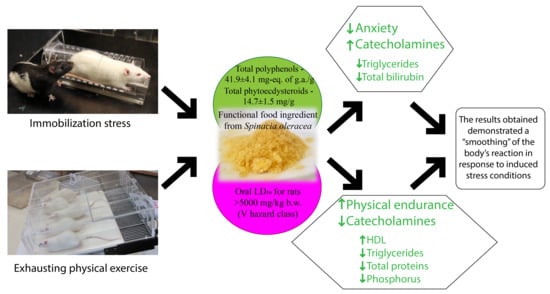
Adaptogenic Properties of a Phytoecdysteroid-Rich Extract from the Leaves of Spinacia oleracea L.
Abstract
:1. Introduction
2. Results
2.1. FFI from Spinacia oleracea L. Leaves: Polyphenol and Flavonoid Profile
2.2. Acute Oral Toxicity of FFI
2.3. Experiment No. 1: Adaptogenic Properties of FFI in a Model of Immobilization-Induced Emotional Stress
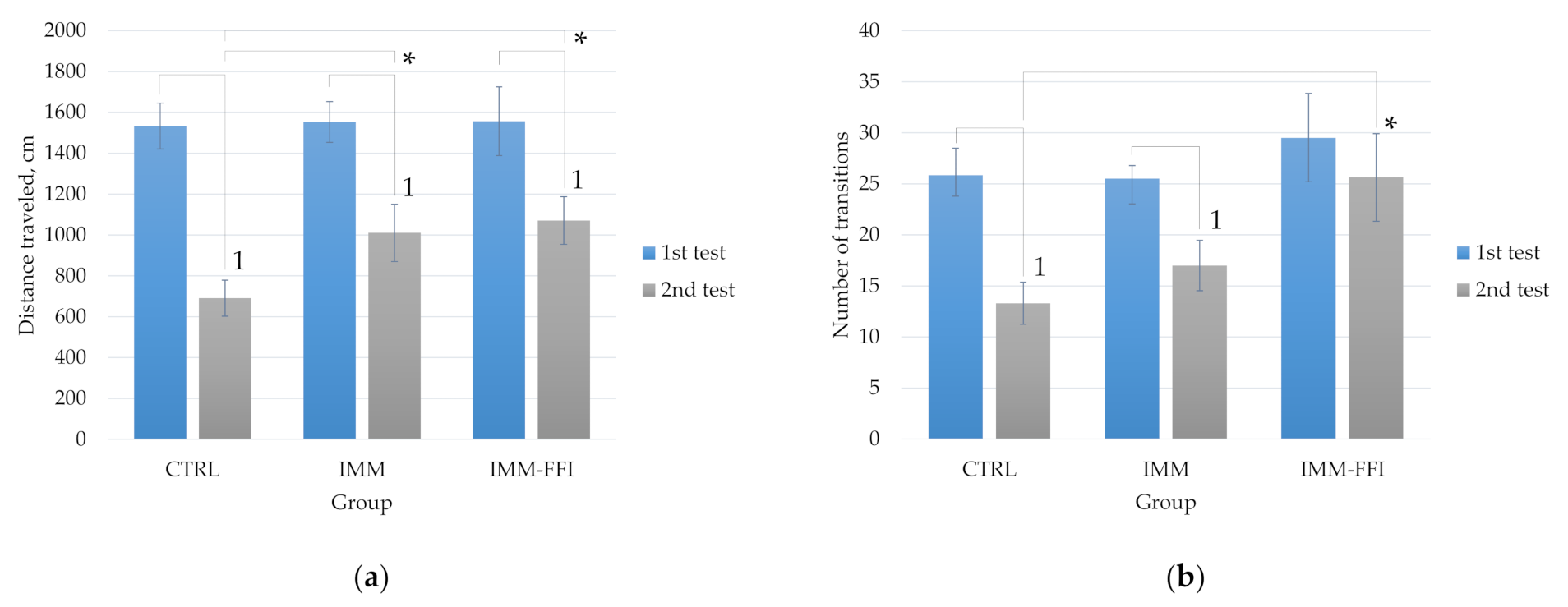
2.3.1. Integral Indicators
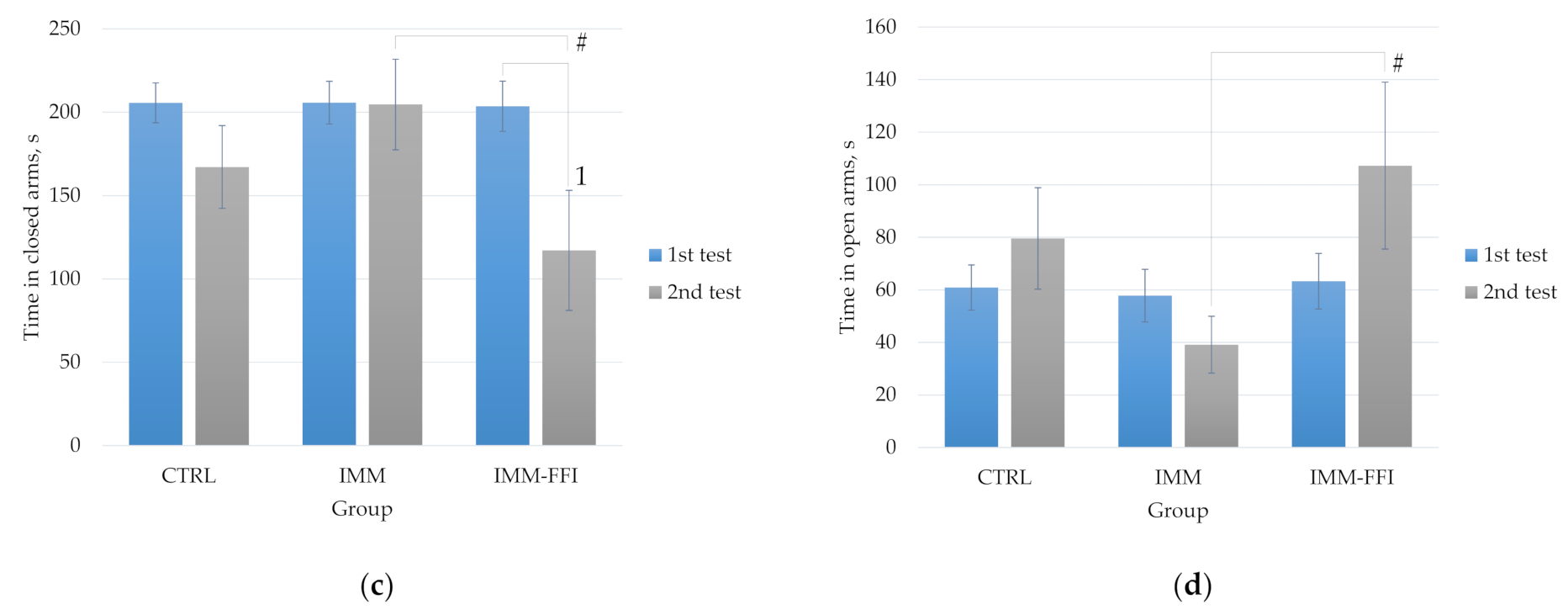
2.3.2. Memory Function and Behavioral Responses
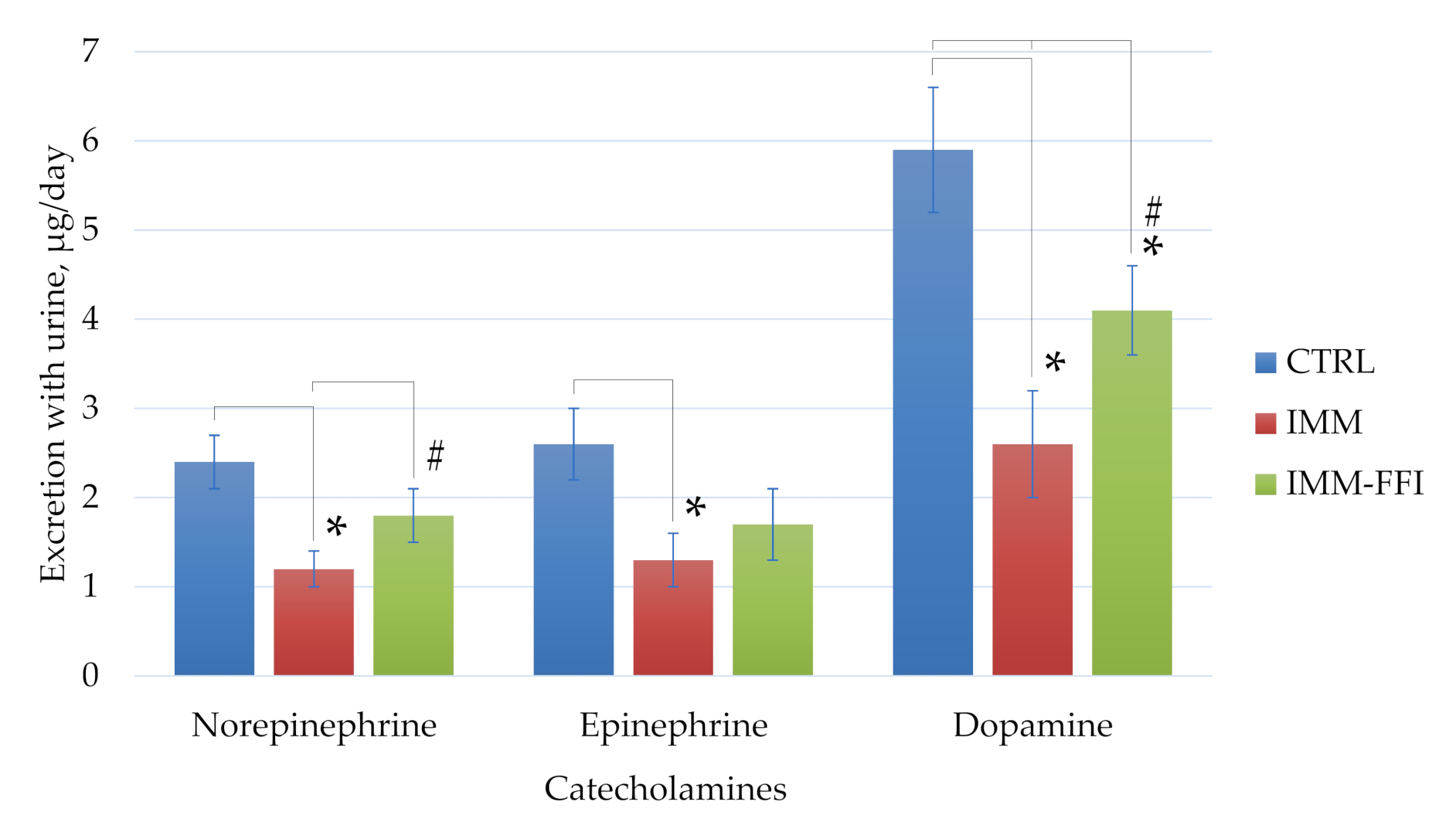
2.3.3. Biochemical Indices
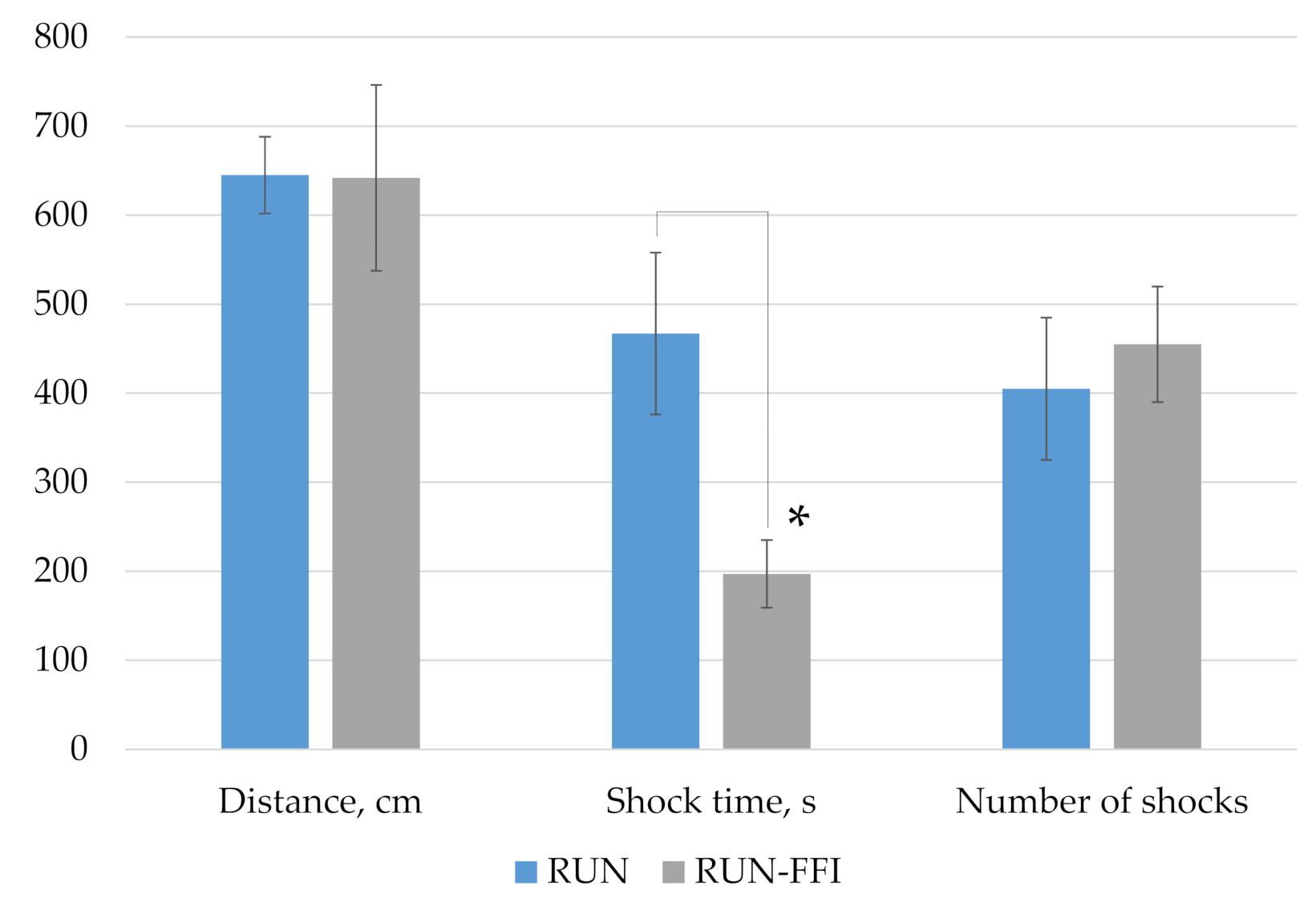
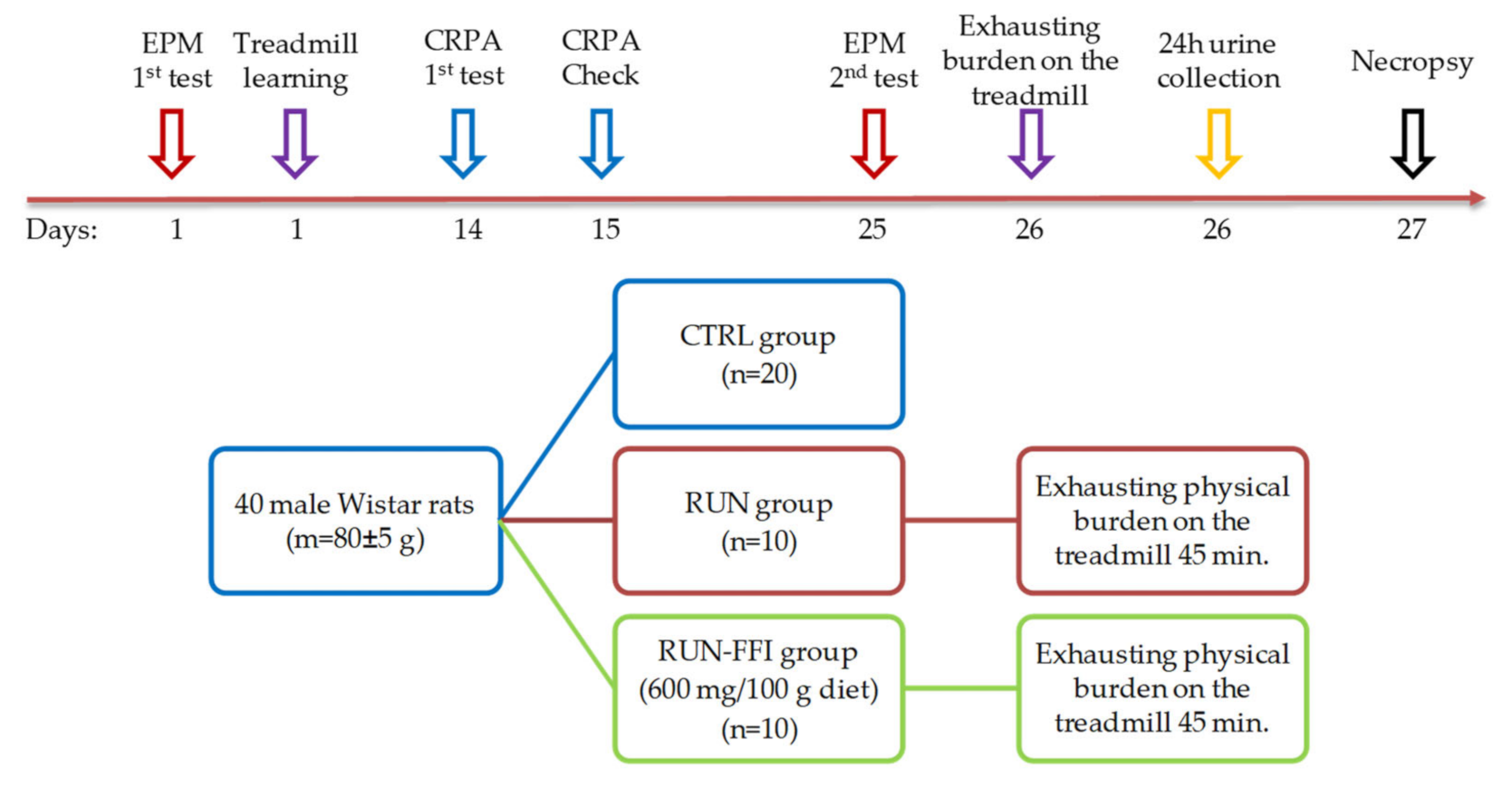
2.4. Experiment No. 2: Adaptogenic Properties of FFI in a Model of Increased Physical Energy Expenditure
