Bioactive Essential Oils from Cuban Plants: An Inspiration to Drug Development
Abstract
:1. Introduction
2. Results and Discussion
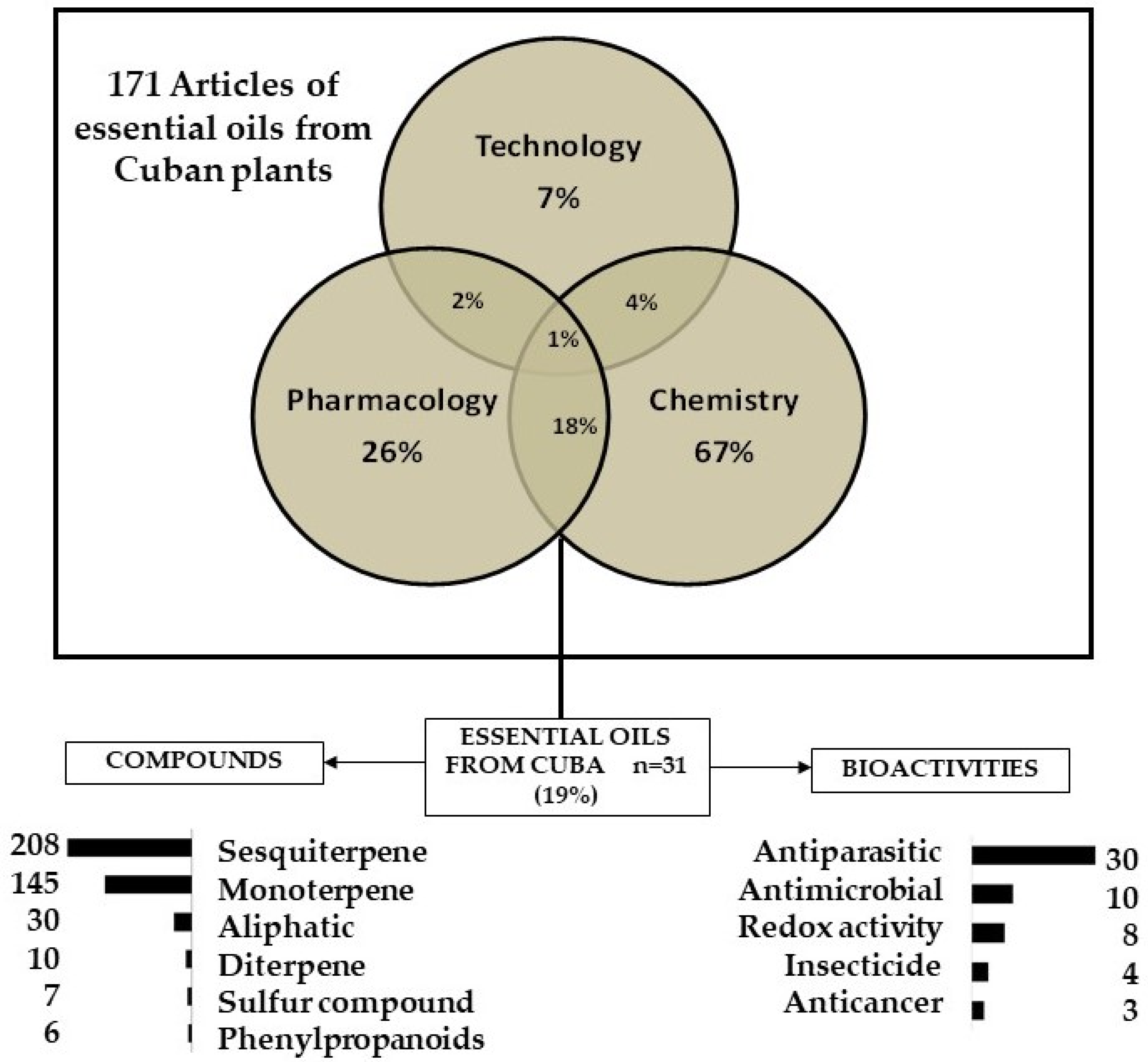
2.1. Study Selection
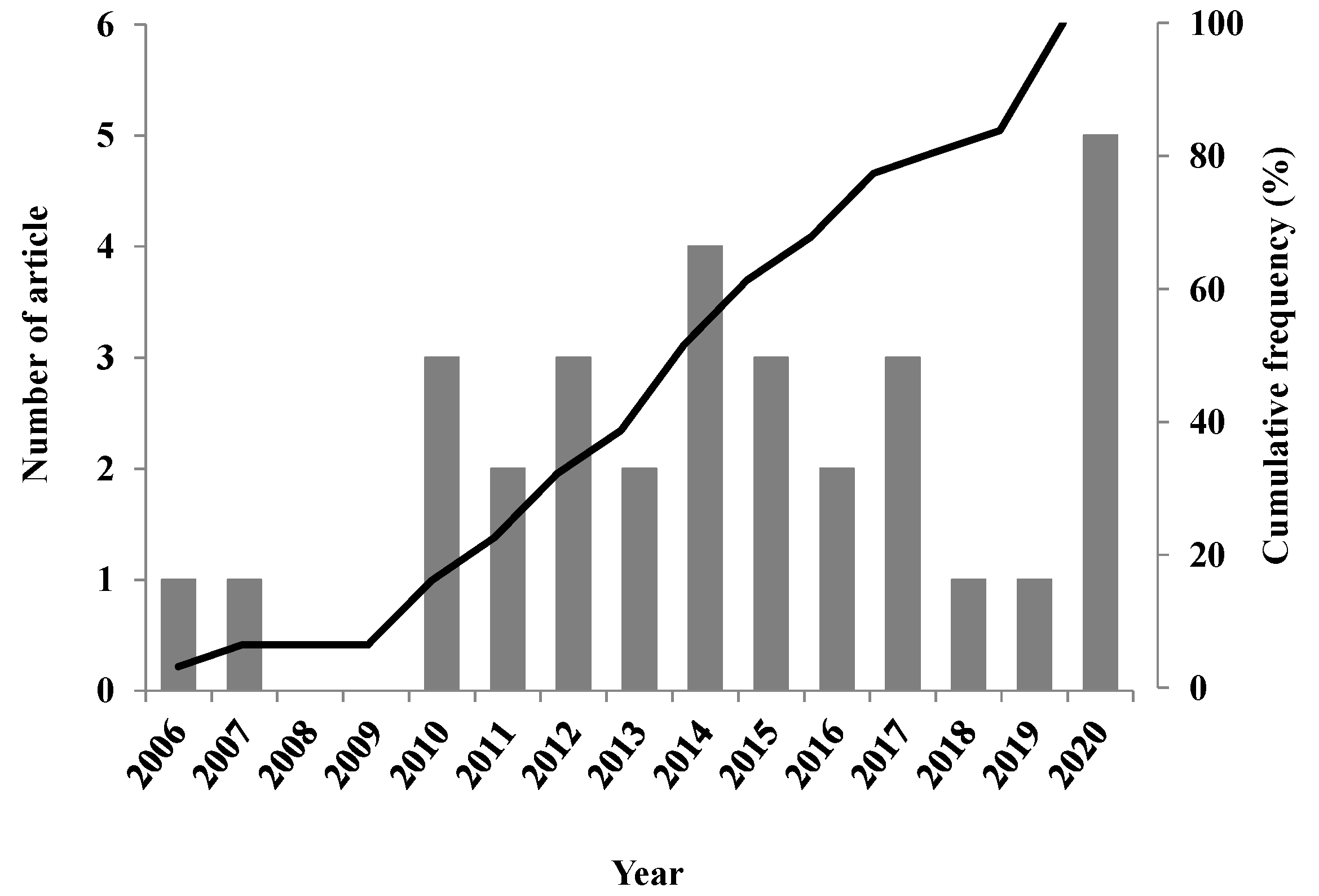
2.2. Bibliometric Analysis of Reports about Essential Oils from Cuban Plants
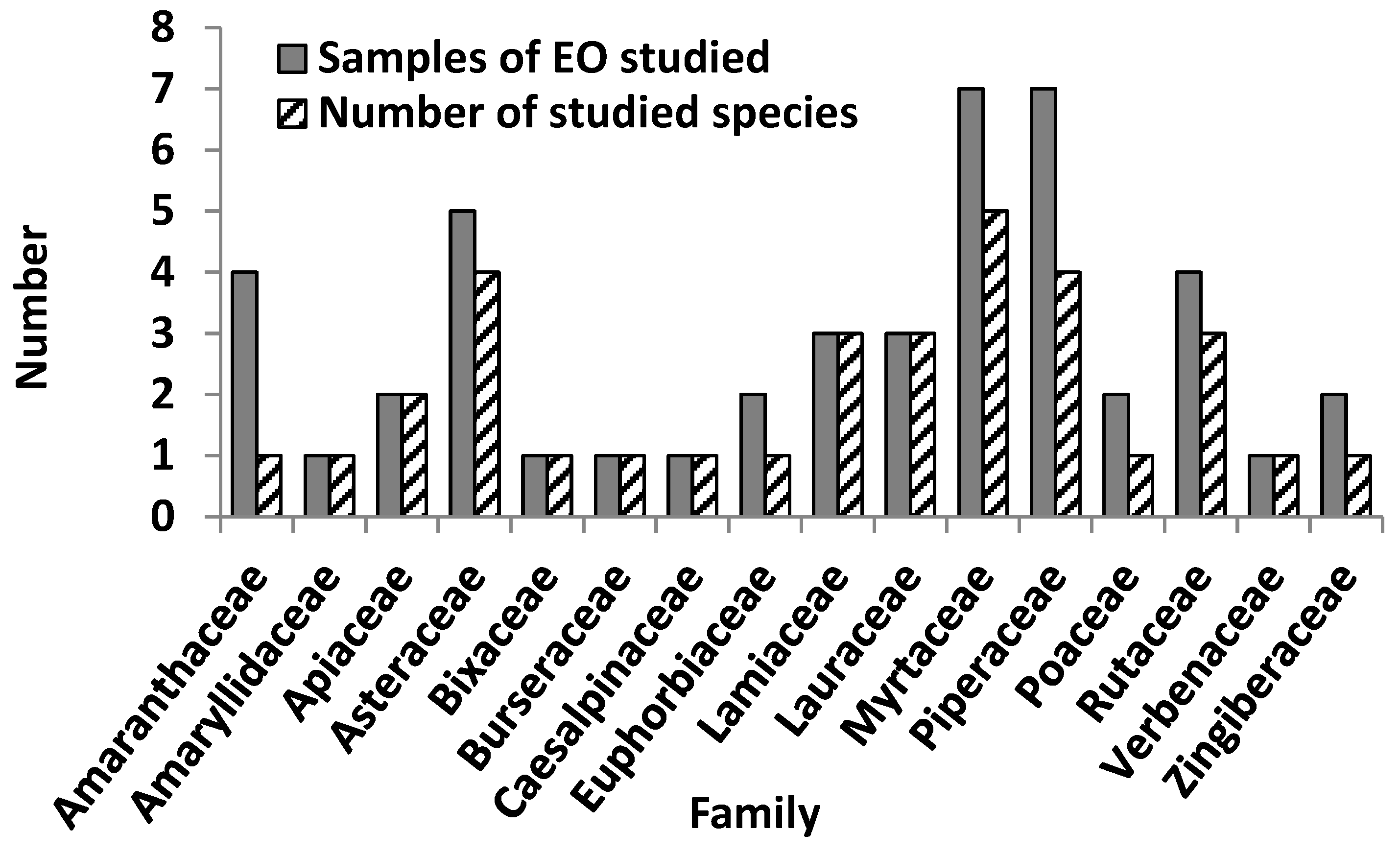
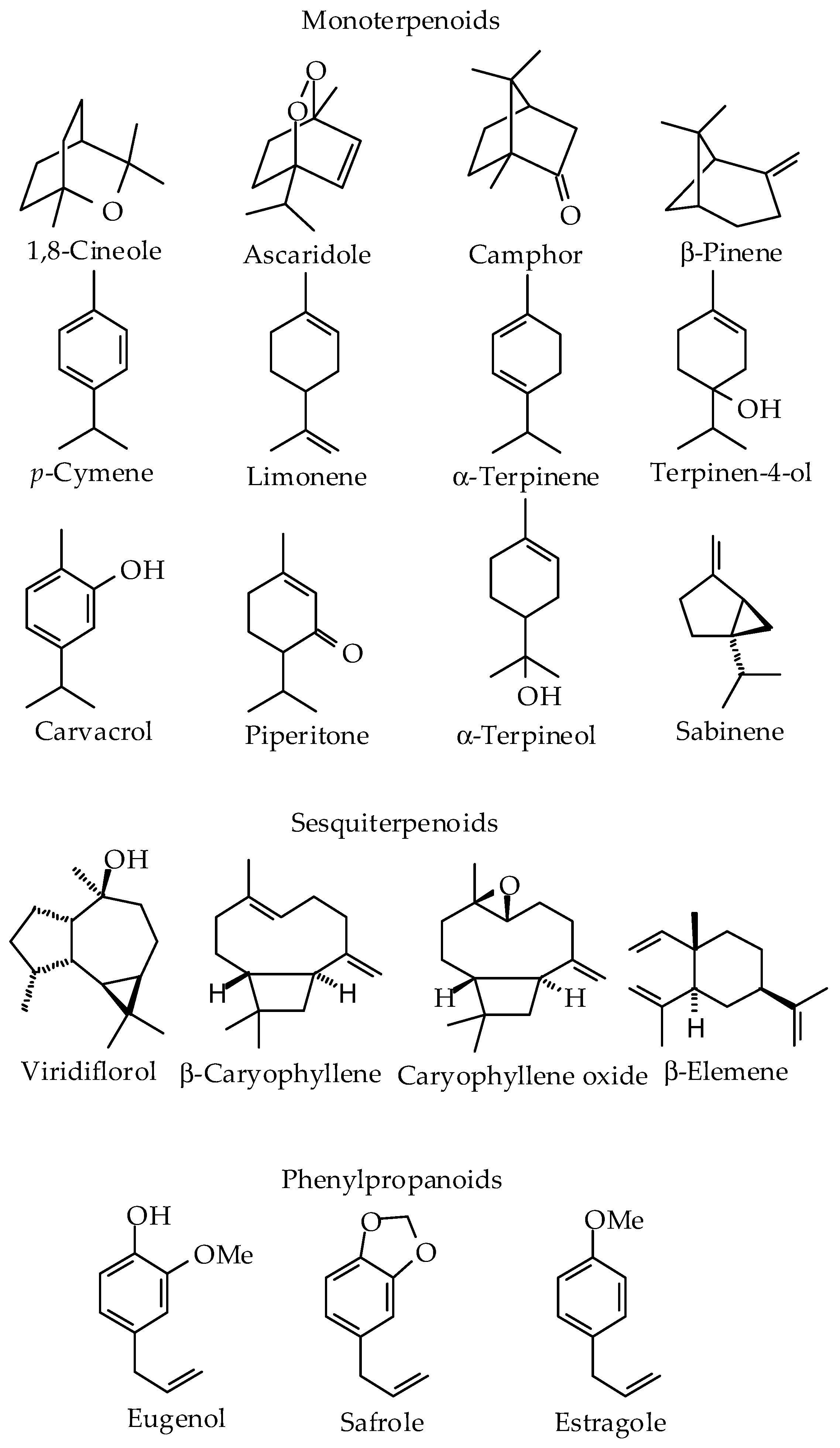
2.3. Chemical and Pharmacological Overview of Essential Oil from Cuban Plants
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarkar, N.; Dey, Y.N.; Kumar, D.R.M. Anti-mycobacterial constituents from medicinal plants: A review. Mini Rev. Med. Chem. 2021, 21, 3037–3051. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lautié, E.; Russo, O.; Ducrot, P.; Boutin, J.A. Unraveling plant natural chemical diversity for drug discovery purposes. Front. Pharmacol. 2020, 11, 397. [Google Scholar] [CrossRef]
- Hancock, R.D.; Hogenhout, S.; Foyer, C.H. Mechanisms of plant-insect interaction. J. Exp. Bot. 2015, 66, 421–424. [Google Scholar] [CrossRef] [Green Version]
- Kieliszek, M.; Edris, A.; Kot, A.M.; Piwowarek, K. Biological activity of some aromatic plants and their metabolites, with an emphasis on health-promoting properties. Molecules 2020, 25, 2478. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef] [Green Version]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [Green Version]
- Dunning, T. Aromatherapy: Overview, safety and quality issues. OA Altern. Med. 2013, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Chiriac, A.P.; Rusu, A.G.; Nita, L.E.; Chiriac, V.M.; Neamtu, I.; Sandu, A. Polymeric carriers designed for encapsulation of essential oils with biological activity. Pharmaceutics 2021, 13, 631. [Google Scholar] [CrossRef]
- Setzer, W.N. Essential oils and anxiolytic aromatherapy. Nat. Prod. Commun. 2009, 4, 1305–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Nogueiras, C.; Spengler, I.; Guerra, J.O.; Ortiz, Y.; Torres, S.; García, T.H.; Romeu, C.R.; Regalado, E.L.; González, T.A.; Perera, W.H.; et al. Contribution to the phytochemical study and biological activity of plants of Cuban flora. Biotecnol. Apl. 2010, 27, 315–318. [Google Scholar]
- Fuentes Fiallo, V.R. Las especies medicinales amenazadas en Cuba. Rev. Jard. Bot. Nac. 2008, 28, 77–81. [Google Scholar]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [Green Version]
- Monzote, L.; Montalvo, A.M.; Almanonni, S.; Scull, R.; Miranda, M.; Abreu, J. Activity of the essential oil from Chenopodium ambrosioides grown in Cuba against Leishmania amazonensis. Chemotherapy 2006, 52, 130–136. [Google Scholar] [CrossRef]
- Monzote, L.; Nance, M.R.; García, M.; Scull, R.; Setzer, W.N. Comparative chemical, cytotoxicity and antileishmanial properties of essential oils from Chenopodium ambrosioides. Nat. Prod. Commun. 2011, 6, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Borrego, S.; Valdés, O.; Vivar, I.; Lavin, P.; Guiamet, P.; Battistoni, P.; Gómez de Saravia, S.; Borges, P. Essential oils of plants as biocides against microorganisms isolated from Cuban and Argentine documentary heritage. ISRN Microbiol. 2012, 2012, 826786. [Google Scholar] [CrossRef] [Green Version]
