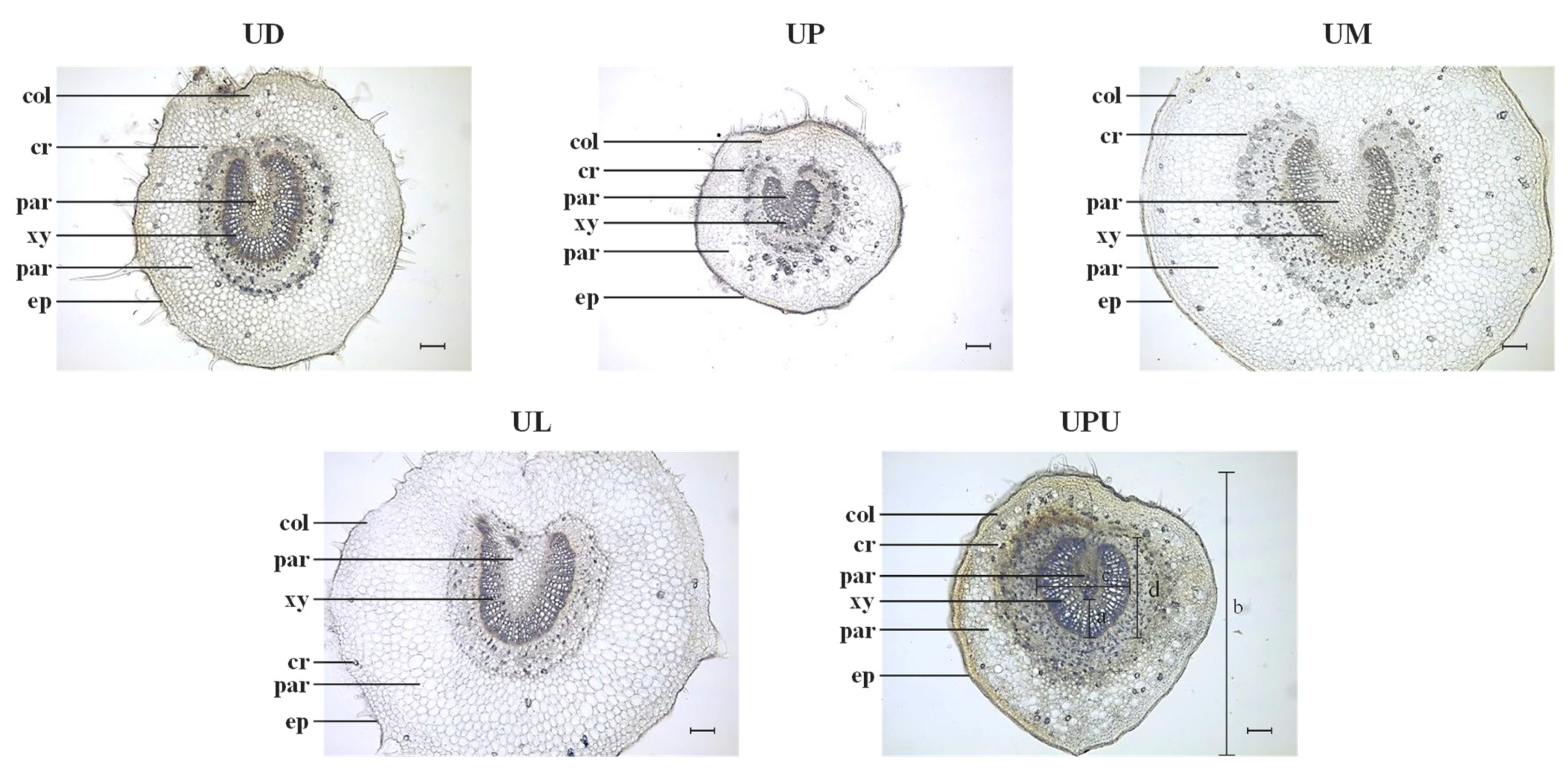
2.2. Anatomical Characteristics of the Leaf Midrib
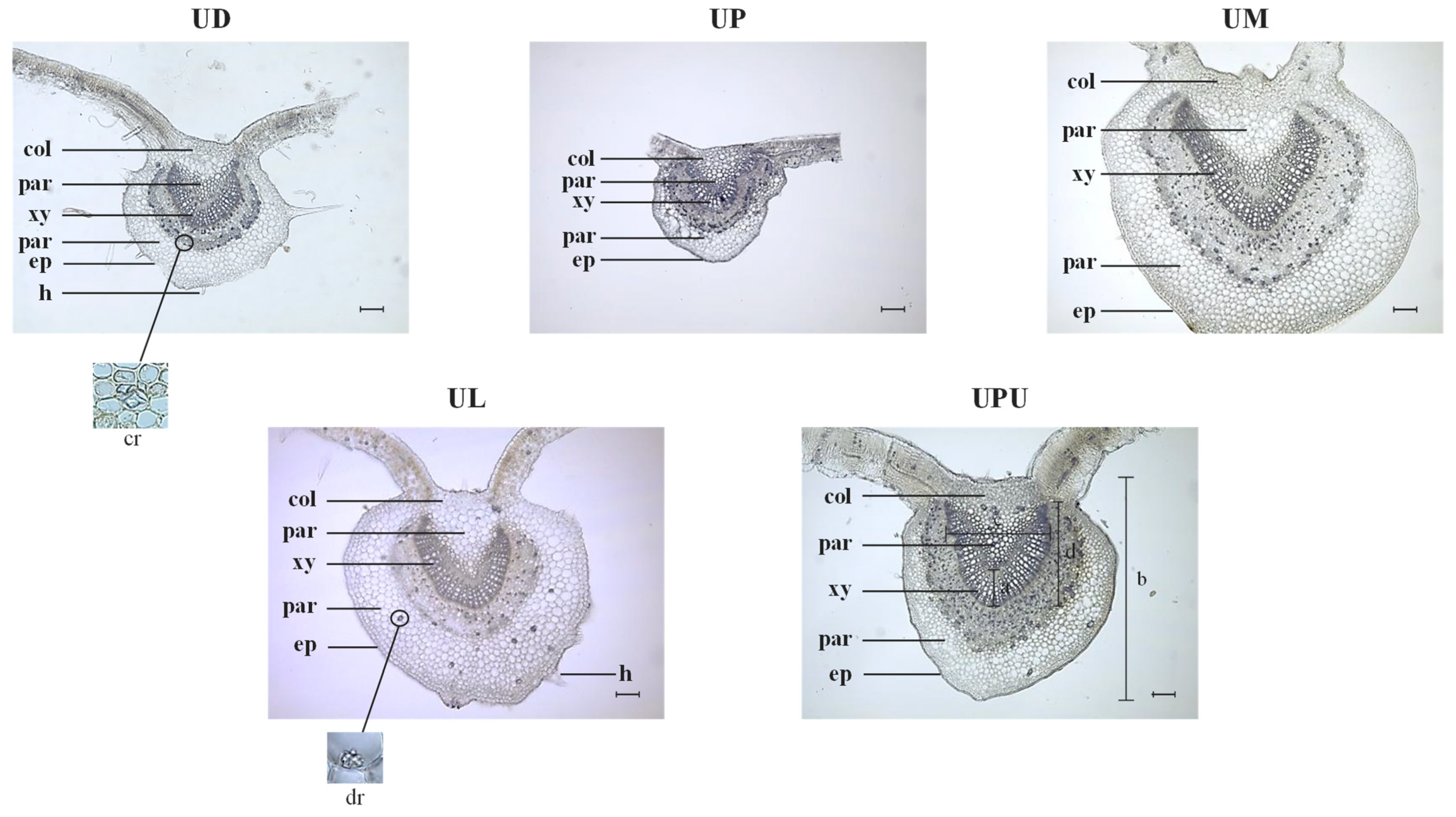
2.3. Anatomical Characteristics of the Petiole
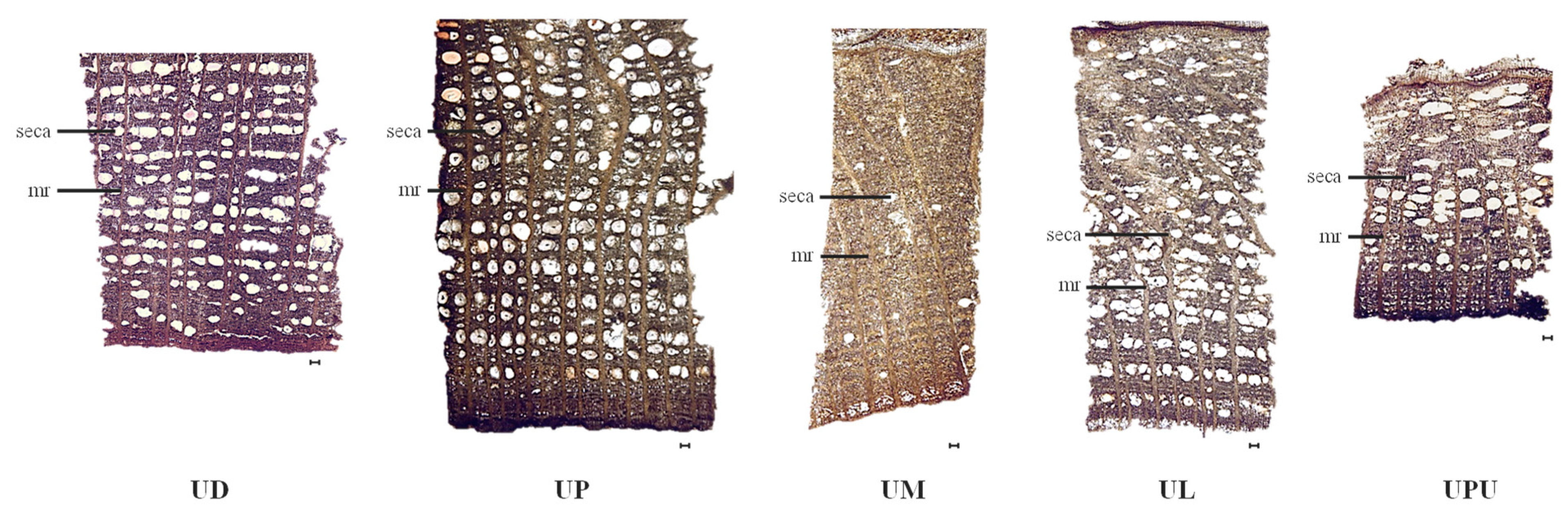
2.4. Anatomical Characteristics of the Stem Bark
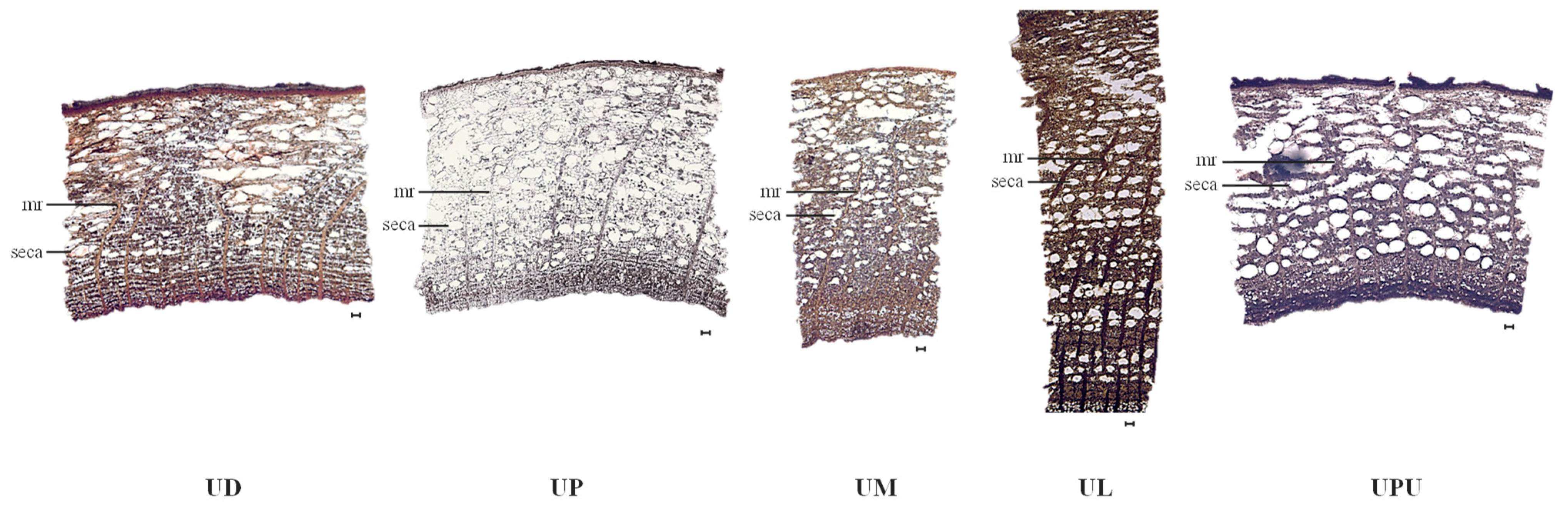
2.5. Anatomical Characteristics of the Root Bark
2.6. Multivariate Statistical Analysis of Anatomical Data
2.7. High-Performance Liquid Chromatography (HPLC) Profiles of the Five Ulmus Species
3. Materials and Methods
3.1. Plant Materials and Reagents
3.2. Anatomical Study
3.3. Sample Extraction and Compound Isolation
3.4. HPLC-DAD Profiling and Quantification
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbasi, S.; Hosseini, S.M.; Khorasani, N.; Karbassi, A. Responses of the morphological traits of elm (Ulmus minor ‘umbraculifera’) leaves to air pollution in urban areas (A case study of Tehran Metropolitan city, Iran). Appl. Ecol. Environ. Res. 2018, 16, 4955–4968. [Google Scholar] [CrossRef]
- Jun, C.D.; Pae, H.O.; Kim, Y.C.; Jeong, S.J.; Yoo, J.C.; Lee, E.J.; Choi, B.M.; Chae, S.W.; Park, R.K.; Chung, H.T. Inhibition of nitric oxide synthesis by butanol fraction of the methanol extract of Ulmus davidiana in murine macrophages. J. Ethnopharmacol. 1998, 62, 129–135. [Google Scholar] [CrossRef]
- Lee, Y.N. Flora of Korea; Gyohaksa: Seoul, Korea, 2006; pp. 236–238. [Google Scholar]
- Lee, Y.; Park, H.; Ryu, H.S.; Chun, M.; Kang, S.; Kim, H.-S. Effects of elm bark (Ulmus davidiana var. japonica) extracts on the modulation of immunocompetence in mice. J. Med. Food 2007, 10, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.S.; Li, G.; Li, Y.; Seo, C.-S.; Lee, Y.-K.; Jung, J.-S.; Song, N.-K.; Bae, H.-B.; Kwak, S.-H.; Chang, H.-W.; et al. Protective constituents against sepsis in mice from the root barks of Ulmus davidiana var. japonica. Arch. Pharm. Res. 2011, 34, 1443–1450. [Google Scholar] [CrossRef]
- Baek, I.; Im, L.-H.; Park, C.; Choi, Y.H. Anti-cancer potentials of Rhus verniciflua stokes, Ulmus davidiana var. japonica Nakai and Arsenium sublimatum in human gastric cancer AGS cells. J. Life Sci. 2015, 25, 849–860. [Google Scholar] [CrossRef][Green Version]
