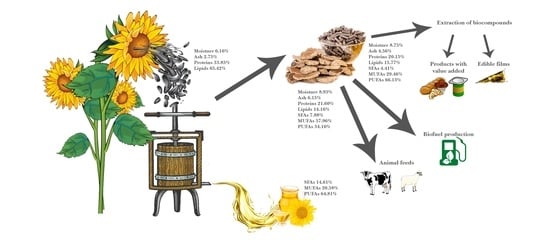
Nutritional Characteristics Assessment of Sunflower Seeds, Oil and Cake. Perspective of Using Sunflower Oilcakes as a Functional Ingredient
Abstract
1. Introduction
2. Results and Discussions
2.1. Chemical Composition of the Seeds, Kernels and Hulls
2.2. Classification of the Sunflower Seeds
2.3. Size, Shape and Gravimetric Properties of Sunflower Seeds and Kernels
2.4. Sunflower Oilcakes Characterization
2.5. Comparison of the Mineral Composition of the Sunflower Seeds, Oil and Oilcake
2.6. Fatty Acids Profile of Sunflower Seeds, Oil and Oilcakes
2.7. Amino Acids Profile of Sunflower Seeds, Oil and Oilcakes
3. Materials and Methods
3.1. Samples
3.2. Chemical Composition
3.2.1. Seeds and Kernels
3.2.2. Oilcakes
3.3. Physical Properties of Seeds and Kernels
3.3.1. Mass and Classification
3.3.2. Geometric Parameters
3.3.3. Gravimetric Parameters
3.4. Comparison of the Seeds, Oil and Oilcakes
3.4.1. Free Amino Acids Determination
3.4.2. Fatty Acids Determination
3.4.3. Mineral Estimation
3.5. Statistical Analysis
4. Challenges and Future Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otles, S.; Despoudi, S.; Bucatariu, C.; Kartal, C. Valorization, and Sustainability in the Food Industry; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 978-0-12-800351-0. [Google Scholar]
- Kot, A.M.; Pobiega, K.; Piwowarek, K.; Kieliszek, M.; Błażejak, S.; Gniewosz, M.; Lipińska, E. Biotechnological Methods of Management and Utilization of Potato Industry Waste—A Review. Potato Res. 2020, 63, 431–447. [Google Scholar] [CrossRef]
- Esposito, B.; Sessa, M.R.; Sica, D.; Malandrino, O. Towards circular economy in the agri-food sector. A systematic literature review. Sustainability 2020, 12, 7401. [Google Scholar] [CrossRef]
- Borrello, M.; Caracciolo, F.; Lombardi, A.; Pascucci, S.; Cembalo, L. Consumers’ perspective on circular economy strategy for reducing food waste. Sustainability 2017, 9, 141. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Olejnik, A.; Rybicka, I.; Zielińska-Dawidziak, M.; Białas, W.; Lewandowicz, G. Membrane filtration-assisted enzymatic hydrolysis affects the biological activity of potato juice. Molecules 2021, 26, 852. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Pobiega, K. The aspects of microbial biomass use in the utilization of selected waste from the agro-food industry. Open Life Sci. 2020, 15, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, P.Ł.; Olejnik, A.; Białas, W.; Kubiak, P.; Siger, A.; Nowicki, M.; Lewandowicz, G. Effect of Thermal Processing on Antioxidant Activity and Cytotoxicity of Waste Potato Juice. Open Life Sci. 2019, 14, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kushwaha, R.; Verma, M.L.; Elsevier, B.V. Recovery and Utilization of Bioactives from Food Processing Waste; Elsevier B.V.: Amsterdam, The Netherlands, 2019; ISBN 9780444643230. [Google Scholar]
- Ancuţa, P.; Sonia, A. Oil press-cakes and meals valorization through circular economy approaches: A review. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, R.; Sharma, S.; Singh, B. Oilseed as Potential Functional Food Ingredient. In Trends & Prospects in Food Technology, Processing and Preservation, 1st ed.; Prodyut Kumar, P., Mahawar, M.K., Abobatta, W., Panja, P., Eds.; Today and Tomorrow’s Printers and Publishers: New Delhi, India, 2018; pp. 25–58. [Google Scholar]
- Adeleke, B.S.; Babalola, O.O. Oilseed crop sunflower (Helianthus annuus) as a source of food: Nutritional and health benefits. Food Sci. Nutr. 2020, 8, 4666–4684. [Google Scholar] [CrossRef]
- Chauhan, V. Nutritional quality analysis of sunflower seed cake (SSC). Pharma Innov. J. 2021, 10, 720–728. [Google Scholar]
- Mirpoor, S.F.; Giosafatto, C.V.L.; Porta, R. Biorefining of seed oil cakes as industrial co-streams for production of innovative bioplastics. A review. Trends Food Sci. Technol. 2021, 109, 259–270. [Google Scholar] [CrossRef]
- Gültekin Subaşı, B.; Vahapoğlu, B.; Capanoglu, E.; Mohammadifar, M.A. A review on protein extracts from sunflower cake: Techno-functional properties and promising modification methods. Crit. Rev. Food Sci. Nutr. 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ahmar, S.; Gill, R.A.; Jung, K.H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and molecular techniques from simple breeding to speed breeding in crop plants: Recent advances and future outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef] [PubMed]
