Effects of Salinity on the Growth and Nutrition of Taro (Colocasia esculenta): Implications for Food Security
Abstract
:1. Introduction
2. Results
2.1. Plant Growth and Biomass
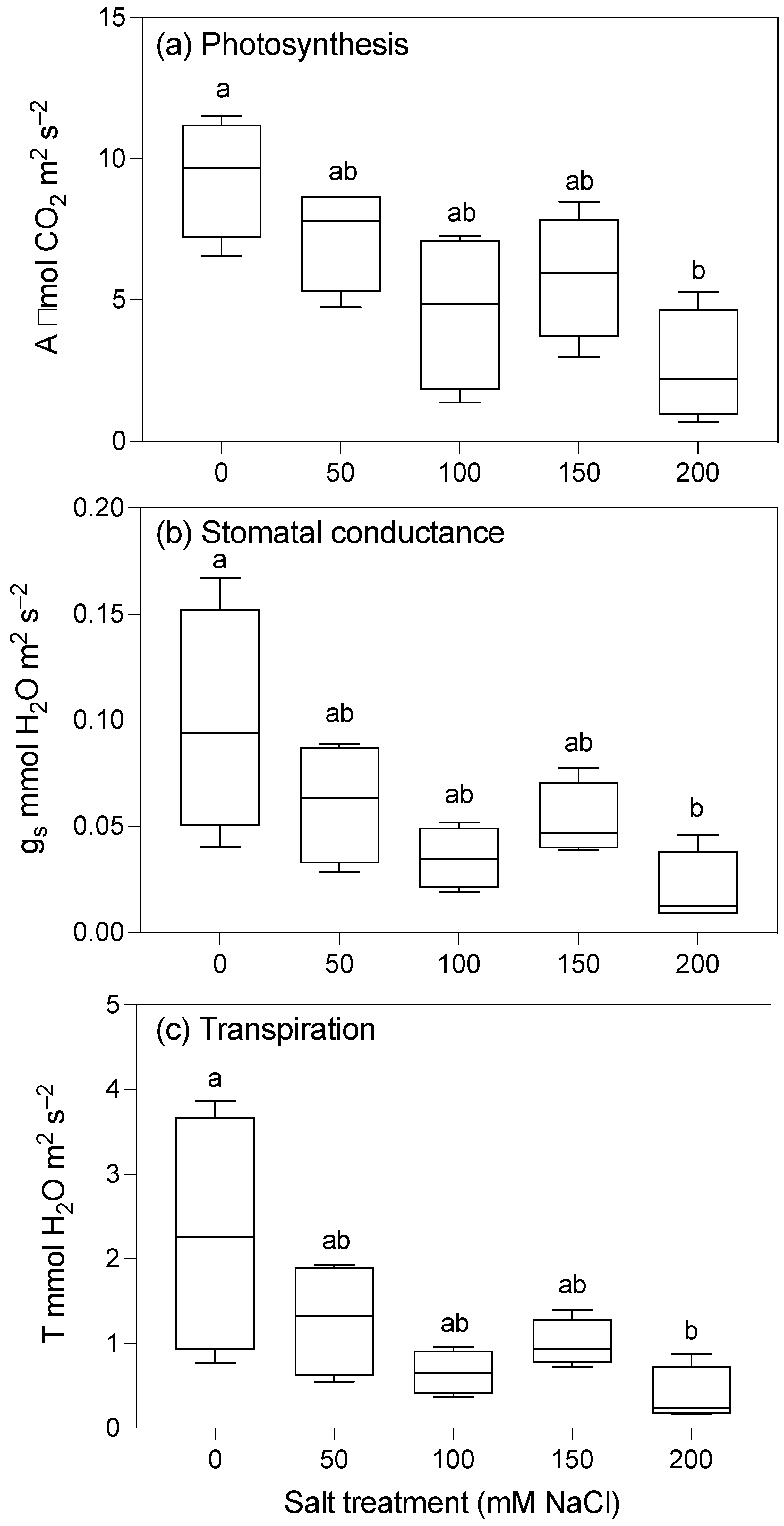
2.2. Photosynthetic Parameters
2.3. Minerals and Micronutrients
2.4. Oxalate Determination
2.5. Specialised Metabolites
3. Discussion
3.1. Sodium Excluded from Upper Parts of Plant but Not Corms
3.2. Growth and Photosynthesis Are Reduced in Plants Grown at Moderate Salinity
3.3. Mineral Nutrient Uptake Decreased with Increasing Salinity
3.4. Calcium Oxalate Crystal Formation and Remobilisation May Increase Survival at High Levels of NaCl
3.5. Secondary Metabolites in Plants Grown with Higher Concentrations of Salt
3.6. Conclusions and Implications for Food Security
4. Materials and Methods
4.1. Plant Material, Glasshouse Conditions, and Treatment Groups
4.2. Phenology, Harvesting, and Sampling Protocol
4.3. Photosynthetic Parameters
4.4. Chemical Analysis of Primary and Secondary Metabolites
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gleadow, R.M.; Pegg, A.; Blomstedt, C.K. Resilience of cassava (Manihot esculenta Crantz) to salinity: Implications for food security in low-lying regions. J. Exp. Bot. 2016, 67, 5403–5413. [Google Scholar] [CrossRef] [Green Version]
- Nunn, P.D. The end of the Pacific? Effects of sea level rise on Pacific Island livelihoods. Singap. J. Trop. Geogr. 2013, 34, 143–171. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.J.; Negrão, S.; Tester, M. Salt resistant crop plants. Curr. Opion. Biotechnol. 2014, 26, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, P. Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 2010, 37, 613. [Google Scholar] [CrossRef]
- FAO. Climate Change and Food Security in Pacific Island Countries; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2008. [Google Scholar]
- Maas, E.V.; Hoffman, G.J. Crop salt tolerance—Current assessment. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Shannon, M.C.; Grieve, C.M. Tolerance of vegetable crops to salinity. Sci. Hortic. 1999, 78, 5–38. [Google Scholar] [CrossRef]
- Bradbury, J.H.; Holloway, W.D. Chemistry of Tropical Root Crops: Significance for Nutrition and Agriculture in the Pacific; Australian Centre for International Agricultural Research, Australian National University: Canberra, Australia, 1988; Volume 6, p. 201. [Google Scholar]
- Kaushal, P.; Kumar, V.; Sharma, H.K. Utilization of taro (Colocasia esculenta): A review. J. Food Sci. Technol. Mysore 2015, 52, 27–40. [Google Scholar] [CrossRef]
- Bradbury, J.H.; Nixon, R.W. The acridity of raphides from the edible aroids. J. Sci. Food Agric. 1998, 76, 608–616. [Google Scholar] [CrossRef]
- Lebot, V. Tropical Root and Tuber Crops: Cassava, Sweet Potato, Yams and Aroids; CABI: Wallingford, UK, 2009; Volume 17, p. 413. [Google Scholar]
- Temesgen, M.; Retta, N. Nutritional potential, health and food security benefits of taro Colocasia esculenta (L.): A review. Food Sci. Qual. Manag. 2015, 36, 23–31. [Google Scholar]
- Hill, S.; Abaidoo, R.; Miyasaka, S. Sodium chloride concentration affects early growth and nutrient accumulation in taro. Hortscience 1998, 33, 1153–1156. [Google Scholar] [CrossRef] [Green Version]