- Monzote, L.; Piñón, A.; Scull, R.; Setzer, W.N. Chemistry and leishmanicidal activity of the essential oil from Artemisia absinthium from Cuba. Nat. Prod. Commun. 2014, 9, 1799–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, Y.I.; Scull, R.; Villa, A.; Satyal, P.; Cos, P.; Monzote, L.; Setzer, W.N. Chemical composition, antimicrobial and antiparasitic screening of the essential oil from Phania matricarioides (Spreng.) Griseb. Molecules 2019, 24, 1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, M.; Scull, R.; Satyal, P.; Setzer, W.N.; Monzote, L. Chemical characterization, antileishmanial activity, and cytotoxicity effects of the essential iil from leaves of Pluchea carolinensis (Jacq.) G. Don. (Asteraceae). Phyther. Res. 2017, 31, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Gutiérrez, Y.; Machin, L.; Staniek, K.; Scull, R.; Satyal, P.; Gille, L.; Setzer, W.N. Antileishmanial activity and influence on mitochondria of the essential oil from Tagetes lucida Cav. And its main component. Sci. Pharm. 2020, 88, 31. [Google Scholar] [CrossRef]
- Regalado, E.L.; Fernández, M.D.; Pino, J.A.; Mendiola, J.; Echemendia, O.A. Chemical composition and biological properties of the leaf essential oil of Tagetes lucida Cav. from Cuba. J. Essent. Oil Res. 2011, 23, 63–67. [Google Scholar] [CrossRef]
- Monzote, L.; Garcia, M.; Scull, R.; Cuellar, A.; Setzer, W.N. Antileishmanial activity of the essential oil from Bixa orellana. Phyther. Res. 2014, 28, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Hill, G.M.; Cuellar, A.; Scull, R.; Setzer, W.N. Chemical composition and anti-proliferative properties of Bursera graveolens essential oil. Nat. Prod. Commun. 2012, 7, 1531–1534. [Google Scholar] [CrossRef] [Green Version]
- García Díaz, J.; Escalona Arranz, J.C.; da Gama Jaén Batista, D.; Monzote Fidalgo, L.; de La Vega Acosta, J.; de Macedo, M.B.; Cos, P. Antileishmanial potentialities of Croton linearis leaf essential oil. Nat. Prod. Commun. 2018, 13, 629–634. [Google Scholar] [CrossRef]
- Rodriguez Amado, J.R.; Lafourcade Prada, A.; Garcia Diaz, J.; Souto, R.N.P.; Escalona Arranz, J.C.; de Souza, T.P. Development, larvicide activity, and toxicity in nontarget species of the Croton linearis Jacq essential oil nanoemulsion. Environ. Sci. Pollut. Res. 2020, 27, 9410–9423. [Google Scholar] [CrossRef] [PubMed]
- Escalona-Arranz, J.; Péres-Roses, R.; Urdaneta-Laffita, I.; Camacho-Pozo, M.; Rodríguez-Amado, J.; Licea-Jiménez, I. Antimicrobial activity of extracts from Tamarindus indica L. leaves. Pharmacogn. Mag. 2010, 6, 242–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chil Núñez, I.; Escalona Arranz, J.C.; Berenguer Rivas, C.A.; Mendonça, P.M.; Mateo Pérez, K.; Dutok Sánchez, C.M.; Cortinhas, L.B.; Silva, C.F.; Carvalho, M.G.; Queiroz, M.M.C. Chemical composition and toxicity of Ocimum sanctum L. var. cubensis essential oil up-growing in the eastern of Cuba. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 1021–1028. [Google Scholar]
- Monzote, L.; Scherbakov, A.M.; Scull, R.; Gutiérrez, Y.I.; Satyal, P.; Cos, P.; Shchekotikhin, A.E.; Gille, L.; Setzer, W.N. Pharmacological assessment of the carvacrol chemotype essential oil from Plectranthus amboinicus growing in Cuba. Nat. Prod. Commun. 2020, 15, 1934578X20962233. [Google Scholar] [CrossRef]
- Pino, J.A.; Rodríguez, D.K.; Beldarraín, T.; Blandariz, S.R. Chemical composition and antibacterial activity of the essential oil of Licaria triandra (Sw.) Kosterm. leaves from Cuba. J. Essent. Oil Res. 2014, 26, 263–266. [Google Scholar] [CrossRef]