- Jocić, S.; Miladinović, D.; Kaya, Y. Breeding and Genetics of Sunflower; AOCS Press: Champaign, IL, USA, 2015; ISBN 9781630670627. [Google Scholar]
- Pal, D. Sunflower (Helianthus annuus L.) Seeds in Health and Nutrition; Elsevier Inc.: Amsterdam, The Netherlands, 2011; ISBN 9780123756886. [Google Scholar]
- Romanić, R. Cold Pressed Sunflower (Helianthus annuus L.) oil. In Cold Pressed Oils; Academic Press: Cambridge, MA, USA, 2020; pp. 197–218. [Google Scholar] [CrossRef]
- Islam, R.T.; Hossain, M.M.; Majumder, K.; Tipu, A.H. In vitro Phytochemical Investigation of Helianthus annuus Seeds. Bangladesh Pharm. J. 2016, 19, 100–105. [Google Scholar] [CrossRef][Green Version]
- Anjum, F.M.; Nadeem, M.; Khan, M.I.; Hussain, S. Nutritional and therapeutic potential of sunflower seeds: A review. Br. Food J. 2012, 114, 544–552. [Google Scholar] [CrossRef]
- Ivanova, P.; Chalova, V.; Koleva, L.; Pishtiyski, I. Amino acid composition and solubility of proteins isolated from sunflower meal produced in Bulgaria. Int. Food Res. J. 2013, 20, 2995–3000. [Google Scholar]
- Sarwar, F. The role of oilseeds nutrition in human health: A critical review. J. Cereals Oilseeds 2013, 4, 97–100. [Google Scholar] [CrossRef]
- Savoire, R.; Lanoisellé, J.L.; Vorobiev, E. Mechanical Continuous Oil Expression from Oilseeds: A Review. Food Bioprocess Technol. 2013, 6, 1–16. [Google Scholar] [CrossRef]
- Ramadan, M.F. Introduction to Cold Pressed Oils: Green Technology, Bioactive Compounds, Functionality, and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128181881. [Google Scholar]
- Poiana, M.; Alexa, E.; Moigradean, D.; Popa, M. The influence of the storage conditions on the oxidative stability and antioxidant properties of sunflower and pumpkin oil. In Proceedings of the 44th Croatian & 4th International Symposium of Agriculture, Opatija, Croatia, 16–20 February 2009; pp. 449–453. [Google Scholar]
- Zoumpoulakis, P.; Sinanoglou, V.J.; Siapi, E.; Heropoulos, G.; Proestos, C. Evaluating modern techniques for the extraction and characterisation of sunflower (Hellianthus annus L.) seeds phenolics. Antioxidants 2017, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Avni, T.C.A.; Anupriya, S.; Rai, P.; Maan, K. Effects of Heating and Storage on Nutritional value of Sunflower Oil. DU J. Undergrad. Res. Innov. 2016, 2, 196–202. [Google Scholar]
- Nadeem, M.; Situ, C.; Mahmud, A.; Khalique, A.; Imran, M.; Rahman, F.; Khan, S. Antioxidant activity of sesame (Sesamum indicum L.) cake extract for the stabilization of olein based butter. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 967–977. [Google Scholar] [CrossRef]
- Nandha, R.; Singh, H.; Garg, K.; Rani, S. Therapeutic Potential of Sunflower Seeds: An Overview. Int. J. Res. Dev. Pharm. Life Sci. 2014, 3, 967–972. [Google Scholar]
- Bochkarev, M.S.; Egorova, E.Y.; Reznichenko, I.Y.; Poznyakovskiy, V.M. Reasons for the ways of using oilcakes in food industry. Foods Raw Mater. 2016, 4, 4–12. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; El-Hack, M.E.A.; Dhama, K. The Practical Application of Sunflower Meal in Poultry Nutrition. Adv. Anim. Vet. Sci. 2015, 3, 634–648. [Google Scholar] [CrossRef]
- Serrapica, F.; Masucci, F.; Raffrenato, E.; Sannino, M.; Vastolo, A.; Barone, C.M.A.; Di Francia, A. High fiber cakes from mediterranean multipurpose oilseeds as protein sources for ruminants. Animals 2019, 9, 918. [Google Scholar] [CrossRef] [PubMed]
- Nang Thu, T.T.; Bodin, N.; Saeger, S.; Larondelle, Y.; Rollin, X. Substitution of fish meal by sesame oil cake (Sesamum indicum L.) in the diet of rainbow trout (Oncorhynchus mykiss W.). Aquac. Nutr. 2011, 17, 80–89. [Google Scholar] [CrossRef]
- Wanjari, N.; Waghmare, J. Phenolic and antioxidant potential of sunflower meal. Pelagia Res. Libr. Adv. Appl. Sci. Res. 2015, 6, 221–229. [Google Scholar]
- Lazaro, E.; Benjamin, Y.; Robert, M. The Effects of Dehulling on Physicochemical Properties of Seed Oil and Cake Quality of Sunflower. Tanzania J. Agric. Sci. 2014, 13, 41–47. [Google Scholar]