2.4.1. Integral Indicators
2.4.2. Memory Function, Behavioral Responses, and Exhausting Physical Exercise on the Treadmill
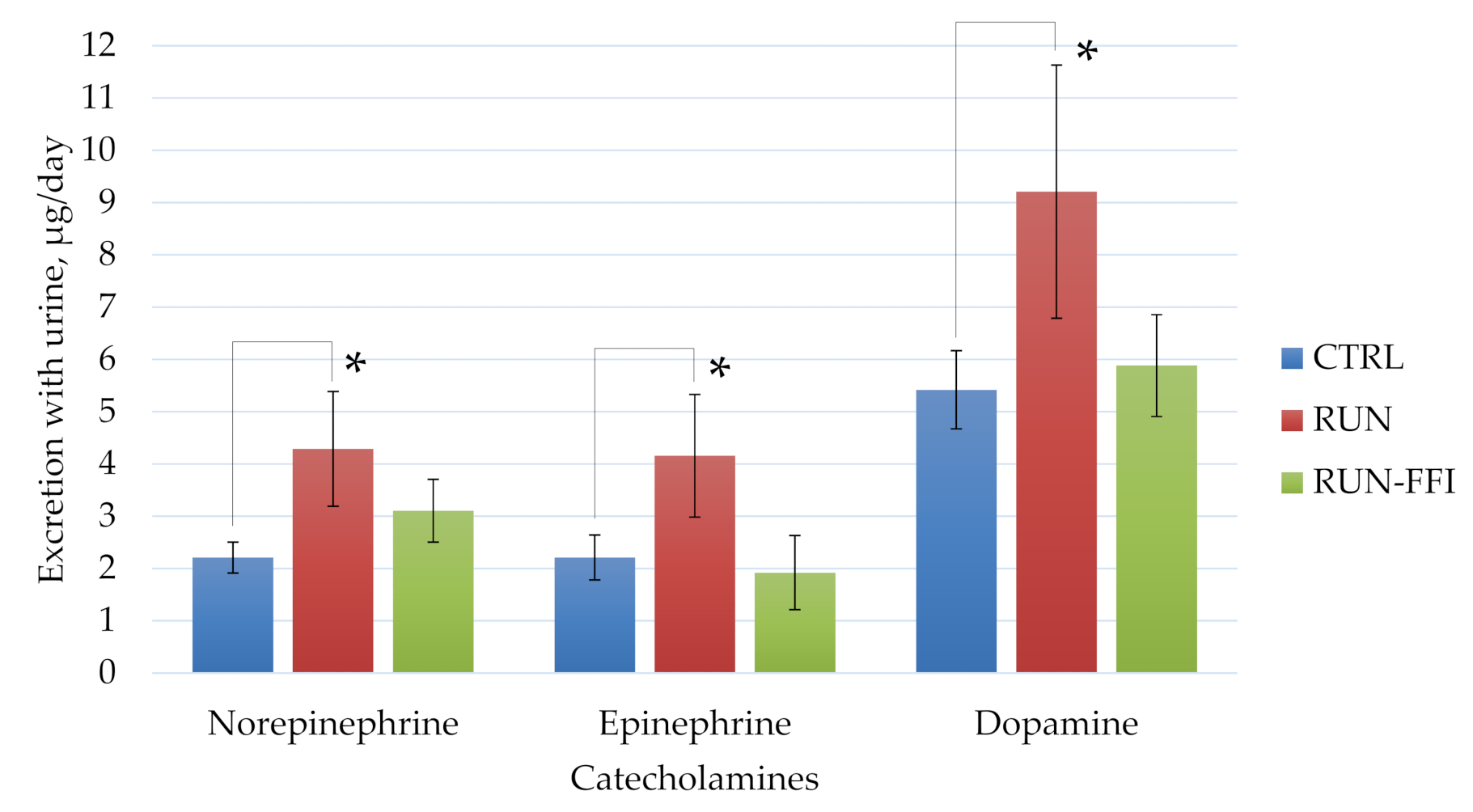
2.4.3. Biochemical Indices
3. Discussion
4. Materials and Methods
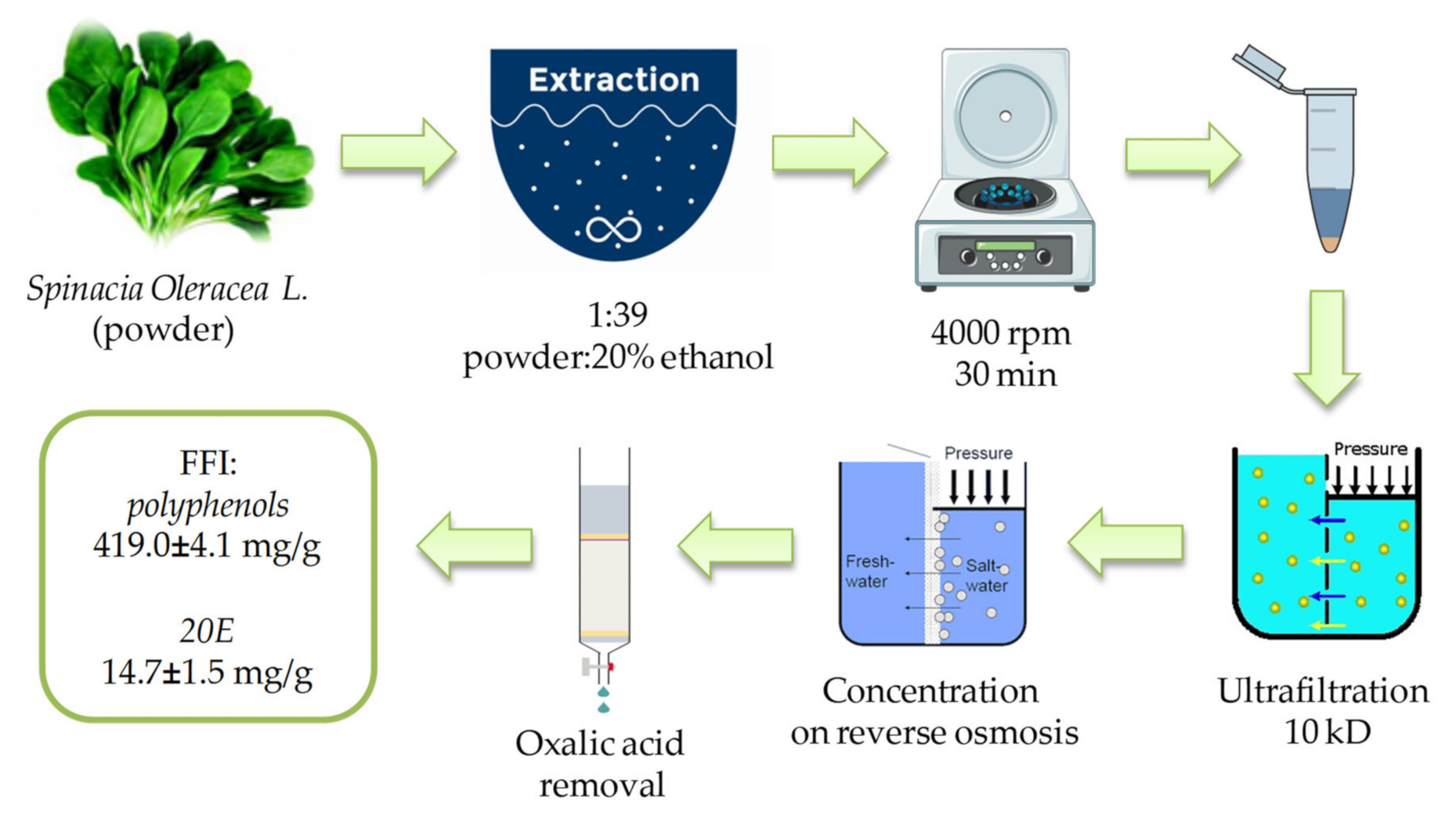
4.1. Preparation and Characterization of the FFI from Spinacia oleracea L. Leaves
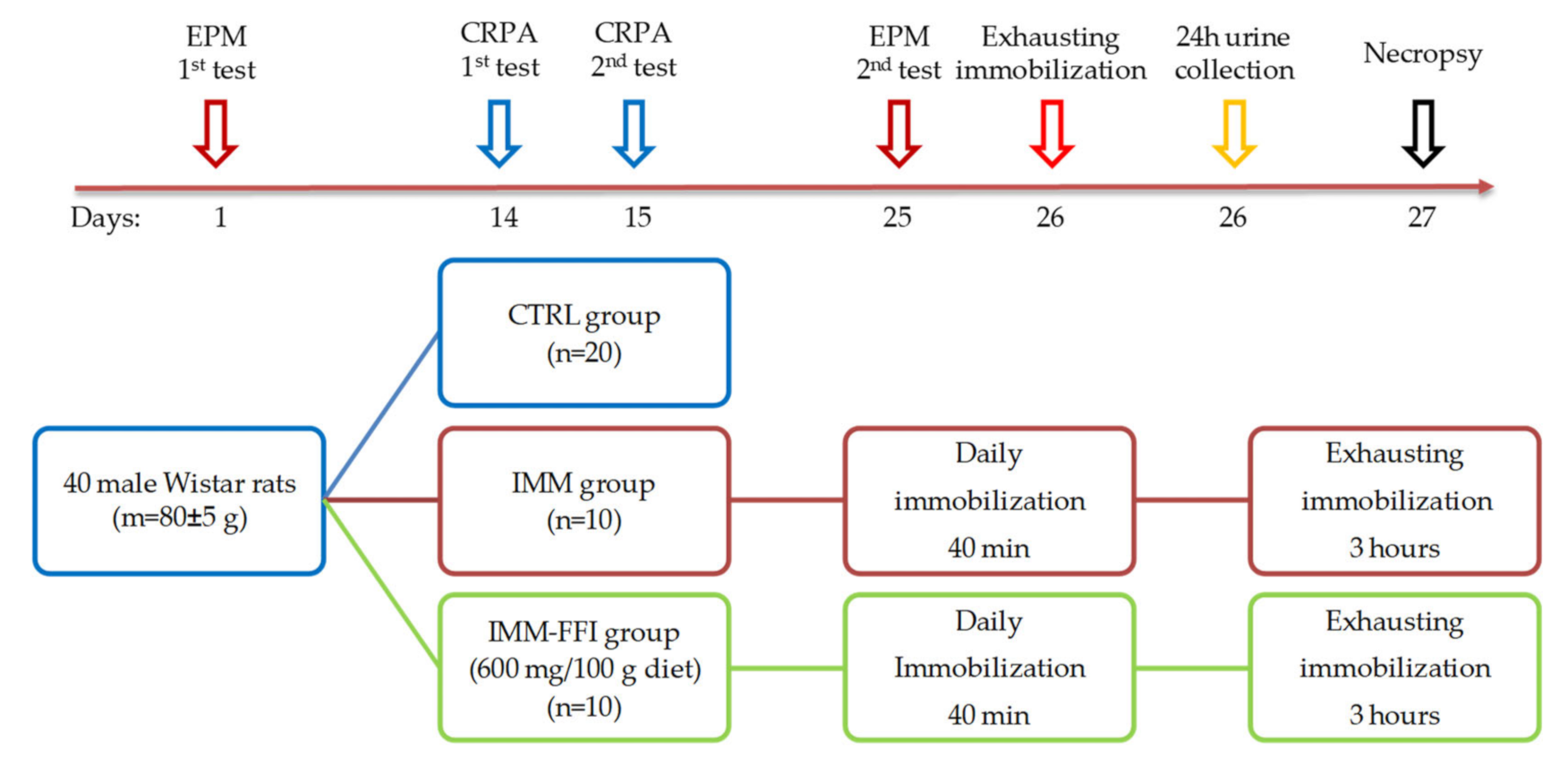
4.2. Animals and Experimental Design
4.2.1. Animals and Ethics
4.2.2. Acute Oral Toxicity: Fixed Dose Procedure
4.2.3. Experiment No. 1: Study of FFI Adaptogenic Properties in a Model of Immobilization-induced Emotional Stress
4.2.4. Experiment No. 2: Study of FFI Adaptogenic Properties in a Model of Increased Physical Energy Expenditure
