1. Introduction
Wheat (
Triticum aestivum L.) is an extremely important cereal crop for human nutrition and animal feed worldwide. Though wheat cultivation is pervasive in a wide spectrum of ecosystems, many parts of the world (particularly in the arid and semiarid regions) suffer from drought stress as a major constraint hindering its expansion from meeting the growing demands day by day for the population [
1,
2].
Frequent climate change scenarios and rareness of fresh water are two major limiting constraints to sustainable agriculture and preserving food security worldwide. These two major problems may be exacerbated in the semiarid regions where the annual precipitation (200 to 750 mm/year) is not sufficient to meet the needs for farming throughout the year [
3,
4]. In plants, drought stress or water deficit can trigger a wide spectrum of successive events at morphological, biochemical, and molecular levels that may contribute to the adaptation or tolerance processes to such conditions [
5,
6,
7,
8]. Among these responses is the excessive release of reactive oxygen species (ROS) that induce oxidative damage to plant cell components, i.e., protein, lipids and, nucleic acids [
9,
10,
11], and the development of several efficient non-enzymatic and enzymatic antioxidant systems [
1,
12,
13]. Plant resilience to drought stress is mostly attained by accumulating compatible solutes, ion homeostasis, and redox management [
5,
14,
15,
16]. Additionally, water shortage can trigger a cascade of interconnected responses that coordinate the physiological metabolisms of plants, such as altering the signaling pathways, gene expression, hormonal homeostasis, and photosynthetic machinery, leading eventually to affecting crop productivity [
8,
17,
18,
19,
20,
21].
Sulfur-containing molecules and a number of non-protein and protein thiols have been evidenced to play a fundamental role in plant tolerance to various abiotic stresses [
22,
23,
24]. These molecules can work together, representing a crucial network of responses that enable plants to cope with environmental stresses [
24]. Therefore, special attention should be given to reveal a complete picture in this respect.
Alpha-lipoic acid (1,2-dithiolane-3-pentanoic acid; ALA) is a dithiol short-chain fatty acid that is ubiquitous both in prokaryotic or eukaryotic organisms [
25]. It has received great attention as a dietary supplement for humans because of its antioxidant and therapeutic properties [
26,
27]. This antioxidant capacity depends on its two sulfhydryl moieties [
25] which enable it to scavenge free radicals and chelate metals [
28]. In cereal crops, a few studies during the seedling stage have reported that exogenous ALA can enhance the photosynthetic performance in maize under drought stress [
29], ameliorate lipid peroxidation, and induce the antioxidant systems of maize under osmotic stress [
25]. Furthermore, it can regulate the ion homeostasis and osmotic potential in wheat under saline conditions [
30]. In addition, it has been found that there is a close connection between the endogenous ALA and the cellular redox status of wheat seedlings grown under excess copper [
31].
Cysteine (Cys) is an essential thiol-containing amino acid that comprises amino group (NH
2), carboxylic acid (COOH) and sulfhydryl group (SH) as reactive centers. This distinct structure enables Cys to be a potent antioxidant and efficient scavenger for reactive oxygen species (ROS). This is due to the presence of the thiol side chain, which smoothly oxidizes, protecting against the oxidative damage induced by biotic and abiotic stresses [
32,
33]. Moreover, Cys is involved in synthesizing a wide array of vital and defensive molecules such as protein, glutathione, phytoalexins, thionins, glucosinolates, metallothioneins, and phytochelatins [
23,
32,
34,
35,
36]. Additionally, Cys is implicated in different sulfur metabolism pathways and the synthesis of methionine [
33]. These responses can regulate plant growth and development via the importance of methionine and/or S-adenosylmethionine (SAM) as precursors for some vital phytohormones, i.e., ethylene and polyamines [
37,
38]. Several lines of evidence have suggested that exogenous Cys can alleviate the deleterious effects of heavy metals in plants through its roles in metal sequestration and detoxification [
35,
36]. Similarly, applied Cys has been shown to delay the senescence of postharvest green leafy vegetables by decreasing the rate of Chl degradation, cellular respiration, and ethylene biosynthesis [
39]. Up to now, there are no conclusive data to elucidate the role of ALA and/or Cys in wheat plants subjected to water deficit. The current research explores novel approaches to learn more about the combined effect of biomolecules on the diverse parameters of wheat plants under water deficit.
