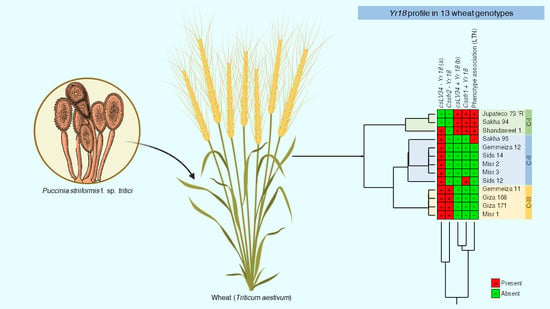
Durability of Adult Plant Resistance Gene Yr18 in Partial Resistance Behavior of Wheat (Triticum aestivum) Genotypes with Different Degrees of Tolerance to Stripe Rust Disease, Caused by Puccinia striiformis f. sp. tritici: A Five-Year Study
Abstract
1. Introduction
2. Results
2.1. Sakha 95 and Misr 3, but Not Other Genotypes, Were Efficient against Pst Races at the Seedling Stage under Greenhouse Conditions
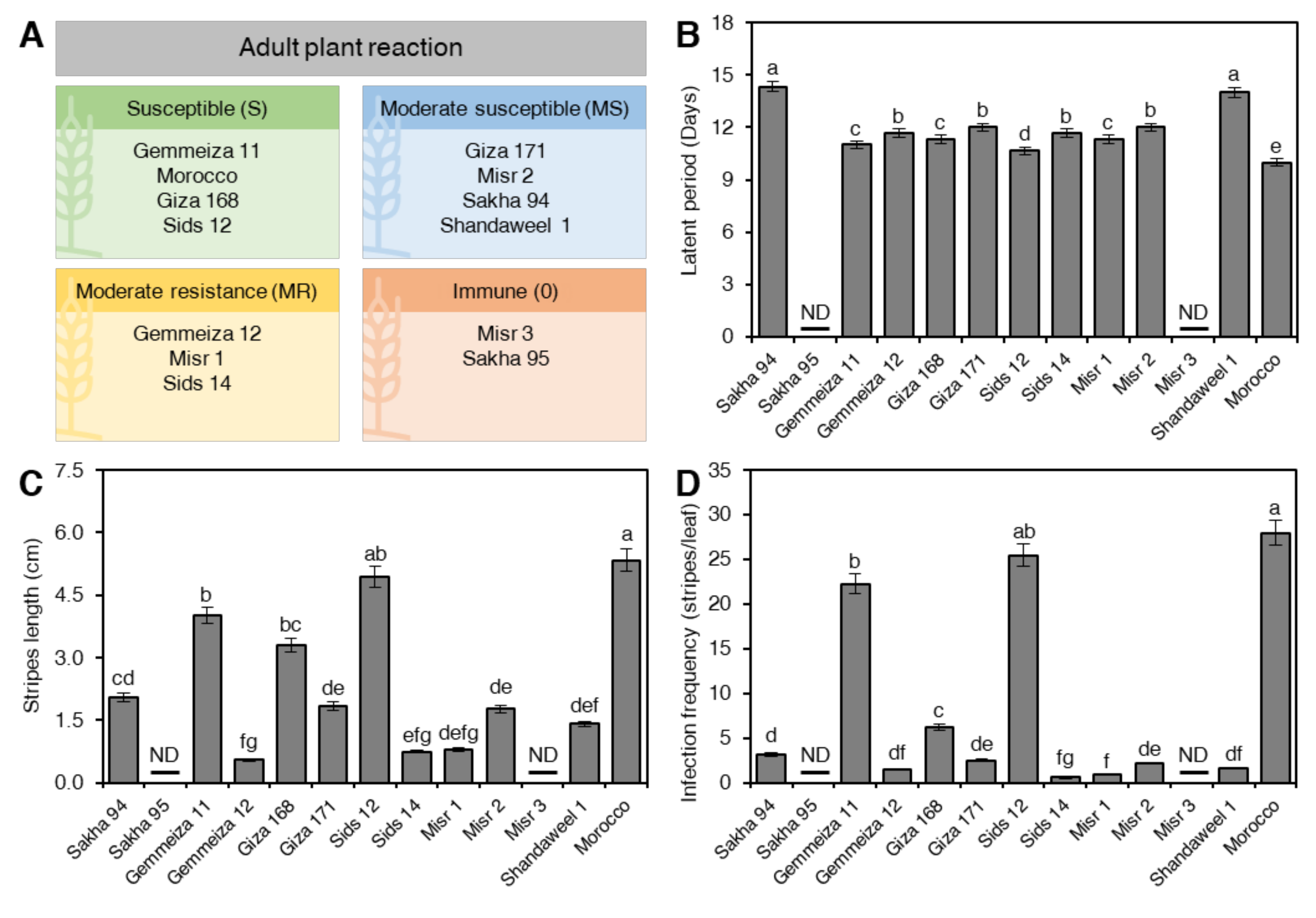
2.2. Assessment of Partial-Resistance/Slow-Rusting Components at Adult Stage under Greenhouse Conditions
2.3. Partial Resistance under Field Conditions during the Period from 2016 to 2021
2.3.1. Final Rust Severity (FRS %)
2.3.2. Area under Disease Curve (AUDPC)
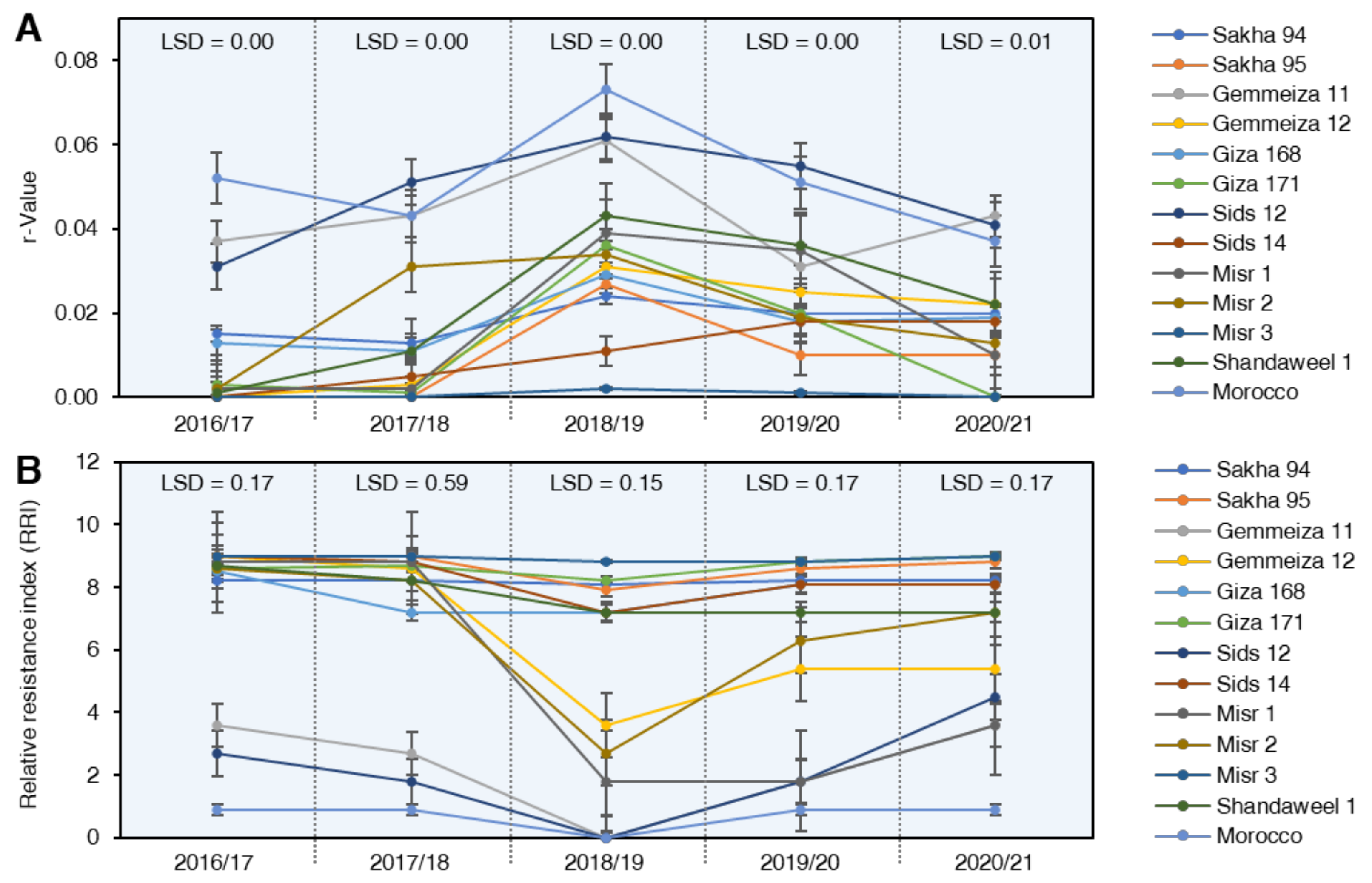
2.3.3. Rate of Disease Increases (r-Value)
2.3.4. Relative Resistance Index (RRI)
2.3.5. Observation of Phenotypic of APR Gene Yr18
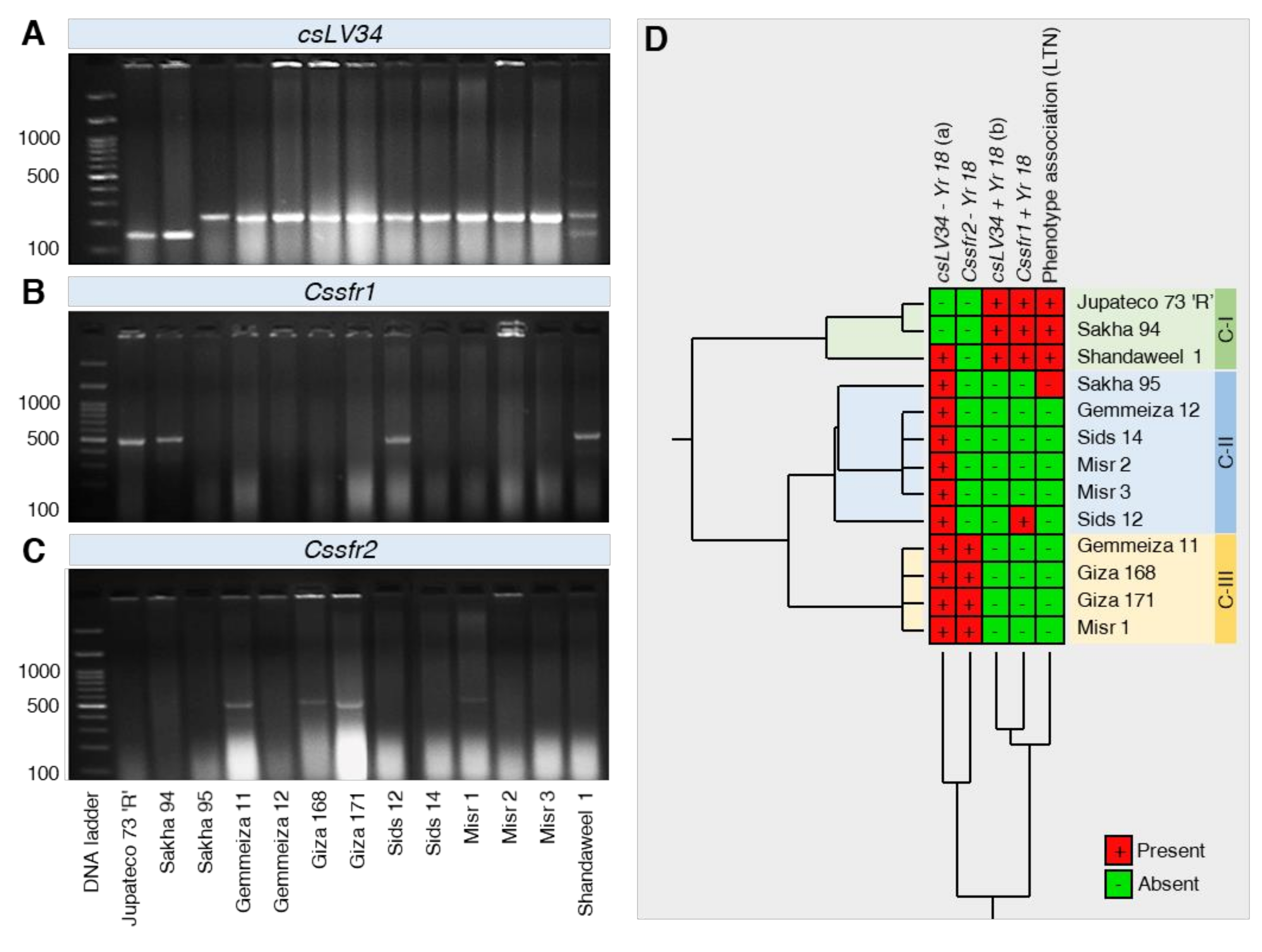
2.3.6. Molecular Analyses to APR Gene Yr18
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Greenhouse Investigations
4.2.1. Seedling Tests

4.2.2. Slow-Rusting Components at Adult Stage
4.3. Field Investigations
4.3.1. Disease Assessment
4.3.2. Molecular Analyses for Yr18