4.3. Assessment of Biochemical Indices
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Head, K.A.; Kelly, G.S. Nutrients and botanicals for treatment of stress: Adrenal fatigue, neurotransmitter imbalance, anxiety, and restless sleep. Altern. Med. Rev. A J. Clin. Ther. 2009, 14, 114–140. [Google Scholar]
- Ray, A.; Gulati, K.; An, R. Stress, Adaptogens and Their Evaluation: An Overview. J. Pharm. Rep. 2016, 1, 1000110. [Google Scholar]
- Volodin, V.V.; Sidorova, I.S.; Mazo, V. 20-Hydroxyecdysone—Plant adaptogen: An anabolic effect, possible use in sports nutrition. Vopr. Pitan. 2013, 82, 24–30. [Google Scholar] [PubMed]
- Todorova, V.; Ivanov, K.; Delattre, C.; Nalbantova, V.; Karcheva-Bahchevanska, D.; Ivanova, S. Plant Adaptogens—History and Future Perspectives. Nutrients 2021, 13, 2861. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, Z.; Bildziukevich, U.; Wimmerová, M.; Macůrková, A.; Lovecká, P.; Wimmer, Z. Plant Adaptogens: Natural Medicaments for 21st Century? ChemistrySelect 2018, 3, 2196–2214. [Google Scholar] [CrossRef]
- Das, N.; Mishra, S.K.; Bishayee, A.; Ali, E.S.; Bishayee, A. The phytochemical, biological, and medicinal attributes of phytoecdysteroids: An updated review. Acta Pharm. Sin. B 2020, 11, 1740–1766. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.G.; Efferth, T.; Shikov, A.N.; Pozharitskaya, O.N.; Kuchta, K.; Mukherjee, P.K.; Banerjee, S.; Heinrich, M.; Wu, W.; Guo, D.; et al. Evolution of the adaptogenic concept from traditional use to medical systems: Pharmacology of stress- and aging-related diseases. Med. Res. Rev. 2021, 41, 630–703. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, E.G.; Carrera, S.P.P.; Gutierrez, R.M.P. Spinacia oleracea Linn Considered as One of the Most Perfect Foods: A Pharmacological and Phytochemical Review. Mini-Rev. Med. Chem. 2019, 19, 1666–1680. [Google Scholar] [CrossRef]
- Do, B.B.; YuS, S.; Vk, M.M.; Vv, B.B. Prospects for the Use of Spinach (Spinacia oleracea L.) Containing Phytoecdysteroids and Polyphenols. Pharmacogn. J. 2020, 12, 246–250. [Google Scholar] [CrossRef] [Green Version]
- Atlasbig. Available online: https://www.atlasbig.com/en-us/countries-spinach-production (accessed on 21 October 2021).