4. Discussion
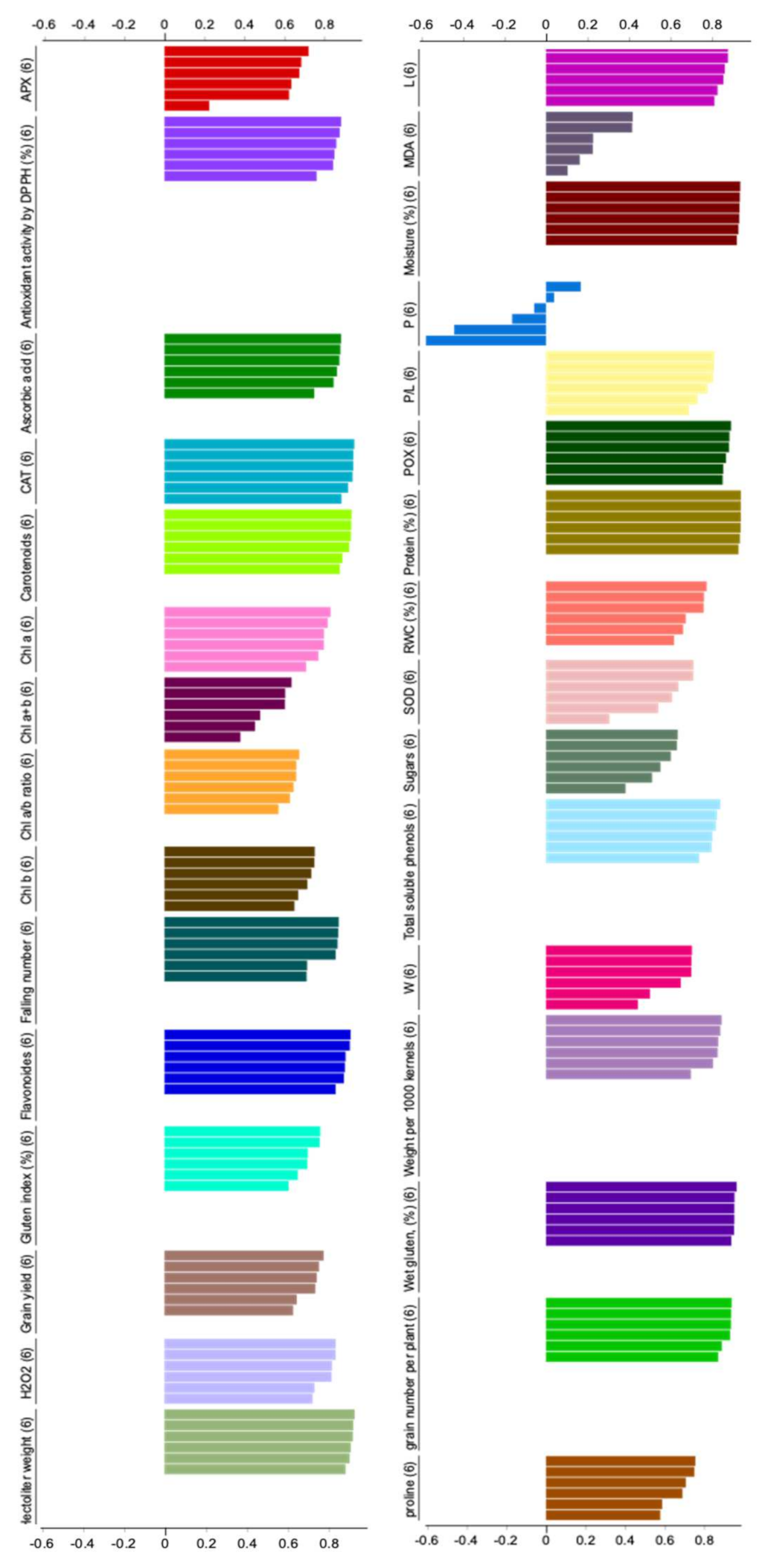
In the present study, exposing wheat plants to deficit irrigation manifestly altered the leaf chlorophyll composition. In this respect, it can be observed that Chl a, Chl b, Chl a+b contents were significantly decreased in the water stressed plants compared to the well watered ones (
Figure 1A–C). This reduction could be attributed to increase the activity of chlorophyllase [
62], as well as water deficiency induced oxidative damage (
Figure 2B,C) which can collapse the membranes and chloroplast structure where the leaf pigments are localized [
63,
64]. In contrast, the Chl a/b ratio (
Figure 1D) in ALA/Cys-untreated plants was significantly increased under water deficit conditions compared to the well watered plants. It is well known that Chl a is the major cofactor for the photochemical reactions in the plastid because it is required for the assembly of pigment–protein complexes, while Chl b can also act as one of the accessory pigments in light-harvesting chlorophyll complexes (LHCs) [
65,
66]. In this study, increasing the Chl a/b ratio may act as a protective and adaptive behavior to maintain the function of photosynthetic apparatus under water stress conditions.