- Maathius, F.J.M. Sodium in plants: Perception, signalling, and regulation of sodium fluxes. J. Exp. Bot. 2014, 65, 849–858. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Munns, R.; James, R.A.; Gilliham, M.; Flowers, T.J.; Colmer, T.D. Tissue tolerance: An essential but elusive trait for salt-tolerant crops. Funct. Plant Biol. 2016, 43, 1103–1113. [Google Scholar] [CrossRef] [Green Version]
- Kotula, L.; Caparros, P.G.; Zörb, C.; Colmer, T.D.; Flowers, T.J. Improving crop salt tolerance using transgenic approaches: An update and physiological analysis. Plant Cell Environ. 2020, 43, 2932–2956. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Selmar, D.; Kleinwächeter, M. Stress enhances the synthesis of secondary plant products: The impact of stress-related over-reduction on the accumulation of natural products. Plant Cell Physiol. 2013, 54, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Oscarsson, K.V.; Savage, G.P. Composition and availability of soluble and insoluble oxalates in raw and cooked taro (Colocasia esculenta var. Schott) leaves. Food Chem. 2007, 101, 559–562. [Google Scholar] [CrossRef]
- Bradbury, M.G.; Egan, S.V.; Bradbury, J.H. Picrate paper kits for determination of total cyanogens in cassava roots and all forms of cyanogens in cassava products. J. Sci. Food Agric. 1999, 79, 593–601. [Google Scholar] [CrossRef]
- Gleadow, R.M.; Møller, B.L. Cyanogenic glycosides: Synthesis, physiology, and phenotypic plasticity. Annu. Rev. Plant Biol. 2014, 65, 155–185. [Google Scholar] [CrossRef]
- Nyman, L.P.; Arditti, J.A. Effects of artificial seawater on morphology and anatomy of taro (Colocasia esculenta var antiquorum, Araceae) tissues in vitro. Environ. Exp. Bot. 1988, 28, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.S.; Choi, W.Y.; Ko, J.C.; Kim, T.S.; Gregorio, G.B. Salinity tolerance of japonica and indica rice (Oryza sativa L.) at the seedling stage. Planta 2003, 216, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; James, R.A.; Lauchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Negrao, S.; Schmockel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nyman, L.P.; Gonzales, C.J.; Arditti, J. In vitro selection for salt tolerance of taro (Colocasia esculenta var antiquorum). Ann. Bot. 1983, 51, 229–236. [Google Scholar] [CrossRef]
- Webb, A. Assessment of Salinity of Groundwater in Swamp Taro (Cyrtosperma Chamissonis) “Pulaka” Pits in Tuvalu; SOPAC: Suva, Fiji, 2007; p. 37. [Google Scholar]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Daryanto, S.; Wang, L.; Jacinthe, P.-A. Drought effects on root and tuber production: A meta-analysis. Agric. Water Manag. 2016, 176, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Levy, D. The response of potatoes (Solunum tuberosum L.) to salinity: Plant growth and tuber yields in the arid desert of Israel. Ann. Appl. Biol. 1992, 120, 547–555. [Google Scholar] [CrossRef]
- Marcelis, L.F.M.; Van Hooijdonk, J. Effect of salinity on growth, water use and nutrient use in radish (Raphanus sativus L.). Plant Soil 1999, 215, 57–64. [Google Scholar] [CrossRef]