- Kim, H.-S.; Cho, J.-H.; Lee, J.-M.; Lee, C.-H.; Jang, J.-B.; Lee, K.-S. Experimental studies on antimetastatic and immunomodulating effects of Ulmus davidiana. J. Orient. Obstet. Gynecol. 2010, 23, 1–11. [Google Scholar]
- Ahn, J.J.; Lee, J.S.; Yang, K.M. Ultrafine particles of Ulmus davidiana var. japonica induce apoptosis of gastric cancer cells via activation of caspase and endoplasmic reticulum stress. Arch. Pharm. Res. 2014, 37, 783–792. [Google Scholar] [CrossRef]
- Zheng, M.S.; Lee, Y.-K.; Li, Y.; Hwangbo, K.; Lee, C.-S.; Kim, J.-R.; Lee, S.K.-S.; Chang, H.-W.; Son, J.-K. Inhibition of DNA topoisomerases I and II and cytotoxicity of compounds from Ulmus davidiana var. japonica. Arch. Pharm. Res. 2010, 33, 1307–1315. [Google Scholar] [CrossRef]
- Kim, S.P.; Lee, S.J.; Nam, S.H.; Friedman, M. Elm tree (Ulmus parvifolia) bark bioprocessed with mycelia of shiitake (Lentinus edodes) mushrooms in liquid culture: Composition and mechanism of protection against allergic asthma in mice. J. Agric. Food Chem. 2016, 64, 773–784. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Kim, S.-B.; Park, K.-H.; Lee, M.-W. Antioxidative and anti-inflammatory effects of phenolic compounds from the roots of Ulmus macrocarpa. Arch. Pharm. Res. 2011, 34, 1459–1466. [Google Scholar] [CrossRef]
- Kang, M.C.; Yumnam, S.; Park, W.S.; So, H.M.; Kim, K.H.; Shin, M.C.; Ahn, M.-J.; Kim, S.Y. Ulmus parvifolia accelerates skin wound healing by regulating the expression of MMPs and TGF-β. J. Clin. Med. 2019, 9, 59. [Google Scholar] [CrossRef]
- Kim, H.-J.; Yeom, S.-H.; Kim, M.-K.; Shim, J.-G. Nitric oxide and prostaglandin E2 synthesis inhibitory activities of flavonoids from the barks of Ulmus macrocarpa. Nat. Prod. Sci. 2004, 10, 344–346. [Google Scholar]
- Zheng, M.S.; Yang, J.-H.; Li, Y.; Li, X.; Chang, H.-W.; Son, J.-K. Anti-inflammatory activity of constituents isolated from Ulmus davidiana var. japonica. Biomol. Ther. 2010, 18, 321–328. [Google Scholar] [CrossRef][Green Version]