- Pino, J.A.; Rodríguez, D.K.; Beldarraín, T.; Blandariz, S.R. Chemical composition and antibacterial activity of the essential oil of Nectandra antillana Meisn. leaves from Cuba. J. Essent. Oil Res. 2014, 26, 359–362. [Google Scholar] [CrossRef]
- Pino, J.A.; Rodríguez, D.K.; Beldarraín, T. Chemical composition and antibacterial activity of the essential oil of Callistemon speciosus (Sims) DC. leaves from Cuba. J. Essent. Oil Res. 2013, 25, 419–423. [Google Scholar] [CrossRef]
- Monzote, L.; Scherbakov, A.M.; Scull, R.; Satyal, P.; Cos, P.; Shchekotikhin, A.E.; Gille, L.; Setzer, W.N. Essential oil from Melaleuca leucadendra: Antimicrobial, antikinetoplastid, antiproliferative and cytotoxic assessment. Molecules 2020, 25, 5514. [Google Scholar] [CrossRef]
- Pino, J.A.; Regalado, E.L.; Rodríguez, J.L.; Fernández, M.D. Phytochemical analysis and in vitro free-radical-scavenging activities of the essential oils from leaf and fruit of Melaleuca leucadendra L. Chem. Biodivers. 2010, 7, 2281–2288. [Google Scholar] [CrossRef]
- Gaínza, Y.A.; Domingues, L.F.; Perez, O.P.; Rabelo, M.D.; López, E.R.; de Souza Chagas, A.C. Anthelmintic activity in vitro of Citrus sinensis and Melaleuca quinquenervia essential oil from Cuba on Haemonchus contortus. Ind. Crops Prod. 2015, 76, 647–652. [Google Scholar] [CrossRef]
- Leyva, M.; Tacoronte, J.E. Chemical composition and lethal effect of essential oil from Pimenta racemosa (Myrtales: Myrtaceae) on Blatella germanica (Dictyoptera: Blattellidae). Rev. Cubana Med. Trop. 2007, 59, 154–158. [Google Scholar]
- Monzote, L.; Scull, R.; Cos, P.; Setzer, W.N. Essential oil from Piper aduncum: Chemical analysis, antimicrobial assessment, and literature review. Medicines 2017, 4, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, E.J.; Saucedo-Hernández, Y.; Vander Heyden, Y.; Simó-Alfonso, E.F.; Ramis-Ramos, G.; Lerma-García, M.J.; Monteagudo, U.; Bravo, L.; Medinilla, M.; de Armas, Y.; et al. Chemical analysis and antioxidant activity of the essential oils of three Piperaceae species growing in the central region of Cuba. Nat. Prod. Commun. 2013, 8, 1325–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, Y.I.; Scull, R.; Delgado, L.; Sánchez, A.; Jiménez, C.A. Parámetros farmacognósticos y actividad antioxidante de Piper aduncum subsp. ossanum Trel según el lugar de colecta. Rev. Ciencias Farm. Aliment. 2016, 2, 1–14. [Google Scholar]
- Monzote, L.; García, M.; Montalvo, A.M.; Scull, R.; Miranda, M. Chemistry, cytotoxicity and antileishmanial activity of the essential oil from Piper auritum. Mem. Inst. Oswaldo Cruz 2010, 105, 168–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, Z.T.; Sánchez, F.F.; Santos, A.R.D.; Amaral, A.C.F.; Ferreira, J.L.P.; Escalona-Arranz, J.C.; de C. Queiroz, M.M. Chemical composition and insecticidal activity of Cymbopogon citratus essential oil from Cuba and Brazil against housefly. Rev. Bras. Parasitol. Vet. 2015, 24, 36–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, E.J.; Ramis-Ramos, G.; Heyden, Y.V.; Simó-Alfonso, E.F.; Lerma-García, M.J.; Saucedo-Hernández, Y.; Monteagudo, U.; Morales, Y.; Holgado, B.; Herrero-Martínez, J.M. Chemical composition, antioxidant properties and antimicrobial activity of the essential oil of Murraya paniculata leaves from the mountains of Central Cuba. Nat. Prod. Commun. 2012, 7, 1527–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, L.M.; Kasangana, P.B.; Ojito-Ramos, K.; Portal, O. Antifungal potential of essential oil from Zanthoxylum pseudodumosum Beurton, an endemic species of Cuba. Curr. Top. Phytochem. 2020, 16, 29–38. [Google Scholar]
