Carotenoids and Apocarotenoids in Planta: Their Role in Plant Development, Contribution to the Flavour and Aroma of Fruits and Flowers, and Their Nutraceutical Benefits
Abstract
1. Introduction
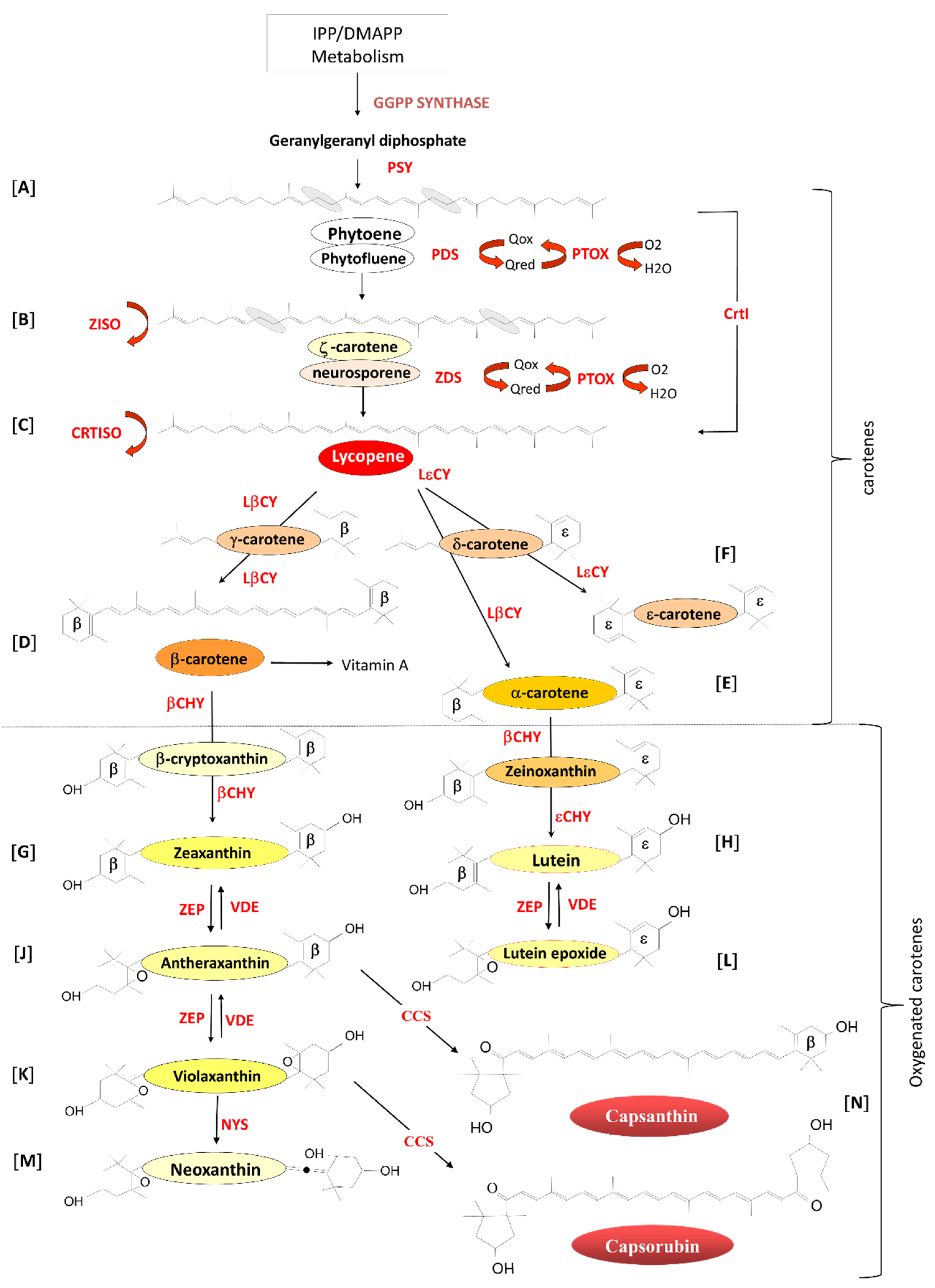
2. Carotenoids
2.1. Carotenoid Biosynthesis in Planta
2.2. Manipulating Carotenoid Content in Planta
2.2.1. ‘Push’ Strategies for Increasing Carotenoid Content in Planta
| Plant | Transgene(s) | Metabolite Analysis | Ref | ||
|---|---|---|---|---|---|
| Tomato fruit | crtB | - | - | phytoene content increased (1.6–3.1-fold). Lycopene (1.8–2.1-fold) and β-carotene (1.6–2.7-fold) were increased | [117] |
| crtL | - | - | β-carotene content increased about threefold, up to 45% of the total carotenoid content | [116] | |
| SlPSY | - | - | phytoene content increased 135%; β-carotene increased 39%; total carotenoids increased by 25% | [118] | |
| AtPDS | - | - | Lycopene and β-carotene increased 31.1% and 42.8%, respectively, and phytoene decreased by up to 70% | [119] | |
| AtZDS | - | - | 18–26% increase in lycopene in fruit | [120] | |
| SlLyc | - | - | Increase in total carotenoids (2.3-fold). β-carotene increased (11.8-fold), and Lycopene decreased (10-fold) | [121] | |
| Cassava tubers | crtB | - | - | ~15-fold increases in carotenoids (as all-trans-β-carotene) (40–60 µg/g DW compared to CN 0.5–1 µg/g DW) | [114] |
| crtB | AtDXS | - | Up to 30-fold carotenoid increase (as all-trans-β-carotene) (25 µg/g DW) compared to CN 0.5–1 µg/g DW) | ||
| Potato tubers | DXS | - | - | 2-fold increase in total carotenoids; 7-fold increase phytoene | [122] |
| crtB | - | - | Carotenoid levels reached 35 μg/g. β-carotene levels in the transgenic tubers reached ~11 μg/g DW | [115] | |
| crtB | AtDxs | - | 37–109 µg/g DW total carotenoids (CN 8 µg/g) | [114] | |
| crtB | crtL | crtY | 20-fold increase (to 114 µg/g DW) with β-carotene 3600-fold higher (47 µg/g DW) | [113] | |
| Canola seed | crtB | - | - | 50-fold increase in carotenoids with α- and β-carotene. Lutein, the predominant carotenoid in CN seeds, remained at similar levels in transgenic seeds | [109] |
| Soybean | crtB | - | - | Accumulate 845 µg/g DW of β carotene. An increase of 1500-fold compared to CN | [123] |
| Wheat | ZmPsy | ctrI | - | Increase in β-carotene from 0.81µg/g DW to 2.3–4.9 µg/g DW in the best lines | [106] |
| Cavendish Banana | MtPsy | - | - | Increase in β-carotene content from 3.1 µg/g DW in fully ripe fruit to up to 8.3 µg/g DW. | [124] |
| ZmPsy | - | - | Increase in β-carotene content from 3.1 µg/g DW in fully ripe fruit to up to 9.0 µg/g DW. | ||
| ZmPsy | ctrI | - | Increase in β-carotene content from 3.1 µg/g DW in fully ripe fruit to up to 13.2 µg/g DW. | ||
| Maize | ZmPsy | ctrI | - | Increase in β-carotene from 0.35 µg/g DW to 15–59 µg/g DW in the best lines. Up to 100-fold increase in total carotenoids | [125] |
| crtB | ctrI | - | Increase β-carotene from 0.39 µg/g DW to 9.8 µg/g DW | [126] | |
| Rice | NpPsy | crtI | - | β-carotene, + small amounts of lutein and zeaxanthin | [110,112] |
| NpPsy | crtI | NpLyc | 1.6 µg/g DW carotenoid in the endosperm | [111] | |
| NpPsy | crtI | - | 0.8–1.2 µg/g DW (up to 68% β-carotene) | ||
| SlPsy | crtI | - | 0.9–1.2 µg/g DW (up to 68% β-carotene) | ||
| CaPsy | crtI | - | 1.1–4.7 µg/g DW (up to 80% β-carotene) | ||
| OsPsy | crtI | - | Up to 18.4 µg/g DW (up to 86% β-carotene) | ||
| ZmPsy | crtI | - | Up to 14.4 µg/g DW (up to 89% β-carotene) | ||
| ZmPsy | crtI | - | Up to 5.5 µg/g DW (up to 39% β-carotene) | [127] | |
| ZmPsy | crtI | AtOr | Up to 25.8 µg/g DW (up to 50% β-carotene) | ||
| Sorghum | AtDxs | ZmPsy | ctrI, PMI | β-carotene levels ranged from 2.5 to 9.1 μg/g DW in the mature seeds compared to CN 0.5 μg/g DW (+10-fold) | [107] |
| HGGT AtDxs | ZmPsy, | ctrI, PMI | all-trans β-carotene levels ranged from 7.3 to 12.3 μg/g DW in the mature seeds compared to CN 0.5 μg/g DW (~19-fold increase) | ||
2.2.2. ‘Pull’ Strategies for Manipulating Carotenoid Storage in Planta
2.2.3. ‘Block’ Strategies for Manipulating Carotenoid Storage in Planta
| Plant | Knockout Targets | Metabolite Analysis | Ref | |
|---|---|---|---|---|
| Arabidopsis | ccd1-1 | - | In seeds, Carotenoids, lutein +21%, β-carotene + 86%, antheraxanthin +20%, violaxanthin +130%, neoxanthin +311% increased relative to WT | [42] |
| ccd1-1 | - | In seeds, Carotenoids, lutein, neoxanthin and violaxanthin increased 170% to 210%, and β-carotene 400% relative to the wild type | [138] | |
| - | ccd4-1 | In seeds, Carotenoids, lutein +230%, violaxanthin +590%, neoxanthin +390%, and β-carotene + 840% compared with the WT | ||
| ccd1-1 | cdd4-1 | In seeds, Combining ccd4-1 and ccd1-1, antheraxanthin, and lutein levels (470, and 240% of wild-type levels, respectively), β-carotene +1710%, violaxanthin +1220%, and neoxanthin +1620 (at 1220, and 1620% of WT | ||
| Peach | - | ccd4 | Mutation in ccd4 in peach results in a yellow peach variety | [141] |
| Potato | - | ccd4 KO | Increased carotenoid content, 2- to 5-fold higher than in WT Lutein and antheraxanthin increased ~900%, violaxanthin by ~400%, and neoxanthin by ~224% in the best lines | [143] |
| Chrysanthemum | - | ccd4 KO | resulted in a change of petal color from white to yellow. During late-stage petal development, wild-type petals completely lost their carotenoids, the petals of RNAi lines contained 3 to 8 μg/g fresh weight of carotenoids | [142,144] |
| Tomato | ccd1 KO | - | No changes observed in tomato fruit | [40] |
2.3. ’Hidden Hunger’ and the Health Benefits of Carotenoids
3. Apocarotenoids
3.1. Apocarotenoid Biosynthesis Is Planta
3.2. Carotenoid Cleavage Dioxygenase 1 (CCD1) Enzymes Cleave a Broad Category of Carotenoids and Apocarotenoids at Multiple Double Bonds in the Cytosol
3.3. Carotenoid Cleavage Dioxygenase 4
3.4. Novel Carotenoid Cleavage Dioxygenases
3.5. Apocarotenoids Are Important to Flavour and Aroma
3.6. Apocarotenoids Are Important Therapeutical Compounds
3.6.1. Bixin
3.6.2. Saffron and Crocetin
3.6.3. Carotenoid-Derived Ionones
3.7. Apocarotenoids Have Roles in Plant Development and Defense
3.7.1. Apocarotenoids Promote Arbuscular Mycorrhizal Symbiosis and Have Antimicrobial Activities
3.7.2. Apocarotenoids Attract and Repel Insects
3.7.3. Developmental Roles of Apocarotenoids
4. Future Prospects and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodwin, T.W. The Biochemistry of Carotenoids, Vol 1, Plants; Chapman and Hall: London, UK, 1980. [Google Scholar]
- Ruiz-Sola, M.Á.; Rodríguez-Concepción, M. Carotenoid Biosynthesis in Arabidopsis: A Colorful Pathway. Arab. Book 2012, 10, e0158. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids: Handbook; Birkhäuser: Basel, Switzerland, 2012. [Google Scholar]
- Paliwal, C.; Ghosh, T.; George, B.; Pancha, I.; Maurya, R.; Chokshi, K.; Ghosh, A.; Mishra, S. Microalgal carotenoids: Potential nutraceutical compounds with chemotaxonomic importance. Algal Res. 2016, 15, 24–31. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2021, 11–51. [Google Scholar] [CrossRef]
- Yabuzaki, J. Carotenoids Database: Structures, chemical fingerprints and distribution among organisms. Database 2017, 2017, bax004. [Google Scholar] [CrossRef] [PubMed]
- Lerfall, J. Carotenoids: Occurrence, Properties and Determination. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 663–669. [Google Scholar] [CrossRef]
- Sun, T.; Tadmor, Y.; Li, L. Pathways for Carotenoid Biosynthesis, Degradation, and Storage. Methods Mol. Biol. 2020, 2083, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.W.; Siervo, M.; Lara, J. Lycopene and tomato and risk of cardiovascular diseases: A systematic review and meta-analysis of epidemiological evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 141–158. [Google Scholar] [CrossRef]
- Langi, P.; Kiokias, S.; Varzakas, T.; Proestos, C. Carotenoids: From Plants to Food and Feed Industries. Methods Mol. Biol. 2018, 1852, 57–71. [Google Scholar] [CrossRef]
- Van Hoang, D.; Pham, N.M.; Lee, A.H.; Tran, D.N.; Binns, C.W. Dietary Carotenoid Intakes and Prostate Cancer Risk: A Case-Control Study from Vietnam. Nutrients 2018, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.-l.; Gao, C.; Chen, L.; Hu, G.-q.; Xie, S.-q. Essential role of autophagy in fucoxanthin-induced cytotoxicity to human epithelial cervical cancer HeLa cells. Acta Pharmacol. Sin. 2013, 34, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Satomi, Y. Antitumor and Cancer-preventative Function of Fucoxanthin: A Marine Carotenoid. Anticancer Res. 2017, 37, 1557–1562. [Google Scholar] [CrossRef]
- Koklesova, L.; Liskova, A.; Samec, M.; Zhai, K.; Abotaleb, M.; Ashrafizadeh, M.; Brockmueller, A.; Shakibaei, M.; Biringer, K.; Bugos, O.; et al. Carotenoids in Cancer Metastasis-Status Quo and Outlook. Biomolecules 2020, 10, 1653. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoids as membrane stabilisers in chloroplasts. Trends Plant Sci. 1998, 3, 147–151. [Google Scholar] [CrossRef]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 108–115. [Google Scholar] [CrossRef]
- Johnson, Q.R.; Mostofian, B.; Fuente Gomez, G.; Smith, J.C.; Cheng, X. Effects of carotenoids on lipid bilayers. Phys. Chem. Chem. Phys. 2018, 20, 3795–3804. [Google Scholar] [CrossRef] [PubMed]
- Mostofian, B.; Johnson, Q.R.; Smith, J.C.; Cheng, X. Carotenoids promote lateral packing and condensation of lipid membranes. Phys. Chem. Chem. Phys. 2020, 22, 12281–12293. [Google Scholar] [CrossRef]
- Simkin, A.J.; Laizet, Y.; Kuntz, M. Plastid lipid associated proteins of the fibrillin family: Structure, localisation, functions and gene expression. In Recent Research Developmetns in Biochemistry; Pandalai, S.G., Ed.; Research Signpost: Trivandrum, India, 2004; Volume 5, pp. 307–316. [Google Scholar]
- Deruere, J.; Romer, S.; d’Harlingue, A.; Backhaus, R.A.; Kuntz, M.; Camara, B. Fibril assembly and carotenoid overaccumulation in chromoplasts: A model for supramolecular lipoprotein structures. Plant Cell 1994, 6, 119–133. [Google Scholar] [CrossRef]
- Simkin, A.J.; Gaffe, J.; Alcaraz, J.P.; Carde, J.P.; Bramley, P.M.; Fraser, P.D.; Kuntz, M. Fibrillin influence on plastid ultrastructure and pigment content in tomato fruit. Phytochemistry 2007, 68, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Frank, H.A.; Cogdell, R.J. Carotenoids in photosynthesis. Photochem. PhotoBiol. 1996, 63, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Uragami, C.; Cogdell, R.J. Carotenoids and Photosynthesis. Subcell. Biochem. 2016, 79, 111–139. [Google Scholar] [CrossRef]
- Ledford, H.K.; Niyogi, K.K. Singlet oxygen and photo-oxidative stress management in plants and algae. Plant Cell Environ. 2005, 28, 1037–1045. [Google Scholar] [CrossRef]
- Telfer, A. What is beta-carotene doing in the photosystem II reaction centre? Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2002, 357, 1431–1439. [Google Scholar] [CrossRef]
- Sandmann, G.; Böger, P. Inhibition of carotenoid biosynthesis by herbicides. In Target Sites of Herbicides Action; Böger, P., Sandmann, G., Eds.; CRC Press: Boca Raton, FL, USA, 1989; pp. 25–44. [Google Scholar]
- Simkin, A.J.; Breitenbach, J.; Kuntz, M.; Sandmann, G. In vitro and in situ inhibition of carotenoid biosynthesis in Capsicum annuum by bleaching herbicides. J. Agric. Food Chem. 2000, 48, 4676–4680. [Google Scholar] [CrossRef] [PubMed]
- Josse, E.M.; Simkin, A.J.; Gaffe, J.; Laboure, A.M.; Kuntz, M.; Carol, P. A plastid terminal oxidase associated with carotenoid desaturation during chromoplast differentiation. Plant Physiol. 2000, 123, 1427–1436. [Google Scholar] [CrossRef]