- Araujo, M.E.V.; Barbosa, E.G.; Gomes, F.A.; Teixeira, I.R.; Lisboa, C.F.; Araújo, R.S.L.; Corrêa, P.C. Physical properties of sesame seeds harvested at different maturation stages and thirds of the plant. Chil. J. Agric. Res. 2018, 78, 495–502. [Google Scholar] [CrossRef]
- Ortiz-Hernandez, A.A.; Araiza-Esquivel, M.; Delgadillo-Ruiz, L.; Ortega-Sigala, J.J.; Durán-Muñoz, H.A.; Mendez-Garcia, V.H.; Yacaman, M.J.; Vega-Carrillo, H.R. Physical characterization of sunflower seeds dehydrated by using electromagnetic induction and low-pressure system. Innov. Food Sci. Emerg. Technol. 2020, 60, 102285. [Google Scholar] [CrossRef]
- Igbozulike, A.O.; Amamgbo, N. Effect of Moisture Content on Physical Properties of Fluted Pumpkin Seeds. J. Biosyst. Eng. 2019, 44, 69–76. [Google Scholar] [CrossRef]
- Malik, M.A.; Saini, C.S. Engineering properties of sunflower seed: Effect of dehulling and moisture content. Cogent Food Agric. 2016, 2. [Google Scholar] [CrossRef]
- Munder, S.; Argyropoulos, D.; Müller, J. Class-based physical properties of air-classified sunflower seeds and kernels. Biosyst. Eng. 2017, 164, 124–134. [Google Scholar] [CrossRef]
- De Figueiredo, A.K.; Baümler, E.; Riccobene, I.C.; Nolasco, S.M. Moisture-dependent engineering properties of sunflower seeds with different structural characteristics. J. Food Eng. 2011, 102, 58–65. [Google Scholar] [CrossRef]
- Costa, C.; Antonucci, F.; Pallottino, F.; Aguzzi, J.; Sun, D.W.; Menesatti, P. Shape Analysis of Agricultural Products: A Review of Recent Research Advances and Potential Application to Computer Vision. Food Bioprocess Technol. 2011, 4, 673–692. [Google Scholar] [CrossRef]
- Mirzabe, A.H.; Khazaei, J.; Chegini, G.R. Physical properties and modeling for sunflower seeds. Agric. Eng. Int. CIGR J. 2012, 14, 190–202. [Google Scholar]
- Seiler, G.J.; Gulya, T.J. Sunflower: Overview, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; ISBN 9780081005965. [Google Scholar]
- Menzel, C. Improvement of starch films for food packaging through a three-principle approach: Antioxidants, cross-linking and reinforcement. Carbohydr. Polym. 2020, 250, 116828. [Google Scholar] [CrossRef] [PubMed]
- Casoni, A.I.; Gutierrez, V.S.; Volpe, M.A. Conversion of sunflower seed hulls, waste from edible oil production, into valuable products. J. Environ. Chem. Eng. 2019, 7, 102893. [Google Scholar] [CrossRef]
- Dobrzaski, B.; Stpniewski, A. Physical Properties of Seeds in Technological Processes. Adv. Agrophys. Res. 2013, 11, 269–294. [Google Scholar] [CrossRef]
- Rodríguez, M.; Nolasco, S.; Izquierdo, N.; Mascheroni, R.; Madrigal, M.S.; Flores, D.C.; Ramos, A.Q. Microwave-assisted extraction of antioxidant compounds from sunflower hulls. Heat Mass Transf. 2019, 55, 3017–3027. [Google Scholar] [CrossRef]
- Çetin, N.; Karaman, K.; Beyzi, E.; Sağlam, C.; Demirel, B. Comparative Evaluation of Some Quality Characteristics of Sunflower Oilseeds (Helianthus annuus L.) Through Machine Learning Classifiers. Food Anal. Methods 2021, 14, 1666–1681. [Google Scholar] [CrossRef]
- Muttagi, G.C.; Joshi, N. Physico-chemical composition of selected sunflower seed cultivars. Int. J. Chem. Stud. 2020, 8, 2095–2100. [Google Scholar] [CrossRef]
- Akkaya, M.R. Prediction of fatty acid composition of sunflower seeds by near-infrared reflectance spectroscopy. J. Food Sci. Technol. 2018, 55, 2318–2325. [Google Scholar] [CrossRef]
- Nadeem, M.; Anjum, F.M.; Arshad, M.U.; Hussain, S. Chemical characteristics and antioxidant activity of different sunflower hybrids and their utilization in bread. Afr. J. Food Sci. 2010, 4, 618–626. [Google Scholar]
- Kolláthová, R.; Varga, B.; Ivanišová, E.; Bíro, D.; Rolinec, M.; Juráček, M.; Šimko, M.; Gálik, B. Mineral Profile Analysis of Oilseeds and Their By-Products As Feeding Sources for Animal Nutrition. Slovak J. Anim. Sci 2019, 52, 9–15. [Google Scholar]
- Jafari, S.; Khazaei, J.; Arabhosseini, A.; Massah, J.; Khoshtaghaza, M.H. Study on Mechanical Properties of Sunflower Seeds. Food Sci. Technol. 2011, 14, 6. [Google Scholar]
- Gupta, R.K.; Das, S.K. Physical properties of sunflower seeds. J. Agric. Eng. Res. 1997, 66, 1–8. [Google Scholar] [CrossRef]
- Sumon, M.M.; Tinggi, S.; Ekonomi, I.; Surabaya, P.; Hossain, A. Comparative Study on Physicochemical Composition of Different Genotypes of Sunflower Seed and Mineral Profile of Oil Cake. Agriculturists 2021, 18, 83–93. [Google Scholar] [CrossRef]