- Huang, C.-C.; Chen, W.-C.; Wang, C.-C.R. Comparison of Taiwan paddy- and upland-cultivated taro (Colocasia esculenta L.) cultivars for nutritive values. Food Chem. 2007, 102, 250–256. [Google Scholar] [CrossRef]
- Mergedus, A.; Kristl, J.; Ivancic, A.; Sober, A.; Sustar, V.; Krizan, T.; Lebot, V. Variation of mineral composition in different parts of taro (Colocasia esculenta) corms. Food Chem. 2015, 170, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.C.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- O’Sullivan, J.N.; Blamey, F.P.C. Research, Development and Extension Needs for Overcoming Nutritional Limitations of Root Crops in the Pacific: A Workshop Summary. In Mineral Nutrient Disorders of Root Crops in the Pacific: Proceedings of a Workshop, Nuku’alofa, Kingdom of Tonga, 17–20 April 1995; Craswell, E.T., Asher, C.J., O’Sullivan, J.N., Eds.; ACIAR: Canberra, Australia, 1996; pp. 5–9. [Google Scholar]
- Reuter, D.J.; Edawrs, D.G.; Wilhelm, N.S. Temperature and Tropical Crops. In Plant Analysis, 2nd ed.; Reuter, D.J., Robinson, J.B., Ditkiewicz, C., Eds.; CSIRO: Melbourne, Australia, 1997. [Google Scholar]
- Hunsche, M.; Buerling, K.; Saied, A.S.; Schmitz-Eiberger, M.; Sohail, M.; Gebauer, J.; Noga, G.; Buerkert, A. Effects of NaCl on surface properties, chlorophyll fluorescence and light remission, and cellular compounds of Grewia tenax (Forssk.) Fiori and Tamarindus indica L. leaves. Plant Growth Regul. 2010, 61, 253–263. [Google Scholar] [CrossRef]
- Król, A.; Amarowicz, R.; Weidner, S. Changes in the composition of phenolic compounds and antioxidant properties of grapevine roots and leaves (Vitis vinifera L.) under continuous of long-term drought stress. Acta Physiol. Plant. 2014, 36, 1491–1499. [Google Scholar] [CrossRef] [Green Version]
- Tooulakou, G.; Giannopoulos, A.; Nikolopoulos, D.; Bresta, P.; Dotsika, E.; Orkoula, M.G.; Kontoyannis, C.G.; Fasseas, C.; Liakopoulos, G.; Klapa, M.I.; et al. Alarm photosynthesis: Calcium oxalate crystals as an internal CO2 source in plants. Plant Physiol. 2016, 171, 2577–2585. [Google Scholar] [CrossRef] [Green Version]
- Waskiewicz, A.; Muzolf-Panek, M.; Golinski, P. Phenolic content changes in plants under salt stress. In Ecophysiology and Responses of Plants under Salt Stress; Ahmad, P., Azooz, M., Prasad, M., Eds.; Springer: New York, NY, USA, 2013; pp. 283–314. [Google Scholar]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef] [Green Version]
- Xiong, L.; Wang, R.-G.; Mao, G.; Koczan, J.M. Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiol. 2006, 142, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- Bradbury, J.H.; Egan, S.V.; Matthews, P.J. Cyanide content of the leaves and stems of edible aroids. Phytochem. Anal. 1995, 6, 268–271. [Google Scholar] [CrossRef]
- Burns, A.E.; Gleadow, R.M.; Zacarias, A.; Cuambe, C.E.; Miller, R.E.; Cavagnaro, T.R. Variations in the chemical composition of cassava (Manihot esculenta Crantz) leaves and roots as affected by genotypic and environmental variation J. Agric. Food Chem. 2012, 60, 4946–4956. [Google Scholar] [CrossRef]