- Wilson, F.; Harrison, K.; Armitage, A.D.; Simkin, A.J.; Harrison, R.J. CRISPR/Cas9-mediated mutagenesis of phytoene desaturase in diploid and octoploid Strawberry. BMC Plant Methods 2019, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Booker, J.; Auldridge, M.; Wills, S.; McCarty, D.; Klee, H.; Leyser, O. MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr. Biol. 2004, 14, 1232–1238. [Google Scholar] [CrossRef]
- Snowden, K.C.; Simkin, A.J.; Janssen, B.J.; Templeton, K.R.; Loucas, H.M.; Simons, J.L.; Karunairetnam, S.; Gleave, A.P.; Clark, D.G.; Klee, H.J. The Decreased apical dominance1/Petunia hybrida CAROTENOID CLEAVAGE DIOXYGENASE8 gene affects branch production and plays a role in leaf senescence, root growth, and flower development. Plant Cell 2005, 17, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.H.; Qin, X.; Loewen, M.C. The biochemical characterization of two carotenoid cleavage enzymes from Arabidopsis indicates that a carotenoid-derived compound inhibits lateral branching. J. Biol. Chem. 2004, 279, 46940–46945. [Google Scholar] [CrossRef]
- Lopez-Obando, M.; Ligerot, Y.; Bonhomme, S.; Boyer, F.-D.; Rameau, C. Strigolactone biosynthesis and signaling in plant development. Development 2015, 142, 3615–3619. [Google Scholar] [CrossRef]
- Vogel, J.T.; Walter, M.H.; Giavalisco, P.; Lytovchenko, A.; Kohlen, W.; Charnikhova, T.; Simkin, A.J.; Goulet, C.; Strack, D.; Bouwmeester, H.J.; et al. SlCCD7 controls strigolactone biosynthesis, shoot branching and mycorrhiza-induced apocarotenoid formation in tomato. Plant J. Cell Mol. Biol. 2010, 61, 300–311. [Google Scholar] [CrossRef]
- Jia, K.P.; Baz, L.; Al-Babili, S. From carotenoids to strigolactones. J. Exp. Bot. 2018, 69, 2189–2204. [Google Scholar] [CrossRef]
- Qin, X.; Zeevaart, J.A.D. The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proc. Natl. Acad. Sci. USA 1999, 96, 15354–15361. [Google Scholar] [CrossRef]
- Parry, A.D.; Babiano, M.J.; Horgan, R. The role of cis-carotenoids in abscisic acid biosynthesis. Planta 1990, 182, 118–128. [Google Scholar] [CrossRef]
- Dong, T.; Park, Y.; Hwang, I. Abscisic acid: Biosynthesis, inactivation, homoeostasis and signalling. Essays Biochem. 2015, 58, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; Schwartz, S.H.; Auldridge, M.; Taylor, M.G.; Klee, H.J. The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles beta-ionone, pseudoionone, and geranylacetone. Plant J. 2004, 40, 882–892. [Google Scholar] [CrossRef]
- Simkin, A.J.; Underwood, B.A.; Auldridge, M.; Loucas, H.M.; Shibuya, K.; Schmelz, E.; Clark, D.G.; Klee, H.J. Circadian regulation of the PhCCD1 carotenoid cleavage dioxygenase controls emission of beta-ionone, a fragrance volatile of petunia flowers. Plant Physiol. 2004, 136, 3504–3514. [Google Scholar] [CrossRef] [PubMed]
- Auldridge, M.E.; Block, A.; Vogel, J.T.; Dabney-Smith, C.; Mila, I.; Bouzayen, M.; Magallanes-Lundback, M.; DellaPenna, D.; McCarty, D.R.; Klee, H.J. Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J. 2006, 45, 982–993. [Google Scholar] [CrossRef]
- Auldridge, M.E.; McCarty, D.R.; Klee, H.J. Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr. Opin. Plant Biol. 2006, 9, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.H.; Qin, X.; Zeevaart, J.A. Characterization of a novel carotenoid cleavage dioxygenase from plants. J. Biol. Chem. 2001, 276, 25208–25211. [Google Scholar] [CrossRef]
- Giberti, S.; Giovannini, D.; Forlani, G. Carotenoid cleavage in chromoplasts of white and yellow-fleshed peach varieties. J. Sci. Food Agric. 2019, 99, 1795–1803. [Google Scholar] [CrossRef]
- Simkin, A.J.; Moreau, H.; Kuntz, M.; Pagny, G.; Lin, C.; Tanksley, S.; McCarthy, J. An investigation of carotenoid biosynthesis in Coffea canephora and Coffea arabica. J. Plant Physiol. 2008, 165, 1087–1106. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.; McCarthy, J.; Petiard, V.; Lin, C.; Tanksley, S. Polynucleotides Encoding Carotenoid and Apocartenoid Biosynthetic Pathway Enzymes in Coffee. Patent WO2007028115A2, 28 August 2007. [Google Scholar]
- Simkin, A.J. Genetic Engineering for Global Food Security: Photosynthesis and Biofortification. Plants 2019, 8, 586. [Google Scholar] [CrossRef]
- Mezzomo, N.; Ferreira, S.R. Carotenoids Functionality, Sources, and Processing by Supercritical Technology: A Review. J. Chem. 2016, 2016, 16. [Google Scholar] [CrossRef]
- Cunningham, F.X.; Gantt, E. Genes and enzymes of carotenoid biosynthesis in plants. Annu. Rev. Plant Phys. 1998, 49, 557–583. [Google Scholar] [CrossRef]
- Hirschberg, J. Carotenoid biosynthesis in flowering plants. Curr. Opin. Plant Biol. 2001, 4, 210–218. [Google Scholar] [CrossRef]
- Ruban, A.V.; Lee, P.J.; Wentworth, M.; Young, A.J.; Horton, P. Determination of the stoichiometry and strength of binding of xanthophylls to the photosystem II light harvesting complexes. J. Biol. Chem. 1999, 274, 10458–10465. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V.; Young, A.J.; Pascal, A.A.; Horton, P. The Effects of Illumination on the Xanthophyll Composition of the Photosystem II Light-Harvesting Complexes of Spinach Thylakoid Membranes. Plant Physiol. 1994, 104, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Thayer, S.S.; Björkman, O. Carotenoid distribution and deepoxidation in thylakoid pigment-protein complexes from cotton leaves and bundle-sheath cells of maize. Photosynth. Res. 1992, 33, 213–225. [Google Scholar] [CrossRef]
- Bartley, G.E.; Viitanen, P.V.; Bacot, K.O.; Scolnik, P.A. A tomato gene expressed during fruit ripening encodes an enzyme of the carotenoid biosynthesis pathway. J. Biol. Chem. 1992, 267, 5036–5039. [Google Scholar] [CrossRef]
- Hugueney, P.; Römer, S.; Kuntz, M.; Camara, B. Characterization and molecular cloning of a flavoprotein catalyzing the synthesis of phytofluene and ζ-carotene in Capsicum chromoplasts. Eur. J. Biochem. 1992, 209, 399–407. [Google Scholar] [CrossRef]
- Bartley, G.E.; Ishida, B.K. Zeta-carotene desaturase from tomato. Plant Physiol. 1999, 121, 1383. [Google Scholar]
- Albrecht, M.; Klein, A.; Hugueney, P.; Sandmann, G.; Kuntz, M. Molecular cloning and functional expression in E. coli of a novel plant enzyme mediating ξ-carotene desaturation. FEBS Lett. 1995, 372, 199–202. [Google Scholar] [CrossRef]
- Chen, Y.; Li, F.; Wurtzel, E.T. Isolation and Characterization of the Z-ISO Gene Encoding a Missing Component of Carotenoid Biosynthesis in Plants. Plant Physiol. 2010, 153, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Ghisla, S.; Hirschberg, J.; Mann, V.; Beyer, P. Plant Carotene Cis-Trans Isomerase CRTISO: A new member of the FAD RED dependent flavoproteins catalysing non-redox reactions. J. Biol. Chem. 2011, 286, 8666–8676. [Google Scholar] [CrossRef]
- Isaacson, T.; Ronen, G.; Zamir, D.; Hirschberg, J. Cloning of tangerine from Tomato Reveals a Carotenoid Isomerase Essential for the Production of β-Carotene and Xanthophylls in Plants. Plant Cell 2002, 14, 333–342. [Google Scholar] [CrossRef]
- Park, H.; Kreunen, S.S.; Cuttriss, A.J.; DellaPenna, D.; Pogson, B.J. Identification of the Carotenoid Isomerase Provides Insight into Carotenoid Biosynthesis, Prolamellar Body Formation, and Photomorphogenesis. Plant Cell 2002, 14, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Carol, P.; Stevenson, D.; Bisanz, C.; Breitenbach, J.; Sandmann, G.; Mache, R.; Coupland, G.; Kuntz, M. Mutations in the Arabidopsis Gene IMMUTANS Cause a Variegated Phenotype by Inactivating a Chloroplast Terminal Oxidase Associated with Phytoene Desaturation. Plant Cell 1999, 11, 57–68. [Google Scholar] [CrossRef]
- Josse, E.-M.; Alcaraz, J.-P.; Labouré, A.-M.; Kuntz, M. In vitro characterization of a plastid terminal oxidase (PTOX). Eur. J. Biochem. 2003, 270, 3787–3794. [Google Scholar] [CrossRef]
- Kuntz, M. Plastid terminal oxidase and its biological significance. Planta 2004, 218, 896–899. [Google Scholar] [CrossRef]
- Kambakam, S.; Bhattacharjee, U.; Petrich, J.; Rodermel, S. PTOX Mediates Novel Pathways of Electron Transport in Etioplasts of Arabidopsis. Mol. Plant 2016, 9, 1240–1259. [Google Scholar] [CrossRef]
- Cunningham, F.X.; Pogson, B.; Sun, Z.; McDonald, K.A.; DellaPenna, D.; Gantt, E. Functional analysis of the beta and epsilon lycopene cyclase enzymes of Arabidopsis reveals a mechanism for control of cyclic carotenoid formation. Plant Cell 1996, 8, 1613–1626. [Google Scholar] [CrossRef] [PubMed]
- Ronen, G.; Cohen, M.; Zamir, D.; Hirschberg, J. Regulation of carotenoid biosynthesis during tomato fruit development: Expression of the gene for lycopene epsilon-cyclase is down-regulated during ripening and is elevated in the mutant Delta. Plant J. 1999, 17, 341–351. [Google Scholar] [CrossRef]
- Cunningham, F.X.; Gantt, E. One ring or two? Determination of ring number in carotenoids by lycopene ɛ-cyclases. Proc. Natl. Acad. Sci. USA 2001, 98, 2905–2910. [Google Scholar] [CrossRef]
- Partensky, F.; Hoepffner, N.; Li, W.; Ulloa, O.; Vaulot, D. Photoacclimation of Prochlorococcus sp. (Prochlorophyta) Strains Isolated from the North Atlantic and the Mediterranean Sea. Plant Physiol. 1993, 101, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Krubasik, P.; Sandmann, G. Molecular evolution of lycopene cyclases involved in the formation of carotenoids with ionone end groups. Biochem. Soc. Trans. 2000, 28, 806–810. [Google Scholar] [CrossRef]
- Hess, W.R.; Rocap, G.; Ting, C.S.; Larimer, F.; Stilwagen, S.; Lamerdin, J.; Chisholm, S.W. The photosynthetic apparatus of Prochlorococcus: Insights through comparative genomics. Photosynth. Res. 2001, 70, 53–71. [Google Scholar] [CrossRef]
- Stickforth, P.; Steiger, S.; Hess, W.R.; Sandmann, G. A novel type of lycopene ε-cyclase in the marine cyanobacterium Prochlorococcus marinus MED4. Arch. MicroBiol. 2003, 179, 409–415. [Google Scholar] [CrossRef]
- Sandmann, G. Carotenoid biosynthesis in microorganisms and plants. Eur. J. Biochem. 1994, 223, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Gantt, E.; Cunningham, F.X. Cloning and Functional Analysis of the β-Carotene Hydroxylase of Arabidopsis thaliana. J. Biol. Chem. 1996, 271, 24349–24352. [Google Scholar] [CrossRef]
- Kim, J.; DellaPenna, D. Defining the primary route for lutein synthesis in plants: The role of Arabidopsis carotenoid beta-ring hydroxylase CYP97A3. Proc. Natl. Acad. Sci. USA 2006, 103, 3474–3479. [Google Scholar] [CrossRef]
- Tian, L.; DellaPenna, D. Characterization of a second carotenoid β-hydroxylase gene from Arabidopsis and its relationship to the LUT1 locus. Plant Mol. Biol. 2001, 47, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Magallanes-Lundback, M.; Musetti, V.; DellaPenna, D. Functional Analysis of β- and ε-Ring Carotenoid Hydroxylases in Arabidopsis. Plant Cell 2003, 15, 1320–1332. [Google Scholar] [CrossRef]
- Tian, L.; DellaPenna, D. Progress in understanding the origin and functions of carotenoid hydroxylases in plants. Arch. Biochem. Biophys 2004, 430, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Musetti, V.; Kim, J.; Magallanes-Lundback, M.; DellaPenna, D. The Arabidopsis LUT1 locus encodes a member of the cytochrome P450 family that is required for carotenoid ε-ring hydroxylation activity. Proc. Natl. Acad. Sci. USA 2004, 101, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K. Carotenoid hydroxylation--P450 finally! Trends Plant Sci. 2004, 9, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Pogson, B.J.; Niyogi, K.K.; Björkman, O.; DellaPenna, D. Altered xanthophyll compositions adversely affect chlorophyll accumulation and nonphotochemical quenching in Arabidopsis mutants. Proc. Natl. Acad. Sci. USA 1998, 95, 13324–13329. [Google Scholar] [CrossRef]