On the other hand, applied ALA and/or Cys were shown to enhance the content of photosynthetic pigments (Chl a, Chl b and Chl a+b) under well watered and water stressed conditions. However, an opposite trend was observed in Chl a/b ratio (
Figure 1). Sulfur-containing biomolecules (ALA and Cys) are considered very potent antioxidants that can protect plants against abiotic stresses [
24,
30]. In this context, exogenous ALA was shown to stimulate photosystem II and gene expression of carbon fixation enzymes (Rubisco and PEP carboxylase) in maize seedlings exposed to drought stress [
29]. These responses were associated with a simultaneous down-regulation in the chlorophyllase gene (Chlase) [
29]. Moreover, Cys has been reported to improve Chl a and Chl b content in oat plants subjected to drought stress [
67]. This effect could be attributed to enhancing the uptake of N in the plants, which is essential for Chl biosynthesis [
68]. In contrast, the Chl a/b ratio (
Figure 1D) differentially responded to ALA treatments under water deficit conditions. It was significantly decreased in the ALA-treated plants compared to the ALA-untreated plants. This effect implies that ALA as an efficient antioxidant has a more positive impact on Chl b than Chl a.
In the present study, water shortage led to an increase of H
2O
2 and the rate of lipid peroxidation as indicated by malondialdehyde (MDA) (
Figure 2B,C). The excessive generation of reactive oxygen species (ROS) under drought stress conditions has been evidenced in several plant species [
69,
70,
71,
72]. This increase of toxic molecules should be restricted by enhancing the overall antioxidant activities and scavenging capacity of plants (
Figure 2A). In this context, the treatments of ALA/Cys revealed beneficial effects as protectant antioxidants by reducing MDA and H
2O
2 in parallel with enhancing the antioxidant capacity by DPPH (
Figure 2A–C). Previous investigations on the antioxidative role of ALA during Pb toxicity revealed ALA-induced accumulation of various antioxidant molecules in wheat plants [
73]. Furthermore, ALA can enhance the photosynthetic performance in maize under drought stress [
29], ameliorate lipid peroxidation, and induce the antioxidant systems of maize under osmotic stress [
25]. Cysteine (Cys) has also been suggested as an important precursor for the synthesis of glutathione in plants [
24].
Carotenoids were significantly decreased in the water stressed plants compared to the well watered plants (
Figure 3A). This response could be explained by that carotenoids are involved in the biosynthesis of ABA, the major signaling hormone in response to drought stress [
74]. In contrast, the treatments of ALA/Cys improved the content of carotenoids under deficit irrigation. This synergistic effect may have occurred to keep the carotenoids that mediate the xanthophyll cycle, leading to dissipating the exceeding light energy and enhancing the photosynthetic capacity under drought stress. In this regard, earlier evidence has confirmed that Cys can hinder energy transfer to prevent photooxidation [
75].