- Blomstedt, C.K.; Gleadow, R.M.; O’Donnell, N.; Naur, P.; Jensen, K.; Laursen, T.; Olsen, C.E.; Stuart, P.; Hamill, J.D.; Møller, B.L.; et al. A combined biochemical screen and TILLING approach identifies mutations in Sorghum bicolor L. Moench resulting in acyanogenic forage production. Plant Biotechnol. J. 2012, 10, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Ballhorn, D.J.; Elias, D.J. Salinity-mediated cyanogenesis in white clover (Trifolium repens) affects trophic interactions. Ann. Bot. 2014, 114, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.-Y.; Lu, C.T.; Wei, M.-L.; Chan, L.-F. Comparison of different models for non-destructive leaf area estimation in taro. Agron. J. 2004, 96, 448–453. [Google Scholar] [CrossRef]
- Crimp, S.; Lisson, S.; Gleadow, R.; Hargreaves, J.; Nauluvula, P.; Melteras, M. Understanding the Response of Taro and Cassava to Climate Change; Australia Centre for International Agricultural Research: Canberra, Australia, 2017. [Google Scholar]
- O’Donnell, N.H.; Møller, B.L.; Neale, A.D.; Hamill, J.D.; Blomstedt, C.K.; Gleadow, R.M. Effects of PEG-induced osmotic stress on growth and dhurrin levels of forage sorghum. Plant Physiol. Biochem. 2013, 73, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Gleadow, R.M.; Rowan, K.S.; Ashton, D.H. Invasion by Pittosporum undulatum of the forests of central Victoria. IV. Shade tolerance. Aust. J. Bot. 1983, 31, 151–160. [Google Scholar] [CrossRef]
- Burns, A.E.; Gleadow, R.M.; Woodrow, I.E. Light alters the allocation of nitrogen to cyanogenic glycosides in Eucalyptus cladocalyx. Oecologia 2002, 133, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Jaspars, E.M.J. Pigmentation of tobacco crown gall tissues cultured in vitro in dependence of composition of medium. Physiol. Plant. 1965, 18, 933–940. [Google Scholar] [CrossRef]
- Uesugi, A.; Kessler, A. Herbivore release drives parallel patterns of evolutionary divergence in invasive plant phenotypes. J. Ecol. 2016, 104, 876–886. [Google Scholar] [CrossRef] [Green Version]
- Woodrow, I.E.; Slocum, D.J.; Gleadow, R.M. Influence of water stress on cyanogenic capacity in Eucalyptus cladocalyx. Funct. Plant Biol. 2002, 29, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Nahrstedt, A.; Kant, J.D.; Hosel, W. Aspects on the biosynthesis of the cyanogenic glucoside triglochinin in Triglochin maritima. Planta Med. 1984, 50, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Gleadow, R.M.; Bjarnholt, N.; Jørgensen, K.; Fox, J.; Miller, R.E. Detection, identification and quantitative measurement of cyanogenic glycosides. In Research Methods in Plant Science. Soil Allelochemicals; Narwal, S.S., Szajdak, L., Sampietro, D.A., Eds.; International Allelopathy Foundation; Studium Press: Houston, TX, USA, 2012; Volume 1. [Google Scholar]
- Takos, A.; Lai, D.; Mikkelsen, L.; Abou Hachem, M.; Shelton, D.; Motawia, M.S.; Olsen, C.E.; Wang, T.L.; Martin, C.; Rook, F. Genetic screening Identifies cyanogenesis-deficient mutants of Lotus japonicus and reveals enzymatic specificity in hydroxynitrile glucoside metabolism. Plant Cell 2010, 22, 1605–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R_Core_Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: http://www.r-project.org/ (accessed on 1 September 2021).