- Lokstein, H.; Tian, L.; Polle, J.E.W.; DellaPenna, D. Xanthophyll biosynthetic mutants of Arabidopsis thaliana: Altered nonphotochemical quenching of chlorophyll fluorescence is due to changes in Photosystem II antenna size and stability. Biochim. Biophys. Acta Bioenerg. 2002, 1553, 309–319. [Google Scholar] [CrossRef]
- Dall’Osto, L.; Fiore, A.; Cazzaniga, S.; Giuliano, G.; Bassi, R. Different roles of alpha- and beta-branch xanthophylls in photosystem assembly and photoprotection. J. Biol. Chem. 2007, 282, 35056–35068. [Google Scholar] [CrossRef] [PubMed]
- Dall’Osto, L.; Lico, C.; Alric, J.; Giuliano, G.; Havaux, M.; Bassi, R. Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biol. 2006, 6, 32. [Google Scholar] [CrossRef]
- García-Plazaola, J.I.; Matsubara, S.; Osmond, C.B. The lutein epoxide cycle in higher plants: Its relationships to other xanthophyll cycles and possible functions. Funct. Plant Biol. 2007, 34, 759–773. [Google Scholar] [CrossRef]
- Li, Z.; Ahn, T.K.; Avenson, T.J.; Ballottari, M.; Cruz, J.A.; Kramer, D.M.; Bassi, R.; Fleming, G.R.; Keasling, J.D.; Niyogi, K.K. Lutein accumulation in the absence of zeaxanthin restores nonphotochemical quenching in the Arabidopsis thaliana npq1 mutant. Plant Cell 2009, 21, 1798–1812. [Google Scholar] [CrossRef] [PubMed]
- Jahns, P.; Wehner, A.; Paulsen, H.; Hobe, S. De-epoxidation of violaxanthin after reconstitution into different carotenoid binding sites of light-harvesting complex II. J. Biol. Chem. 2001, 276, 22154–22159. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, S.; Krause, G.H.; Aranda, J.; Virgo, A.; Beisel, K.G.; Jahns, P.; Winter, K. Sun-shade patterns of leaf carotenoid composition in 86 species of neotropical forest plants. Funct. Plant Biol. 2009, 36, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Standfuss, J.; Terwisscha van Scheltinga, A.C.; Lamborghini, M.; Kuhlbrandt, W. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 A resolution. EMBO J. 2005, 24, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Nussaume, L.; Quesada, A.; Gonneau, M.; Sotta, B.; Hugueney, P.; Frey, A.; Marion-Poll, A. Molecular identification of zeaxanthin epoxidase of Nicotiana plumbaginifolia, a gene involved in abscisic acid biosynthesis and corresponding to the ABA locus of Arabidopsis thaliana. EMBO J. 1996, 15, 2331–2342. [Google Scholar] [CrossRef]
- Bouvier, F.; d’Harlingue, A.; Hugueney, P.; Marin, E.; Marion-Poll, A.; Camara, B. Xanthophyll Biosynthesis: Cloning, expression, functional reconstitution, and regulation of β-cyclohexenyl carotenoid epoxidase from pepper (Capsicum annuum). J. Biol. Chem. 1996, 271, 28861–28867. [Google Scholar] [CrossRef]
- Esteban, R.; Moran, J.F.; Becerril, J.M.; García-Plazaola, J.I. Versatility of carotenoids: An integrated view on diversity, evolution, functional roles and environmental interactions. Environ. Exp. Bot. 2015, 119, 63–75. [Google Scholar] [CrossRef]
- Brüggemann, W.; Bergmann, M.; Nierbauer, K.-U.; Pflug, E.; Schmidt, C.; Weber, D. Photosynthesis studies on European evergreen and deciduous oaks grown under Central European climate conditions: II. Photoinhibitory and light-independent violaxanthin deepoxidation and downregulation of photosystem II in evergreen, winter-acclimated European Quercus taxa. Trees 2009, 23, 1091–1100. [Google Scholar] [CrossRef]
- Hieber, A.D.; Bugos, R.C.; Yamamoto, H.Y. Plant lipocalins: Violaxanthin de-epoxidase and zeaxanthin epoxidase. Biochim. Biophys. Acta 2000, 1482, 84–91. [Google Scholar] [CrossRef]
- Rabinowitch, H.D.; Budowski, P.; Kedar, N. Carotenoids and epoxide cycles in mature-green tomatoes. Planta 1975, 122, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, F.; D’Harlingue, A.; Backhaus, R.A.; Kumagai, M.H.; Camara, B. Identification of neoxanthin synthase as a carotenoid cyclase paralog. Eur. J. Biochem. 2000, 267, 6346–6352. [Google Scholar] [CrossRef] [PubMed]
- Al-Babili, S.; Hugueney, P.; Schledz, M.; Welsch, R.; Frohnmeyer, H.; Laule, O.; Beyer, P. Identification of a novel gene coding for neoxanthin synthase from Solanum tuberosum. FEBS Lett. 2000, 485, 168–172. [Google Scholar] [CrossRef]
- Kuntz, M.; Chen, H.C.; Simkin, A.J.; Römer, S.; Shipton, C.A.; Drake, R.; Schuch, W.; Bramley, P.M. Upregulation of two ripening-related genes from a non-climacteric plant (pepper) in a transgenic climacteric plant (tomato). Plant J. 1998, 13, 351–361. [Google Scholar] [CrossRef]
- Bouvier, F.; Hugueney, P.; D’Harlingue, A.; Kuntz, M.; Camara, B. Xanthophyll biosynthesis in chromoplasts: Isolation and molecular cloning of an enzyme catalyzing the conversion of 5,6-epoxycarotenoid into ketocarotenoid. Plant J. 1994, 6, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V.; Kuntz, M.; Camara, B.; Palloix, A. The capsanthin-capsorubin synthase gene: A candidate gene for the y locus controlling the red fruit colour in pepper. Plant Mol. Biol. 1998, 36, 785–789. [Google Scholar] [CrossRef]
- Ronen, G.; Carmel-Goren, L.; Zamir, D.; Hirschberg, J. An alternative pathway to β-carotene formation in plant chromoplasts discovered by map-based cloning of Beta and old-gold color mutations in tomato. Proc. Natl. Acad. Sci. USA 2000, 97, 11102–11107. [Google Scholar] [CrossRef]
- Hugueney, P.; Badillo, A.; Chen, H.-C.; Klein, A.; Hirschberg, J.; Camara, B.; Kuntz, M. Metabolism of cyclic carotenoids: A model for the alteration of this biosynthetic pathway in Capsicum annuum chromoplasts. Plant J. 1995, 8, 417–424. [Google Scholar] [CrossRef]
- Fujisawa, M.; Misawa, N. Enrichment of carotenoids in flaxseed by introducing a bacterial phytoene synthase gene. Methods Mol. Biol. 2010, 643, 201–211. [Google Scholar] [CrossRef]
- Fujisawa, M.; Watanabe, M.; Choi, S.K.; Teramoto, M.; Ohyama, K.; Misawa, N. Enrichment of carotenoids in flaxseed (Linum usitatissimum) by metabolic engineering with introduction of bacterial phytoene synthase gene crtB. J. Biosci. Bioeng. 2008, 105, 636–641. [Google Scholar] [CrossRef]
- Cong, L.; Wang, C.; Chen, L.; Liu, H.; Yang, G.; He, G. Expression of phytoene synthase1 and Carotene Desaturase crtI Genes Result in an Increase in the Total Carotenoids Content in Transgenic Elite Wheat (Triticum aestivum L.). J. Agric. Food Chem. 2009, 57, 8652–8660. [Google Scholar] [CrossRef]
- Che, P.; Zhao, Z.-Y.; Glassman, K.; Dolde, D.; Hu, T.X.; Jones, T.J.; Gruis, D.F.; Obukosia, S.; Wambugu, F.; Albertsen, M.C. Elevated vitamin E content improves all-trans β-carotene accumulation and stability in biofortified sorghum. Proc. Natl. Acad. Sci. USA 2016, 113, 11040–11045. [Google Scholar] [CrossRef] [PubMed]
- Lipkie, T.E.; De Moura, F.F.; Zhao, Z.-Y.; Albertsen, M.C.; Che, P.; Glassman, K.; Ferruzzi, M.G. Bioaccessibility of Carotenoids from Transgenic Provitamin A Biofortified Sorghum. J. Agric. Food Chem. 2013, 61, 5764–5771. [Google Scholar] [CrossRef] [PubMed]
- Shewmaker, C.K.; Sheehy, J.A.; Daley, M.; Colburn, S.; Ke, D.Y. Seed-specific overexpression of phytoene synthase: Increase in carotenoids and other metabolic effects. Plant J. Cell Mol. Biol. 1999, 20, 401–412X. [Google Scholar] [CrossRef]
- Ye, X.; Al-Babili, S.; Kloti, A.; Zhang, J.; Lucca, P.; Beyer, P.; Potrykus, I. Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 2000, 287, 303–305. [Google Scholar] [CrossRef]
- Paine, J.A.; Shipton, C.A.; Chaggar, S.; Howells, R.M.; Kennedy, M.J.; Vernon, G.; Wright, S.Y.; Hinchliffe, E.; Adams, J.L.; Silverstone, A.L.; et al. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat. Biotechnol. 2005, 23, 482–487. [Google Scholar] [CrossRef]
- Al-Babili, S.; Hoa, T.T.; Schaub, P. Exploring the potential of the bacterial carotene desaturase CrtI to increase the beta-carotene content in Golden Rice. J. Exp. Bot. 2006, 57, 1007–1014. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diretto, G.; Al-Babili, S.; Tavazza, R.; Papacchioli, V.; Beyer, P.; Giuliano, G. Metabolic engineering of potato carotenoid content through tuber-specific overexpression of a bacterial mini-pathway. PLoS ONE 2007, 2, e350. [Google Scholar] [CrossRef] [PubMed]
- Beyene, G.; Solomon, F.R.; Chauhan, R.D.; Gaitan-Solis, E.; Narayanan, N.; Gehan, J.; Siritunga, D.; Stevens, R.L.; Jifon, J.; Van Eck, J.; et al. Provitamin A biofortification of cassava enhances shelf life but reduces dry matter content of storage roots due to altered carbon partitioning into starch. Plant Biotechnol. J. 2017, 16, 1186–1200. [Google Scholar] [CrossRef]
- Ducreux, L.J.M.; Morris, W.L.; Hedley, P.E.; Shepherd, T.; Davies, H.V.; Millam, S.; Taylor, M.A. Metabolic engineering of high carotenoid potato tubers containing enhanced levels of β-carotene and lutein. J. Exp. Bot. 2004, 56, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Römer, S.; Fraser, P.D.; Kiano, J.W.; Shipton, C.A.; Misawa, N.; Schuch, W.; Bramley, P.M. Elevation of the provitamin A content of transgenic tomato plants. Nat. Biotechnol. 2000, 18, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.D.; Romer, S.; Shipton, C.A.; Mills, P.B.; Kiano, J.W.; Misawa, N.; Drake, R.G.; Schuch, W.; Bramley, P.M. Evaluation of transgenic tomato plants expressing an additional phytoene synthase in a fruit-specific manner. Proc. Natl. Acad. Sci. USA 2002, 99, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.D.; Enfissi, E.M.; Halket, J.M.; Truesdale, M.R.; Yu, D.; Gerrish, C.; Bramley, P.M. Manipulation of phytoene levels in tomato fruit: Effects on isoprenoids, plastids, and intermediary metabolism. Plant Cell 2007, 19, 3194–3211. [Google Scholar] [CrossRef]
- McQuinn, R.P.; Wong, B.; Giovannoni, J.J. AtPDS overexpression in tomato: Exposing unique patterns of carotenoid self-regulation and an alternative strategy for the enhancement of fruit carotenoid content. Plant Biotechnol. J. 2018, 16, 482–494. [Google Scholar] [CrossRef]
- McQuinn, R.P.; Gapper, N.E.; Gray, A.G.; Zhong, S.; Tohge, T.; Fei, Z.; Fernie, A.R.; Giovannoni, J.J. Manipulation of ZDS in tomato exposes carotenoid- and ABA-specific effects on fruit development and ripening. Plant Biotechnol. J. 2020, 18, 2210–2224. [Google Scholar] [CrossRef]
- D’Ambrosio, C.; Giorio, G.; Marino, I.; Merendino, A.; Petrozza, A.; Salfi, L.; Stigliani, A.L.; Cellini, F. Virtually complete conversion of lycopene into β-carotene in fruits of tomato plants transformed with the tomato lycopene β-cyclase (tlcy-b) cDNA. Plant Sci. 2004, 166, 207–214. [Google Scholar] [CrossRef]
- Morris, W.L.; Ducreux, L.J.M.; Hedden, P.; Millam, S.; Taylor, M.A. Overexpression of a bacterial 1-deoxy-D-xylulose 5-phosphate synthase gene in potato tubers perturbs the isoprenoid metabolic network: Implications for the control of the tuber life cycle. J. Exp. Bot. 2006, 57, 3007–3018. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Parrott, W.A.; Hildebrand, D.F.; Berg, R.H.; Cooksey, A.; Pendarvis, K.; He, Y.; McCarthy, F.; Herman, E.M. Transgenic soya bean seeds accumulating β-carotene exhibit the collateral enhancements of oleate and protein content traits. Plant Biotechnol. J. 2015, 13, 590–600. [Google Scholar] [CrossRef]
- Paul, J.Y.; Khanna, H.; Kleidon, J.; Hoang, P.; Geijskes, J.; Daniells, J.; Zaplin, E.; Rosenberg, Y.; James, A.; Mlalazi, B.; et al. Golden bananas in the field: Elevated fruit pro-vitamin A from the expression of a single banana transgene. Plant Biotechnol. J. 2017, 15, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Zhu, C.; Farre, G.; Ramessar, K.; Bassie, L.; Breitenbach, J.; Perez Conesa, D.; Ros, G.; Sandmann, G.; Capell, T.; et al. Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc. Natl. Acad. Sci. USA 2009, 106, 7762–7767. [Google Scholar] [CrossRef] [PubMed]