On the other hand, flavonoids and total soluble phenols revealed a significant increase in the water stressed plants compared to the well watered ones (
Figure 3B,C). This over-accumulation of secondary metabolites can enhance plant tolerance to drought stress and alleviate the induced oxidative damage due to their higher antioxidant capacity [
76,
77]. In addition to their antioxidant properties, phenolic compounds can be involved in plant tolerance to drought stress as a sink for carbon under stress conditions [
78]. Furthermore, flavonoids are widely distributed secondary metabolites that are synthesized through the phenylpropanoid pathway, transforming phenylalanine into 4-coumaroyl-CoA, which finally enters the flavonoid biosynthesis pathway [
79]. The carbon skeleton of all secondary metabolites including the compound-mediated phenylpropanoid pathway basically relies on photosynthesis, since several changes in the stressed plants happen in carbon metabolism to achieve the balance between the biomass production and formation of defensive secondary compounds [
76]. In this study, flavonoids and total soluble phenols were relatively enhanced by the combined ALA/Cys treatments. This enhancement could be attributed to the increase of the rate of photosynthesis by both compounds [
29,
80].
Alpha lipoic acid (ALA) is soluble in water and lipid phases, connects the activity of antioxidants in the cell membrane (α-tocopherol) with other antioxidants in the cytoplasm, i.e., ascorbic acid (AsA) and glutathione (GSH), leading to reinforcing the antioxidant power of plants [
31]. Moreover, Cys is an important precursor of GSH biosynthesis in plants [