- Cartford, M.C.; Gemma, C.; Bickford, P. Eighteen-Month-Old Fischer 344 Rats Fed a Spinach-Enriched Diet Show Improved Delay Classical Eyeblink Conditioning and Reduced Expression of Tumor Necrosis Factor α (TNFα) and TNFβ in the Cerebellum. J. Neurosci. 2002, 22, 5813–5816. [Google Scholar] [CrossRef] [Green Version]
- Pezeshki-Nia, S.; Asle-Rousta, M.; Mahmazi, S. Spinacia oleracea L. extract attenuates hippocampal expression of TNF-α and IL-1β in rats exposed to chronic restraint stress. Med. J. Islam. Repub. Iran 2020, 34, 68–73. [Google Scholar] [CrossRef]
- Panda, V.; Shinde, P.; Dande, P. Consumption of Spinacia oleracea (spinach) and aerobic exercise controls obesity in rats by an inhibitory action on pancreatic lipase. Arch. Physiol. Biochem. 2020, 126, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, R.M.P.; Velazquez, E.G. Glucopyranoside flavonoids isolated from leaves of Spinacia oleracea (spinach) inhibit the formation of advanced glycation end products (AGEs) and aldose reductase activity (RLAR). Biomed. Pharmacother. 2020, 128, 110299. [Google Scholar] [CrossRef]
- Panda, V.; Shinde, P. Appetite suppressing effect of Spinacia oleracea in rats: Involvement of the short term satiety signal cholecystokinin. Appetite 2017, 113, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.; Kothari, P.; Tripathi, A.K.; Singh, S.; Adhikary, S.; Ahmad, N.; Kumar, S.; Dev, K.; Mishra, V.K.; Shukla, S.; et al. Spinacia oleracea extract attenuates disease progression and sub-chondral bone changes in monosodium iodoacetate-induced osteoarthritis in rats. BMC Complement. Altern. Med. 2018, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Amirinejad, A.; Hekmatdoost, A.; Ebrahimi, A.; Ranjbaran, F.; Shidfar, F. The effects of hydroalcoholic extract of spinach on prevention and treatment of some metabolic and histologic features in a rat model of nonalcoholic fatty liver disease. J. Sci. Food Agric. 2020, 100, 1787–1796. [Google Scholar] [CrossRef]
- Roberts, J.L.; Moreau, R. Functional properties of spinach (Spinacia oleracea L.) phytochemicals and bioactives. Food Funct. 2016, 7, 3337–3353. [Google Scholar] [CrossRef]
- Lomnitski, L.; Bergman, M.; Nyska, A.; Ben-Shaul, V.; Grossman, S. Composition, Efficacy, and Safety of Spinach Extracts. Nutr. Cancer 2003, 46, 222–231. [Google Scholar] [CrossRef]
- Son, H.; Jung, S.; Shin, J.H.; Kang, M.J.; Kim, H.J. Anti-Stress and Anti-Depressive Effects of Spinach Extracts on a Chronic Stress-Induced Depression Mouse Model through Lowering Blood Corticosterone and Increasing Brain Glutamate and Glutamine Levels. J. Clin. Med. 2018, 7, 406. [Google Scholar] [CrossRef] [Green Version]
- Gorgi, H.M.; Safakhah, H.A.; Haghighi, S. Anxiolytic effects of the aqueous extracts of spinach leaves in mice. Sci. J. Kurd. Univ. Med. Sci. 2010, 15, 43–50. [Google Scholar]
- Kaur, D.; Kamboj, A.; Shri, R.; Gujral, I. Comparative evaluation of anxiolytic effects of various extracts of oats (Avena Sativa), rice bran (Oryza Sativa) and spinach (Spinacia oleracea) in experimental animals. Int. J. Pharm. Sci. Res. 2016, 7, 4110–4116. [Google Scholar] [CrossRef]
- Fiorito, S.; Preziuso, F.; Epifano, F.; Scotti, L.; Bucciarelli, T.; Taddeo, V.A.; Genovese, S. Novel biologically active principles from spinach, goji and quinoa. Food Chem. 2019, 276, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Jaime, L.; Vázquez, E.; Fornari, T.; Hazas, M.D.C.L.D.L.; Garcia-Risco, M.R.; Santoyo, S.; Reglero, G. Extraction of functional ingredients from spinach (Spinacia oleracea L.) using liquid solvent and supercritical CO2extraction. J. Sci. Food Agric. 2014, 95, 722–729. [Google Scholar] [CrossRef] [Green Version]
- Zorin, S.N.; Petrov, N.A.; Perova, I.B.; Malinkin, A.D.; Bokov, D.O.; Bessonov, V.V. Development of a method for producing purified spinach extract with a high content of 20-hydroxyecdysone and polyphenols. Int. J. Pharm. Qual. Assur. 2021, 12, 1–6. [Google Scholar]
- Flandreau, E.I.; Tóth, M.A. Animal Models of PTSD: A Critical Review. Curr. Top. Behav. Neurosci. 2018, 38, 47–68. [Google Scholar] [CrossRef]
- Verbitsky, A.; Dopfel, D.; Zhang, N. Rodent models of post-traumatic stress disorder: Behavioral assessment. Transl. Psychiatry 2020, 10, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Caixeta, D.C.; Teixeira, R.R.; Peixoto, L.; Machado, H.L.; Baptista, N.B.; De Souza, A.V.; Vilela, D.D.; Franci, C.R.; Espindola, F.S. Adaptogenic potential of royal jelly in liver of rats exposed to chronic stress. PLoS ONE 2018, 13, e0191889. [Google Scholar] [CrossRef] [Green Version]