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT Food and Agriculture Organization of the United Nations. 2019. Available online: http://www.fao.org/faostat/en/#home (accessed on 13 February 2020).
- Wally, A.; Specialist, A.; Akingbe, O.O.; Abdi, A. Report Name: Grain and Feed Annual Egyptian Wheat Imports Hold Steady Despite Increased Local Production. 2020. Available online: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Grain%20and%20Feed%20Annual_Cairo_Egypt_03-15-2020 (accessed on 13 February 2020).
- McIntosh, R.A. Breeding Wheat for Resistance to Biotic Stresses; Springer Science and Business Media LLC.: New York, NY, USA, 1997; pp. 71–86. [Google Scholar]
- Wellings, C.R. Global status of stripe rust: A review of historical and current threats. Euphytica 2011, 179, 129–141. [Google Scholar] [CrossRef]
- Brown, J.K.M.; Hovmøller, M.S. Aerial Dispersal of Pathogens on the Global and Continental Scales and Its Impact on Plant Disease. Science 2002, 297, 537–541. [Google Scholar] [CrossRef]
- Chen, X. Epidemiology and control of stripe rust [Puccinia striiformis f. sp. tritici] on wheat. Can. J. Plant Pathol. 2005, 27, 314–337. [Google Scholar] [CrossRef]
- Jin, Y.; Szabo, L.J.; Carson, M. Century-Old Mystery of Puccinia striiformis Life History Solved with the Identification of Berberis as an Alternate Host. Phytopathology 2010, 100, 432–435. [Google Scholar] [CrossRef]