- Aluru, M.; Xu, Y.; Guo, R.; Wang, Z.; Li, S.; White, W.; Wang, K.; Rodermel, S. Generation of transgenic maize with enhanced provitamin A content. J. Exp. Bot. 2008, 59, 3551–3562. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Capell, T.; Berman, J.; Medina, V.; Sandmann, G.; Christou, P.; Zhu, C. Bottlenecks in carotenoid biosynthesis and accumulation in rice endosperm are influenced by the precursor-product balance. Plant Biotechnol. J. 2016, 14, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Van Eck, J.; Zhou, X.; Lopez, A.B.; O’Halloran, D.M.; Cosman, K.M.; Conlin, B.J.; Paolillo, D.J.; Garvin, D.F.; Vrebalov, J.; et al. The cauliflower Or gene encodes a DnaJ cysteine-rich domain-containing protein that mediates high levels of beta-carotene accumulation. Plant Cell 2006, 18, 3594–3605. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, M.; Sun, Z.; Yuan, H.; Zeng, S.; Thannhauser, T.W.; Vrebalov, J.; Ma, Q.; Xu, Y.; Fei, Z.; Van Eck, J.; et al. Ectopic expression of ORANGE promotes carotenoid accumulation and fruit development in tomato. Plant Biotechnol. J. 2019, 17, 33–49. [Google Scholar] [CrossRef]
- Li, L.; Yang, Y.; Xu, Q.; Owsiany, K.; Welsch, R.; Chitchumroonchokchai, C.; Lu, S.; Van Eck, J.; Deng, X.-X.; Failla, M.; et al. The Or Gene Enhances Carotenoid Accumulation and Stability During Post-Harvest Storage of Potato Tubers. Mol. Plant 2012, 5, 339–352. [Google Scholar] [CrossRef]
- Lopez, A.B.; Van Eck, J.; Conlin, B.J.; Paolillo, D.J.; O’Neill, J.; Li, L. Effect of the cauliflower Or transgene on carotenoid accumulation and chromoplast formation in transgenic potato tubers. J. Exp. Bot. 2008, 59, 213–223. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.S.; Jung, Y.J.; Kim, S.H.; Ji, C.Y.; Wang, Z.; Jeong, J.C.; Lee, H.S.; Lee, S.Y.; Kwak, S.S. Orange protein has a role in phytoene synthase stabilization in sweetpotato. Sci. Rep. 2016, 6, 33563. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.; Zorrilla-López, U.; Medina, V.; Farré, G.; Sandmann, G.; Capell, T.; Christou, P.; Zhu, C. The Arabidopsis ORANGE (AtOR) gene promotes carotenoid accumulation in transgenic corn hybrids derived from parental lines with limited carotenoid pools. Plant Cell Rep. 2017, 36, 933–945. [Google Scholar] [CrossRef]
- Zhou, X.; Welsch, R.; Yang, Y.; Álvarez, D.; Riediger, M.; Yuan, H.; Fish, T.; Liu, J.; Thannhauser, T.W.; Li, L. Arabidopsis Or proteins are the major posttranscriptional regulators of phytoene synthase in controlling carotenoid biosynthesis. Proc. Natl. Acad. Sci. USA 2015, 112, 3558–3563. [Google Scholar] [CrossRef] [PubMed]
- Chayut, N.; Yuan, H.; Ohali, S.; Meir, A.; Sa’ar, U.; Tzuri, G.; Zheng, Y.; Mazourek, M.; Gepstein, S.; Zhou, X.; et al. Distinct Mechanisms of the ORANGE Protein in Controlling Carotenoid Flux. Plant Physiol. 2017, 173, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Osorio, C.E. The Role of Orange Gene in Carotenoid Accumulation: Manipulating Chromoplasts Toward a Colored Future. Front. Plant Sci. 2019, 10, 1235. [Google Scholar] [CrossRef]
- Park, S.C.; Kim, S.H.; Park, S.; Lee, H.U.; Lee, J.S.; Park, W.S.; Ahn, M.J.; Kim, Y.H.; Jeong, J.C.; Lee, H.S.; et al. Enhanced accumulation of carotenoids in sweetpotato plants overexpressing IbOr-Ins gene in purple-fleshed sweetpotato cultivar. Plant Physiol. Biochem. 2015, 86, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Jorge, S.; Ha, S.-H.; Magallanes-Lundback, M.; Gilliland, L.U.; Zhou, A.; Lipka, A.E.; Nguyen, Y.-N.; Angelovici, R.; Lin, H.; Cepela, J.; et al. Carotenoid cleavage dioxygenase4 Is a Negative Regulator of β-Carotene Content in Arabidopsis Seeds. Plant Cell 2013, 25, 4812–4826. [Google Scholar] [CrossRef]
- Frusciante, S.; Diretto, G.; Bruno, M.; Ferrante, P.; Pietrella, M.; Prado-Cabrero, A.; Rubio-Moraga, A.; Beyer, P.; Gomez-Gomez, L.; Al-Babili, S.; et al. Novel carotenoid cleavage dioxygenase catalyzes the first dedicated step in saffron crocin biosynthesis. Proc. Natl. Acad. Sci. USA 2014, 111, 12246–12251. [Google Scholar] [CrossRef]
- Rubio, A.; Rambla, J.L.; Santaella, M.; Gomez, M.D.; Orzaez, D.; Granell, A.; Gomez-Gomez, L. Cytosolic and plastoglobule-targeted carotenoid dioxygenases from Crocus sativus are both involved in beta-ionone release. J. Biol. Chem. 2008, 283, 24816–24825. [Google Scholar] [CrossRef] [PubMed]
- Falchi, R.; Vendramin, E.; Zanon, L.; Scalabrin, S.; Cipriani, G.; Verde, I.; Vizzotto, G.; Morgante, M. Three distinct mutational mechanisms acting on a single gene underpin the origin of yellow flesh in peach. Plant J. 2013, 76, 175–187. [Google Scholar] [CrossRef]
- Ohmiya, A.; Kishimoto, S.; Aida, R.; Yoshioka, S.; Sumitomo, K. Carotenoid Cleavage Dioxygenase (CmCCD4a) Contributes to White Color Formation in Chrysanthemum Petals. Plant Physiol. 2006, 142, 1193–1201. [Google Scholar] [CrossRef]
- Campbell, R.; Ducreux, L.J.M.; Morris, W.L.; Morris, J.A.; Suttle, J.C.; Ramsay, G.; Bryan, G.J.; Hedley, P.E.; Taylor, M.A. The metabolic and developmental roles of carotenoid cleavage dioxygenase4 from potato. Plant Physiol. 2010, 154, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Ohmiya, A.; Sumitomo, K.; Aida, R. Yellow jimba: Suppression of carotenoid cleavage dioxygenase (CmCCD4a) expression turns white Chrysanthemum petals yellow. J. Jpn. Soc. Hortic. Sci. 2009, 78, 450–455. [Google Scholar] [CrossRef][Green Version]
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P.; Benítez-González, A.; Stinco, C.M. A comprehensive review on the colorless carotenoids phytoene and phytofluene. Arch. Biochem. Biophys. 2015, 572, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P.; Stinco, C.M. The colourless carotenoids phytoene and phytofluene: From dietary sources to their usefulness for the functional foods and nutricosmetics industries. J. Food Compos. Anal. 2018, 67, 91–103. [Google Scholar] [CrossRef]
- Olson, J.A. Provitamin A Function of Carotenoids: The Conversion of β-Carotene into Vitamin A. J. Nutr. 1989, 119, 105–108. [Google Scholar] [CrossRef]
- West, C.E.; Rombout, J.H.; van der Zijpp, A.J.; Sijtsma, S.R. Vitamin A and immune function. Proc. Nutr. Soc. 1991, 50, 251–262. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A. Vitamin A: Biomarkers of nutrition for development. Am. J. Clin. Nutr. 2011, 94, 658S–665S. [Google Scholar] [CrossRef] [PubMed]
- Rando, R.R. The chemistry of vitamin A and vision. Angew. Chem. Int. 1990, 29, 461–480. [Google Scholar] [CrossRef]
- von Lintig, J.; Vogt, K. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J. Biol. Chem. 2000, 275, 11915–11920. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J. Hidden hunger: Approaches to tackling micronutrient deficiencies. In Nourishing Millions: Stories of Change in Nutrition; Gillespie, S., Hodge, J., Yosef, S., Pandya-Lorch, R., Eds.; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2016; pp. 35–46. [Google Scholar]
- Gannon, B.; Kaliwile, C.; Arscott, S.A.; Schmaelzle, S.; Chileshe, J.; Kalungwana, N.; Mosonda, M.; Pixley, K.V.; Masi, C.; Tanumihardjo, S.A. Biofortified orange maize is as efficacious as a vitamin A supplement in Zambian children even in the presence of high liver reserves of vitamin A: A community-based, randomized placebo-controlled trial. Am. J. Clin. Nutr. 2014, 100, 1541–1550. [Google Scholar] [CrossRef]
- Palmer, A.C.; Healy, K.; Barffour, M.A.; Siamusantu, W.; Chileshe, J.; Schulze, K.J.; West, K.P., Jr.; Labrique, A.B. Provitamin A Carotenoid-Biofortified Maize Consumption Increases Pupillary Responsiveness among Zambian Children in a Randomized Controlled Trial. J. Nutr. 2016, 146, 2551–2558. [Google Scholar] [CrossRef] [PubMed]
- Regis, E. Golden Rice: The Imperiled Birth of a GMO Superfood; Johns Hopkins University Press: Baltimore, MD, USA, 2019. [Google Scholar]
- Liu, X.; Song, M.; Gao, Z.; Cai, X.; Dixon, W.; Chen, X.; Cao, Y.; Xiao, H. Stereoisomers of Astaxanthin Inhibit Human Colon Cancer Cell Growth by Inducing G2/M Cell Cycle Arrest and Apoptosis. J. Agric. Food Chem. 2016, 64, 7750–7759. [Google Scholar] [CrossRef] [PubMed]
- Shareck, M.; Rousseau, M.-C.; Koushik, A.; Siemiatycki, J.; Parent, M.-E. Inverse Association between Dietary Intake of Selected Carotenoids and Vitamin C and Risk of Lung Cancer. Front. Oncol. 2017, 7, 23. [Google Scholar] [CrossRef]
- Ansari, M.; Ansari, S. Lycopene and prostate cancer. Future Oncol. 2005, 1, 425–430. [Google Scholar] [CrossRef]
- Rafi, M.M.; Kanakasabai, S.; Gokarn, S.V.; Krueger, E.G.; Bright, J.J. Dietary Lutein Modulates Growth and Survival Genes in Prostate Cancer Cells. J. Med. Food 2015, 18, 173–181. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Kushiro, M.; Zhang, H.; Sugawara, T.; Miyashita, K.; Nagao, A. Carotenoids affect proliferation of human prostate cancer cells. J. Nutr. 2001, 131, 3303–3306. [Google Scholar] [CrossRef] [PubMed]
- Mayne, S.T.; Cartmel, B.; Lin, H.; Zheng, T.; Goodwin, W.J., Jr. Low plasma lycopene concentration is associated with increased mortality in a cohort of patients with prior oral, pharynx or larynx cancers. J. Am. Coll. Nutr. 2004, 23, 34–42. [Google Scholar] [CrossRef]
- De Waart, F.; Schouten, E.; Stalenhoef, A.; Kok, F. Serum carotenoids, α-tocopherol and mortality risk in a prospective study among Dutch elderly. Int. J. Epidemiol. 2001, 30, 136–143. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, Y.; Li, Y.; Lu, K.; Shen, Y.; Guo, Y.; Qi, Q.; Wang, M.; Zhang, S. NrF2/ARE and NF-κB pathway regulation may be the mechanism for lutein inhibition of human breast cancer cell. Future Oncol. 2018, 14, 719–726. [Google Scholar] [CrossRef]
- Gloria, N.F.; Soares, N.; Brand, C.; Oliveira, F.L.; Borojevic, R.; Teodoro, A.J. Lycopene and beta-carotene induce cell-cycle arrest and apoptosis in human breast cancer cell lines. Anticancer Res. 2014, 34, 1377–1386. [Google Scholar]
- Upadhyaya, K.R.; Radha, K.S.; Madhyastha, H.K. Cell cycle regulation and induction of apoptosis by beta-carotene in U937 and HL-60 leukemia cells. J. Biochem. Mol. Biol. 2007, 40, 1009–1015. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nara, E.; Hayashi, H.; Kotake, M.; Miyashita, K.; Nagao, A. Acyclic carotenoids and their oxidation mixtures inhibit the growth of HL-60 human promyelocytic leukemia cells. Nutr. Cancer 2001, 39, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Niranjana, R.; Gayathri, R.; Nimish Mol, S.; Sugawara, T.; Hirata, T.; Miyashita, K.; Ganesan, P. Carotenoids modulate the hallmarks of cancer cells. J. Funct. Foods 2015, 18, 968–985. [Google Scholar] [CrossRef]
- Cha, J.H.; Kim, W.K.; Ha, A.W.; Kim, M.H.; Chang, M.J. Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr. Res. Pr. 2017, 11, 90–96. [Google Scholar] [CrossRef]
- Lee, H.S.; Jung, J.I.; Kang, Y.H.; YoonPark, J.H.; Khachik, F. Effect of Lycopene on the Insulin-like Growth Factor-I Receptor Signaling Pathway in Human Colon Cancer HT-29 Cells. J. Korean Soc. Food Sci. Nutr. 2003, 32, 437–443. [Google Scholar] [CrossRef]
- Huang, R.F.; Wei, Y.J.; Inbaraj, B.S.; Chen, B.H. Inhibition of colon cancer cell growth by nanoemulsion carrying gold nanoparticles and lycopene. Int. J. Nanomed. 2015, 10, 2823–2846. [Google Scholar] [CrossRef]
- Sahin, K.; Tuzcu, M.; Sahin, N.; Akdemir, F.; Ozercan, I.; Bayraktar, S.; Kucuk, O. Inhibitory effects of combination of lycopene and genistein on 7,12- dimethyl benz(a)anthracene-induced breast cancer in rats. Nutr. Cancer 2011, 63, 1279–1286. [Google Scholar] [CrossRef]
- Sahin, K.; Yenice, E.; Tuzcu, M.; Orhan, C.; Mizrak, C.; Ozercan, I.H.; Sahin, N.; Yilmaz, B.; Bilir, B.; Ozpolat, B.; et al. Lycopene Protects Against Spontaneous Ovarian Cancer Formation in Laying Hens. J. Cancer Prev. 2018, 23, 25–36. [Google Scholar] [CrossRef]