- Satyal, P.; Crouch, R.A.; Monzote, L.; Cos, P.; Awadh Ali, N.A.; Alhaj, M.A.; Setzer, W.N. The chemical diversity of Lantana camara: Analyses of essential oil samples from Cuba, Nepal, and Yemen. Chem. Biodivers. 2016, 13, 336–342. [Google Scholar] [CrossRef]
- Mendiola, J.; Pino, J.A.; Fernández-Calienes, A.; Mendoza, D.; Herrera, P. Chemical composition and in vitro antiplasmodial activity of essential oils of leaves and flowers of Alpinia zerumbet grown in Cuba. Pharmacologyonline 2015, 2, 1–5. [Google Scholar]
- Ross, I.A. Medicinal Plants of the World, Volume 1; Humana Press: Totowa, NJ, USA, 1999. [Google Scholar]
- Ross, I.A. Medicinal Plants of the World, Volume 2; Humana Press: Totowa, NJ, USA, 2001. [Google Scholar]
- Moore, B.D.; Andrew, R.L.; Külheim, C.; Foley, W.J. Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol. 2014, 201, 733–750. [Google Scholar] [CrossRef]
- Sangwan, N.S.; Farooqi, A.H.A.; Shabih, F.; Sangwan, R.S. Regulation of essential oil production in plants. Plant Growth Regul. 2001, 34, 3–21. [Google Scholar] [CrossRef]
- Bednarek, P.; Osbourn, A. Plant-microbe interactions: Chemical diversity in plant defense. Science 2009, 324, 746–748. [Google Scholar] [CrossRef]
- Gutiérrez, Y.; Montes, R.; Scull, R.; Sánchez, A.; Cos, P.; Monzote, L.; Setzer, W.N. Chemodiversity associated with cytotoxicity and antimicrobial activity of Piper aduncum var. ossanum. Chem. Biodivers. 2016, 13, 1715–1719. [Google Scholar] [CrossRef]
- Luna, E.C.; Luna, I.S.; Scotti, L.; Monteiro, A.F.M.; Scotti, M.T.; De Moura, R.O.; De Araújo, R.S.A.; Monteiro, K.L.C.; de Aquino, T.M.; Ribeiro, F.F.; et al. Active essential oils and their components in use against neglected diseases and arboviruses. Oxid. Med. Cell. Longev. 2019, 2019, 6587150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Morais, M.C.; de Souza, J.V.; da Silva Maia Bezerra Filho, C.; Dolabella, S.S.; de Sousa, D.P. Trypanocidal essential oils: A review. Molecules 2020, 25, 4568. [Google Scholar] [CrossRef] [PubMed]
- de Lara da Silva, C.E.; Oyama, J.; Ferreira, F.B.P.; de Paula Lalucci-Silva, M.P.; Lordani, T.V.A.; de Lara da Silva, R.C.; de Souza Terron Monich, M.; Teixeira, J.J.V.; Lonardoni, M.V.C. Effect of essential oils on Leishmania amazonensis: A systematic review. Parasitology 2020, 147, 1392–1407. [Google Scholar] [CrossRef]
- Montalvo, A.M.; Fraga, J.; Blanco, O.; González, D.; Monzote, L.; Soong, L.; Capó, V. Imported leishmaniasis cases in Cuba (2006–2016): What have we learned. Trop. Dis. Travel Med. Vaccines 2018, 4, 7. [Google Scholar] [CrossRef]
- Herrera, G.; Barragán, N.; Luna, N.; Martínez, D.; De Martino, F.; Medina, J.; Niño, S.; Páez, L.; Ramírez, A.; Vega, L.; et al. An interactive database of Leishmania species distribution in the Americas. Sci. Data 2020, 7, 110. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Oliveira, P.; Otero, P.; Pereira, A.G.; Chamorro, F.; Carpena, M.; Echave, J.; Fraga-Corral, M.; Simal-Gandara, J.; Prieto, M.A. Status and challenges of plant-anticancer compounds in cancer treatment. Pharmaceuticals 2021, 14, 157. [Google Scholar] [CrossRef]
- Dias, C.N.; Moraes, D.F.C. Essential oils and their compounds as Aedes aegypti L. (Diptera: Culicidae) larvicide: Review. Parasitol. Res. 2014, 113, 565–592. [Google Scholar] [CrossRef] [PubMed]