| Growth Parameter | 0 mM | 50 mM | 100 mM | 150 mM | 200 mM | df | F | p |
|---|---|---|---|---|---|---|---|---|
| Total biomass (g) | 32.05 ± 3.60 a | 24.04 ± 2.35 b | 17.44 ± 1.50 bc | 10.93 ± 0.94 c | 14.10 ± 2.10 bc | 4 | 14.54 | 0.001 * |
| Leaf mass (g) | 3.26 ± 0.52 a | 2.70 ± 0.35 ab | 1.83 ± 0.17 b | 0.91 ± 0.15 c | 0.35 ± 0.05 c | 4 | 21.03 | 0.001 * |
| Petiole mass (g) | 5.72 ± 1.14 a | 4.84 ± 0.66 ab | 2.75 ± 0.31 bc | 1.28 ± 0.19 cd | 0.60 ± 0.12 d | 4 | 19.32 | 0.001 * |
| Root mass (g) | 2.77 ± 0.18 a | 2.34 ± 0.38 ab | 1.66 ± 0.14 bc | 1.20 ± 0.19 c | 1.05 ± 0.11 c | 4 | 12.39 | 0.001 * |
| Corm mass (g) | 20.31 ± 2.41 a | 14.19 ± 1.56 b | 11.20 ± 1.32 b | 7.55 ± 0.74 b | 12.11 ± 1.98 b | 4 | 8.21 | 0.001 * |
| Above-ground mass (g) | 8.97 ± 1.64 a | 7.53 ± 1.00 ab | 4.58 ± 0.47 bc | 2.19 ± 0.33 c | 0.95 ± 0.16 d | 4 | 20.57 | 0.001 * |
| Below-ground mass (g) | 23.08 ± 2.46 a | 16.51 ± 1.80 b | 12.86 ± 1.38 b | 8.74 ± 0.79 b | 13.15 ± 2.01 b | 4 | 9.76 | 0.001 * |
| Plant height (cm) | 69.06 ± 3.14 a | 59.58 ± 5.58 b | 57.58 ± 1.97 ab | 40.32 ± 3.16 c | 28.50 ± 3.06 c | 4 | 21.40 | 0.001 * |
| Leaf number | 3.67 ± 0.16 a | 3.00 ± 0.29 ab | 3.28 ± 0.20 ab | 2.71 ± 0.27 bc | 1.72 ± 0.14 d | 4 | 10.41 | 0.001 * |
| No. senescent | 3.22 ± 0.15 a | 2.72 ± 0.30 a | 2.83 ± 0.20 a | 1.18 ± 0.21 b | 0.94 ± 0.17 a | 4 | 5.66 | 0.001 * |
| No. fully expanded | 0.11 ± 0.08 a | 0.22 ± 0.10 a | 0.11 ± 0.08 a | 0.82 ± 0.15 b | 0.33 ± 0.11 b | 4 | 23.16 | 0.001 * |
| Leaf area (cm2) | 1007.07 ± 144.03 a | 821.67 ± 108.30 ab | 511.18 ± 53.77 b | 143.73 ± 22.83 c | 79.86 ± 15.90 c | 4 | 31.06 | 0.001 * |
| Root/shoot ratio | 3.38 ± 0.57 a | 5.19 ± 2.30 a | 3.37 ± 0.51 a | 5.70 ± 0.96 a | 20.71 ± 3.55 b | 4 | 11.28 | 0.001 * |
| RGR (g g−1 day−1) | 0.003 ± 0.00 a | 0.001 ± 0.00 ab | 0.000 ± 0.00 bc | −0.002 ± 0.00 c | −0.002 ± 0.00 c | 4 | 13.40 | 0.001 * |
| LAR (cm2 g−1) | 32.31 ± 2.57 | 36.47 ± 3.70 | 31.00 ± 3.06 | 14.93 ± 3.32 | 6.56 ± 1.75 | 4 | 1.60 | 0.181 |
| NAR (g cm2 day−1) | 0.0002 ± 0.00 a | 0.0002 ± 0.00 ab | 0.0002 ± 0.00 a | 0.0003 ± 0.00 ab | 0.0004 ± 0.00 b | 4 | 4.791 | 0.002 * |