24]. In the current work, the treatments of ALA/Cys increased the concentration of AsA under both investigated levels of irrigation (
Figure 3D). These findings indicate that the treatments of ALA/Cys can play a modulatory role in the operation of the GSH-AsA cycle under the circumstances of this study [
81].
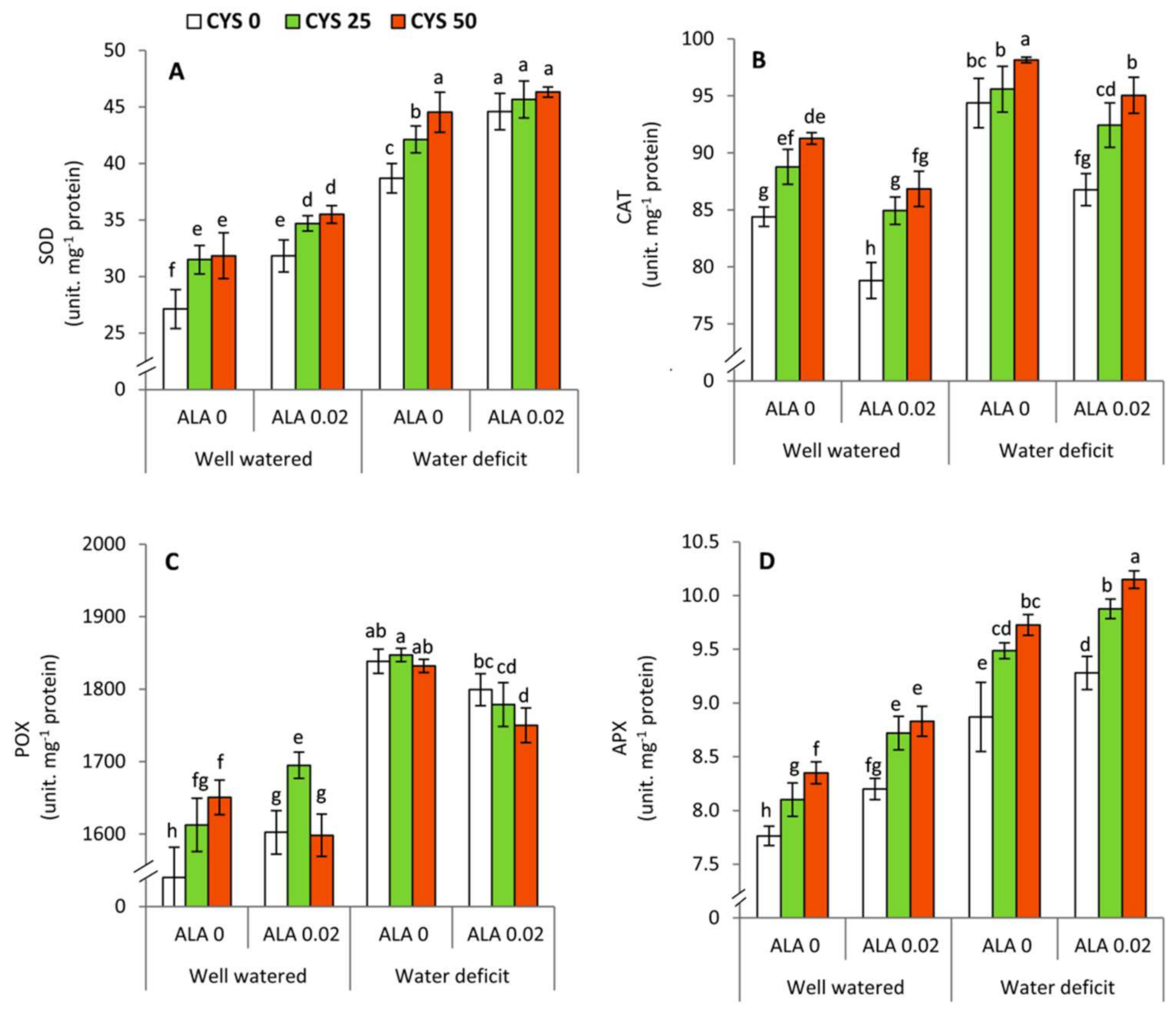
The activities of antioxidant enzymes including superoxide dismutase (SOD), catalase (CAT), peroxidase (POX) and ascorbate peroxidase (APX) were observed to be significantly affected by deficit irrigation and ALA/Cys treatments (
Table 5;
Figure 4). Interestingly, ALA and Cys exhibited differential effects on the activity of antioxidative enzymes, whereas SOD and APX activity were positively upregulated by ALA treatment while CAT and POX activity were decreased. Thus, ALA functions through the activation of the major ROS scavenging system that includes APX and SOD. Reduction of H
2O
2 content was accompanied by ALA and Cys-mediated elevation in APX, POX, and CAT activity. Further investigations are necessary to reveal the mechanism of ALA and Cys signaling in the modulation of antioxidative enzymes in specific organelles in response to water stress in wheat leaves. Interestingly, Cys treatment during water deficit elevated the activity of all antioxidative enzymes, which was all the higher for POX. Thus, the present work highlights the integrative and synergistic role of ALA and Cys application in the upregulation of the enzymatic antioxidative defense systems. It is further advocated that both ALA and Cys are beneficial to instigate redox management and ROS scavenging activity [
25,
30,
67,
73]. Exogenously applied ALA and Cys have been suggested to mitigate the oxidative stress and confer osmotic tolerance to wheat and soybean plants subjected to NaCl stress [