- Pawar, V.D.; Patil, J.N.; Sakhale, B.K.; Agarkar, B.S. Studies on selected functional properties of defatted sunflower meal and its high protein products. J. Food Sci. Technol. 2001, 38, 47–51. [Google Scholar]
- Taha, F.S.; Mohamed, G.F.; Mohamed, S.H.; Mohamed, S.S.; Kamil, M.M. Optimization of the Extraction of Total Phenolic Compounds from Sunflower Meal and Evaluation of the Bioactivities of Chosen Extracts. Am. J. Food Technol. 2011, 6, 1002–1020. [Google Scholar] [CrossRef]
- Rosa, P.M.; Antoniassi, R.; Freitas, S.C.; Bizzo, H.R.; Zanotto, D.L.; Oliveira, M.F.; Castiglioni, V.B.R. Chemical composition of brazilian sunflower varieties. Helia 2009, 32, 145–156. [Google Scholar] [CrossRef]
- Žilic, S.; Barac, M.; Pešic, M.; Crevar, M.; Stanojevic, S.; Nišavic, A.; Saratlic, G.; Tolimir, M. Characterization of sunflower seed and kernel proteins. Helia 2010, 33, 103–114. [Google Scholar] [CrossRef]
- Santalla, E.M.; Mascheroni, R.H. Note: Physical Properties of High Oleic Sunflower Seeds. Food Sci. Technol. Int. 2003, 9, 435–442. [Google Scholar] [CrossRef]
- Abdullah, M.H.R.O.; Ch’ng, P.E.; Lim, T.H. Some Physical Properties of Parkia Speciosa Seeds. Int. Conf. Food Eng. Biotechnol. 2011, 9, 43–47. [Google Scholar]
- Krajewska, M.; Ślaska-Grzywna, B.; Andrejko, D. Physical Properties of Seeds of the Selected Oil Plants. Agric. Eng. 2016, 20, 69–77. [Google Scholar] [CrossRef]
- Coşkuner, Y.; Gökbudak, A. Dimensional specific physical properties of fan palm fruits, seeds and seed coats (Washingtonia robusta). Int. Agrophys. 2016, 30, 301–309. [Google Scholar] [CrossRef]
- Aviara, N.A.; Gwandzang, M.I.; Haque, M.A. Physical properties of guna seeds. J. Agric. Eng. Res. 1999, 73, 105–111. [Google Scholar] [CrossRef]
- Niveditha, V.R.; Sridhar, K.R.; Balasubramanian, D. Physical and mechanical properties of seeds and kernels of canavalia of coastal sand dunes. Int. Food Res. J. 2013, 20, 1547–1554. [Google Scholar]
- Seifi, M.R.; Alimardani, R. Moisture-Dependent Physical Properties of Sunflower (SHF8190). Mod. Appl. Sci. 2010, 4, 135–143. [Google Scholar] [CrossRef][Green Version]
- Babić, L.J.; Radojčin, M.; Pavkov, I.; Babić, M. The physical and compressive load properties of sunflower (Helianthus annuus L.) fruit. Helia 2012, 35, 95–112. [Google Scholar] [CrossRef][Green Version]
- Popović, S.; Hromiš, N.; Šuput, D.; Bulut, S.; Romanić, R.; Lazić, V. Valorization of By-Products From the Production of Pressed Edible Oils to Produce Biopolymer Films. In Cold Pressed Oils; Academic Press: Cambridge, MA, USA, 2020; pp. 15–30. [Google Scholar] [CrossRef]
- Sobczak, P.; Zawislak, K.; Starek, A.; Zukiewicz-Sobczak, W.; Sagan, A.; Zdybel, B.; Andrejko, D. Compaction process as a concept of press-cake production from organic waste. Sustainability 2020, 12, 1567. [Google Scholar] [CrossRef]
- Adesina, S.A. Effect of processing on the proximate composition of sunflower (Helianthus annuus) seeds. Agro-Science 2019, 17, 27. [Google Scholar] [CrossRef]
- Rani, R.; Badwaik, L.S. Functional Properties of Oilseed Cakes and Defatted Meals of Mustard, Soybean and Flaxseed. Waste Biomass Valorization 2021, 12, 5639–5647. [Google Scholar] [CrossRef]
- Sinkovič, L.; Kolmanič, A. Elemental composition and nutritional characteristics of cucurbita pepo subsp. Pepo seeds, oil cake and pumpkin oil. J. Elem. 2021, 26, 97–107. [Google Scholar] [CrossRef]
- Sunil, L.; Appaiah, P.; Prasanth Kumar, P.K.; Gopala Krishna, A.G. Preparation of food supplements from oilseed cakes. J. Food Sci. Technol. 2015, 52, 2998–3005. [Google Scholar] [CrossRef]
- Cozea, A.; Ionescu, N.; Popescu, M.; Neagu, M.; Gruia, R. Comparative study concerning the composition of certain oil cakes with phytotherapeutical potential. Rev. Chim. 2016, 67, 422–425. [Google Scholar]
- Hussain, S.; Jõudu, I.; Bhat, R. Dietary fiber from underutilized plant resources-A positive approach for valorization of fruit and vegetable wastes. Sustainability 2020, 12, 5401. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Maphosa, Y.; Jideani, V.A. Dietary fiber extraction for human nutrition—A review. Food Rev. Int. 2016, 32, 98–115. [Google Scholar] [CrossRef]