- Thies, F.; Mills, L.M.; Moir, S.; Masson, L.F. Cardiovascular benefits of lycopene: Fantasy or reality? Proc. Nutr. Soc. 2017, 76, 122–129. [Google Scholar] [CrossRef]
- Alvi, S.S.; Iqbal, D.; Ahmad, S.; Khan, M.S. Molecular rationale delineating the role of lycopene as a potent HMG-CoA reductase inhibitor: In vitro and in silico study. Nat. Prod. Res. 2016, 30, 2111–2114. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, V.; Rodríguez-Rodríguez, R.; Martínez-Garza, Ú.; Rosell-Cardona, C.; Lamuela-Raventós, R.M.; Marrero, P.F.; Haro, D.; Relat, J. Mediterranean Tomato-Based Sofrito Sauce Improves Fibroblast Growth Factor 21 (FGF21) Signaling in White Adipose Tissue of Obese ZUCKER Rats. Mol. Nutr. Food Res. 2018, 62, 1700606. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar] [CrossRef] [PubMed]
- Costa-Rodrigues, J.; Pinho, O.; Monteiro, P.R.R. Can lycopene be considered an effective protection against cardiovascular disease? Food Chem. 2018, 245, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis 2017, 257, 100–108. [Google Scholar] [CrossRef]
- Chung, R.W.S.; Leanderson, P.; Lundberg, A.K.; Jonasson, L. Lutein exerts anti-inflammatory effects in patients with coronary artery disease. Atherosclerosis 2017, 262, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Taguchi, C.; Saita, E.; Suzuki-Sugihara, N.; Nishiyama, H.; Wang, W.; Masuda, Y.; Kondo, K. Additional consumption of one egg per day increases serum lutein plus zeaxanthin concentration and lowers oxidized low-density lipoprotein in moderately hypercholesterolemic males. Food Res. Int. 2017, 99, 944–949. [Google Scholar] [CrossRef]
- Leermakers, E.T.; Darweesh, S.K.; Baena, C.P.; Moreira, E.M.; Melo van Lent, D.; Tielemans, M.J.; Muka, T.; Vitezova, A.; Chowdhury, R.; Bramer, W.M.; et al. The effects of lutein on cardiometabolic health across the life course: A systematic review and meta-analysis1,2. Am. J. Clin. Nutr. 2016, 103, 481–494. [Google Scholar] [CrossRef]
- Christensen, K.; Gleason, C.E.; Mares, J.A. Dietary carotenoids and cognitive function among US adults, NHANES 2011–2014. Nutr. Neurosci. 2020, 23, 554–562. [Google Scholar] [CrossRef]
- Krishnaraj, R.N.; Kumari, S.S.S.; Mukhopadhyay, S.S. Antagonistic molecular interactions of photosynthetic pigments with molecular disease targets: A new approach to treat AD and ALS. J. Recept. Signal. Transduct. 2016, 36, 67–71. [Google Scholar] [CrossRef]
- Min, J.; Min, K. Serum Lycopene, Lutein and Zeaxanthin, and the Risk of Alzheimer’s Disease Mortality in Older Adults. Dement. Geriatr. Cogn. Disord. 2014, 37, 246–256. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Carotenoids: How Effective Are They to Prevent Age-Related Diseases? Molecules 2019, 24, 1801. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Madrid, R.; Carballo-Uicab, V.M.; Cárdenas-Conejo, Y.; Aguilar-Espinosa, M.; Siva, R. 1—Overview of carotenoids and beneficial effects on human health. In Carotenoids: Properties, Processing and Applications; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–40. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharm. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.-C.; Joseph, L.M.; Deng, W.-T.; Liu, L.; Li, Q.-B.; Cline, K.; McCarty, D.R. Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J. 2003, 35, 44–56. [Google Scholar] [CrossRef]
- Hou, X.; Rivers, J.; León, P.; McQuinn, R.P.; Pogson, B.J. Synthesis and Function of Apocarotenoid Signals in Plants. Trends Plant Sci. 2016, 21, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; Kuntz, M.; Moreau, H.; McCarthy, J. Carotenoid profiling and the expression of carotenoid biosynthetic genes in developing coffee grain. Plant Physiol. Biochem. 2010, 48, 434–442. [Google Scholar] [CrossRef]
- Buttery, R.G.; Teranishi, R.; Ling, L.C.; Flath, R.A.; Stern, D.J. Quantitative studies on origins of fresh tomato aroma volatiles. J. Agric. Food Chem. 1988, 36, 1247–1250. [Google Scholar] [CrossRef]
- Bouvier, F.; Isner, J.-C.; Dogbo, O.; Camara, B. Oxidative tailoring of carotenoids: A prospect towards novel functions in plants. Trends Plant Sci. 2005, 10, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.C.; Schwartz, S.H.; Zeevaart, J.A.D.; McCarty, D.R. Genetic control of abscisic acid biosynthesis in maize. Proc. Natl. Acad. Sci. USA 1997, 94, 12235–12240. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.H.; Tan, B.C.; Gage, D.A.; Zeevaart, J.A.D.; McCarty, D.R. Specific Oxidative Cleavage of Carotenoids by VP14 of Maize. Science 1997, 276, 1872–1874. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Hsu, P.K.; Dubeaux, G.; Takahashi, Y.; Schroeder, J.I. Signaling mechanisms in abscisic acid-mediated stomatal closure. Plant J. 2021, 105, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Aliche, E.B.; Screpanti, C.; De Mesmaeker, A.; Munnik, T.; Bouwmeester, H.J. Science and application of strigolactones. New Phytol. 2020, 227, 1001–1011. [Google Scholar] [CrossRef]
- Waters, M.T.; Gutjahr, C.; Bennett, T.; Nelson, D.C. Strigolactone Signaling and Evolution. Annu. Rev. Plant Biol. 2017, 68, 291–322. [Google Scholar] [CrossRef]
- Dun, E.A.; Brewer, P.B.; Beveridge, C.A. Strigolactones: Discovery of the elusive shoot branching hormone. Trends Plant Sci. 2009, 14, 364–372. [Google Scholar] [CrossRef]
- Wang, J.Y.; Haider, I.; Jamil, M.; Fiorilli, V.; Saito, Y.; Mi, J.; Baz, L.; Kountche, B.A.; Jia, K.-P.; Guo, X.; et al. The apocarotenoid metabolite zaxinone regulates growth and strigolactone biosynthesis in rice. Nat. Commun. 2019, 10, 810. [Google Scholar] [CrossRef]
- Froehlich, J.; Itoh, A.; Howe, G. Tomato allene oxide synthase and fatty acid hydroperoxide lyase, two cytochrome P450s involved in oxylipin metabolism, are targeted to different membranes of chloroplast envelope. Plant Physiol. 2001, 125, 306–317. [Google Scholar] [CrossRef]
- Block, M.A.; Dorne, A.; Joyard, J.; Douce, R. Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts. J. Biol. Chem. 1983, 258, 13281–13286. [Google Scholar] [CrossRef]
- Markwell, J.; Bruce, B.; Keegstra, K. Isolation of a carotenoid-containing sub-membrane particle from the chloroplastic envelope outer membrane of pea (Pisum sativum). J. Biol. Chem. 1992, 267, 13933–13937. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, G.; Gu, T.; Ding, J.; Li, Y. Bioinformatic and expression analyses on carotenoid dioxygenase genes in fruit development and abiotic stress responses in Fragaria vesca. Mol. Genet. Genom. 2017, 292, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Huang, N.; Jiang, S.; Li, K.; Zhuang, Z.; Wang, Q.; Lu, S. Cloning and functional characterization of two carotenoid cleavage dioxygenases for ionone biosynthesis in chili pepper (Capsicum annuum L.) fruits. Sci. Hortic. 2021, 288, 110368. [Google Scholar] [CrossRef]
- Yahyaa, M.; Bar, E.; Dubey, N.K.; Meir, A.; Davidovich-Rikanati, R.; Hirschberg, J.; Aly, R.; Tholl, D.; Simon, P.W.; Tadmor, Y.; et al. Formation of Norisoprenoid Flavor Compounds in Carrot (Daucus carota L.) Roots: Characterization of a Cyclic-Specific Carotenoid Cleavage Dioxygenase 1 Gene. J. Agric. Food Chem. 2013, 61, 12244–12252. [Google Scholar] [CrossRef]
- Ilg, A.; Beyer, P.; Al-Babili, S. Characterization of the rice carotenoid cleavage dioxygenase 1 reveals a novel route for geranial biosynthesis. FEBS J. 2009, 276, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Ibdah, M.; Azulay, Y.; Portnoy, V.; Wasserman, B.; Bar, E.; Meir, A.; Burger, Y.; Hirschberg, J.; Schaffer, A.A.; Katzir, N.; et al. Functional characterization of CmCCD1, a carotenoid cleavage dioxygenase from melon. Phytochemistry 2006, 67, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Nawade, B.; Shaltiel-Harpaz, L.; Yahyaa, M.; Bosamia, T.C.; Kabaha, A.; Kedoshim, R.; Zohar, M.; Isaacson, T.; Ibdah, M. Analysis of apocarotenoid volatiles during the development of Ficus carica fruits and characterization of carotenoid cleavage dioxygenase genes. Plant Sci. 2020, 290, 110292. [Google Scholar] [CrossRef]
- Timmins, J.J.B.; Kroukamp, H.; Paulsen, I.T.; Pretorius, I.S. The Sensory Significance of Apocarotenoids in Wine: Importance of Carotenoid Cleavage Dioxygenase 1 (CCD1) in the Production of β-Ionone. Molecules 2020, 25, 2779. [Google Scholar] [CrossRef]
- Mathieu, S.; Terrier, N.; Procureur, J.; Bigey, F.; Günata, Z. A Carotenoid Cleavage Dioxygenase from Vitis vinifera L.: Functional characterization and expression during grape berry development in relation to C13-norisoprenoid accumulation. J. Exp. Bot. 2005, 56, 2721–2731. [Google Scholar] [CrossRef]
- Lashbrooke, J.G.; Young, P.R.; Dockrall, S.J.; Vasanth, K.; Vivier, M.A. Functional characterisation of three members of the Vitis vinifera L. carotenoid cleavage dioxygenase gene family. BMC Plant Biol. 2013, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-T.; Jia, L.-D.; Duan, M.-Z.; Chen, X.; Qiao, C.-L.; Ma, J.-Q.; Zhang, C.; Jing, F.-Y.; Zhang, S.-S.; Yang, B.; et al. Genome-wide identification and expression profiling of the carotenoid cleavage dioxygenase (CCD) gene family in Brassica napus L. PLoS ONE 2020, 15, e0238179. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.-C.; Horváth, G.; Molnár, P.; Turcsi, E.; Deli, J.; Schrader, J.; Sandmann, G.; Schmidt, H.; Schwab, W. Substrate promiscuity of RdCCD1, a carotenoid cleavage oxygenase from Rosa damascena. Phytochemistry 2009, 70, 457–464. [Google Scholar] [CrossRef]
- Vogel, J.T.; Tan, B.-C.; McCarty, D.R.; Klee, H.J. The Carotenoid Cleavage Dioxygenase 1 Enzyme Has Broad Substrate Specificity, Cleaving Multiple Carotenoids at Two Different Bond Positions. J. Biol. Chem. 2008, 283, 11364–11373. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Kurtzer, R.; Eisenreich, W.; Schwab, W. The carotenase AtCCD1 from Arabidopsis thaliana is a dioxygenase. J. Biol. Chem. 2006, 281, 9845–9851. [Google Scholar] [CrossRef]
- Mathieu, S.; Bigey, F.; Procureur, J.; Terrier, N.; Günata, Z. Production of a recombinant carotenoid cleavage dioxygenase from grape and enzyme assay in water-miscible organic solvents. Biotechnol. Lett. 2007, 29, 837–841. [Google Scholar] [CrossRef]
- Suzuki, M.; Matsumoto, S.; Mizoguchi, M.; Hirata, S.; Takagi, K.; Hashimoto, I.; Yamano, Y.; Ito, M.; Fleischmann, P.; Winterhalter, P.; et al. Identification of (3S, 9R)- and (3S, 9S)-megastigma-6,7-dien-3,5,9-triol 9-O-beta-D-glucopyranosides as damascenone progenitors in the flowers of Rosa damascena. Mill. Biosci. Biotechnol. Biochem. 2002, 66, 2692–2697. [Google Scholar] [CrossRef]
- Bezman, Y.; Bilkis, I.; Winterhalter, P.; Fleischmann, P.; Rouseff, R.L.; Baldermann, S.; Naim, M. Thermal oxidation of 9′-cis-neoxanthin in a model system containing peroxyacetic acid leads to the potent odorant beta-damascenone. J. Agric. Food Chem. 2005, 53, 9199–9206. [Google Scholar] [CrossRef] [PubMed]
- Skouroumounis, G.; Massy-Westropp, R.; Sefton, M.; Williams, P. Precursors of damascenone in fruit juices. Tetrahedron. Lett. 1992, 33, 3533–3536. [Google Scholar] [CrossRef]
- Carballo-Uicab, V.M.; Cárdenas-Conejo, Y.; Vallejo-Cardona, A.A.; Aguilar-Espinosa, M.; Rodríguez-Campos, J.; Serrano-Posada, H.; Narváez-Zapata, J.A.; Vázquez-Flota, F.; Rivera-Madrid, R. Isolation and functional characterization of two dioxygenases putatively involved in bixin biosynthesis in annatto (Bixa orellana L.). PeerJ 2019, 7, e7064. [Google Scholar] [CrossRef]
- Cárdenas-Conejo, Y.; Carballo-Uicab, V.; Lieberman, M.; Aguilar-Espinosa, M.; Comai, L.; Rivera-Madrid, R. De novo transcriptome sequencing in Bixa orellana to identify genes involved in methylerythritol phosphate, carotenoid and bixin biosynthesis. BMC Genom. 2015, 16, 877. [Google Scholar] [CrossRef]