- Roumanille, R.; Vernus, B.; Brioche, T.; Descossy, V.; Van Ba, C.T.; Campredon, S.; Philippe, A.G.; Delobel, P.; Bertrand-Gaday, C.; Chopard, A.; et al. Acute and chronic effects of Rhaponticum carthamoides and Rhodiola rosea extracts supplementation coupled to resistance exercise on muscle protein synthesis and mechanical power in rats. J. Int. Soc. Sports Nutr. 2020, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Poleschuk, T.S.; Sultanov, R.M.; Ermolenko, E.; Shulgina, L.V.; Kasyanov, S.P. Protective action of alkylglycerols under stress. Stress 2019, 23, 213–220. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure; OECD Publishing: Paris, France, 2008. [Google Scholar] [CrossRef]
- Botros, M.; Sikaris, K.A. The De Ritis Ratio: The Test of Time. Clin. Biochem. Rev. 2013, 34, 117–130. [Google Scholar]
- Grebenok, R.J.; Ripa, P.V.; Adler, J.H. Occurrence and levels of ecdysteroids in spinach. Lipids 1991, 26, 666–668. [Google Scholar] [CrossRef]
- Gorelick, J.; Iraqi, R.H.; Bernstein, N. Ecdysteroid Content and Therapeutic Activity in Elicited Spinach Accessions. Plants 2020, 9, 727. [Google Scholar] [CrossRef]
- Rojo, M.Á.; Garrosa, M.; Jiménez, P.; Girbés, T.; Garcia-Recio, V.; Cordoba-Diaz, M.; Cordoba-Diaz, D. Unexpected Toxicity of Green Tea Polyphenols in Combination with the Sambucus RIL Ebulin. Toxins 2020, 12, 542. [Google Scholar] [CrossRef]
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and safety of polyphenol consumption. Am. J. Clin. Nutr. 2005, 81, 326S–329S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, N.; Yoshida, H.; Mizushina, Y. Chapter 26—Spinach and Health: Anticancer Effect; Watson, R.R., Preedy, V.R., Eds.; Academic Press: San Diego, MA, USA, 2010; pp. 393–405. ISBN 978-0-12-374628-3. [Google Scholar]
- Shukla, A.; Bigoniya, P.; Nagar, A. Anti-inflammatory potential of Spinacia oleracea leaf extract. J. Nat. Pharm. 2011, 2, 80. [Google Scholar] [CrossRef]
- Sharanova, N.E.; Kirbaeva, N.V.; Toropygin, I.Y.; Khryapova, E.V.; Koplik, E.V.; Soto, C.K.; Pertsov, S.S.; Vasiliev, A.V. Effect of Acute Emotional Stress on Proteomic Profile of Selected Brain Areas and Lysosomal Proteolysis in Rats with Different Behavioral Activity. Bull. Exp. Biol. Med. 2016, 161, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Pertsov, S.S.; Koplik, E.V.; Kalinichenko, L.S.; Alekseeva, I.V. Effects of Melatonin on Lipid Peroxidation in Blood in Rats with Different Behavioral Characteristics in Acute Emotional Stress. Neurosci. Behav. Physiol. 2016, 46, 133–137. [Google Scholar] [CrossRef]
- Mzhelskaya, K.V.; Shipelin, V.A.; Shumakova, A.A.; Musaeva, A.D.; Soto, J.S.; Riger, N.A.; Trusov, N.V.; Kirbaeva, N.V.; Apryatin, S.A.; Gmoshinski, I.V. Effects of quercetin on the neuromotor function and behavioral responses of Wistar and Zucker rats fed a high-fat and high-carbohydrate diet. Behav. Brain Res. 2020, 378, 112270. [Google Scholar] [CrossRef] [PubMed]
- Apryatin, S.A.; Shipelin, V.A.; Trusov, N.V.; Mzhelskaya, K.V.; Evstratova, V.S.; Kirbaeva, N.V.; Soto, J.S.; Fesenko, Z.S.; Gainetdinov, R.R.; Gmoshinski, I.V. Comparative analysis of the influence of a high-fat/high-carbohydrate diet on the level of anxiety and neuromotor and cognitive functions in Wistar and DAT-KO rats. Physiol. Rep. 2019, 7, e13987. [Google Scholar] [CrossRef] [Green Version]
- Poole, D.C.; Copp, S.W.; Colburn, T.D.; Craig, J.C.; Allen, D.L.; Sturek, M.; O’Leary, D.S.; Zucker, I.H.; Musch, T.I. Guidelines for animal exercise and training protocols for cardiovascular studies. Am. J. Physiol. Circ. Physiol. 2020, 318, H1100–H1138. [Google Scholar] [CrossRef]
- De Souza, R.F.; Augusto, R.L.; De Moraes, S.R.A.; De Souza, F.B.; Gonçalves, L.V.D.P.; Pereira, D.D.; Moreno, G.M.M.; De Souza, F.M.A.; Andrade-Da-Costa, B.L.D.S. Ultra-Endurance Associated With Moderate Exercise in Rats Induces Cerebellar Oxidative Stress and Impairs Reactive GFAP Isoform Profile. Front. Mol. Neurosci. 2020, 13, 157. [Google Scholar] [CrossRef]
- Millin, P.M.; Rickert, G.T. Effect of a Strawberry and Spinach Dietary Supplement on Spatial Learning in Early and Late Middle-Aged Female Rats. Antioxidants 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, M.; Moyna, N.M.; Zderic, T.W.; O’Gorman, D.J.; McCaffrey, N.; Carson, B.P.; Hamilton, M.T. Lipoprotein particle distribution and skeletal muscle lipoprotein lipase activity after acute exercise. Lipids Health Dis. 2012, 11, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casadio, M. Urinary Catecholamines as Markers in Overtraining Syndrome BT—Urinary Biomarkers: Methods and Proto-Cols; Salvi, S., Casadio, V., Eds.; Springer US: New York, NY, USA, 2021; pp. 185–192. ISBN 978-1-0716-1354-2. [Google Scholar]