- Nevara, G.A.; Kharidah, S.; Muhammad, S.; Zawawi, N.; Mustapha, N.A.; Karim, R. Dietary Fiber: Fractionation, Characterization and Potential Sources from Defatted Oilseeds. Foods 2021, 10, 754. [Google Scholar] [CrossRef] [PubMed]
- Bhise, S.R.; Kaur, A.; Manikantan, M.R.; Singh, B. Development of textured defatted sunflower meal by extrusion using response surface methodology. Acta Aliment. 2015, 44, 251–258. [Google Scholar] [CrossRef]
- Bhise, S.; Kaur, A. The effect of extrusion conditions on the functional properties of defatted cake of sunflower-maize based expanded snacks. Int. J. Food Ferment. Technol. 2015, 5, 247. [Google Scholar] [CrossRef]
- Brachet, M.; Arroyo, J.; Bannelier, C.; Cazals, A.; Fortun-Lamothe, L. Hydration capacity: A new criterion for feed formulation. Anim. Feed Sci. Technol. 2015, 209, 174–185. [Google Scholar] [CrossRef]
- Lin, M.J.; Humbert, E.S.; Sosulski, F.W. Functional Properties Sunflower Meal Products of. J. Food Sci. 1974, 39, 368–370. [Google Scholar] [CrossRef]
- Sosulski, F.; Fleming, S.E. Chemical, functional, and nutritional properties of sunflower protein products. J. Am. Oil Chem. Soc. 1977, 54, A100. [Google Scholar] [CrossRef]
- Grasso, S.; Omoarukhe, E.; Wen, X.; Papoutsis, K.; Methven, L. The use of upcycled defatted sunflower seed flour as a functional ingredient in biscuits. Foods 2019, 8, 305. [Google Scholar] [CrossRef]
- Amza, T.; Amadou, I.; Zhu, K.X.; Zhou, H.M. Effect of extraction and isolation on physicochemical and functional properties of an underutilized seed protein: Gingerbread plum (Neocarya macrophylla). Food Res. Int. 2011, 44, 2843–2850. [Google Scholar] [CrossRef]
- White, N.D.G.; Jayas, D.S. Physical properties of canola and sunflower meal pellets. Can. Biosyst. Eng. 2001, 43, 349–352. [Google Scholar]
- Dabbour, M.; He, R.; Ma, H.; Musa, A. Optimization of ultrasound assisted extraction of protein from sunflower meal and its physicochemical and functional properties. J. Food Process Eng. 2018, 41, e12799. [Google Scholar] [CrossRef]
- Cai, T.; Chang, K.C.; Lunde, H. Physicochemical Properties and Yields of Sunflower Protein Enzymatic Hydrolysates As Affected by Enzyme and Defatted Sunflower Meal. J. Agric. Food Chem. 1996, 44, 3500–3506. [Google Scholar] [CrossRef]
- Melo, D.; Álvarez-Ortí, M.; Nunes, M.A.; Costa, A.S.G.; Machado, S.; Alves, R.C.; Pardo, J.E.; Oliveira, M.B.P.P. Whole or defatted sesame seeds (Sesamum indicum L.)? The effect of cold pressing on oil and cake quality. Foods 2021, 10, 2108. [Google Scholar] [CrossRef] [PubMed]
- Arrutia, F.; Binner, E.; Williams, P.; Waldron, K.W. Oilseeds beyond oil: Press cakes and meals supplying global protein requirements. Trends Food Sci. Technol. 2020, 100, 88–102. [Google Scholar] [CrossRef]
- Jannathulla, R.; Dayal, J.S.; Ambasankar, K.; Muralidhar, M. Effect of Aspergillus niger fermented soybean meal and sunflower oil cake on growth, carcass composition and haemolymph indices in Penaeus vannamei Boone, 1931. Aquaculture 2018, 486, 1–8. [Google Scholar] [CrossRef]
- Zentek, J.; Knorr, F.; Mader, A. Reducing Waste in Fresh Produce Processing and Households through Use of Waste as Animal Feed; Woodhead Publishing Limited: Cambridge, UK, 2013; ISBN 9781782420187. [Google Scholar]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Njuguna, D.G.; Wanyoko, J.K.; Kinyanjui, T.; Wachira, F.N. Mineral Elements in the Kenyan Tea Seed Oil Cake. Int. J. Res. Chem. Environ. 2013, 3, 253–261. [Google Scholar]
- Chaves, E.S.; dos Santos, E.J.; Araujo, R.G.O.; Oliveira, J.V.; Frescura, V.L.A.; Curtius, A.J. Metals and phosphorus determination in vegetable seeds used in the production of biodiesel by ICP OES and ICP-MS. Microchem. J. 2010, 96, 71–76. [Google Scholar] [CrossRef]
- Goiri, I.; Zubiria, I.; Benhissi, H.; Atxaerandio, R.; Ruiz, R.; Mandaluniz, N.; Garcia-Rodriguez, A. Use of cold-pressed sunflower cake in the concentrate as a low-input local strategy to modify the milk fatty acid profile of dairy cows. Animals 2019, 9, 803. [Google Scholar] [CrossRef]
- Zubiria, I.; Garcia-Rodriguez, A.; Atxaerandio, R.; Ruiz, R.; Benhissi, H.; Mandaluniz, N.; Lavín, J.L.; Abecia, L.; Goiri, I. Effect of feeding cold-pressed sunflower cake on ruminal fermentation, lipid metabolism and bacterial community in dairy cows. Animals 2019, 9, 755. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.Ø.; Skrede, A.; Mydland, L.T.; Øverland, M. Fractionation of rapeseed meal by milling, sieving and air classification—Effect on crude protein, amino acids and fiber content and digestibility. Anim. Feed Sci. Technol. 2017, 230, 143–153. [Google Scholar] [CrossRef]