- Fernández Andreu, C.M.; Martínez Machín, G.; Perurena Lancha, M.R.; Illnait Zaragozí, M.T.; Velar Martínez, R.; San Juan Galán, J.L. Contributions of the mycology laboratory at Pedro Kouri Tropical Medicine Institute to the development of the specialty in Cuba. Rev. Cubana Med. Trop. 2017, 69, 1–18. [Google Scholar]
- Carioli, G.; Bertuccio, P.; Malvezzi, M.; Rodriguez, T.; Levi, F.; Boffetta, P.; La Vecchia, C.; Negri, E. Cancer mortality predictions for 2019 in Latin America. Int. J. Cancer 2020, 147, 619–632. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Firn, R.D.; Jones, C.G. Natural products—A simple model to explain chemical diversity. Nat. Prod. Rep. 2003, 20, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Clemons, P.A.; Wilson, J.A.; Dančík, V.; Muller, S.; Carrinski, H.A.; Wagner, B.K.; Koehler, A.N.; Schreiber, S.L. Quantifying structure and performance diversity for sets of small molecules comprising small-molecule screening collections. Proc. Natl. Acad. Sci. USA 2011, 108, 6817–6822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Journal | Number of Articles (%) | Impact Factor | Published | Aims/Scope |
|---|---|---|---|---|
| Chemistry & Biodiversity | 3 (10) | 2.408 a | Wiley-VHCA AG, Zurich, Switzerland | All aspects of biologically relevant chemistry research |
| Chemotherapy | 1 (3) | 2.544 a | Karger Publisher, Basel, Switzerland | All aspects of antimicrobial and antitumor chemotherapy |
| Current Topics in Phytochemistry | 1 (3) | Not available | Research Trends, Trivandrum, India | All aspects of pure and applied plant chemistry, biochemistry, molecular biology and related interdisciplinary aspects |
| Environmental Science and Pollution Research | 1 (3) | 4.223 a | Springer, New York, NY, USA | All areas of environmental science and related subject with emphasis on chemical compounds |
| Industrial Crops and Products | 1 (3) | 5.645 a | Elsevier, Amsterdam, The Netherlands | Research on cultivated plants (crops) of industrial interest (non-food, non-feed) |
| International Journal of Pharmacognosy and Phytochemical Research | 1 (3) | Not available | DYR Labs, Devipura, India | Research in the fields of pharmacognosy, phytochemistry, and ethnopharmacology |
| ISRN Microbiology | 1 (3) | Not available | Hindawi Publishing Corporation, Cairo, Egypt | Microbiological phenomena and microbiology |
| Journal of Essential Oil Research | 4 (13) | 1.963 a | Taylor and Francis Ltd., United Kingdom | Publication of essential oil research and analysis. |
| Medicines | 1 (3) | Not available | MDPI, Basel, Switzerland | All areas of medical disciplines and sub-specialties |
| Memórias do Instituto Oswaldo Cruz | 1 (3) | 2.070 b | Fundação Oswaldo Cruz, Rio de Janeiro, Brazil | Research related to medicine (miscellaneous) or microbiology (medical) |
| Molecules | 2 (7) | 4.411 a | MDPI, Basel, Switzerland | Provides an advanced forum for science of chemistry and all interfacing disciplines |
| Natural Product Communications | 7 (23) | 0.986 a | SAGE Publishing, Newbury Park, CA, USA | All aspects of natural products |
| Pharmacognosy Magazine | 1 (3) | 1.31 b | Wolters Kluwer Health, Mumbai, India | All aspects of pharmacognosy, and related fields |
| Pharmacologyonline | 1 (3) | 0.205 b | Societa Italo-Latinoamericana di Etnomedicina, Fisciano, Italy | Pharmacology, ethnopharmacology and medicinal plants |