- Lee, M.K.; Sung, S.H.; Lee, H.S.; Cho, J.H.; Kim, Y.C. Lignan and neolignan glycosides from Ulmus davidiana var. japonica. Arch. Pharm. Res. 2001, 24, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Kim, Y.C. Five novel neuroprotective triterpene esters of Ulmus davidiana var. japonica. J. Nat. Prod. 2001, 64, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.H.; Rim, G.R. Studies on the constituents of Ulmus parvifolia. Kor. J. Pharmacogn. 1995, 26, 1–7. [Google Scholar]
- Kim, S.H.; Hwang, K.T.; Park, J.C. Isolation of flavonoids and determination of rutin from the leaves of Ulmus parvifolia. Kor. J. Pharmacogn. 1992, 23, 229–234. [Google Scholar]
- So, H.M.; Yu, J.S.; Khan, Z.; Subedi, L.; Ko, Y.-J.; Lee, I.K.; Park, W.S.; Chung, S.J.; Ahn, M.-J.; Kim, S.Y.; et al. Chemical constituents of the root bark of Ulmus davidiana var. japonica and their potential biological activities. Bioorg. Chem. 2019, 91, 103145. [Google Scholar] [CrossRef]
- Kwoun, Y.M.; Lee, J.H.; Lee, M.W. Phenolic compounds from bark of Ulmus macrocarpa and its antioxidative activities. Kor. J. Pharmacogn. 2002, 33, 404–410. [Google Scholar]
- Wheeler, E.; Manchester, S. Review of the wood anatomy of extant Ulmaceae as context for new reports of late Eocene Ulmus woods. Bull. Geosci. 2007, 82, 329–342. [Google Scholar] [CrossRef]
- Sweitzer, E.M. The comparative anatomy of Ulmaceae. J. Arnold Arbor. 1971, 52, 523–585. [Google Scholar] [CrossRef]
- Yamamoto, F.; Angeles, G.; Kozlowski, T.T. Effect of ethrel on stem anatomy of Ulmus americana seedlings. IAWA J. 1987, 8, 3–9. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Gao, Z.; Li, Y. Wood anatomy of 12 species and 2 varieties from Ulmus of China. J. Henan For. Sci. Technol. 2007, 27, 1–19. [Google Scholar]
- Kokate, C.K. Practical Pharmacognosy; Vallabh Prakashan: Delhi, India, 1994; pp. 117–118. [Google Scholar]
- Salisbury, E.J. On the causes and ecological significance of stomatal frequency, with special reference to the woodland flora. Philos. Trans. R. Soc. London B 1927, 216, 1–65. [Google Scholar]