- Chetrariu, A.; Dabija, A. Quality Characteristics of Spelt Pasta Enriched with Spent Grain. Agronomy 2021, 11, 1824. [Google Scholar] [CrossRef]
- Omowaye-Taiwo, O.A.; Fagbemi, T.N.; Ogunbusola, E.M.; Badejo, A.A. Effect of germination and fermentation on the proximate composition and functional properties of full-fat and defatted cucumeropsis mannii seed flours. J. Food Sci. Technol. 2015, 52, 5257–5263. [Google Scholar] [CrossRef] [PubMed]
- Onipe, O.O.; Beswa, D.; Jideani, A.I.O. Effect of size reduction on colour, hydration and rheological properties of wheat bran. Food Sci. Technol. 2017, 37, 389–396. [Google Scholar] [CrossRef]
- Coțovanu, I.; Batariuc, A.; Mironeasa, S. Characterization of quinoa seeds milling fractions and their effect on the rheological properties of wheat flour dough. Appl. Sci. 2020, 10, 7225. [Google Scholar] [CrossRef]
- Iyenagbe, D.O.; Malomo, S.A.; Idowu, A.O.; Badejo, A.A.; Fagbemi, T.N. Effects of thermal processing on the nutritional and functional properties of defatted conophor nut (Tetracarpidium conophorum) flour and protein isolates. Food Sci. Nutr. 2017, 5, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Konak, M.; Çarman, K.; Aydin, C. Physical properties of chick pea seeds. Biosyst. Eng. 2002, 82, 73–78. [Google Scholar] [CrossRef]
- Dabadé, D.S.; Jacxsens, L.; Miclotte, L.; Abatih, E.; Devlieghere, F.; De Meulenaer, B. Survey of multiple biogenic amines and correlation to microbiological quality and free amino acids in foods. Food Control 2021, 120, 107497. [Google Scholar] [CrossRef]
- Gifty, A.G.; De Meulenaer, B.; Olango, T.M. Variation in tuber proximate composition, sugars, fatty acids and amino acids of eight Oromo dinich (Plectranthus edulis) landraces experimentally grown in Ethiopia. J. Food Compos. Anal. 2018, 67, 191–200. [Google Scholar] [CrossRef]
- Dulf, F.V. Fatty acids in berry lipids of six sea buckthorn (Hippophae rhamnoides L., subspecies carpatica) cultivars grown in Romania. Chem. Cent. J. 2012, 6, 106. [Google Scholar] [CrossRef] [PubMed]


| Sample | Moisture, % | Ash, % | Proteins, % | Lipids, % |
|---|---|---|---|---|
| Seed | 6.16 ± 0.04 b | 2.73 ± 0.04 b | 33.85 ± 0.88 b | 65.42 ± 0.4 a |
| Kernel | 4.60 ± 0.03 c | 3.31 ± 0.11 a | 23.73 ± 1.31 a | 32.50 ± 2.21 b |
| Hull | 7.88 ± 0.09 a | 2.45 ± 0.11 c | 7.82 ± 0.22 c | 8.81 ± 0.12 c |
| Parameters | Ratio Value | Correlation Coefficient |
|---|---|---|
| L/W | 2.04 | 0.693 * |
| L/T | 3.34 | 0.567 * |
| W/T | 1.64 | 0.828 * |
| l/w | 2.17 | 0.431 * |
| l/t | 3.65 | 0.828 * |
| w/t | 1.68 | 0.404 * |
| L/M | 182.65 | 0.625 * |
| W/M | 89.69 | 0.805 * |
| T/M | 54.67 | 0.793 * |
| De/M | 96.24 | 0.841 * |
| ψ/M | 8.67 | 0.615 * |
| l/m | 183.77 | 0.599 * |
| t/m | 50.43 | 0.511 * |
| w/m | 84.81 | 0.626 * |
| Dek/m | 9.21 | 0.723 * |
| Ψk/m | 1.07 | 0.259 * |
| L/m | 23.91 | 0.617 * |
| W/m | 11.74 | 0.809 * |
| T/m | 7.16 | 0.791 * |
| De/m | 12.60 | 0.840 * |
| ψ/m | 1.14 | 0.623 * |
| M/m | 1.31 | 0.965 * |
| L/w | 2.82 | 0.538 * |
| L/l | 1.30 | 0.453 * |
| W/w | 1.39 | 0.679 * |
| T/t | 1.42 | 0.603 * |
| Type | Parameters | Unsorted | Classification Based on Mass | ||
|---|---|---|---|---|---|
| Class I | Class II | Class III | |||
| Shape and spatial dimension | |||||
| Seed | L, mm | 11.16 ± 0.03 b | 10.27 ± 0.74 d | 10.83 ± 0.71 c | 12.20 ± 0.53 a |
| W, mm | 5.48 ± 0.02 b | 4.13 ± 0.10 d | 5.25 ± 0.29 c | 6.53 ± 0.29 a | |
| T, mm | 3.34 ± 0.01 b | 2.42 ± 0.26 d | 3.21 ± 0.24 c | 4.01 ± 0.25 a | |
| De, mm | 5.88 ± 0.07 b | 4.67 ± 0.18 d | 5.66 ± 0.19 c | 6.83 ± 0.19 a | |
| Ψ, - | 0.53 ± 0.01 b | 0.46 ± 0.03 c | 0.52 ± 0.03 b | 0.56 ± 0.02 a | |
| V, mm3 | 113.88 ± 5.12 b | 46.83 ± 3.53 d | 95.60 ± 5.26 c | 178.80 ± 6.94 a | |
| S, mm2 | 110.15 ± 2.54 b | 68.59 ± 5.16 d | 100.84 ± 6.11 c | 146.47 ± 8.27 a | |
| Ap, mm2 | 48.48 ± 0.89 b | 33.33 ± 2.56 d | 44.65 ± 3.71 c | 62.56 ± 4.49 a | |
| Kernel | L, mm | 8.58 ± 0.63 b | 7.52 ± 0.48 c | 8.55 ± 0.51 b | 9.15 ± 0.41 a |
| W, mm | 3.96 ± 0.25 b | 3.47 ± 0.21 c | 3.8 ± 0.28 b | 4.44 ± 0.28 a | |