- Rodríguez-Ávila, N.L.; Narváez-Zapata, J.A.; Ramírez-Benítez, J.E.; Aguilar-Espinosa, M.L.; Rivera-Madrid, R. Identification and expression pattern of a new carotenoid cleavage dioxygenase gene member from Bixa orellana. J. Exp. Bot. 2011, 62, 5385–5395. [Google Scholar] [CrossRef] [PubMed]
- Meng, N.; Yan, G.-L.; Zhang, D.; Li, X.-Y.; Duan, C.-Q.; Pan, Q.-H. Characterization of two Vitis vinifera carotenoid cleavage dioxygenases by heterologous expression in Saccharomyces cerevisiae. Mol. Biol. Rep. 2019, 46, 6311–6323. [Google Scholar] [CrossRef]
- Simkin, A.J.; Miettinen, K.; Claudel, P.; Burlat, V.; Guirimand, G.; Courdavault, V.; Papon, N.; Meyer, S.; Godet, S.; St-Pierre, B.; et al. Characterization of the plastidial geraniol synthase from Madagascar periwinkle which initiates the monoterpenoid branch of the alkaloid pathway in internal phloem associated parenchyma. Phytochemistry 2013, 85, 36–43. [Google Scholar] [CrossRef]
- Lewis, W.D.; Lilly, S.; Jones, K.L. Lymphoma: Diagnosis and Treatment. Am. Fam. Physician 2020, 101, 34–41. [Google Scholar]
- Floss, D.S.; Schliemann, W.; Schmidt, J.; Strack, D.; Walter, M.H. RNA Interference-Mediated Repression of MtCCD1 in Mycorrhizal Roots of Medicago truncatula Causes Accumulation of C27 Apocarotenoids, Shedding Light on the Functional Role of CCD1. Plant Physiol. 2008, 148, 1267–1282. [Google Scholar] [CrossRef]
- Floss, D.S.; Walter, M.H. Role of carotenoid cleavage dioxygenase 1 (CCD1) in apocarotenoid biogenesis revisited. Plant Signal. Behav. 2009, 4, 172–175. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Scott, J.W.; Shewmaker, C.K.; Schuch, W. Flavor Trivia and Tomato Aroma: Biochemistry and Possible Mechanisms for Control of Important Aroma Components. Hort. Sci. 2000, 35, 1013–1022. [Google Scholar] [CrossRef]
- Buttery, R.G.; Teranishi, R.; Ling, L.C.; Turnbaugh, J.G. Quantitative and sensory studies on tomato paste volatiles. J. Agric. Food Chem. 1990, 38, 336–340. [Google Scholar] [CrossRef]
- Waché, Y.; Bosser-DeRatuld, A.; Lhuguenot, J.-C.; Belin, J.-M. Effect of cis/trans Isomerism of β-Carotene on the Ratios of Volatile Compounds Produced during Oxidative Degradation. J. Agric. Food Chem. 2003, 51, 1984–1987. [Google Scholar] [CrossRef]
- Simkin, A.J.; Zhu, C.; Kuntz, M.; Sandmann, G. Light-dark regulation of carotenoid biosynthesis in pepper (Capsicum annuum) leaves. J. Plant Physiol. 2003, 160, 439–443. [Google Scholar] [CrossRef]
- Fraser, P.D.; Truesdale, M.R.; Bird, C.R.; Schuch, W.; Bramley, P.M. Carotenoid Biosynthesis during Tomato Fruit Development (Evidence for Tissue-Specific Gene Expression). Plant Physiol. 1994, 105, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.-C.; Molnár, P.; Schwab, W. Cloning and functional characterization of carotenoid cleavage dioxygenase 4 genes. J. Exp. Bot. 2009, 60, 3011–3022. [Google Scholar] [CrossRef] [PubMed]
- Rottet, S.; Devillers, J.; Glauser, G.; Douet, V.; Besagni, C.; Kessler, F. Identification of Plastoglobules as a Site of Carotenoid Cleavage. Front. Plant Sci. 2016, 7, 1855. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.X.; Gantt, E. A portfolio of plasmids for identification and analysis of carotenoid pathway enzymes: Adonis aestivalis as a case study. Photosynth. Res. 2007, 92, 245–259. [Google Scholar] [CrossRef]
- Brandi, F.; Bar, E.; Mourgues, F.; Horváth, G.; Turcsi, E.; Giuliano, G.; Liverani, A.; Tartarini, S.; Lewinsohn, E.; Rosati, C. Study of ‘Redhaven’ peach and its white-fleshed mutant suggests a key role of CCD4 carotenoid dioxygenase in carotenoid and norisoprenoid volatile metabolism. BMC Plant Biol. 2011, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Zhang, L.; Matsuta, A.; Matsutani, K.; Yamawaki, K.; Yahata, M.; Wahyudi, A.; Motohashi, R.; Kato, M. Enzymatic Formation of β-Citraurin from β-Cryptoxanthin and Zeaxanthin by Carotenoid Cleavage Dioxygenase4 in the Flavedo of Citrus Fruit. Plant Physiol. 2013, 163, 682–695. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Alquézar, B.; Alós, E.; Medina, V.; Carmona, L.; Bruno, M.; Al-Babili, S.; Zacarías, L. A novel carotenoid cleavage activity involved in the biosynthesis of Citrus fruit-specific apocarotenoid pigments. J. Exp. Bot. 2013, 64, 4461–4478. [Google Scholar] [CrossRef]
- Kato, M.; Ikoma, Y.; Matsumoto, H.; Sugiura, M.; Hyodo, H.; Yano, M. Accumulation of Carotenoids and Expression of Carotenoid Biosynthetic Genes during Maturation in Citrus Fruit. Plant Physiol. 2004, 134, 824–837. [Google Scholar] [CrossRef]
- Rodrigo, M.-J.; Marcos, J.F.; Zacarías, L. Biochemical and Molecular Analysis of Carotenoid Biosynthesis in Flavedo of Orange (Citrus sinensis L.) during Fruit Development and Maturation. J. Agric. Food Chem. 2004, 52, 6724–6731. [Google Scholar] [CrossRef]
- Rey, F.; Zacarías, L.; Rodrigo, M.J. Carotenoids, Vitamin C, and Antioxidant Capacity in the Peel of Mandarin Fruit in Relation to the Susceptibility to Chilling Injury during Postharvest Cold Storage. Antioxidants 2020, 9, 1296. [Google Scholar] [CrossRef]
- Yamagishi, M.; Kishimoto, S.; Nakayama, M. Carotenoid composition and changes in expression of carotenoid biosynthetic genes in tepals of Asiatic hybrid lily. Plant Breed. 2010, 129, 100–107. [Google Scholar] [CrossRef]
- Ahrazem, O.; Rubio-Moraga, A.; Berman, J.; Capell, T.; Christou, P.; Zhu, C.; Gómez-Gómez, L. The carotenoid cleavage dioxygenase CCD2 catalysing the synthesis of crocetin in spring crocuses and saffron is a plastidial enzyme. New Phytol. 2016, 209, 650–663. [Google Scholar] [CrossRef] [PubMed]
- Eugster, C.H.; Hürlimann, H.; Leuenberger, H.J. Crocetindialdehyd und Crocetinhalbaldehyd als Blütenfarbstoffe von Jacquinia angustifolia. Helv. Chim. Acta 1969, 52, 89–90. [Google Scholar] [CrossRef]
- Tandon, J.S.; Katti, S.B.; Rüedi, P.; Eugster, C.H. Crocetin-dialdehyde from Coleus forskohlii BRIQ., Labiatae. Helv. Chim. Acta 1979, 62, 2706–2707. [Google Scholar] [CrossRef]
- Zhong, Y.; Pan, X.; Wang, R.; Xu, J.; Guo, J.; Yang, T.; Zhao, J.; Nadeem, F.; Liu, X.; Shan, H.; et al. ZmCCD10a Encodes a Distinct Type of Carotenoid Cleavage Dioxygenase and Enhances Plant Tolerance to Low Phosphate. Plant Physiol. 2020, 184, 374–392. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.H.; Fester, T.; Strack, D. Arbuscular mycorrhizal fungi induce the non-mevalonate methylerythritol phosphate pathway of isoprenoid biosynthesis correlated with accumulation of the ‘yellow pigment’ and other apocarotenoids. Plant J. 2000, 21, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.H.; Floss, D.S.; Strack, D. Apocarotenoids: Hormones, mycorrhizal metabolites and aroma volatiles. Planta 2010, 232, 1–17. [Google Scholar] [CrossRef]
- Buttery, R.G.; Seifert, R.M.; Guadagni, D.G.; Ling, L.C. Characterization of additional volatile components of tomato. J. Agric. Food Chem. 1971, 19, 524–529. [Google Scholar] [CrossRef]
- Buttery, R.G.; Teranishi, R.; Ling, L.C. Fresh tomato aroma volatiles: A quantitative study. J. Agric. Food Chem. 1987, 35, 540–544. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Nisperos-Carriedo, M.O.; Baker, R.; Scott, J.W. Quantitative analysis of flavor parameters in six Florida tomato cultivars (Lycopersicon esculentum Mill). J. Agric. Food Chem. 1991, 39, 1135–1140. [Google Scholar] [CrossRef]
- Kjeldsen, F.; Christensen, L.P.; Edelenbos, M. Changes in Volatile Compounds of Carrots (Daucus carota L.) during Refrigerated and Frozen Storage. J. Agric. Food Chem. 2003, 51, 5400–5407. [Google Scholar] [CrossRef] [PubMed]
- Lutz, A.; Winterhalter, P. Bio-oxidative cleavage of carotenoids: Important route to physiological active plant constituents. Tetrahedron Lett. 1992, 33, 5169–5172. [Google Scholar] [CrossRef]
- Winterhalter, P.; Schreier, P. The generation of norisoprenoid volatiles in starfruit (Averrhoa carambola L.): A review. Food Rev. Int. 1995, 11, 237–254. [Google Scholar] [CrossRef]
- Mahattanatawee, K.; Rouseff, R.; Valim, M.F.; Naim, M. Identification and Aroma Impact of Norisoprenoids in Orange Juice. J. Agric. Food Chem. 2005, 53, 393–397. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Sitrit, Y.; Bar, E.; Azulay, Y.; Meir, A.; Zamir, D.; Tadmor, Y. Carotenoid pigmentation affects the volatile composition of tomato and watermelon fruits, as revealed by comparative genetic analyses. J. Agric. Food Chem. 2005, 53, 3142–3148. [Google Scholar] [CrossRef]
- Oyedeji, A.O.; Ekundayo, O.; Koenig, W.A. Essential Oil Composition of Lawsonia inermis L. Leaves from Nigeria. J. Essent. Oil Res. 2005, 17, 403–404. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Sendra, E.; Pérez-Alvarez, J.A.; Fernández-López, J.; Amensour, M.; Abrini, J. Identification of Flavonoid Content and Chemical Composition of the Essential Oils of Moroccan Herbs: Myrtle (Myrtus communis L.), Rockrose (Cistus ladanifer L.) and Montpellier cistus (Cistus monspeliensis L.). J. Essent. Oil Res. 2011, 23, 1–9. [Google Scholar] [CrossRef]
- Cooper, C.M.; Davies, N.W.; Menary, R.C. C-27 Apocarotenoids in the Flowers of Boronia megastigma (Nees). J. Agric. Food Chem. 2003, 51, 2384–2389. [Google Scholar] [CrossRef] [PubMed]
- Paparella, A.; Shaltiel-Harpaza, L.; Ibdah, M. β-Ionone: Its Occurrence and Biological Function and Metabolic Engineering. Plants 2021, 10, 754. [Google Scholar] [CrossRef]
- Demole, E.; Enggist, P.; Saeuberli, U.; Stoll, M.; Kováts, E.S. Structure et synthèse de la damascénone (triméthyl-2,6,6-trans-crotonoyl-1-cyclohexadiène-1,3), constituant odorant de l’essence de rose bulgare (rosa damascena Mill. Helv. Chim. Acta 1970, 53, 541–551. [Google Scholar] [CrossRef]
- Renold, W.; Naf-Muller, R.; Keller, U.; Wilhelm, B.; Ohloff, G. Investigation of tea aroma. Part 1. New volatile black tea constituents. Helv. Chim. Acta 1974, 57, 1301–1308. [Google Scholar] [CrossRef]
- Kumazawa, K.; Masuda, H. Change in the flavor of black tea drink during heat processing. J. Agric. Food Chem. 2001, 49, 3304–3309. [Google Scholar] [CrossRef]
- Buttery, R.G.; Teranishi, R.; Ling, L.C. Identification of damascenone in tomato volatiles. Chem. Ind. 1988, 7, 238. [Google Scholar]
- Roberts, D.; Morehai, A.P.; Acree, T.E. Detection and partialcharacterization of eight â-damascenone precursors in apples (Malus domestica Borkh. Cv. Empire). J. Agric. Food Chem. 1994, 42, 345–349. [Google Scholar] [CrossRef]
- Lin, J.; Rouseff, R.L.; Barros, S.; Naim, M. Aroma Composition Changes in Early Season Grapefruit Juice Produced from Thermal Concentration. J. Agric. Food Chem. 2002, 50, 813–819. [Google Scholar] [CrossRef]
- Winterhalter, P.; Gök, R. TDN and β-Damascenone: Two Important Carotenoid Metabolites in Wine. In Carotenoid Cleavage Products; American Chemical Society: Washington, DC, USA, 2013; Volume 1134, pp. 125–137. [Google Scholar]
- Sefton, M.A.; Skouroumounis, G.K.; Elsey, G.M.; Taylor, D.K. Occurrence, Sensory Impact, Formation, and Fate of Damascenone in Grapes, Wines, and Other Foods and Beverages. J. Agric. Food Chem. 2011, 59, 9717–9746. [Google Scholar] [CrossRef]
- Schreier, P.; Drawert, F.; Schmid, M. Changes in the composition of neutral volatile components during the production of apple brandy. J. Sci. Food Agric. 1978, 29, 728–736. [Google Scholar] [CrossRef]
- Chevance, F.; Guyot-Declerck, C.; Dupont, J.; Collin, S. Investigation of the beta-damascenone level in fresh and aged commercial beers. J. Agric. Food Chem. 2002, 50, 3818–3821. [Google Scholar] [CrossRef]