- Wellings, C.R. Puccinia striiformis in Australia: A review of the incursion, evolution, and adaptation of stripe rust in the period 1979–2006. Aust. J. Agric. Res. 2007, 58, 567–575. [Google Scholar] [CrossRef]
- Milus, E.A.; Kristensen, K.; Hovmøller, M.S. Evidence for Increased Aggressiveness in a Recent Widespread Strain of Puccinia striiformis f. sp.tritici Causing Stripe Rust of Wheat. Phytopathology 2009, 99, 89–94. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Pretorius, Z.A. Borlaug Global Rust Initiative provides momentum for wheat rust research. Euphytica 2011, 179, 1–2. [Google Scholar] [CrossRef]
- Sharma-Poudyal, D.; Chen, X.M.; Wan, A.M.; Zhan, G.M.; Kang, Z.S.; Cao, S.Q.; Jin, S.L.; Morgounov, A.; Akin, B.; Mert, Z.; et al. Virulence Characterization of International Collections of the Wheat Stripe Rust Pathogen, Puccinia striiformis f. sp. tritici. Plant Dis. 2013, 97, 379–386. [Google Scholar] [CrossRef]
- Sharma, R.C.; Nazari, K.; Amanov, A.; Ziyaev, Z.; Jalilov, A.U. Reduction of Winter Wheat Yield Losses Caused by Stripe Rust through Fungicide Management. J. Phytopathol. 2016, 164, 671–677. [Google Scholar] [CrossRef]
- Shahin, A.; Ashmawy, M.; El-Orabey, W.; Esmail, S. Yield Losses in Wheat Caused by Stripe Rust (Puccinia striiformis) in Egypt. Am. J. Life Sci. 2020, 8, 127. [Google Scholar] [CrossRef]