- Leroux, O. Collenchyma, a versatile mechanical tissue with dynamic cell walls. Ann. Bot. 2012, 110, 1083–1098. [Google Scholar] [CrossRef]
- Fahn, A.; Every, R.F. Ultrastructure of the secretory ducts of Rhus glabra L. Amer. J. Bot. 1974, 61, 1–14. [Google Scholar] [CrossRef]
- Abd El-Razek, M.H. NMR assignments of four catechin epimers. Asian J. Chem. 2007, 19, 4867–4872. [Google Scholar]
- Na, M.K.; An, R.B.; Lee, S.M.; Min, B.S.; Kim, Y.H.; Bae, K.H.; Kang, S.S. Antioxidant compounds from the stem bark of Sorbus commixta. Nat. Prod. Sci. 2002, 8, 26–29. [Google Scholar]
- Lee, A.H.; Lee, M.W. Tannins from Rubus coreanum. Kor. J. Pharmacogn. 1995, 26, 27–30. [Google Scholar]
- Inoshiri, S.; Sasaki, M.; Kohda, H.; Otsuka, H.; Yamasaki, K. Aromatic glycosides from Berchemia racemosa. Phytochemistry 1987, 26, 2811–2814. [Google Scholar] [CrossRef]
- Foo, L.Y.; Karchesy, J.J. Polyphenolic glycosides from Douglas fir inner bark. Phytochemistry 1989, 28, 1237–1240. [Google Scholar]
- Son, B.W.; Park, J.H.; Zee, O.-P. Catechin glycoside from Ulmus davidiana. Arch. Pharm. Res. 1989, 12, 219–222. [Google Scholar] [CrossRef]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Jung, M.J.; Heo, S.-I.; Wang, M.-H. HPLC analysis and antioxidant activity of Ulmus davidiana and some flavonoids. Food Chem. 2010, 120, 313–318. [Google Scholar] [CrossRef]
- Park, Y.J.; Kim, D.M.; Jeong, M.H.; Yu, J.S.; So, H.M.; Bang, I.J.; Kim, H.R.; Kwon, S.-H.; Kim, K.H.; Chung, K.H. (–)-Catechin-7-O-β-D-apiofuranoside inhibits hepatic stellate cell activation by suppressing the STAT3 signaling pathway. Cells 2019, 9, 30. [Google Scholar] [CrossRef]
- Cui, E.-J.; Song, N.-Y.; Shrestha, S.; Chung, I.-S.; Kim, J.-Y.; Jeong, T.-S.; Baek, N.-I. Flavonoid glycosides from cowpea seeds (Vigna sinensis K.) inhibit LDL oxidation. Food Sci. Biotechnol. 2012, 21, 619–624. [Google Scholar] [CrossRef]
- Bate-Smith, E.C.; Richens, R.H. Flavonoid chemistry and taxonomy in Ulmus. Biochem. Syst. 1973, 1, 141–146. [Google Scholar] [CrossRef]


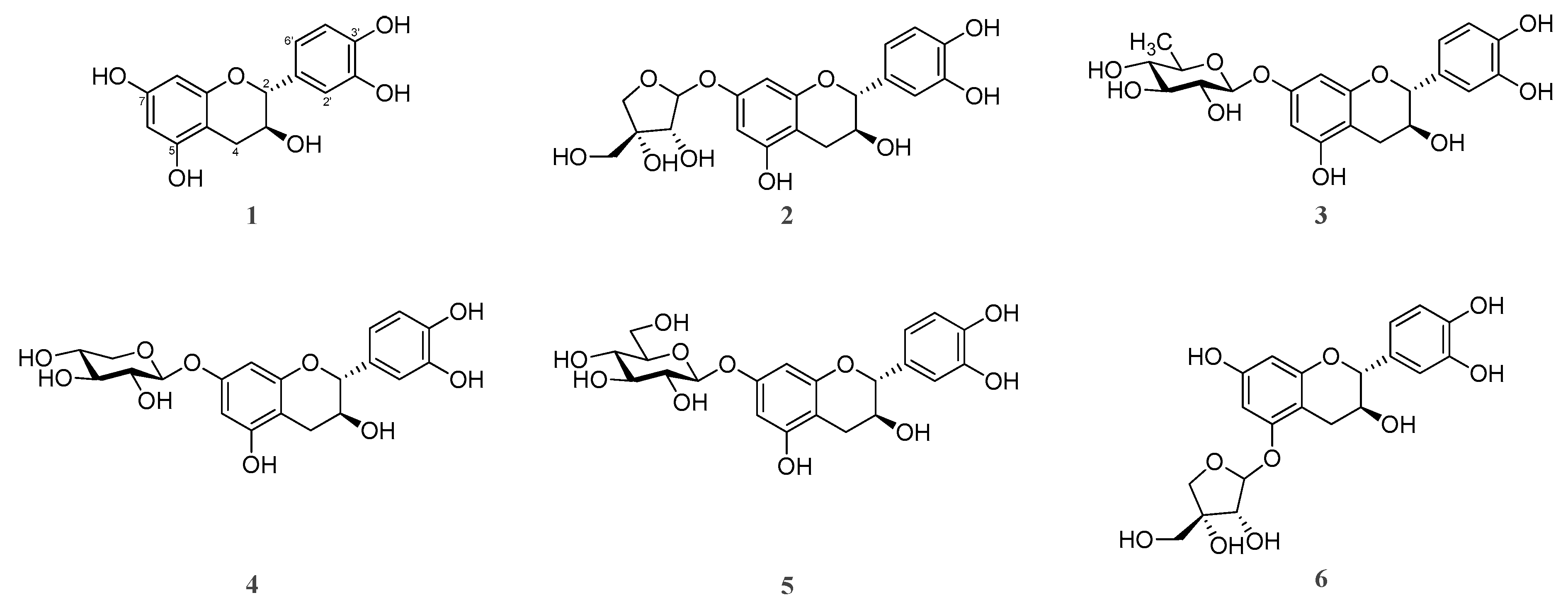
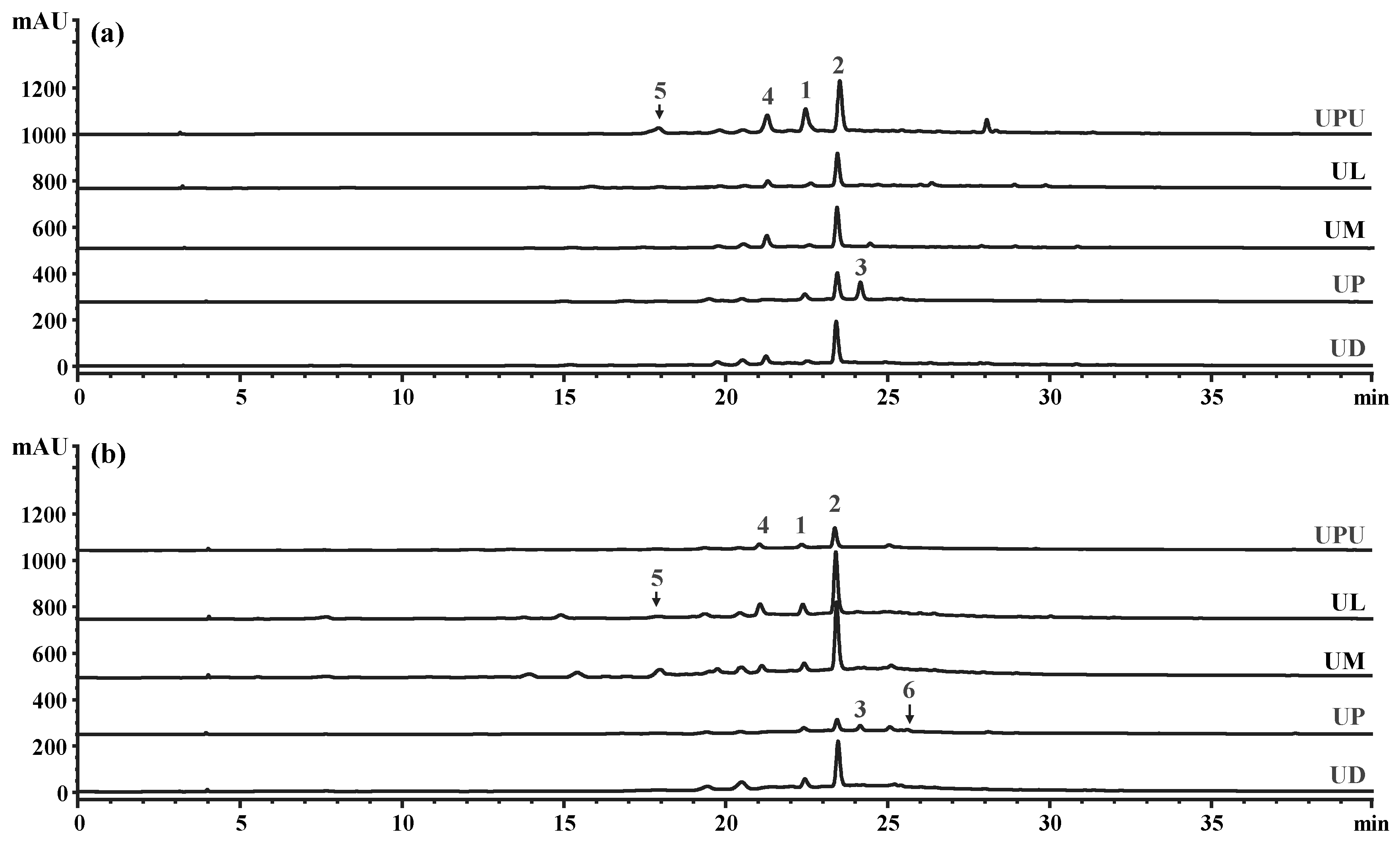
| Parameters | U. davidiana var. japonica | U. parvifolia | U. macrocarpa | U. laciniata | U. pumila |