| A (µmol CO2 m−2 s−1) | 9.36 ± 1.06 a | 7.25 ± 0.94 ab | 4.59 ± 1.41 ab | 5.84 ± 1.12 ab | 2.60 ± 1.00 b | 4 | 4.99 | 0.012 * |
| gs (mmol H2O m−2 s−1) | 0.10 ± 0.03 a | 0.06 ± 0.01 ab | 0.04 ± 0.01 ab | 0.05 ± 0.01 ab | 0.02 ± 0.01 b | 4 | 4.99 | 0.028 * |
| T (mmol H2O m−2 s−1) | 2.29 ± 0.72 a | 1.28 ± 0.35 ab | 0.66 ± 0.13 ab | 1.00 ± 0.14 ab | 0.38 ± 0.17 b | 4 | 3.84 | 0.012 * |
| Fv/Fm | 0.88 ± 0.01 | 0.87 ± 0.00 | 0.84 ± 0.05 | 0.79 ± 0.04 | 0.67 ± 0.11 | 4 | 1.24 | 0.367 |
| Total Chlorophyll (mg g−1) | 12.33 ± 1.06 | 9.46 ± 1.33 | 10.08 ± 0.44 | 10.41 ± 0.87 | 10.30 ± 0.98 | 4 | 1.95 | 0.210 |
| Leaf | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 mM | 50 mM | 100 mM | 150 mM | 200 mM | df | F | p | |
| Nitrogen | 37.00 ± 1.64 | 43.35 ± 2.59 | 43.03 ± 2.56 | 44.45 ± 3.53 | 43.42 ± 3.63 | 4 | 1.13 | 0.367 |
| Phosphorus | 4.07 ± 0.41 | 4.82 ± 0.70 | 4.55 ± 0.33 | 5.02 ± 0.44 | 4.23 ± 0.50 | 4 | 0.61 | 0.658 |
| Potassium | 35.40 ± 2.24 | 33.10 ± 2.63 | 32.28 ± 2.10 | 35.82 ± 2.63 | 33.67 ± 3.78 | 4 | 0.24 | 0.915 |
| Sulphur | 2.10 ± 0.08 | 2.47 ± 0.15 | 2.48 ± 0.12 | 2.52 ± 0.23 | 2.42 ± 0.22 | 4 | 1.09 | 0.385 |
| Carbon | 431.17 ± 3.13 | 425.83 ± 1.92 | 430.50 ± 4.25 | 424.83 ± 10.35 | 419.67 ± 10.49 | 4 | 0.12 | 0.975 |
| Calcium | 16.15 ± 0.89 | 15.22 ± 1.06 | 15.43 ± 1.53 | 17.63 ± 3.38 | 14.37 ± 4.08 | 4 | 0.29 | 0.880 |
| Magnesium | 4.28 ± 0.23 | 3.73 ± 0.28 | 3.52 ± 0.20 | 4.03 ± 0.41 | 3.93 ± 0.75 | 4 | 0.46 | 0.768 |
| Sodium | 0.03 ± 0.02 | 0.01 ± 0.00 | 0.06 ± 0.03 | 0.04 ± 0.03 | 0.07 ± 0.05 | 4 | 0.78 | 0.549 |
| Copper | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 4 | 2.79 | 0.052 |
| Zinc | 0.03 ± 0.01 a | 0.04 ± 0.01 ab | 0.07 ± 0.01 ab | 0.07 ± 0.01 ab | 0.08 ± 0.01 b | 4 | 4.06 | 0.013 * |
| Manganese | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.11 ± 0.01 | 0.12 ± 0.02 | 0.10 ± 0.02 | 4 | 0.20 | 0.933 |
| Iron | 0.16 ± 0.03 | 0.06 ± 0.01 | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.04 ± 0.01 | 4 | 2.01 | 0.128 |
| Corm | ||||||||
| 0 mM | 50 mM | 100 mM | 150 mM | 200 mM | df | F | p | |
| Nitrogen | 3.38 ± 0.47 a | 6.05 ± 1.10 ab | 5.38 ± 2.03 ac | 20.75 ± 4.06 b | 20.05 ± 7.40 bc | 4 | 5.47 | 0.003 * |