- Reeves, P.G. Components of the AIN-93 Diets as Improvements in the AIN-76A Diet. J. Nutr. 1997, 127, 838S–841S. [Google Scholar] [CrossRef] [PubMed]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide Laboratory for the Care and Use of Animals; National Academies Press (US): Washington, DC, USA, 2011; ISBN 978-0-309-15400-0. [Google Scholar]

| Flavonoid | Content, % |
|---|---|
| The sum of flavonoids | 44.0 ± 2.0 |
| Patuletin-3-glucosyl-(1→6)-apiosyl-(1→2)-glucoside | 5.5 ± 0.8 |
| Patuletin-3-glucosyl-(1→6)-glucoside | 0.9 ± 0.3 |
| Patuletin-3-(2″feruloylglucosyl)-(1→6)-apiosyl-(1→2)-glucoside | 3.1 ± 0.4 |
| Patuletin-3-(2″feruloylglucosyl)-(1→6)-glucoside | 1.3 ± 0.3 |
| Axilyarin-4′-glucuronide (spinatoside) | 3.8 ± 0.6 |
| 5,3′,4′-trihydroxy-3-methoxy-6:7-methylenedioxyflavone-4′-glucuronide | 23.4 ± 0.4 |
| 5,4′-dihydroxy-3-methoxy-6:7-methylenedioxyflavone-4′-β-D-glucuronide | 1.5 ± 0.2 |
| 5,4′-dihydroxy-3,3′-dimethoxy-6:7-methylenedioxyflavone-4′-glucuronide | 1.0 ± 0.2 |
| Indicator | Group | ||
|---|---|---|---|
| CTRL | IMM | IMM-FFI | |
| Body weight, g | 104 ± 3 | 103 ± 3 | 105 ± 2 |
| Time in open arms, s | 61 ± 9 | 58 ± 10 | 63 ± 11 |
| Time in closed arms, s | 206 ± 12 | 206 ± 13 | 204 ± 15 |
| Distance, cm | 1533 ± 112 | 1553 ± 100 | 1556 ± 168 |
| Number of transitions | 26 ± 3 | 26 ± 1 | 30 ± 4 |
| First Test CRPA Formation | Second Test after 24 h Short-Term Memory | |||
|---|---|---|---|---|
| Latency, s | Non-Entered Animals (Excluded from the Test) | Latency, s | Number of Animals Entered | |
| CTRL | 40 ± 10 | 2 (20%) | 122 ± 18 | 3 (30%) |
| IMM | 34 ± 8 | 0 (0%) | 105 ± 25 | 4 (40%) |
| IMM-FFI | 48 ± 12 | 1 (10%) | 145 ± 23 | 2 (20%) |
| Indicator | Group | ||
|---|---|---|---|
| CTRL | IMM | IMM-FFI | |
| HDL, mmol/l | 0.58 ± 0.03 | 0.66 ± 0.08 | 0.60 ± 0.05 |
| LDL mmol/l | 0.17 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.02 |
| Triglycerides, mmol/l | 0.68 ± 0.05 | 0.84 ± 0.09 | 0.46 ± 0.05 1,2 |
| Cholesterol, mmol/l | 1.24 ± 0.02 | 1.31 ± 0.07 | 1.24 ± 0.09 |
| AlAT, U/l | 45.5 ± 1.6 | 42.5 ± 2.5 | 48.7 ± 3.4 |
| AsAT, U/l | 119.6 ± 4.7 | 116.5 ± 9.0 | 132 ± 11.6 |
| Total bilirubin, mmol/l | 2.96 ± 0.14 | 3.57 ± 0.30 1 | 2.65 ± 0.13 2 |
| Globulin, g/l | 19.3 ± 0.4 | 17.9 ± 0.4 1 | 19.3 ± 0.8 |
| Albumin, g/l | 24.4 ± 0.2 | 24.8 ± 0.3 | 24.1 ± 0.7 |
| Total protein, g/l | 43.6 ± 0.5 | 42.5 ± 0.6 | 43.4 ± 1.5 |
| Phosphorus, mmol/l | 1.95 ± 0.04 | 1.92 ± 0.06 | 1.89 ± 0.07 |
| Indicator | Group | ||
|---|---|---|---|
| CTRL | RUN | RUN-FFI | |
| Body weight, g | 104 ± 3 | 103 ± 3 | 104 ± 3 |
| Time in open arms, s | 61 ± 9 | 58 ± 10 | 62 ± 7 |
| Time in closed arms, s | 206 ± 12 | 206 ± 13 | 206 ± 11 |
| Distance, cm | 1533 ± 112 | 1553 ± 100 | 1553 ± 157 |
| Number of transitions | 26 ± 3 | 25 ± 3 | 27 ± 3 |
| Indicator | Group | ||
|---|---|---|---|
| CTRL | RUN | RUN-FFI | |
| HDL, mmol/l | 0.58 ± 0.03 | 0.65 ± 0.05 | 0.69 ± 0.03 1 |
| LDL mmol/l | 0.17 ± 0.01 | 0.18 ± 0.01 | 0.25 ± 0.03 1 |
| Triglycerides, mmol/l | 0.68 ± 0.05 | 0.83 ± 0.08 | 0.45 ± 0.03 1,2 |
| Cholesterol, mmol/l | 1.24 ± 0.02 | 1.50 ± 0.07 1 | 1.45 ± 0.03 1 |
| AlAT, U/l | 45.6 ± 1.6 | 47.0 ± 2.4 | 57.3 ± 3.3 1,2 |
| AsAT, U/l | 119.7 ± 4.7 | 140.0 ± 8.2 1 | 147.4 ± 12.1 1 |
| AsAT/AlAT | 2.67 ± 0.12 | 3.03 ± 0.66 | 2.56 ± 0.29 |
| Total bilirubin, mmol/l | 2.96 ± 0.14 | 2.81 ± 0.19 | 2.59 ± 0.21 |
| Globulin, g/l | 19.3 ± 0.4 | 21.5 ± 0.7 1 | 20.2 ± 0.3 1,2 |
| Albumin, g/l | 24.3 ± 0.2 | 25.1 ± 0.3 1 | 24.6 ± 0.4 |
| Total protein, g/l | 43.6 ± 0.5 | 46.7 ± 0.9 1 | 44.8 ± 0.6 |
| Phosphorus, mmol/l | 1.95 ± 0.04 | 2.08 ± 0.04 1 | 1.88 ± 0.06 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidorova, Y.S.; Shipelin, V.A.; Petrov, N.A.; Zorin, S.N.; Mazo, V.K. Adaptogenic Properties of a Phytoecdysteroid-Rich Extract from the Leaves of Spinacia oleracea L. Plants 2021, 10, 2555. https://doi.org/10.3390/plants10122555
Sidorova YS, Shipelin VA, Petrov NA, Zorin SN, Mazo VK. Adaptogenic Properties of a Phytoecdysteroid-Rich Extract from the Leaves of Spinacia oleracea L. Plants. 2021; 10(12):2555. https://doi.org/10.3390/plants10122555
Chicago/Turabian StyleSidorova, Yuliya S., Vladimir A. Shipelin, Nikita A. Petrov, Sergey N. Zorin, and Vladimir K. Mazo. 2021. "Adaptogenic Properties of a Phytoecdysteroid-Rich Extract from the Leaves of Spinacia oleracea L." Plants 10, no. 12: 2555. https://doi.org/10.3390/plants10122555
APA StyleSidorova, Y. S., Shipelin, V. A., Petrov, N. A., Zorin, S. N., & Mazo, V. K. (2021). Adaptogenic Properties of a Phytoecdysteroid-Rich Extract from the Leaves of Spinacia oleracea L. Plants, 10(12), 2555. https://doi.org/10.3390/plants10122555