|---|---|---|---|---|---|
| Stomatal length (μm) | 21.3 ± 1.7 c | 26.0 ± 1.2 b | 18.8 ± 1.4 d | 26.8 ± 2.6 ab | 30.0 ± 1.1 a |
| Stomatal width (μm) | 16.9 ± 0.9 d | 19.3 ± 1.1 c | 14.4 ± 1.5 e | 21.2 ± 1.4 b | 25.7 ± 1.2 a |
| Stomatal index (%) | 11.4 ± 0.2 d | 13.5 ± 0.1 b | 18.8 ± 0.1 a | 12.5 ± 0.1 c | 19.2 ± 0.2 a |
| Frequency of stomata | 34.4 ± 5.1 a | 18.1 ± 0.7 b | 29.0 ± 2.2 a | 16.8 ± 0.9 c | 11.0 ± 0.8 d |
| Parameters | U. davidiana var. japonica | U. parvifolia | U. macrocarpa | U. laciniata | U. pumila |
|---|---|---|---|---|---|
| Width of epidermal cells in adaxial part (μm) | 11.5 ± 0.7 d | 20.1 ± 0.8 a | 16.0 ± 2.2 cb | 17.3 ± 1.2 b | 13.6 ± 0.7 c |
| Length of epidermal cells in adaxial part (μm) | 9.9 ± 0.9 b | 15.3 ± 1.5 a | 14.9 ± 2.1 a | 15.6 ± 1.6 a | 10.5 ± 1.4 b |
| Width of epidermal cells in abaxial part (μm) | 13.8 ±1.3 b | 15.6 ± 1.4 ab | 17.4 ± 5.6 ab | 18.9 ± 1.4 a | 14.0 ± 3.3 b |
| Length of epidermal cells in abaxial part (μm) | 12.1 ± 1.1 b | 12.1 ± 0.5 b | 14.2 ± 3.5 ab | 14.9 ± 0.6 a | 11.4 ± 2.1 b |
| Diameter of collenchyma cells (μm) | 15.0 ± 1.6 d | 21.5 ± 1.6 c | 32.0 ± 7.2 ab | 38.6 ± 4.0 a | 26.0 ± 3.6 b |
| Diameter of parenchyma cells in cortex (μm) | 20.2 ± 2.6 b | 19.4 ± 1.6 b | 22.8 ± 2.0 ab | 25.6 ± 2.2 a | 24.1 ± 0.8 a |
| Diameter of parenchyma cells in pith (μm) | 23.7 ± 1.6 b | 22.2 ± 2.7 bc | 28.2 ± 3.9 a | 29.8 ± 2.8 a | 20.1 ± 0.9 c |
| Ratio of vascular bundle thickness to midrib diameter * | 0.10 ± 0.01 b | 0.17 ± 0.01 a | 0.10 ± 0.01 b | 0.10 ± 0.01 b | 0.16 ± 0.01 a |
| Ratio of vascular bundle width to vascular bundle height * | 1.02 ± 0.18 a | 1.05 ± 0.02 a | 1.12 ± 0.27 a | 1.14 ± 0.18 a | 0.94 ± 0.07 a |
| Parameters | U. davidiana var. japonica | U. parvifolia | U. macrocarpa | U. laciniata | U. pumila |
|---|---|---|---|---|---|
| Width of epidermal cells in adaxial part (μm) | 16.4 ± 1.2 ab | 15.5 ± 0.7 b | 18.8 ± 3.2 a | 18.3 ± 5.0 a | 13.5 ± 1.0 c |
| Length of epidermal cells in adaxial part (μm) | 14.4 ± 1.6 a | 9.3 ± 0.5 b | 13.9 ± 1.3 a | 16.6 ± 4.1 a | 9.4 ± 1.1 b |