30,
68].
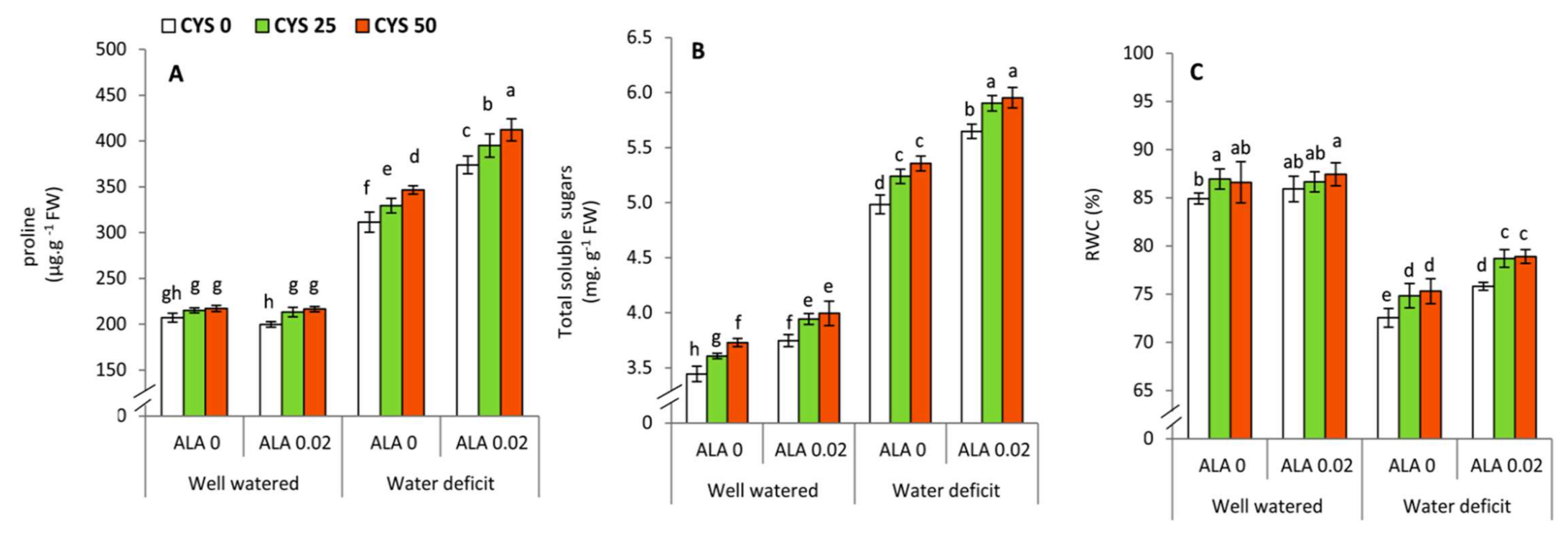
In the present work, ALA and Cys treatments also increased the proline, total soluble sugars and leaf relative water content (RWC) of drought stressed wheat plants (
Table 6,
Figure 5). Several lines of evidence demonstrated that to regulate the osmotic potential and enhance water uptake under osmotic stress (drought or salinity), plants usually accumulate considerable concentrations of some organic molecules such as proline and sugars that function as osmotic regulators and enhance plant water relation [
64,
82,
83].
It is noteworthy that the improved osmotic regulation in leaves was also associated with the amelioration of yield attributes (
Table 7,
Figure 6) including number of spikes/m
2, number of grains/plant and grain yield (ton/ha). Several previous studies reported that water deficit has a destructive effect on the yield of wheat plants [
84,
85]. Generally, water stress can affect many aspects related to the field performance of grain crops such as the photosynthesis and translocation of carbohydrates, which are responsible for the filling of grains [
86,
87]. In this study, besides the amelioration of drought induced oxidative damage, the treatments of ALA/Cys were evidenced to enhance photosynthetic pigments, accumulation of osmolytes and improving the non-enzymatic and enzymatic antioxidants. These positive effects could explain the increase of grain yield attributes.
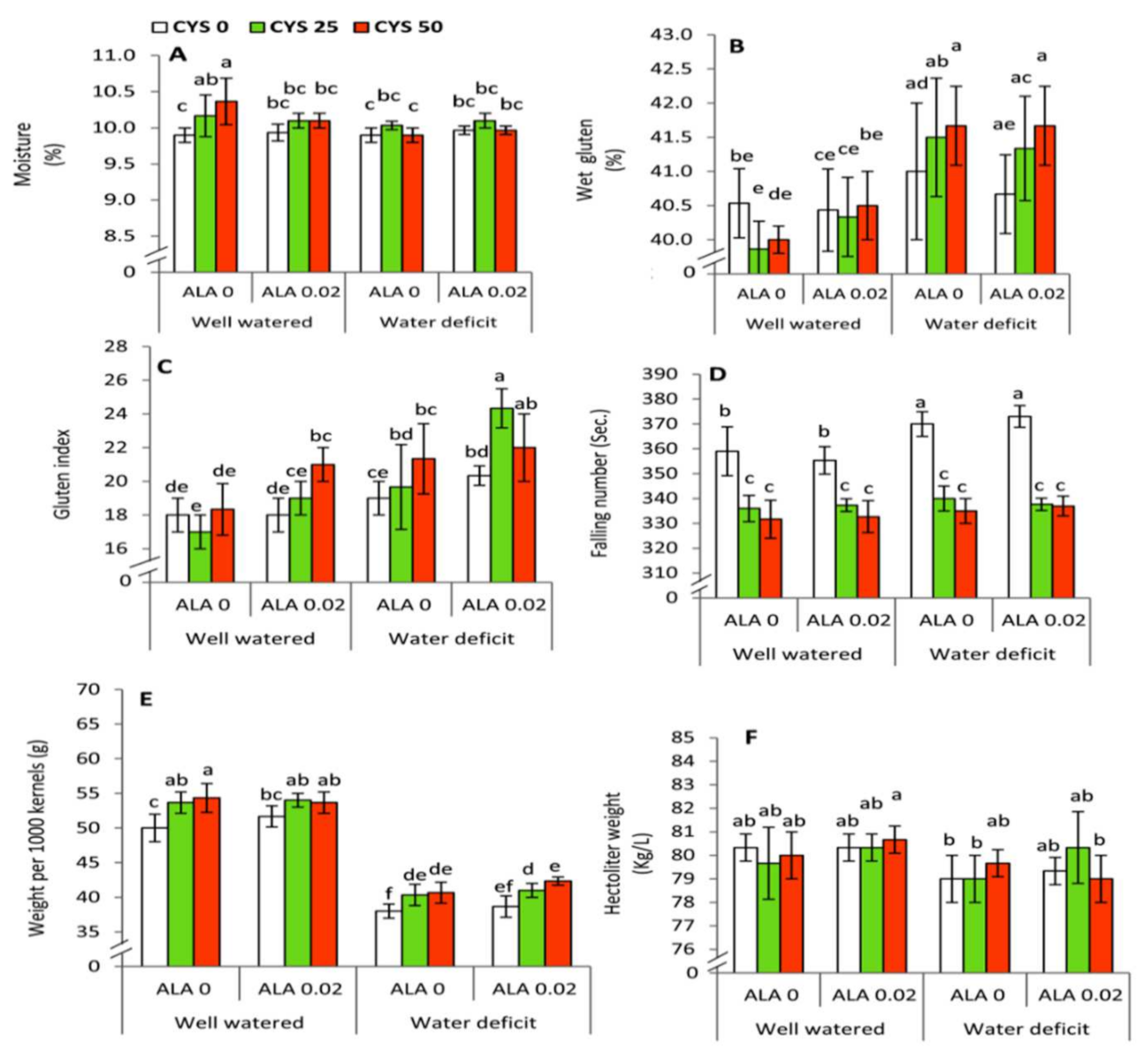