- Zhang, P.; McIntosh, R.A.; Hoxha, S.; Dong, C. Wheat stripe rust resistance genes Yr5 and Yr7 are allelic. Theor. Appl. Genet. 2009, 120, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, R.M. Breeding for general and/or specific plant disease resistance. In Proceedings of the Third International Wheat Genetics Symposium, Canberra, Australia, 5–9 August 1968; pp. 236–272. [Google Scholar]
- Parlevliet, J.E. Components of Resistance that Reduce the Rate of Epidemic Development. Annu. Rev. Phytopathol. 1979, 17, 203–222. [Google Scholar] [CrossRef]
- Nayar, S.K.; Prashar, M.; Bhardwaj, S.C.; Jain, K.; Nagarajan, S. Race specific adult plant resistance against brown rust in Indian wheat (Triticum aestivum). Indian J. Agric. Sci. 2005, 75, 712–714. [Google Scholar]
- Datta, D.; Nayar, S.K.; Prashar, M.; Bhardwaj, S.C. Inheritance of temperature-sensitive leaf rust resistance and adult plant stripe rust resistance in common wheat cultivar PBW343. Euphytica 2008, 166, 277–282. [Google Scholar] [CrossRef]
- Knott, D.R. The Wheat Rusts—Breeding for Resistance. In Distant Hybridization of Crop Plants; Knott, D.R., Ed.; Springer: Berlin, Germany; New York, NY, USA, 1989; Volume 12, ISBN 0387504591. [Google Scholar]