| Phytotherapy Research | 2 (7) | 5.878 a | John Wiley & Sons, Hoboken, NJ, USA | Publication on medicinal plant research |
| Revista Brasileira de Parasitologia Veterinária | 1 (3) | 1.024 a | Colégio Brasileiro De Parasitologia Veterinária, Jaboticabal, São Paulo, Brazil | Brazilian research in the areas of helminthology, protozoology, entomology and agents transmitted by arthropods, related to animal health |
| Revista Cubana de Medicina Tropical | 1 (3) | 0.304 b | Centro Nacional de Información de Ciencias Médicas, Havana, Cuba | Publish scientific articles specialized in tropical medicine, microbiology, parasitology, epidemiology and other related specialties |
| Scientia Pharmaceutica | 1 (3) | Not available | MDPI, Basel, Switzerland | All fields of pharmaceutical sciences and related disciplines |
| Family | Plant Species | Collection Site a (Growth Conditions) | Organ Used (Yield) | Main Chemical Compounds f | Ref. |
|---|---|---|---|---|---|
| Amaranthaceae | Dysphania ambrosioides (L.) Mosyakin & Clemants b | IFAL, Havana (NR) | Fresh aerial parts (NR) | Carvacrol (62.4%) and ascaridole (22.5%) | [18] |
| Caimito, Artemisa (NR) | Fresh aerial parts (NR) | α-Terpinene (20.7%), p-cymene (21.3%) and ascaridole (35.1%) | [19] | ||
| Dried aerial parts (NR) | α-Terpinene (19.7%), p-cymene (20.2%) and ascaridole (47.1%) | ||||
| Fermented in water aerial parts (NR) | α-Terpinene (17.0%), p-cymene (21.1%) and ascaridole (30.5%) | ||||
| Amaryllidaceae | Allium sativum L. | - | - | Di-2-propenyl trisulfide (31.9%), methyl 2-propenyl trisulfide (21.7%) and di-2-propenyl disulfide (20.7%) | [20] |
| Apiaceae | Cuminum cyminum L. | - | - | Cuminaldehyde (43.3%), cuminal (20.4%) f and β-pinene (12.8%), | [20] |
| Pimpinella anisum L. | - | - | Anethole (80.8%) | [20] | |
| Asteraceae | Artemisia absinthium L. | IFAL, Havana (NR) | Leaves (NR) | trans-Sabinyl acetate (36.7%) | [21] |
| Phania matricarioides (Spreng.) Griseb. | Bauta, Artemisa (NR) | Fresh aerial parts (0.1%) | Lavandulyl acetate (40.1%) and thymyl isobutyrate (13.9%) | [22] | |
| Pluchea carolinensis (Jacq.) G. Don. | La Lisa, Havana (NR) | Fresh aerial parts (NR) | Selin-11-en-4α-ol (51.0%) | [23] | |
| Tagetes lucida Cav. | IFAL, Havana (NR) | Fresh aerial parts (NR) | Estragole (97%) | [24] | |
| IIIA, Havana (Cultivated) | Leaves (0.79%) | Estragole (96.8%) | [25] | ||
| Bixaceae | Bixa orellana L. | La Lisa, Havana (NR) | Seed (NR) | Ishwarane (18.6%) and geranylgeraniol (9.1%) | [26] |
| Burseraceae | Bursera graveolens (Kunth) Triana & Planch. | La Lisa, Havana (NR) | Fresh aerial part (NR) | Limonene (26.5%) and β-elemene (14.1%) | [27] |
| Euphorbiaceae | Croton linearis Jacq. | Reserva Ecológica Siboney-Juticí, Santiago de Cuba e (Wild) | Leaves (1.6%) | Guaiol (7.9%), guaia-3,10(14)-dien-11-ol (4.5%), selina-4(15),7(11)-diene (4.2%), and β-elemene (4.1%) | [28] |
| Leaves (1.5%) | 1,8-Cineole (26.7%) and sabinene (9.4%) | [29] | |||
| Fabaceae | Tamarindus indica L. | Santiago de Cuba e (Wild) | Leaves (NR) | Benzyl benzoate (40.9%), limonene (24.7%), and hexadecanol (11.9%) | [30] |
| Lamiaceae | Ocimum tenuiflorum L. c | San Luis, Santiago de Cuba (Wild) | Leaves (0.5%) | Eugenol (22.0%), β-caryophyllene (20.8%), bicyclogermacrene (20.4%) | [31] |
| Origanum vulgare L., | - | - | Thymol (38.0%), cis-β-terpineol (16.5%), and terpinen-4-ol (10.2%) | [20] | |
| Plectranthus amboinicus (Lour.) Spreng | IFAL, Havana (Cultivated) | Fresh aerial parts (0.70–0.75%) | Carvacrol (71.0%) and p-cymene (9.7%) | [32] | |