| Phosphorus | 1.87 ± 0.15 a | 1.78 ± 0.24 a | 2.03 ± 0.48 a | 6.82 ± 1.00 b | 5.50 ± 1.47 b | 4 | 9.06 | 0.001 * |
| Potassium | 10.22 ± 1.18 | 10.62 ± 1.20 | 12.15 ± 2.01 | 8.95 ± 1.04 | 9.13 ± 0.99 | 4 | 0.92 | 0.472 |
| Sulphur | 0.37 ± 0.06 | 0.53 ± 0.10 | 0.47 ± 0.13 | 1.55 ± 0.21 | 1.57 ± 0.56 | 4 | 1.06 | 0.399 |
| Carbon | 437.50 ± 14.92 | 417.67 ± 21.87 | 424.17 ± 2.98 | 414.67 ± 2.93 | 416.33 ± 4.15 | 4 | 0.58 | 0.684 |
| Calcium | 1.28 ± 0.26 a | 2.13 ± 0.48 ab | 1.90 ± 0.35 ab | 4.28 ± 0.88 b | 3.03 ± 0.74 ab | 4 | 3.76 | 0.019 * |
| Magnesium | 1.13 ± 0.11 a | 1.07 ± 0.17 a | 0.93 ± 0.14 a | 2.50 ± 0.24 b | 1.82 ± 0.43 ab | 4 | 6.68 | 0.001 * |
| Sodium | 0.06 ± 0.02 a | 3.82 ± 2.08 ab | 2.55 ± 0.79 ab | 9.58 ± 2.62 b | 6.58 ± 2.34 ab | 4 | 4.05 | 0.013 * |
| Copper | 0.003 ± 0.00 a | 0.005 ± 0.00 a | 0.003 ± 0.00 a | 0.010 ± 0.00 b | 0.006 ± 0.00 ab | 4 | 5.70 | 0.003 * |
| Zinc | 0.04 ± 0.01 | 0.0676 ± 0.0143 | 0.06 ± 0.010 | 0.18 ± 0.07 | 0.07 ± 0.02 | 4 | 2.38 | 0.080 |
| Manganese | 0.01 ± 0.00 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | 4 | 2.25 | 0.097 |
| Iron | 0.03 ± 0.01 a | 0.08 ± 0.01 ab | 0.11 ± 0.02 ab | 0.18 ± 0.04 b | 0.11 ± 0.02 b | 4 | 5.72 | 0.004* |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lloyd, G.R.; Uesugi, A.; Gleadow, R.M. Effects of Salinity on the Growth and Nutrition of Taro (Colocasia esculenta): Implications for Food Security. Plants 2021, 10, 2319. https://doi.org/10.3390/plants10112319
Lloyd GR, Uesugi A, Gleadow RM. Effects of Salinity on the Growth and Nutrition of Taro (Colocasia esculenta): Implications for Food Security. Plants. 2021; 10(11):2319. https://doi.org/10.3390/plants10112319
Chicago/Turabian StyleLloyd, Georgia R., Akane Uesugi, and Roslyn M. Gleadow. 2021. "Effects of Salinity on the Growth and Nutrition of Taro (Colocasia esculenta): Implications for Food Security" Plants 10, no. 11: 2319. https://doi.org/10.3390/plants10112319
APA StyleLloyd, G. R., Uesugi, A., & Gleadow, R. M. (2021). Effects of Salinity on the Growth and Nutrition of Taro (Colocasia esculenta): Implications for Food Security. Plants, 10(11), 2319. https://doi.org/10.3390/plants10112319