- Nayar, S.K.; Jain, S.K.; Prashar, M.; Bhardwaj, S.C.; Kumar, S. Appearance of new pathotype of Puccinia recondita tritici virulent on Lr9 in India. Indian Phytopathol. 2003, 56, 196–198. [Google Scholar]
- Kilpatrick, R.A. New wheat cultivars and longevity of rust resistance, 1971–1975. US Dep. Agric. Res. Serv. 1975, NE 64, 1. [Google Scholar]
- McIntosh, R.A. Close genetic linkage of genes conferring adult-plant resistance to leaf rust and stripe rust in wheat. Plant Pathol. 2007, 41, 523–527. [Google Scholar] [CrossRef]
- Dyck, P.L. The association of a gene for leaf rust resistance with the chromosome 7D suppressor of stem rust resistance in common wheat. Genome 1987, 29, 467–469. [Google Scholar] [CrossRef]
- Spielmeyer, W.; McIntosh, R.A.; Kolmer, J.; Lagudah, E. Powdery mildew resistance and Lr34/Yr18 genes for durable resistance to leaf and stripe rust cosegregate at a locus on the short arm of chromosome 7D of wheat. Theor. Appl. Genet. 2005, 111, 731–735. [Google Scholar] [CrossRef]
- McIntosh, R.; Dubcovsky, J.; Rogers, W.; Morris, C.; Appels, R.; Xia, X.; Designators, W.L. Catalogue of Gene Symbols for Wheat: 2017 Supplement Morphological and Physiological Traits. 2014. Available online: https://shigen.nig.ac.jp/wheat/komugi/genes/macgene/supplement2017.pdf (accessed on 13 February 2020).