| Lauraceae | Laurus nobilis L. | - | - | 1,8-Cineole (26.7%), eugenol (18.5%), linalool (18.5%), and sabinene (11.8%) | [20] |
| Licaria triandra (Sw.) Kosterm. | Sierra de Meneses y Cueto, Sancti Spiritus (Wild) | Leaves (0.15%) | β-Pinene (18.2%), α-pinene (14.8%), and β-eudesmol (11.4%) | [33] | |
| Nectandra hihua (Ruiz & Pav.) Rohwer d | Sierra de Meneses y Cueto, Sancti Spiritus (Wild) | Leaves (0.39%) | Caryophyllene oxide (16.0%) and β-caryophyllene (9.9%) | [34] | |
| Myrtaceae | Callistemon speciosus (Sims) Sweet | Candelaria, Pinar del Río (Wild) | Leaves (0.93%) | 1,8-Cineole (57.0%) and α-terpineol (20.4%) | [35] |
| Melaleuca leucadendra L. | NBG, Havana (Cultivated) | Fresh aerial parts (0.8%) | 1,8-Cineole (61.0%) and α-terpineol (15.6%), | [36] | |
| Ciénaga de Zapata, Matanzas (Wild) | Leaves (0.7%) | 1,8-Cineole (43.0%) and viridiflorol (24.2%) | [37] | ||
| Fruit (0.4%) | Viridiflorol (47.6%) | ||||
| Melaleuca quinquenervia (Cav.) S.T. Blake | - | - | Longifolene (32.9%) and 1,8-cineole (25.4%) | [38] | |
| Pimenta racemosa (Mill.) J.W. Moore | Pinar del Río (Wild) | Leaves (NR) | Terpinen-4-ol (20.7%), 1,8-cineole (20.4%), eugenol (10.7%), and α-terpineol (10.0%) | [39] | |
| Syzygium aromaticum (L.) Merr. & L.M. Perry | - | - | Eugenol (67.0%) and eugenyl acetate (18.1%) | [20] | |
| Piperaceae | Piper aduncum L. | IFAL, Havana (NR) | Fresh aerial parts (NR) | Piperitone (23.7%), camphor (17.1%), and viridiflorol (14.5%) | [40] |
| Topes de Collantes, Sancti Spiritus (Wild) | Leaves (1.3%) | Piperitone (34.0%) and camphor (17.1%) | [41] | ||
| Piper aduncum subsp. ossanum Trel. | Bauta, Artemisa (Wild) | Leaves (0.48%) | Piperitone (20.1%), camphor (13.9%) and viridiflorol (13.0%) | [42] | |
| Caimito, Artemisa (Wild) | Leaves (0.37%) | Piperitone (%), camphor (19.0%) and viridiflorol (18.8%) | |||
| Piper auritum Kunth | IFAL, Havana (NR) | Fresh aerial part (NR) | Safrole (87%) | [43] | |
| Topes de Collantes, Sancti Spiritus (Wild) | Leaves (2.5%) | Safrole (71.8%) | [41] | ||
| Piper umbellatum L. | Topes de Collantes, Sancti Spiritus (Wild) | Leaves (2.0%) | Safrole (26.4%) and camphor (9.6%) | [41] | |
| Poaceae | Cymbopogon citratus (DC.) Stapf | Moa, Holguín (Wild) | Leaves (NR) | Geranial (51.1%) and neral (35.2%) | [44] |
| Rutaceae | Citrus sinensis (L.) Osbeck | - | - | Limonene (82.7%) | [20] |
| - | - | Limonene (96.0%) | [38] | ||
| Murraya paniculata (L.) Jack | Topes de Collantes, Sancti Spiritus (Cultivated) | Leaves (0.2%) | β-Caryophyllene (29.8%) | [45] | |
| Zanthoxylum pseudodumosum Beurton | Camajuaní, Santa Clara (Wild) | Leaves (0.43%) | β-Caryophyllene (32.0%) and germacrene D (14.9%) | [46] | |
| Verbenaceae | Lantana camara L. | Caimito, Artemisa (NR) | Fresh aerial part (0.3%) | (E)-Nerolidol (16.6%) and (E)-β-farnesene (11.3%) | [47] |
| Zingiberaceae | Alpinia zerumbet (Pers.) B.L. Burtt & R.M. Smith | NBG, Havana (Cultivated) | Leaves (0.25%) | Terpinen-4-ol (19.0%) and caryophyllene oxide (18.2%) | [48] |
| Flowers (0.20%) | Terpinen-4-ol (14.1%) and viridiflorol (32.2%) |
| Plant Species | Pharmacological Property | Model | Species/Activity (Main Compound Assayed) | Ref. |
|---|---|---|---|---|
| A. absinthium | Antileishmanial activity | In vitro and In vivo |
| [21] |
| A. sativum | Antibacterial activity | In vitro |
| [20] |