| Width of epidermal cells in abaxial part (μm) | 15.2 ± 2.1 b | 16.1 ± 0.4 b | 21.5 ± 3.2 a | 24.7 ± 3.5 a | 14.0 ± 2.2 b |
| Length of epidermal cells in abaxial part (μm) | 13.5 ± 2.3 b | 12.5 ± 0.4 b | 15.1 ± 2.5 ab | 19.4 ± 3.1 a | 10.2 ± 1.2 c |
| Diameter of collenchyma cells (μm) | 22.2 ± 0.7 c | 17.7 ± 5.5 c | 31.5 ± 2.2 b | 37.3 ± 2.3 a | 17.9 ± 2.3 c |
| Diameter of parenchyma cells in cortex (μm) | 20.3 ± 1.5 a | 14.2 ± 3.3 b | 17.5 ± 0.6 ab | 22.3 ± 5.5 a | 16.8 ± 1.9 ab |
| Diameter of parenchyma cells in pith (μm) | 29.1 ± 1.9 c | 23.5 ± 1.1 d | 38.6 ± 2.4 b | 42.9 ± 1.7 a | 26.7 ± 3.7 cd |
| Ratio of vascular bundle thickness to petiole diameter * | 0.08 ± 0.01 b | 0.10 ± 0.01 a | 0.07 ± 0.01 b | 0.07 ± 0.01 b | 0.11 ± 0.02 a |
| Ratio of vascular bundle width to vascular bundle height * | 0.71 ± 0.03 c | 1.03 ± 0.02 a | 0.94 ± 0.05 b | 0.80 ± 0.09 c | 0.94 ± 0.01 b |
| Parameters | U. davidiana var. japonica | U. parvifolia | U. macrocarpa | U. laciniata | U. pumila |
|---|---|---|---|---|---|
| Number of layers in medullary rays | 2.2 ± 0.2 b | 3.2 ± 0.4 a | 4.1 ± 1.2 a | 3.2 ± 0.4 a | 3.5 ± 0.1 a |
| Frequency of medullary rays (in 1 mm2) | 4.3 ± 0.7 a | 3.3 ± 0.5 b | 2.9 ± 0.4 b | 2.9 ± 0.5 b | 2.8 ± 0.3 b |
| Length of medullary ray cells (µm) | 35.9 ± 1.6 b | 47.0 ± 7.2 a | 20.2 ± 7.0 c | 51.4 ± 6.6 a | 37.4 ± 3.0 b |
| Width of medullary ray cells (µm) | 12.6 ± 0.9 b | 13.3 ± 1.7 b | 17.0 ± 1.8 a | 15.3 ± 0.5 ab | 11.7 ± 2.0 b |
| Ratio of secretary canals (%, in 1 mm2) | 20.7 ± 2.8 ab | 23.4 ± 4.0 a | 2.5 ± 0.5 c | 17.6 ± 3.0 b | 20.0 ± 0.9 ab |
| Frequency of secretary canals (in 1 mm2) | 18.4 ± 2.5 a | 20.5 ± 2.7 a | 2.7 ± 0.6 c | 3.2 ± 0.4 c | 15.5 ± 0.6 b |
| Length of secretary canals (µm) | 145.9 ± 9.6 b | 167.9 ± 11.2 a | 89.6 ± 11.0 e | 108.6 ± 11.8 d | 133.6 ± 1.9 c |
| Width of secretary canals (µm) | 169.4 ± 41.3 b | 237.4 ± 28.8 a | 129.8 ± 5.5 c | 191.9 ± 6.5 ab | 224.5 ± 27.3 a |
| Parameters | U. davidiana var. japonica | U. parvifolia | U. macrocarpa | U. laciniata | U. pumila |
|---|---|---|---|---|---|