- Guido, L.F.; Carneiro, J.R.; Santos, J.R.; Almeida, P.J.; Rodrigues, J.A.; Barros, A.A. Simultaneous determination of E-2-nonenal and beta-damascenone in beer by reversed-phase liquid chromatography with UV detection. J. Chromatogr. A 2004, 1032, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Poisson, L.; Schieberle, P. Characterization of the Most Odor-Active Compounds in an American Bourbon Whisky by Application of the Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2008, 56, 5813–5819. [Google Scholar] [CrossRef] [PubMed]
- Czerny, M.; Grosch, W. Potent odorants of raw Arabica coffee. Their changes during roasting. J. Agric. Food Chem. 2000, 48, 868–872. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Scott, J.W.; Einstein, M.A.; Malundo, T.M.M.; Carr, B.T.; Shewfelt, R.L.; Tandon, K.S. Relationship between Sensory and Instrumental Analysis for Tomato Flavor. J. Am. Soc. Hortic. Sci. 1998, 123, 906. [Google Scholar] [CrossRef]
- Tandon, K.; Baldwin, E.A.; Shewfelt, R. Aroma perception of individual volatile compounds in fresh tomatoes (Lycopersicon esculentum, Mill.) as affected by the medium of evaluation. Postharvest Biol. Technol. 2000, 20, 261–268. [Google Scholar] [CrossRef]
- Pino, J.A.; Mesa, J.; Muñoz, Y.; Martí, M.P.; Marbot, R. Volatile Components from Mango (Mangifera indica L.) Cultivars. J. Agric. Food Chem. 2005, 53, 2213–2223. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and Distribution of Floral Scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Baldermann, S.; Kato, M.; Kurosawa, M.; Kurobayashi, Y.; Fujita, A.; Fleischmann, P.; Watanabe, N. Functional characterization of a carotenoid cleavage dioxygenase 1 and its relation to the carotenoid accumulation and volatile emission during the floral development of Osmanthus fragrans Lour. J. Exp. Bot. 2010, 61, 2967–2977. [Google Scholar] [CrossRef]
- Dendy, D.A.V. The assay of annatto preparations by thin-layer chromatography. J. Sci. Food Agric. 1966, 17, 75–76. [Google Scholar] [CrossRef]
- Scotter, M. The chemistry and analysis of annatto food colouring: A review. Food Addit. Contam. Part A 2009, 26, 1123–1145. [Google Scholar] [CrossRef]
- Tibodeau, J.D.; Isham, C.R.; Bible, K.C. Annatto constituent cis-bixin has selective antimyeloma effects mediated by oxidative stress and associated with inhibition of thioredoxin and thioredoxin reductase. Antioxid. Redox Signal. 2010, 13, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, D.M.; Li, J.; Deng, S.H.; Liu, T.; Zhang, T.; He, M.; Zhao, Y.Y.; Xu, Y. Bixin Attenuates Experimental Autoimmune Encephalomyelitis by Suppressing TXNIP/NLRP3 Inflammasome Activity and Activating NRF2 Signaling. Front. Immunol. 2020, 11, 593368. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Júnior, R.G.; Bonnet, A.; Braconnier, E.; Groult, H.; Prunier, G.; Beaugeard, L.; Grougnet, R.; da Silva Almeida, J.R.G.; Ferraz, C.A.A.; Picot, L. Bixin, an apocarotenoid isolated from Bixa orellana L., sensitizes human melanoma cells to dacarbazine-induced apoptosis through ROS-mediated cytotoxicity. Food Chem. Toxicol. 2019, 125, 549–561. [Google Scholar] [CrossRef]
- Alavizadeh, S.H.; Hosseinzadeh, H. Bioactivity assessment and toxicity of crocin: A comprehensive review. Food Chem. Toxicol. 2014, 64, 65–80. [Google Scholar] [CrossRef]
- Noorbala, A.A.; Akhondzadeh, S.; Tahmacebi-Pour, N.; Jamshidi, A.H. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: A double-blind, randomized pilot trial. J. Ethnopharmacol. 2005, 97, 281–284. [Google Scholar] [CrossRef]
- Magesh, V.; DurgaBhavani, K.; Senthilnathan, P.; Rajendran, P.; Sakthisekaran, D. In vivo protective effect of crocetin on benzo(a)pyrene-induced lung cancer in Swiss albino mice. Phytother. Res. 2009, 23, 533–539. [Google Scholar] [CrossRef]
- Aung, H.H.; Wang, C.Z.; Ni, M.; Fishbein, A.; Mehendale, S.R.; Xie, J.T.; Shoyama, C.Y.; Yuan, C.S. Crocin from Crocus sativus possesses significant anti-proliferation effects on human colorectal cancer cells. Exp. Oncol. 2007, 29, 175–180. [Google Scholar]
- Khavari, A.; Bolhassani, A.; Alizadeh, F.; Bathaie, S.Z.; Balaram, P.; Agi, E.; Vahabpour, R. Chemo-immunotherapy using saffron and its ingredients followed by E7-NT (gp96) DNA vaccine generates different anti-tumor effects against tumors expressing the E7 protein of human papillomavirus. Arch. Virol. 2015, 160, 499–508. [Google Scholar] [CrossRef]
- Moshiri, M.; Vahabzadeh, M.; Hosseinzadeh, H. Clinical Applications of Saffron (Crocus sativus) and its Constituents: A Review. Drug Res. 2015, 65, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Pashirzad, M.; Shafiee, M.; Avan, A.; Ryzhikov, M.; Fiuji, H.; Bahreyni, A.; Khazaei, M.; Soleimanpour, S.; Hassanian, S.M. Therapeutic potency of crocin in the treatment of inflammatory diseases: Current status and perspective. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Singh, G.; Kaur, H.; Saxena, A.K.; Ishar, M.P.S. Synthesis of β-ionone derived chalcones as potent antimicrobial agents. Bioorg. Med. Chem. Lett. 2012, 22, 6343–6346. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, S.N.; Bhat, B.A.; Pandey, S.; Chandra, N.; Gupta, S. Chemotherapy of leishmaniasis. Part VII: Synthesis and bioevaluation of substituted terpenyl pyrimidines. Eur. J. Med. Chem. 2007, 42, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.G.; Wyllie, S.G.; Markham, J.L.; Leach, D.N. The role of structure and molecular properties of terpenoids in determining their antimicrobial activity. Flavour Fragr. J. 1999, 14, 322–332. [Google Scholar] [CrossRef]
- Wilson, D.M.; Gueldner, R.C.; Mckinney, J.K.; Lievsay, R.H.; Evans, B.D.; Hill, R.A. Effect of β-lonone onaspergillus flavus andaspergillus parasiticus growth, sporulation, morphology and aflatoxin production. J. Am. Oil Chem. Soc. 1981, 58, A959–A961. [Google Scholar] [CrossRef]
- Ansari, M.; Emami, S. β-Ionone and its analogs as promising anticancer agents. Eur. J. Med. Chem. 2016, 123, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.-W.; Wang, K.; Chang, X.-X.; Jin, F.-F.; Wang, Q.; Jiang, X.-F.; Liu, J.-R.; Wu, Y.-H.; Yang, C. Beta-ionone-inhibited proliferation of breast cancer cells by inhibited COX-2 activity. Arch. Toxicol. 2019, 93, 2993–3003. [Google Scholar] [CrossRef] [PubMed]
- Balbi, A.; Anzaldi, M.; Mazzei, M.; Miele, M.; Bertolotto, M.; Ottonello, L.; Dallegri, F. Synthesis and biological evaluation of novel heterocyclic ionone-like derivatives as anti-inflammatory agents. Bioorg. Med. Chem. 2006, 14, 5152–5160. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Liu, T.; Chen, J.; Yang, Z.; Xu, S.; Fan, Y.; Zeng, J.; Chen, Y.; Ma, Z.; Gao, Y.; et al. Activation of PSGR with β-ionone suppresses prostate cancer progression by blocking androgen receptor nuclear translocation. Cancer Lett. 2019, 453, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Elson, C.E. Apoptosis and Cell-Cycle Arrest in Human and Murine Tumor Cells Are Initiated by Isoprenoids. J. Nutr. 1999, 129, 804–813. [Google Scholar] [CrossRef]
- Janakiram, N.B.; Cooma, I.; Mohammed, A.; Steele, V.E.; Rao, C.V. β-Ionone inhibits colonic aberrant crypt foci formation in rats, suppresses cell growth, and induces retinoid X receptor-α in human colon cancer cells. Mol. Cancer Ther. 2008, 7, 181–190. [Google Scholar] [CrossRef]
- Dong, H.-W.; Zhang, S.; Sun, W.-G.; Liu, Q.; Ibla, J.C.; Soriano, S.G.; Han, X.-H.; Liu, L.-X.; Li, M.-S.; Liu, J.-R. β-Ionone arrests cell cycle of gastric carcinoma cancer cells by a MAPK pathway. Arch. Toxicol. 2013, 87, 1797–1808. [Google Scholar] [CrossRef]
- Liu, J.-R.; Sun, X.-R.; Dong, H.-W.; Sun, C.-H.; Sun, W.-G.; Chen, B.-Q.; Song, Y.-Q.; Yang, B.-F. β-Ionone suppresses mammary carcinogenesis, proliferative activity and induces apoptosis in the mammary gland of the Sprague-Dawley rat. Int. J. Cancer 2008, 122, 2689–2698. [Google Scholar] [CrossRef]
- Aloum, L.; Alefishat, E.; Adem, A.; Petroianu, G. Ionone Is More than a Violet’s Fragrance: A Review. Molecules 2020, 25, 5822. [Google Scholar] [CrossRef]
- Luetragoon, T.; Pankla Sranujit, R.; Noysang, C.; Thongsri, Y.; Potup, P.; Suphrom, N.; Nuengchamnong, N.; Usuwanthim, K. Anti-Cancer Effect of 3-Hydroxy-β-Ionone Identified from Moringa oleifera Lam. Leaf on Human Squamous Cell Carcinoma 15 Cell Line. Molecules 2020, 25, 3563. [Google Scholar] [CrossRef]
- Neuhaus, E.M.; Zhang, W.; Gelis, L.; Deng, Y.; Noldus, J.; Hatt, H. Activation of an Olfactory Receptor Inhibits Proliferation of Prostate Cancer Cells. J. Biol. Chem. 2009, 284, 16218–16225. [Google Scholar] [CrossRef]
- Sanz, G.; Leray, I.; Dewaele, A.; Sobilo, J.; Lerondel, S.; Bouet, S.; Grébert, D.; Monnerie, R.; Pajot-Augy, E.; Mir, L.M. Promotion of Cancer Cell Invasiveness and Metastasis Emergence Caused by Olfactory Receptor Stimulation. PLoS ONE 2014, 9, e85110. [Google Scholar] [CrossRef]
- Scannerini, S.; Bonfante-Fasolo, P. Unusual plasties in an endomycorrhizal root. Can. J. Bot. 1977, 55, 2471–2474. [Google Scholar] [CrossRef]
- Maier, W.; Hammer, K.; Dammann, U.; Schulz, B.; Strack, D. Accumulation of sesquiterpenoid cyclohexenone derivatives induced by an arbuscular mycorrhizal fungus in members of the Poaceae. Planta 1997, 202, 36–42. [Google Scholar] [CrossRef]
- Fiorilli, V.; Vannini, C.; Ortolani, F.; Garcia-Seco, D.; Chiapello, M.; Novero, M.; Domingo, G.; Terzi, V.; Morcia, C.; Bagnaresi, P.; et al. Omics approaches revealed how arbuscular mycorrhizal symbiosis enhances yield and resistance to leaf pathogen in wheat. Sci. Rep. 2018, 8, 9625. [Google Scholar] [CrossRef]
- Zouari, I.; Salvioli, A.; Chialva, M.; Novero, M.; Miozzi, L.; Tenore, G.C.; Bagnaresi, P.; Bonfante, P. From root to fruit: RNA-Seq analysis shows that arbuscular mycorrhizal symbiosis may affect tomato fruit metabolism. BMC Genom. 2014, 15, 221. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Porcel, R.; Azcón, C.; Aroca, R. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: New challenges in physiological and molecular studies. J. Exp. Bot. 2012, 63, 4033–4044. [Google Scholar] [CrossRef]
- Maier, W.; Peipp, H.; Schmidt, J.; Wray, V.; Strack, D. Levels of a Terpenoid Glycoside (Blumenin) and Cell Wall-Bound Phenolics in Some Cereal Mycorrhizas. Plant Physiol. 1995, 109, 465–470. [Google Scholar] [CrossRef]
- Maier, W.; Schmidt, J.; Nimtz, M.; Wray, V.; Strack, D. Secondary products in mycorrhizal roots of tobacco and tomato. Phytochemistry 2000, 54, 473–479. [Google Scholar] [CrossRef]
- Fiorilli, V.; Wang, J.Y.; Bonfante, P.; Lanfranco, L.; Al-Babili, S. Apocarotenoids: Old and New Mediators of the Arbuscular Mycorrhizal Symbiosis. Front. Plant Sci. 2019, 10, 1186. [Google Scholar] [CrossRef]
- Fester, T.; Maier, W.; Strack, D. Accumulation of secondary compounds in barley and wheat roots in response to inoculation with an arbuscular mycorrhizal fungus and co-inoculation with rhizosphere bacteria. Mycorrhiza 1999, 8, 241–246. [Google Scholar] [CrossRef]
- Wang, M.; Schäfer, M.; Li, D.; Halitschke, R.; Dong, C.; McGale, E.; Paetz, C.; Song, Y.; Li, S.; Dong, J.; et al. Blumenols as shoot markers of root symbiosis with arbuscular mycorrhizal fungi. Elife 2018, 7, e37093. [Google Scholar] [CrossRef]
- Mikhlin, E.D.; Radina, V.P.; Dmitrovskii, A.A.; Blinkova, L.P.; Butova, L.G. Antifungal and antimicrobic activity of β-ionone and vitamin A derivatives. Prikl. Biokhim. Mikrobiol. 1983, 19, 795–803. (In Russian) [Google Scholar] [PubMed]
- Utama, I.M.; Wills, R.B.; Ben-Yehoshua, S.; Kuek, C. In vitro efficacy of plant volatiles for inhibiting the growth of fruit and vegetable decay microorganisms. J. Agric. Food Chem. 2002, 50, 6371–6377. [Google Scholar] [CrossRef]
- Schiltz, P. Action inhibitrice de la β-ionone au cours du développement de Peronospora tabacina. Ann. Tab. Sect. 2 1974, 11, 207–216. [Google Scholar]