| A. zerumbet | Antiplasmodial activity | In vitro |
| [48] |
| B. graveolens | Antileishmanial activity and antitumoral effect | In vitro |
| [27] |
| B. orellana | Antileishmanial activity | In vitro and in vivo |
| [26] |
| C. citratus | Antifungal and insecticide effects | In vivo |
| [44] |
| C. cyminum | No relevant activity | In vitro | Antimicrobial assessment | [20] |
| C. linearis | Antileishmanial, antitrypanosomal and larvicidal effect | In vitro |
| [28] |
| In vivo |
| [29] | ||
| C. sinensis | Antibacterial activity | In vitro |
| [20] |
| Anthelmintic effects | In vitro and in vivo |
| [38] | |
| C. speciosus | Antibacterial activity | In vitro |
| [35] |
| D. ambrosioides | Antileishmanial activity | In vitro and in vivo |
| [18] |
| In vitro |
| [19] | ||
| L. camara | Antibacterial activity | In vitro |
| [47] |
| L. nobilis | No relevant activity | In vitro | Antimicrobial assessment | [20] |
| L. triandra | Antibacterial activity | In vitro |
| [33] |
| N. hihua | Antibacterial activity | In vitro |
| [34] |
| M. leucadendra | Antileishmanial, antitrypanosomal, antitumoral and antioxidant activity | In vitro |
| [36] |
| In vivo |
| |||
| In vitro | Antioxidant activity | [37] | ||
| M. paniculata | Antibacterial and antioxidant effect | In vitro |
| [45] |
| M. quinquenervia | Anthelmintic effects | In vitro and in vivo |
| [38] |
| O. tenuiflorum | No relevant activity | In vitro and in vivo |
| [31] |
| O. vulgare | Antibacterial activity | In vitro |
| [20] |
| P. aduncum | Antiprotozoal and antibacterial activity |
| [41] | |
| In vitro |
| [40] | ||
| P. amboinicus | Antiprotozoal and antitumoral activity | In vitro |
| [32] |
| P. anisum | Antifungal against | In vitro |
| [20] |
| P. auritum | Antileishmanial and antioxidant activity | In vitro |
| [43] |
| In vitro |
| [41] | ||
| P. carolinensis | Antileishmanial activity | In vitro and in vivo |
| [23] |
| P. matricarioides | Antiprotozoal activity | In vitro |
| [22] |
| P. ossanum | Antiprotozoal activity | In vitro |
| [54] |
| P. racemosa | Insecticidal effect | In vivo |
| [39] |
| P. umbellatum | No relevant effect | In vitro |
| [41] |
| S. aromaticum | Antibacterial activity | In vitro |
| [20] |
| T. indica | Antibacterial and antifungal activity | In vitro |
| [30] |
| T. lucida | Antileishmanial activity | In vitro |
| [24] |
| Antiplasmodial and antibacterial activity | In vitro |
| [25] | |
| Z. pseudodumosum | Antifungal activity | In vitro |
| [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monzote, L.; García, J.; González, R.; Scotti, M.T.; Setzer, W.N. Bioactive Essential Oils from Cuban Plants: An Inspiration to Drug Development. Plants 2021, 10, 2515. https://doi.org/10.3390/plants10112515
Monzote L, García J, González R, Scotti MT, Setzer WN. Bioactive Essential Oils from Cuban Plants: An Inspiration to Drug Development. Plants. 2021; 10(11):2515. https://doi.org/10.3390/plants10112515
Chicago/Turabian StyleMonzote, Lianet, Jesús García, Rosalia González, Marcus Tullius Scotti, and William N. Setzer. 2021. "Bioactive Essential Oils from Cuban Plants: An Inspiration to Drug Development" Plants 10, no. 11: 2515. https://doi.org/10.3390/plants10112515
APA StyleMonzote, L., García, J., González, R., Scotti, M. T., & Setzer, W. N. (2021). Bioactive Essential Oils from Cuban Plants: An Inspiration to Drug Development. Plants, 10(11), 2515. https://doi.org/10.3390/plants10112515