| Number of layers in medullary rays | 2.0 ± 0.2 b | 2.6 ± 0.4 ab | 2.2 ± 0.3 b | 3.2 ± 0.7 a | 2.2 ± 0.1 b |
| Frequency of medullary rays (in 1 mm2) | 2.9 ± 0.3 b | 2.5 ± 0.5 bc | 1.7 ± 0.04 c | 3.6 ± 0.5 a | 2.1 ± 0.2 c |
| Length of medullary ray cells (µm) | 40.6 ± 2.5 b | 41.2 ± 2.5 b | 41.8 ± 5.0 b | 41.5 ± 5.4 b | 54.5 ± 5.7 a |
| Width of medullary ray cells (µm) | 16.2 ± 1.3 bc | 19.0 ± 0.6 a | 18.3 ± 2.9 ab | 15.0 ± 0.5 c | 16.1 ± 0.7 b |
| Ratio of secretary canal (%, in 1 mm2) | 16.8 ± 0.4 c | 21.4 ± 1.4 b | 24.2 ± 5.6 ab | 16.2 ± 1.4 c | 30.8 ± 0.2 a |
| Frequency of secretary canals (in 1 mm2) | 13.9 ± 0.9 c | 18.9 ± 1.1 b | 36.6 ± 8.1 a | 19.6 ± 2.4 b | 21.4 ± 2.6 b |
| Length of secretary canals (µm) | 116.6 ± 5.6 c | 157.3 ± 5.4 b | 100.8 ± 11.5 d | 103.7 ± 3.1 d | 171.5 ± 1.7 a |
| Width of secretary canals (µm) | 192.0 ± 2.3 b | 243.2 ± 10.0 a | 163.3 ± 7.7 c | 163.5 ± 2.7 c | 248.4 ± 15.5 a |
| Parts | Species | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| Stem bark | UD | 1.31 ± 0.06 c | 22.72 ± 2.39 a | 1.07 ± 0.19 b | 1.52 ± 0.19 b | 0.67 ± 0.00 d | 0.32 ± 0.05 b |
| UP | 2.78 ± 0.21 b | 14.08 ± 1.03 b | 6.81 ± 0.51 a | 0.49 ± 0.04 d | 1.06 ± 0.07 b | 0.25 ± 0.01 c | |
| UM | 1.40 ± 0.09 c | 21.21 ± 3.31 a | 1.19 ± 0.05 b | 1.83 ± 0.20 b | 0.75± 0.12 d | 0.31 ± 0.01 b | |
| UL | 1.44 ± 0.03 c | 15.95 ± 0.33 b | 0.65 ± 0.02 c | 0.81 ± 0.06 c | 0.93 ± 0.07 c | 0.21 ± 0.01 d | |
| UPU | 9.72 ± 1.08 a | 23.62 ± 0.71 a | 0.62 ± 0.00 c | 2.92 ± 0.34 a | 5.65 ± 0.57 a | 0.45 ± 0.04 a | |
| Root bark | UD | 2.96 ± 0.11 a | 20.02 ± 0.48 b | 0.56 ± 0.02 c | 0.24 ± 0.01 e | 0.66 ± 0.02 e | 0.46 ± 0.01 a |
| UP | 1.15 ± 0.07 c | 4.40 ± 1.38 d | 1.63 ± 0.17 a | 0.13 ± 0.02 d | 0.74 ± 0.10 d | 0.18 ± 0.03 d | |
| UM | 2.63 ± 0.03 b | 26.61 ± 0.95 a | 0.51 ± 0.05 c | 0.93 ± 0.07 b | 1.83 ± 0.14 a | 0.32 ± 0.01 b | |
| UL | 3.21 ± 0.10 a | 24.89 ± 0.26 a | 0.57 ± 0.04 c | 1.78 ± 0.10 a | 1.50 ± 0.15 b | 0.25 ± 0.02 c | |
| UPU | 1.26 ± 0.01 c | 7.94 ± 0.26 c | 1.13 ± 0.01 b | 0.51 ± 0.02 c | 0.95 ± 0.15 c | 0.32 ± 0.05 b |
| Botanical Name | Collection Place (Latitude, Longitude) | Specimen No. |