- Salt, S.D.; Tuzun, S.; Kuć, J. Effects of β-ionone and abscisic acid on the growth of tobacco and resistance to blue mold. Mimicry of effects of stem infection by Peronospora tabacina Adam. Physiol. Mol. Plant Pathol. 1986, 28, 287–297. [Google Scholar] [CrossRef]
- Cáceres, L.A.; Lakshminarayan, S.; Yeung, K.K.; McGarvey, B.D.; Hannoufa, A.; Sumarah, M.W.; Benitez, X.; Scott, I.M. Repellent and Attractive Effects of α-, β-, and Dihydro-β- Ionone to Generalist and Specialist Herbivores. J. Chem. Ecol. 2016, 42, 107–117. [Google Scholar] [CrossRef]
- Gruber, M.Y.; Xu, N.; Grenkow, L.; Li, X.; Onyilagha, J.; Soroka, J.J.; Westcott, N.D.; Hegedus, D.D. Responses of the Crucifer Flea Beetle to Brassica Volatiles in an Olfactometer. Environ. Entomol. 2009, 38, 1467–1479. [Google Scholar] [CrossRef]
- Faria, L.R.R.; Zanella, F.C.V. Beta-ionone attracts Euglossa mandibularis (Hymenoptera, Apidae) males in western Paraná forests. Rev. Bras. Entomol. 2015, 59, 260–264. [Google Scholar] [CrossRef]
- Halloran, S.; Collignon, R.M.; Mcelfresh, J.S.; Millar, J.G. Fuscumol and Geranylacetone as Pheromone Components of Californian Longhorn Beetles (Coleoptera: Cerambycidae) in the Subfamily Spondylidinae. Environ. Entomol. 2018, 47, 1300–1305. [Google Scholar] [CrossRef]
- Keesey, I.W.; Knaden, M.; Hansson, B.S. Olfactory specialization in Drosophila suzukii supports an ecological shift in host preference from rotten to fresh fruit. J. Chem. Ecol. 2015, 41, 121–128. [Google Scholar] [CrossRef]
- Bolton, L.G.; Piñero, J.C.; Barrett, B.A. Electrophysiological and Behavioral Responses of Drosophila suzukii (Diptera: Drosophilidae) Towards the Leaf Volatile β-cyclocitral and Selected Fruit-Ripening Volatiles. Environ. Entomol. 2019, 48, 1049–1055. [Google Scholar] [CrossRef]
- Murata, M.; Kobayashi, T.; Seo, S. α-Ionone, an Apocarotenoid, Induces Plant Resistance to Western Flower Thrips, Frankliniella occidentalis, Independently of Jasmonic Acid. Molecules 2019, 25, 17. [Google Scholar] [CrossRef]
- Quiroz, A.; Pettersson, J.; Pickett, J.A.; Wadhams, L.J.; Niemeyer, H.M. Semiochemicals mediating spacing behaviour of bird cherry-oat aphid, Rhopalosiphum padi, feeding on cereals. J. Chem. Ecol. 1997, 23, 2599–2607. [Google Scholar] [CrossRef]
- Du, Y.; Poppy, G.M.; Powell, W.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Identification of Semiochemicals Released During Aphid Feeding That Attract Parasitoid Aphidius ervi. J. Chem. Ecol. 1998, 24, 1355–1368. [Google Scholar] [CrossRef]
- Wei, S.; Hannoufa, A.; Soroka, J.; Xu, N.; Li, X.; Zebarjadi, A.; Gruber, M. Enhanced β-ionone emission in Arabidopsis over-expressing AtCCD1 reduces feeding damage in vivo by the crucifer flea beetle. Environ. Entomol. 2011, 40, 1622–1630. [Google Scholar] [CrossRef]
- Dickinson, A.J.; Lehner, K.; Mi, J.; Jia, K.-P.; Mijar, M.; Dinneny, J.; Al-Babili, S.; Benfey, P.N. β-Cyclocitral is a conserved root growth regulator. Proc. Natl. Acad. Sci. USA 2019, 116, 10563–10567. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Ksas, B.; Havaux, M. Decoding β-Cyclocitral-Mediated Retrograde Signaling Reveals the Role of a Detoxification Response in Plant Tolerance to Photooxidative Stress. Plant Cell 2018, 30, 2495–2511. [Google Scholar] [CrossRef]
- Ramel, F.; Birtic, S.; Ginies, C.; Soubigou-Taconnat, L.; Triantaphylidès, C.; Havaux, M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 5535–5540. [Google Scholar] [CrossRef]
- Shumbe, L.; D’Alessandro, S.; Shao, N.; Chevalier, A.; Ksas, B.; Bock, R.; Havaux, M. METHYLENE BLUE SENSITIVITY 1 (MBS1) is required for acclimation of Arabidopsis to singlet oxygen and acts downstream of β-cyclocitral. Plant Cell Environ. 2017, 40, 216–226. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Havaux, M. Sensing β-carotene oxidation in photosystem II to master plant stress tolerance. New Phytol. 2019, 223, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. An endogenous growth inhibitor, 3-hydroxy-β-ionone. I. Its role in light-induced growth inhibition of hypocotyls of Phaseolus vulgaris. Physiol. Plant. 1992, 86, 583–586. [Google Scholar] [CrossRef]
- Kato-noguchi, H.; Kosemura, S.; Yamamura, S.; Hasegawa, K. A growth inhibitor, R-(−)-3-hydroxy-β-ionone, from lightgrown shoots of a dwarf cultivar of Phaseolus vulgaris. Phytochemistry 1993, 33, 553–555. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Seki, T. Allelopathy of the moss Rhynchostegium pallidifolium and 3-hydroxy-β-ionone. Plant Signal. Behav. 2010, 5, 702–704. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raines, C.A.; Cavanagh, A.P.; Simkin, A.J. Chapter 9. Improving carbon fixation. In Photosynthesis in Action, 1st ed.; Ruban, A., Murchie, E., Foyer, C., Eds.; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Simkin, A.J.; Faralli, M.; Ramamoorthy, S.; Lawson, T. Photosynthesis in non-foliar tissues: Implications for yield. Plant J. 2020, 101, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; Lopez-Calcagno, P.E.; Raines, C.A. Feeding the world: Improving photosynthetic efficiency for sustainable crop production. J. Experimantal. Bot. 2019, 70, 1119–1140. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Simkin, A.J.; Kelly, G.; Granot, D. Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour. New Phytol. 2014, 203, 1064–1081. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Vialet-Chabrand, S. Speedy stomata, photosynthesis and plant water use efficiency. New Phytol. 2019, 221, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.S.A.; Vialet-Chabrand, S.R.M.; Lawson, T. Diurnal Variation in Gas Exchange: The Balance between Carbon Fixation and Water Loss. Plant Physiol. 2017, 174, 614–623. [Google Scholar] [CrossRef]
- Vialet-Chabrand, S.; Matthews, J.S.; Simkin, A.J.; Raines, C.A.; Lawson, T. Importance of Fluctuations in Light on Plant Photosynthetic Acclimation. Plant Physiol. 2017, 173, 2163–2179. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified Crops Generated by Breeding, Agronomy, and Transgenic Approaches Are Improving Lives of Millions of People around the World. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef]
- Aglawe, S.B.; Barbadikar, K.M.; Mangrauthia, S.K.; Madhav, M.S. New breeding technique “genome editing” for crop improvement: Applications, potentials and challenges. 3 Biotech. 2018, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Georges, F.; Ray, H. Genome editing of crops: A renewed opportunity for food security. GM Crop. Food 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Exposito-Rodriguez, M.; Laissue, P.P.; Lopez-Calcagno, P.E.; Mullineaux, P.M.; Raines, C.A.; Simkin, A.J. Development of pGEMINI, a plant gateway destination vector allowing the simultaneous integration of two cDNA via a single LR-clonase reaction. Plants 2017, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Engler, C.; Gruetzner, R.; Kandzia, R.; Marillonnet, S. Golden gate shuffling: A one-pot DNA shuffling method based on type IIs restriction enzymes. PLoS ONE 2009, 4, e5553. [Google Scholar] [CrossRef]
- Engler, C.; Kandzia, R.; Marillonnet, S. A one pot, one step, precision cloning method with high throughput capability. PLoS ONE 2008, 3, e3647. [Google Scholar] [CrossRef] [PubMed]
- Engler, C.; Youles, M.; Gruetzner, R.; Ehnert, T.M.; Werner, S.; Jones, J.D.; Patron, N.J.; Marillonnet, S. A golden gate modular cloning toolbox for plants. ACS Synth. Biol. 2014, 3, 839–843. [Google Scholar] [CrossRef]
- Marillonnet, S.; Werner, S. Assembly of multigene constructs using golden gate cloning. In Glyco-Engineering: Methods and Protocols; Castilho, A., Ed.; Springer: New York, NY, USA, 2015; pp. 269–284. [Google Scholar] [CrossRef]
- Alotaibi, S.S.; Sparks, C.A.; Parry, M.A.J.; Simkin, A.J.; Raines, C.A. Identification of Leaf Promoters for Use in Transgenic Wheat. Plants 2018, 7, 27. [Google Scholar] [CrossRef]
- Mukherjee, S.; Stasolla, C.; Brule-Babel, A.; Ayele, B.T. Isolation and characterization of rubisco small subunit gene promoter from common wheat (Triticum aestivum L.). Plant Signal. Behav. 2015, 10, e989033. [Google Scholar] [CrossRef]
- Alotaibi, S.S.; Alyassi, H.; Alshehawi, A.; Gaber, A.; Hassan, M.M.; Aljuaid, B.S.; Simkin, A.J.; Raines, C.A. Functional analysis of SBPase gene promoter in transgenic wheat under different growth conditions. Biotechnology 2019, 1, 15–23. [Google Scholar]
- Simkin, A.J.; Qian, T.; Caillet, V.; Michoux, F.; Ben Amor, M.; Lin, C.; Tanksley, S.; McCarthy, J. Oleosin gene family of Coffea canephora: Quantitative expression analysis of five oleosin genes in developing and germinating coffee grain. J. Plant Physiol. 2006, 163, 691–708. [Google Scholar] [CrossRef]
- Simkin, A.J.; McCarthy, J.; Petiard, V.; Tanksley, S.; Lin, C. Oleosin Genes and Promoters from Coffee. Patent WO2,007,005,928, 11 January 2007. [Google Scholar]
- Davison, J. GM plants: Science, politics and EC regulations. Plant Sci. 2010, 178, 94–98. [Google Scholar] [CrossRef]
- Davison, J.; Ammann, K. New GMO regulations for old: Determining a new future for EU crop biotechnology. GM Crop. Food 2017, 8, 13–34. [Google Scholar] [CrossRef]
- Smart, R.D.; Blum, M.; Wesseler, J. Trends in Approval Times for Genetically Engineered Crops in the United States and the European Union. J. Agric. Econ. 2017, 68, 182–198. [Google Scholar] [CrossRef]
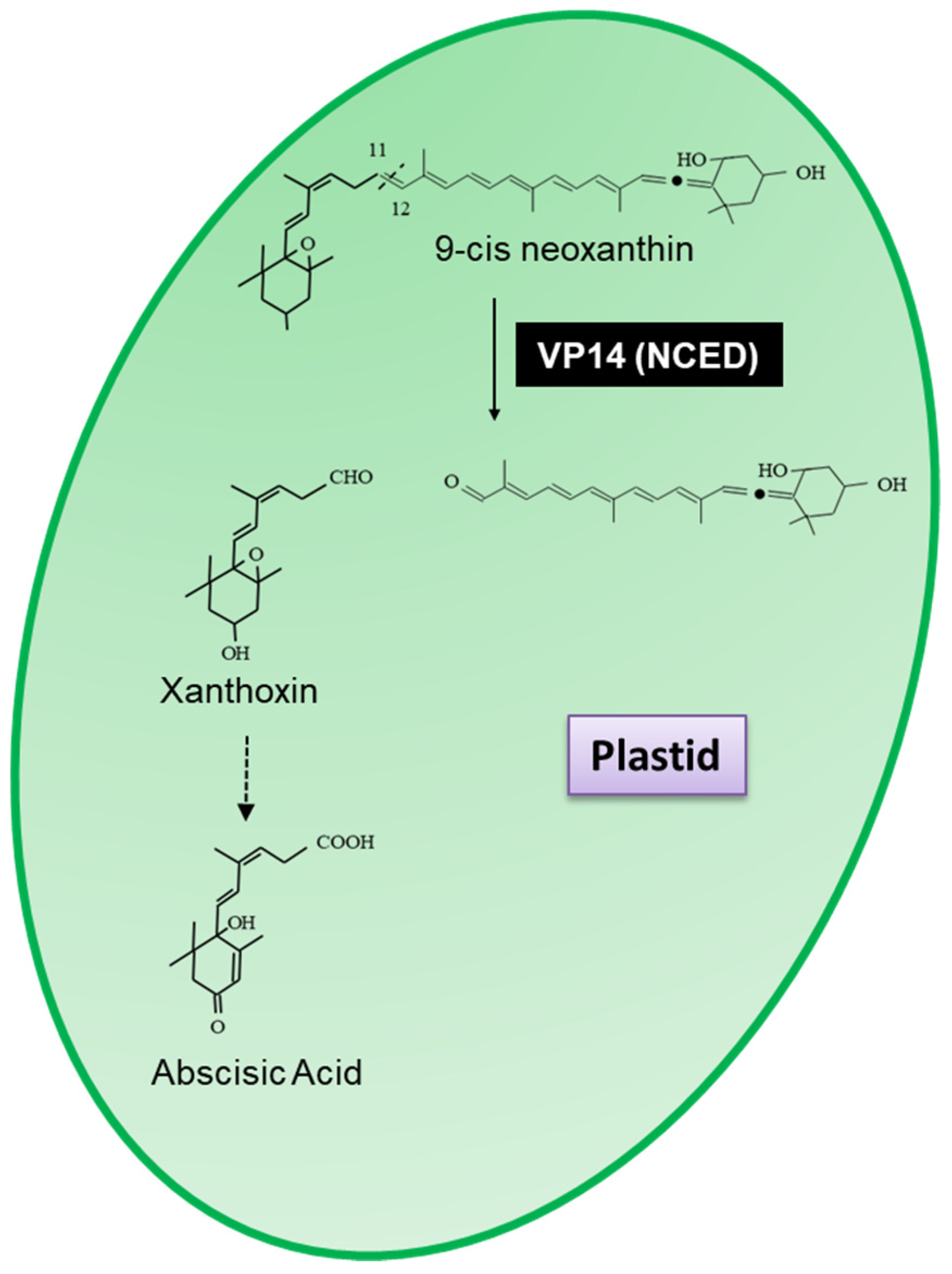
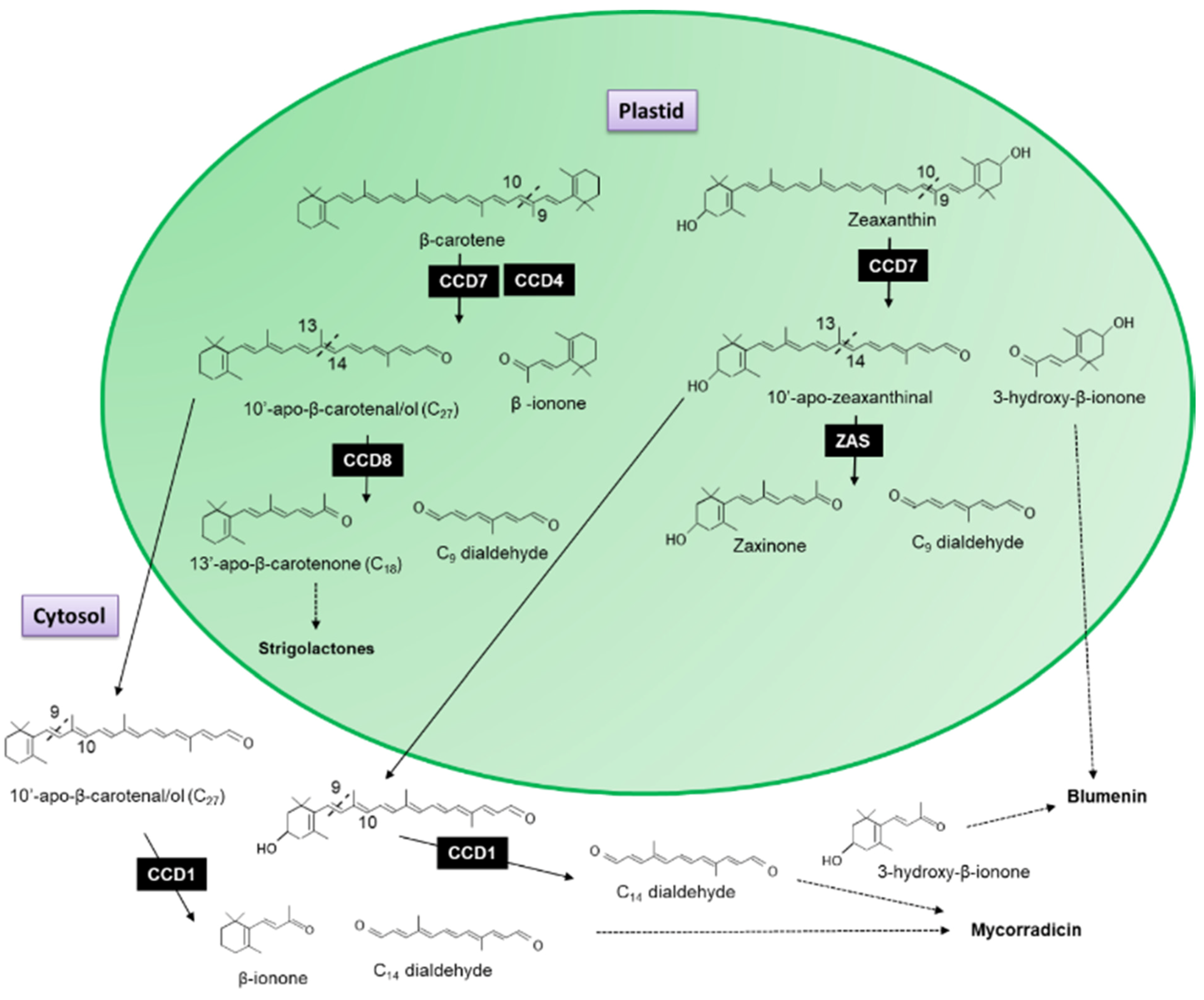
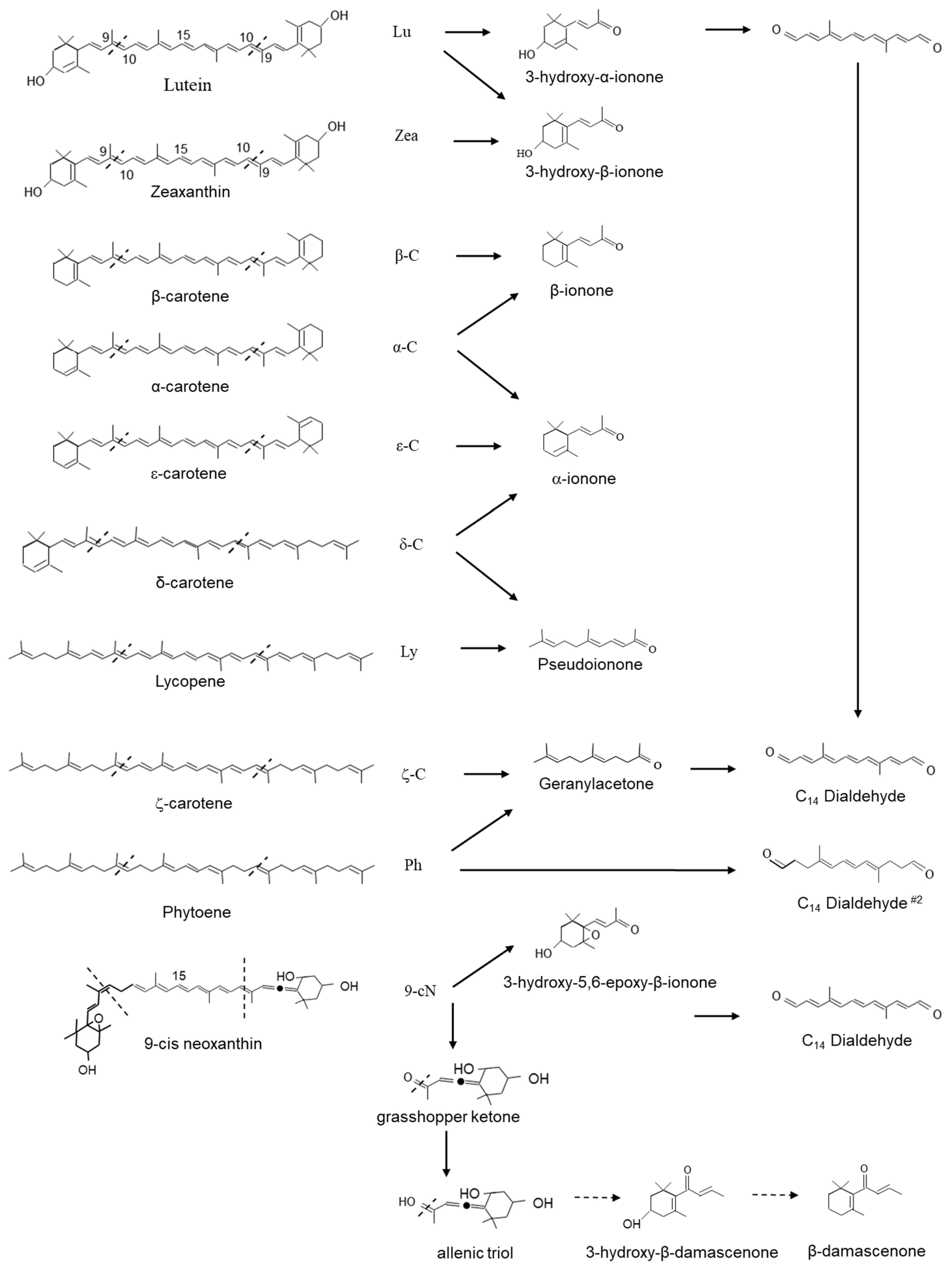

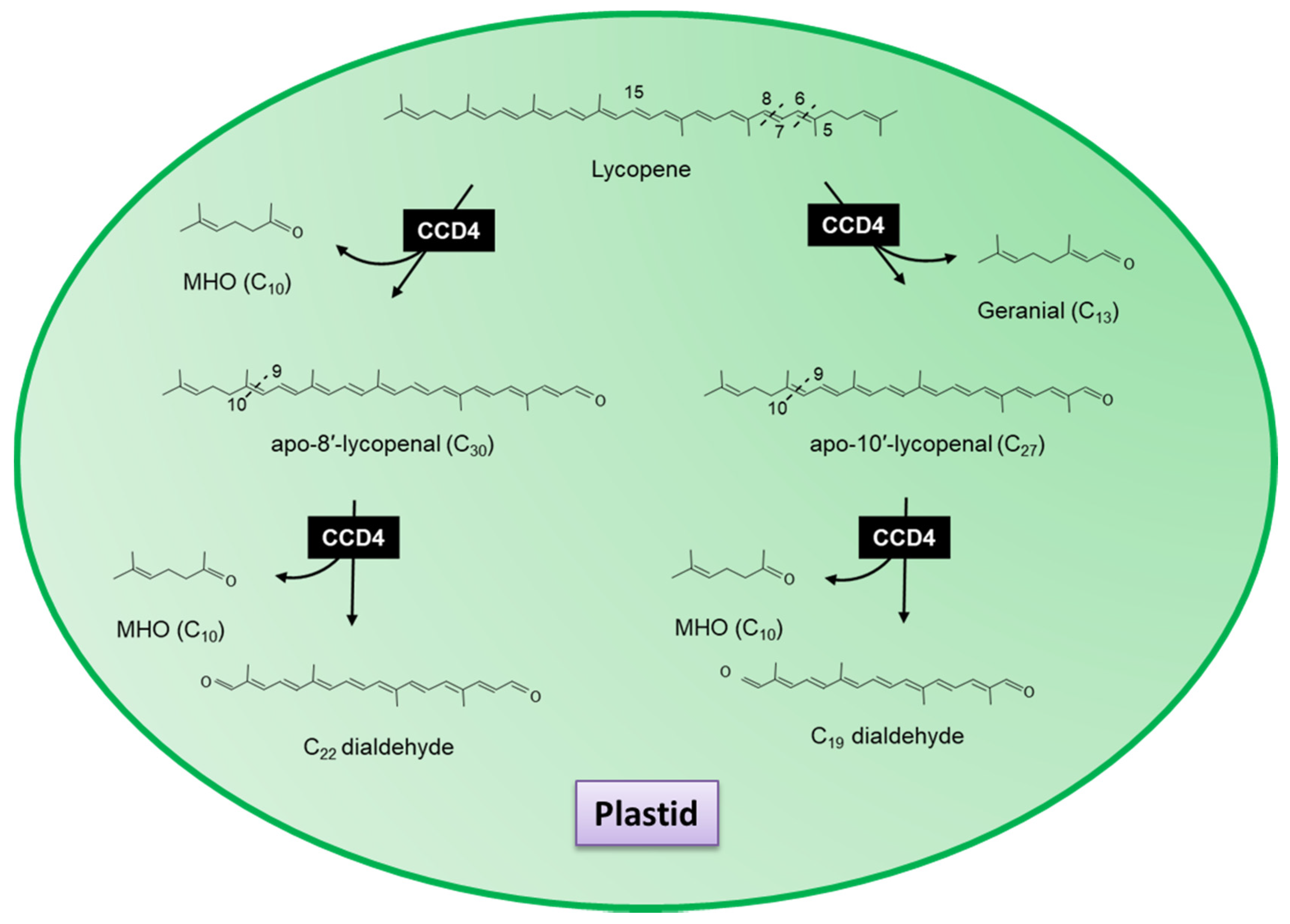

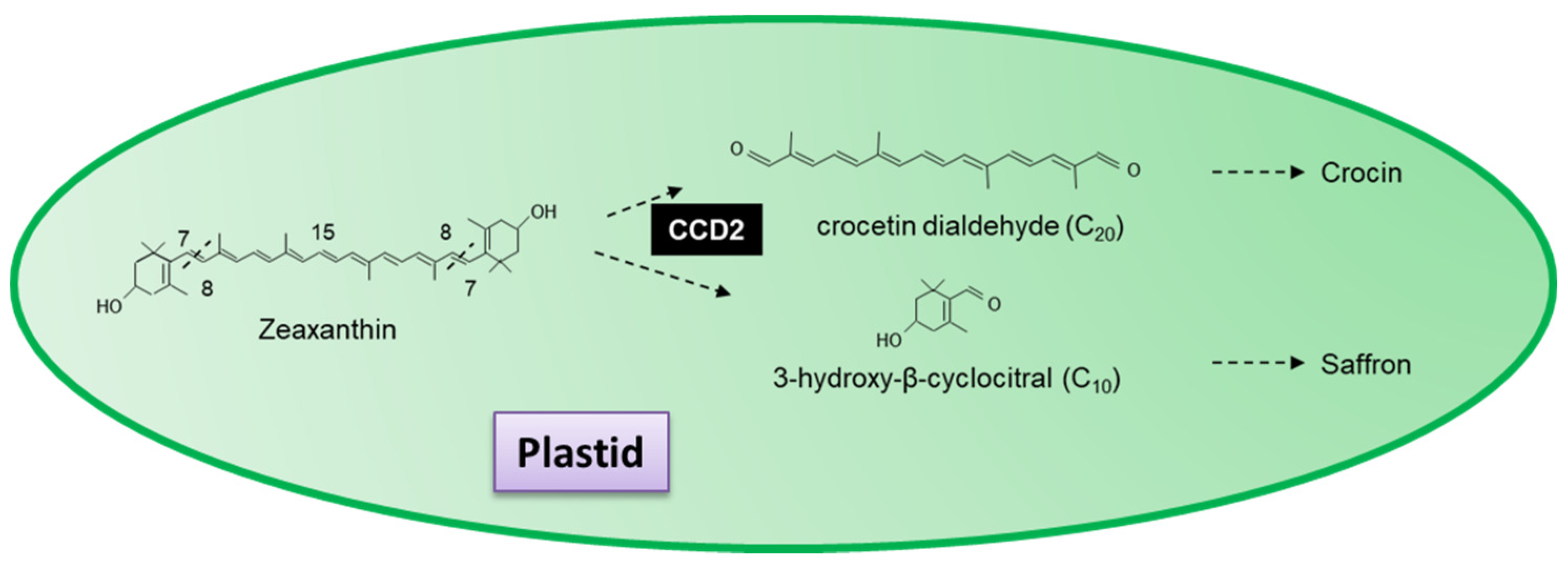
| Plant | Transgene | Metabolite Analysis | Ref |
|---|---|---|---|
| Tomato fruit | AtOr | Increases in Lycopene (1.6-fold), α-carotene 2.6-fold) and β-carotene (2.7-fold) | [129] |
| CaFib | Increases in Lycopene (2.2-fold) and β-carotene (1.6-fold) | [22] | |
| Cassava tubers | BoOr | ~2-fold increases in carotenoids (as all-trans-β-carotene) (3–4 µg/g DW) compared to CN 0.5–1 µg/g DW) | [114] |
| Sweetpotato | IbOr | Total carotenoid levels (up to 7-fold) in their storage roots compared to wild type (WT). The levels of zeaxanthin were ∼12 times elevated, whereas β-carotene increased ∼1.75 times | [137] |
| Potato tubers | BoOr | Total carotenoid 6-old higher than CN. Increase from 4 µg/g DW to 22 µg/g DW | [128] |
| Total carotenoids increased from 5.51 µg/g DW to 31 10 µg/g DW in the best lines, representing a 5.6-fold increase. | [131] | ||
| Rice seed | AtOr | Control rice seed contain no carotenoids. In conjunction with the over-expression of PSY and CrtI, Or expressing lines accumulated upto 25.8 µg/g DW total carotenoids (10.5 µg/g DW β-carotene) | [127] |
| Maize seed | AtOr | 32-fold higher than wild-type controls ~25 µg/g DW | [133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simkin, A.J. Carotenoids and Apocarotenoids in Planta: Their Role in Plant Development, Contribution to the Flavour and Aroma of Fruits and Flowers, and Their Nutraceutical Benefits. Plants 2021, 10, 2321. https://doi.org/10.3390/plants10112321
Simkin AJ. Carotenoids and Apocarotenoids in Planta: Their Role in Plant Development, Contribution to the Flavour and Aroma of Fruits and Flowers, and Their Nutraceutical Benefits. Plants. 2021; 10(11):2321. https://doi.org/10.3390/plants10112321
Chicago/Turabian StyleSimkin, Andrew J. 2021. "Carotenoids and Apocarotenoids in Planta: Their Role in Plant Development, Contribution to the Flavour and Aroma of Fruits and Flowers, and Their Nutraceutical Benefits" Plants 10, no. 11: 2321. https://doi.org/10.3390/plants10112321
APA StyleSimkin, A. J. (2021). Carotenoids and Apocarotenoids in Planta: Their Role in Plant Development, Contribution to the Flavour and Aroma of Fruits and Flowers, and Their Nutraceutical Benefits. Plants, 10(11), 2321. https://doi.org/10.3390/plants10112321