ALA and Cys treatments improved moisture percentage and gluten index in grains obtained from water stressed plants. Cys treatment further improved kernel weight (per 1000 kernels) under water deficit conditions. Analysis of alveographic characteristics indicated that ALA and Cys applications were effective in improving flour and dough quality of wheat grains. Total amino acid content, water relation, and growth characteristics are known to be associated with physiochemical properties, as well as grain quality in drought stressed wheat plants [
88].
Additionally, Cys and ALA treatments (sulfur-containing molecules) significantly improved grain attributes. In recent years, investigations have revealed the emerging role of ALA (dithiol) as a potential antioxidant in plants [
89]. Reduced ALA possesses 2 sulfhydryl moieties which function as potential ROS scavenging sites. ALA is an important sulfur-bearing compound that exerts a plethora of effects in plants subjected to abiotic stress [
73]. ALA further activates a number of mitochondria-localized metabolic enzymes [
90]. Sulfur compounds are important regulators of signaling pathways associated with abiotic stress tolerance in plants [
91,
92]. In the present work, application of the two sulfur-containing priming molecules (ALA and Cys) led to several synergistic effects that enhanced the tolerance of wheat plants to water shortage.
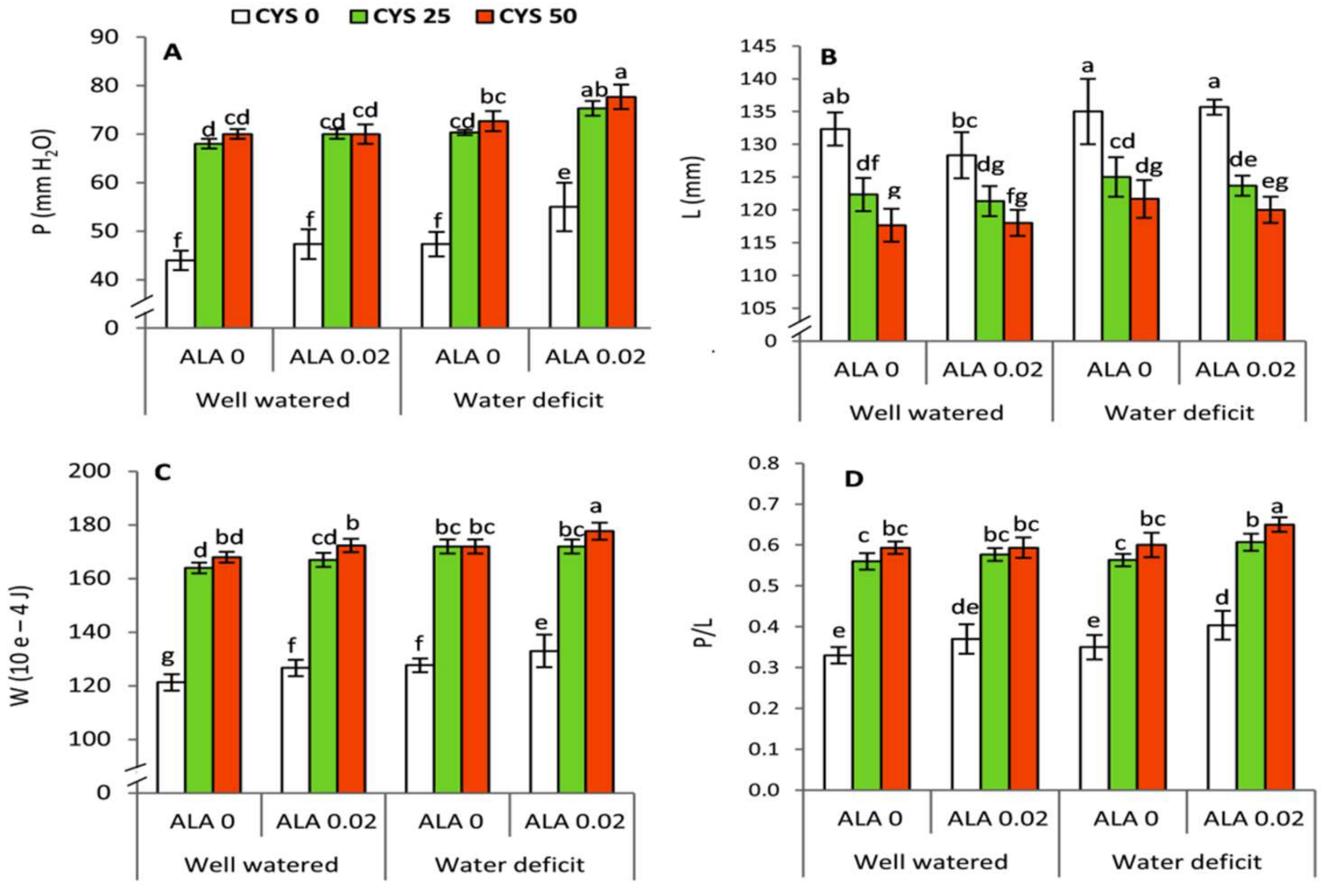
Various physiochemical attributes have been improved in the presence of ALA and Cys treatment during water stress in wheat plants. Future investigations are necessary to establish the possible role of ALA and Cys in the modulation of the glutathione-ascorbate cycle in water stressed wheat plants. It is noteworthy that application of the two biomolecules has led to an improvement in the gluten index and quality of grains (
Table 8;