|---|---|---|
| Ulmusdavidiana var. japonica (Rehder) Nakai | Jinju (35°09′03.2″ N, 128°17′40.3″ E) Sancheong (35°19′18.7″ N,127°45′19.9″ E) Hadong (35°14′17.5″ N, 127°42′19.0″ E) Pocheon (37°45′23.7″ N, 127°10′03.8″ E) | PGSC-451–456 |
| Ulmus parvifolia Jacq. | Jinju (35°12′54.9″ N, 128°04′07.3″ E) (35°13′00.3″ N, 128°04′09.0″ E) (35°09′27.4″ N, 128°17′43.9″ E) Busan (35°22′02.4″ N, 129°13′64.1″ E) (35°22′02.6″ N, 129°13′20.7″ E) | PGSC-461–464 |
| Ulmus macrocarpa Hance | Bonghwa (36°47′15.3″ N, 128°54′24.0″ E) Yeongwol (37°12′31.1″ N, 128°25′58.1″ E) Pocheon (37°45′23.3″ N, 127°09′58.7″ E) | PGSC-471–475 |
| Ulmus laciniata (Trautv.) Mayr | Hadong (35°14′59.2″ N, 127°42′29.1″ E) (35°15′00.9″ N, 127°42′30.2″ E) Pocheon (37°45′19.0″ N, 127°09′51.9″ E) | PGSC-481–484 |
| Ulmus pumila L. | Jeongseon (37°18′53.3″ N, 128°37′30.0″ E) (37°21′47.4″ N, 128°36′34.6″ E) Pocheon (37°45′18.0″ N, 127°09′57.5″ E) | PGSC-491–494 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, W.-S.; Kim, H.-J.; Khalil, A.A.K.; Kang, D.-M.; Akter, K.-M.; Kwon, J.-M.; Kim, Y.-u.; Piao, X.-L.; Koo, K.-A.; Ahn, M.-J. Anatomical and Chemical Characterization of Ulmus Species from South Korea. Plants 2021, 10, 2617. https://doi.org/10.3390/plants10122617
Park W-S, Kim H-J, Khalil AAK, Kang D-M, Akter K-M, Kwon J-M, Kim Y-u, Piao X-L, Koo K-A, Ahn M-J. Anatomical and Chemical Characterization of Ulmus Species from South Korea. Plants. 2021; 10(12):2617. https://doi.org/10.3390/plants10122617
Chicago/Turabian StylePark, Woo-Sung, Hye-Jin Kim, Atif Ali Khan Khalil, Dong-Min Kang, Kazi-Marjahan Akter, Ji-Min Kwon, Yong-ung Kim, Xiang-Lan Piao, Kyung-Ah Koo, and Mi-Jeong Ahn. 2021. "Anatomical and Chemical Characterization of Ulmus Species from South Korea" Plants 10, no. 12: 2617. https://doi.org/10.3390/plants10122617
APA StylePark, W.-S., Kim, H.-J., Khalil, A. A. K., Kang, D.-M., Akter, K.-M., Kwon, J.-M., Kim, Y.-u., Piao, X.-L., Koo, K.-A., & Ahn, M.-J. (2021). Anatomical and Chemical Characterization of Ulmus Species from South Korea. Plants, 10(12), 2617. https://doi.org/10.3390/plants10122617