| T, mm | 2.35 ± 0.16 b | 2.05 ± 0.18 c | 2.22 ± 0.17 b | 2.69 ± 0.19 a | |
| Dek, mm | 4.30 ± 0.13 b | 3.8 ± 0.15 d | 4.15 ± 0.11 c | 4.78 ± 0.12 a | |
| Ψk, - | 0.50 ± 0.03 b | 0.50 ± 0.03 b | 0.49 ± 0.03 b | 0.52 ± 0.02 a | |
| Vk, mm3 | 41.05 ± 3.56 b | 26.99 ± 1.15 d | 34.99 ± 3.31 c | 57.02 ± 4.94 a | |
| Sk, mm2 | 58.39 ± 3.61 b | 44.37 ± 3.8 d | 54.20 ± 2.86 c | 71.66 ± 3.57 a | |
| Apk, mm2 | 26.76 ± 2.35 b | 20.49 ± 1.51 c | 25.50 ± 2.18 b | 31.88 ± 2.60 a | |
| Gravimetric properties | |||||
| Seed | M, g | 0.0611 ± 0.002 b | 0.0395 ± 0.001 c | 0.0569 ± 0.003 b | 0.07856 ± 0.007 a |
| pb, Kg/m3 | 404.54 ± 2.76 b | 395.23 ± 2.53 c | 415.08 ± 2.49 b | 425.47 ± 3.13 a | |
| pt, Kg/m3 | 704.81 ± 1.15 a | 708.07 ± 4.63 a | 691.22 ± 1.3 b | 650.33 ± 2.25 c | |
| φ, Kg/m3 | 42.60 ± 0.77 b | 44.18 ± 0.95 a | 39.95 ± 0.93 c | 34.58± 0.18 d | |
| Kernel | m, g | 0.0467 ± 0.003 b | 0.0292 ± 0.002 c | 0.0403 ± 0.003 b | 0.0591 ± 0.004 a |
| pb, Kg/m3 | 525.29 ± 4.03 b | 414.81 ± 5.29 d | 484.00 ± 3.05 c | 598.08 ± 4.43 a | |
| pt, Kg/m3 | 1072.13 ± 0.75 b | 1079.69 ± 0.45 a | 1074.41 ± 1.21 c | 1068.60 ± 0.73 d | |
| φ, Kg/m3 | 51.02 ± 0.03 c | 61.58 ± 0.02 a | 54.95 ± 0.05 b | 44.03 ± 0.04 d | |
| Parameters | SFOC/PE | SFOC/C |
|---|---|---|
| PHYSICO-CHEMICAL PROPERTIES | ||
| Moisture (%) | 8.75 ± 0.10 a | 8.93 ± 0.11 a |
| Dry matter (%) | 91.25 ± 0.10 a | 91.07 ± 0.11 a |
| Proteins (%) | 20.15 ± 1.57 a | 21.60 ± 1.87 a |
| Fat (%) | 15.77 ± 0.45 a | 14.16 ± 0.04 b |
| Ash (%) | 4.56 ± 0.11 b | 6.15 ± 0.04 a |
| Crude fiber (%) | 31.88 ± 0.79 a | 12.64 ± 0.05 b |
| Carbohydrates (%) | 18.89 ± 0.23 a | 36.52 ± 1.11 b |
| FUNCTIONAL PROPERTIES | ||
| Bulk density (g/mL) | 0.4196 ± 0.002 a | 0.4204 ± 0.001 a |
| WHC (g/g) | 2.58 ± 0.11 a | 2.33 ± 0.07 b |
| OHC (g/g) | 1.34 ± 0.13 a | 1.18 ± 0.08 a |
| WRC (g/g) | 4.67 ± 0.04 a | 5.51 ± 0.06 a |
| SC (%) | 3.56 ± 0.06 a | 3.19 ± 0.17 a |
| EC (%) | 32.17 ± 1.15 a | 30.62 ± 2.14 a |
| ES (%) | 29.87 ± 1.24 a | 27.92 ± 0.57 a |
| COLOUR PROPERTIES | ||
| L* | 42.23 ± 0.01 b | 46.29 ± 0.01 a |
| a* | 1.17 ± 0.01 b | 1.57 ± 0.01 a |
| b* | 6.11 ± 0.01 b | 8.90 ± 0.01 a |
| Parameters | SFS mg/Kg | SFOC/PE mg/Kg | SFOC/C mg/Kg | SFO mg/Kg |
|---|---|---|---|---|
| Li | 1.80 ± 0.01 a | 0.34 ± 0.01 c | 1.40 ± 0.0 b | 0.20 ± 0.01 c |
| Be | 20.89 ± 0.14 c | 32.96 ± 0.55 a | 31.48 ± 0.06 b | 0.72 ± 0.03 d |
| Mg | 3.89 ± 0.241 b | 4.76 ± 0.131 a | - | 3.44 ± 0.15 c |
| Ca | 573.02 ± 4.73 c | 1163.32 ± 10.01 b | 1522.08 ± 5.5 a | - |
| Ti | 7.04 ± 0.25 c | 16.10 ± 0.26 b | 18.38 ± 6.22 a | 0.03 ± 0.0 d |
| Cr | 35.70 ± 0.1 c | 52.79 ± 0.38 b | 58.24 ± 1.81 a | 0.50 ± 0.01 d |
| Mn | 36.91 ± 0.38 c | 57.62 ± 0.21 b | 65.73 ± 3.46 a | 0.78 ± 0.03 d |
| Fe (II) | 6.66 ± 0.13 a | 5.26 ± 0.20 b | 4.71 ± 2.71 c | 0.01 ± 0. d |
| Fe (III) | 6.40 ± 0.44 a | 3.35 ± 0.17 b | 2.51 ± 0.03 c | - |
| Co | 11.46 ± 0.69 a | 7.63 ± 0.41 b | 5.59 ± 0.35 c | - |
| Ni | 21.29 ± 1.30 c | 29.38 ± 1.27 b | 30.63 ± 1.40 a | 0.19 ± 0.01 d |
| Cu | 32.57 ± 1.79 c | 61.15 ± 4.12 b | 71.25 ± 3.36 a | 0.21 ± 0.01 d |
| Zn | 57.83 ± 2.54 c | 94.78 ± 2.28 b | 90.11 ± 4.36 a | 0.09 ± 0.0 d |
| As | - | - | - | - |
| Se | 1.22 ± 0.021 c | 1.99 ± 0.071 b | 3.18 ± 0.181 a | 1.17 ± 0.0 d |
| Sr | - | 72.97 ± 2.42 a | 35.42 ± 1.99 b | - |
| Mo | 0.34 ± 0.0 c | 0.43 ± 0.01 c | 1.62 ± 0.55 a | 0.90 ± 0.01 b |
| Cd | 0.16 ± 0.00 c | 0.23 ± 0.01 b | 0.26 ± 0.0 a | - |
| Sb | - | 0.02 ± 0.0 a | - | - |
| Ce | 0.33 ± 0.01 1 d | 1.02 ± 0.01 1 b | 1.85 ± 0.05 1 a | 6.39 ± 0.35 c |
| Tl | 523.84 ± 9.11 b | 587.97 ± 17.80 a | 417.12 ± 7.31 d | 175.69 ± 4.56 c |
| Fatty Acid 1 | Type | SFS 2 µg/mL | Relative Level 3 % | SFOC/PE µg/mL | Relative Level % | SFOC/C µg/mL | Relative Level % | SFO µg/mL | Relative Level % |
|---|---|---|---|---|---|---|---|---|---|
| C14:0 | SFA | 2.25 ± 0.20 c | 0.43 ± 0.02 A | 5.05 ± 0.02 a | 0.16 ± 0.04 C | 3.78 ± 0.02 b | 0.31 ± 0.00 B | - | - |
| C15:1 | MUFA | 47.98 ± 0.20 b | 27.60 ± 0.50 B | - | - | 148.28 ± 0.36 a | 36.82 ± 0.09 A | - | - |
| C16:0 | SFA | 38.46 ± 0.11 d | 2.44 ± 0.12 C | 210.89 ± 1.35 a | 2.18 ± 0.12 D | 201.91 ± 0.54 b | 5.52 ± 0.32 A | 41.07 ± 0.04 c | 3.41 ± 0.12 B |