Figure 7). Thus, using ALA and Cys are effective in the grain filling stage of wheat plants under water stress. This effect may indicate the presence of long distance signaling of ALA and Cys from leaves to grains. Correlation analysis revealed significant interaction among irrigation, seed soaking, and ALA/Cys treatments in wheat plants raised in the absence and presence of drought stress. Thus, ALA and Cys are effective priming molecules and bear agronomic importance in improving wheat yield in the arid cultivable regions. Priming for drought or salinity allows stress tolerance and offers several benefits in many crops [
93]. This improvement was extended to the grain processing technology by affecting the alveographic parameters (
Table 8 and
Table 9). Analysis of alveographic characteristics indicated that ALA and Cys application were effective in improving flour and dough quality of wheat grains. In our present work, Cys and ALA treatment (sulfur-containing molecules) significantly improved grain attributes. Various physiochemical attributes of grains have improved in the presence of ALA and Cys treatment during water stress in wheat plants, and alveographic parameters of wheat flour dough also was improved. This improvement extended to the grain processing technology by affecting the alveographic parameters (technological quality parameters). Such parameters are strongly related to the flour yield and flour properties by enhancing the viscoelastic properties of dough [
94]. In this context, it has been found that altering water relation and total amino acid content is concomitant with substantial changes in the physiochemical properties and grain quality of drought stressed wheat plants [
88].