| C16:1 | MUFA | - | - | 0.96 ± 0.00 c | 0.03 ± 0.00 C | 123.01 ± 0.95 a | 10.08 ± 0.53 A | 3.08 ± 0.07 b | 0.77 ± 0.04 B |
| C17:1 | MUFA | - | - | 1.03 ± 0.04 b | 0.06 ± 0.00 B | 4.38 ± 0.02 a | 0.72 ± 0.04 A | - | - |
| C18:0 | SFA | 26.47 ±0.07 c | 2.51 ± 0.06 B | 114.30 ± 0.98 a | 1.77 ± 0.15 C | 26.91 ± 0.18 c | 1.10 ± 0.09 D | 83.77 ± 0.54 b | 10.45 ± 0.34 A |
| C18:1 (w-9) | MUFA | 49.47 ± 0.20 d | 14.10 ± 0.32 C | 629.87 ± 0.35 a | 29.32 ± 0.95 A | 84.06 ± 0.54 b | 10.34 ± 0.45 D | 52.97 ± 0.32 c | 19.81 ± 0.54 B |
| C18:2 (all-trans 9,12) (w-6 t) | PUFA | 3.98 ± 0.01 b | 0.76 ± 0.03 A | 2.90 ± 0.07 c | 0.09 ± 0.04 C | 8.17 ± 0.03 a | 0.67 ± 0.04 B | - | - |
| C18:2 (all-cis 9,12) (w-6) | PUFA | 262.34 ± 0.11 c | 50.32 ± 1.49 C | 2102.26 ± 5.55 a | 65.88 ± 1.55 A | 396.30 ± 0.17 b | 32.81 ± 1.85 D | 255.59 ± 1.32 d | 64.35 ± 0.14 B |
| C18:3 (w-3) | PUFA | 1.70 ± 0.02 c | 0.33 ± 0.04 B | 5.35 ± 0.15 a | 0.17 ± 0.00 C | 2.17 ± 0.12 b | 0.18 ± 0.04 C | 1.83 ± 0.42 c | 0.46 ± 0.02 A |
| C20:1 (w-9) | MUFA | - | - | 1.25 ±0.04 a | 0.04 ± 0.00 A | - | - | - | - |
| C20:4 (w-6) | PUFA | - | - | - | - | 6.07 ± 0.22 a | 0.50 ± 0.03 A | - | - |
| C21:0 | SFA | 7.99 ± 0.07 b | 1.53 ± 0.00 A | 6.68 ± 0.98 c | 0.21 ± 0.07 D | 11.50 ± 0.54 a | 0.95 ± 0.04 B | 3.00 ± 0.22 d | 0.75 ± 0.04 C |
| C23:0 | SFA | - | - | 2.86 ± 0.07 a | 0.09 ± 0.04 A | - | - | - | - |
| C18:2 w-6/C18:3 w-3 | 152.49 ± 0.98 C | 387.53 ± 4.55 A | 182.28 ± 1.95 B | 139.89 ± 0.00 D | |||||
| C18:1 w-9/C18:2 w-6 | 0.28 ± 0.01 C | 0.45 ± 0.01 A | 0.32 ± 0.001 B | 0.31 ± 0.00 B | |||||
| ΣSFAs (%) | 6.90 ± 0.04 C | 4.41 ± 0.09 D | 7.88 ± 0.07 B | 14.61 ± 0.04 A | |||||
| ΣUFAs (%) | 93.1 ± 0.54 B | 95.59 ± 0.98 A | 92.12 ± 1.55 C | 85.39 ± 1.49 D | |||||
| ΣMUFAs (%) | 41.69 ± 1.54 B | 29.46 ± 0.54 C | 57.96 ± 0.32 A | 20.58 ± 0.17 D | |||||
| ΣPUFAs (%) | 51.41 ± 1.32 C | 66.13 ± 0.25 A | 34.16 ± 0.15 D | 64.81 ± 0.35 B | |||||
| ΣSFAs/ΣUFAs | 0.07 ± 0.00 C | 0.05 ± 0.00 C | 0.09 ± 0.00 B | 0.17 ± 0.00 A | |||||
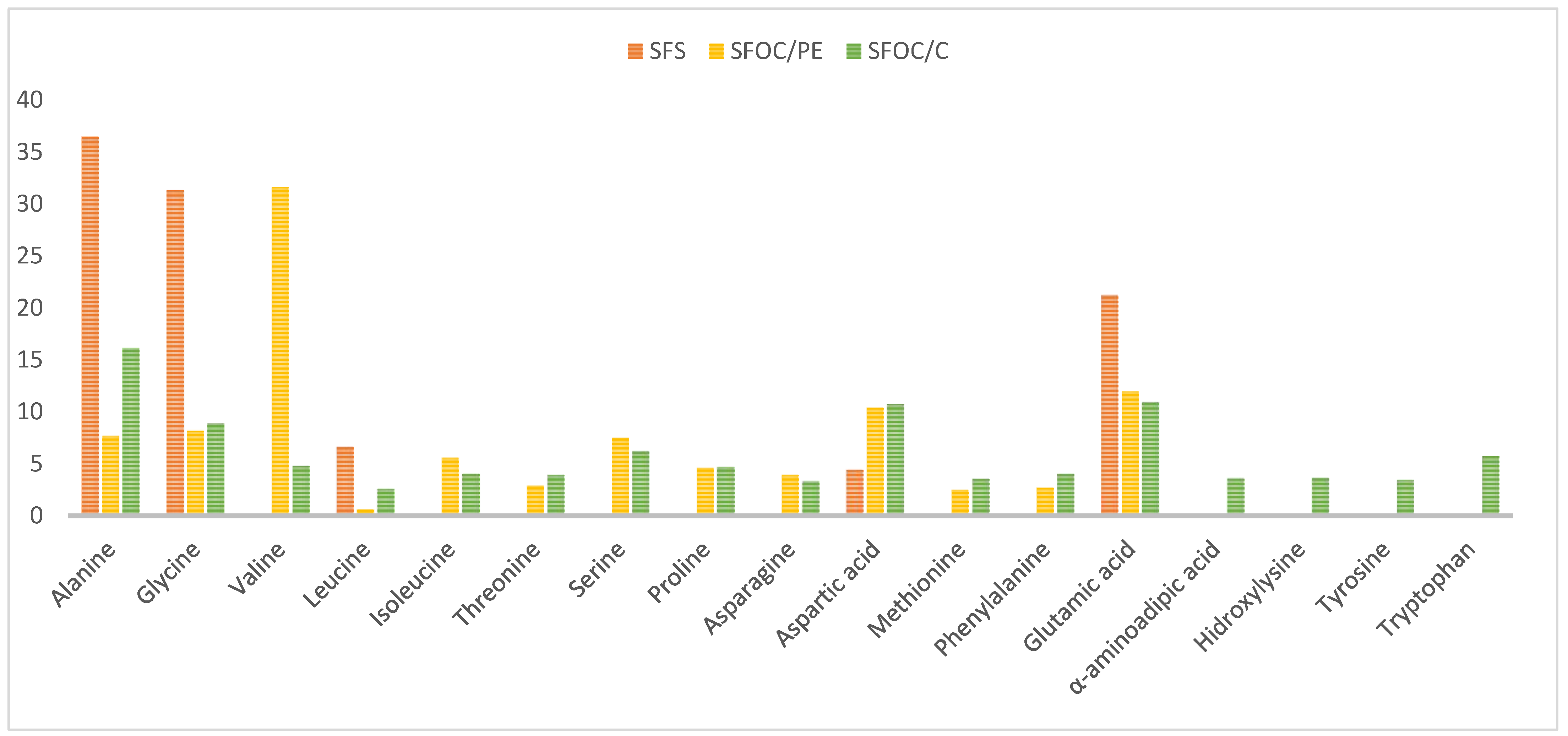
| Parameters | SFS nmol/g | SFOC/PE nmol/g | SFOC/C nmol/g |
|---|---|---|---|
| Alanine | 2110.4 ± 18.21 b | 2187.18 ± 36.93 b | 3073.51 ± 43.48 a |
| Glycine | 1810.93 ± 0.0 a | 2329.15 ± 0.0 a | 1696.04 ± 324.38 a |
| Valine * | - | 8987.78 ± 3.40 a | 905.97 ± 22.10 b |
| Leucine * | 383.84 ± 0.0 a | 164.77 ± 4.20 b | 486.66 ± 8.00 a |
| Isoleucine * | - | 1584.28 ± 14.59 a | 757.88 ± 2.04 b |
| Threonine * | - | 827.21 ± 17.53 a | 742.32 ± 8.33 b |
| Serine | - | 2124.69 ± 12.66 a | 1181.12 ± 26.93 b |
| Proline | - | 1313.13 ± 8.66 a | 887.04 ± 12.66 b |
| Asparagine | - | 1102.81 ± 10.90 a | 629.15 ± 3.76 b |
| Aspartic acid | 255.53 ± 3.58 c | 2949.61 ± 137.36 a | 2045.89 ± 19.58 b |
| Methionine * | - | 696.83 ± 3.92 a | 675.17 ± 1.90 b |
| Phenylalanine * | - | 768.82 ± 1.56 a | 757.25 ± 8.43 b |
| Glutamic acid | 1229.56 ± 0.0 a | 3402.01 ± 0.0 b | 2082.36 ± 39.83 a |
| α-aminoadipic acid | - | - | 680.19 ± 18.43 a |
| Hidroxylysine | - | - | 686.60 ± 33.33 a |
| Tyrosine | - | - | 650.22 ± 0.32 a |
| Tryptophan * | - | - | 1093.97 ± 14.53 a |
| Total, nmol | 5790.26 c | 28438.27 a | 19031.34 b |
| Essential AA, % | 6.63 c | 45.82 a | 28.48 b |
| Non essential AA, % | 93.37 a | 54.18 c | 71.52 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petraru, A.; Ursachi, F.; Amariei, S. Nutritional Characteristics Assessment of Sunflower Seeds, Oil and Cake. Perspective of Using Sunflower Oilcakes as a Functional Ingredient. Plants 2021, 10, 2487. https://doi.org/10.3390/plants10112487
Petraru A, Ursachi F, Amariei S. Nutritional Characteristics Assessment of Sunflower Seeds, Oil and Cake. Perspective of Using Sunflower Oilcakes as a Functional Ingredient. Plants. 2021; 10(11):2487. https://doi.org/10.3390/plants10112487
Chicago/Turabian StylePetraru, Ancuţa, Florin Ursachi, and Sonia Amariei. 2021. "Nutritional Characteristics Assessment of Sunflower Seeds, Oil and Cake. Perspective of Using Sunflower Oilcakes as a Functional Ingredient" Plants 10, no. 11: 2487. https://doi.org/10.3390/plants10112487
APA StylePetraru, A., Ursachi, F., & Amariei, S. (2021). Nutritional Characteristics Assessment of Sunflower Seeds, Oil and Cake. Perspective of Using Sunflower Oilcakes as a Functional Ingredient. Plants, 10(11), 2487. https://doi.org/10.3390/plants10112487