- Singh, R.P. Association between Gene Lr34 for Leaf Rust Resistance and Leaf Tip Necrosis in Wheat. Crop. Sci. 1992, 32, 874–878. [Google Scholar] [CrossRef]
- Krattinger, S.G.; Lagudah, E.S.; Spielmeyer, W.; Singh, R.P.; Huerta-Espino, J.; McFadden, H.; Bossolini, E.; Selter, L.L.; Keller, B. A Putative ABC Transporter Confers Durable Resistance to Multiple Fungal Pathogens in Wheat. Science 2009, 323, 1360–1363. [Google Scholar] [CrossRef]
- Suenaga, K.; Singh, R.P.; Huerta-Espino, J.; William, H.M. Microsatellite Markers for Genes Lr34/Yr18 and Other Quantitative Trait Loci for Leaf Rust and Stripe Rust Resistance in Bread Wheat. Phytopathology 2003, 93, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Schnurbusch, T.; Paillard, S.; Schori, A.; Messmer, M.; Schachermayr, G.; Winzeler, M.; Keller, B. Dissection of quantitative and durable leaf rust resistance in Swiss winter wheat reveals a major resistance QTL in the Lr34 chromosomal region. Theor. Appl. Genet. 2004, 108, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Lagudah, E.S.; McFadden, H.; Singh, R.P.; Huerta-Espino, J.; Bariana, H.S.; Spielmeyer, W. Molecular genetic characterization of the Lr34/Yr18 slow rusting resistance gene region in wheat. Theor. Appl. Genet. 2006, 114, 21–30. [Google Scholar] [CrossRef]
- Lagudah, E.S.; Krattinger, S.G.; Herrera-Foessel, S.; Singh, R.P.; Huerta-Espino, J.; Spielmeyer, W.; Brown-Guedira, G.; Selter, L.L.; Keller, B. Gene-specific markers for the wheat gene Lr34/Yr18/Pm38 which confers resistance to multiple fungal pathogens. Theor. Appl. Genet. 2009, 119, 889–898. [Google Scholar] [CrossRef]
- McNeal, F.H.; Konzak, C.F.; Smith, E.P.; Tate, W.; Russell, T.S. A uniform system for recording and processing cereal research data. U.S. Dep. Agric. Res. Serv. Bull. 1971, 18, 34–121. [Google Scholar]
- Esmail, S.M.; Draz, I.S.; Ashmawy, M.A.; El-Orabey, W.M. Emergence of new aggressive races of Puccinia striiformis f. sp. tritici causing yellow rust epiphytotic in Egypt. Physiol. Mol. Plant Pathol. 2021, 114, 101612. [Google Scholar] [CrossRef]
- Pretorius, Z.A.; Boshoff, W.; Kema, G.H. First Report of Puccinia striiformis f. sp. tritici on Wheat in South Africa. Plant Dis. 1997, 81, 424. [Google Scholar] [CrossRef]
- Khodarahmi, M.; Ghannadha, M.R.; Saidi, A.; Torabi, M.; Karimzadeh, G. Evaluation of resistance components to three races of Puccinia striiformis in wheat genotypes. In Proceedings of the 1st Regional Yellow Rust Conference for Central and West Asia and North Africa, Karaj, Iran, 8–14 May 2001; p. 38. [Google Scholar]
- Torabi, M. Effects of pustule density and leaf age on the latent period of Puccinia recondita in wheat. Vor. Pflanzenzucht 1992, 24, 71–73. [Google Scholar]
- Singh, R.P.; Rajaram, S. Breeding for disease resistance in wheat. FAO Plant Prod. Prot. Ser. 2002, 1, 567. [Google Scholar]
- Omara, R.I.; Abu Aly, A.A.M.; Abou-Zeid, M.A. Characterization of Partial Resistance to Stripe Rust (Puccinia striiformis f. sp. tritici) in some Egyptian Wheat Cultivars. J. Plant Prot. Pathol. 2018, 9, 111–119. [Google Scholar] [CrossRef][Green Version]
- Singh, V.K.; Mathuria, R.C.; Singh, G.P.; Singh, P.K.; Singh, S.; Gogoi, R.; Aggarwal, R. Characterization of yellow rust resistance genes by using gene postulation and assessment of adult plant resistance in some Indian wheat genotypes. Res. Crop. 2015, 16, 742. [Google Scholar] [CrossRef]
- Kolmer, J.A.; Singh, R.P.; Garvin, D.F.; Viccars, L.; William, H.M.; Huerta-Espino, J.; Ogbonnaya, F.C.; Raman, H.; Orford, S.; Bariana, H.S.; et al. Analysis of the Lr34/Yr18 Rust Resistance Region in Wheat Germplasm. Crop. Sci. 2008, 48, 1841–1852. [Google Scholar] [CrossRef]
- Singh, R.P.; Huerta-Espino, J.; William, H.M. Genetics and breeding for durable resistance to leaf and stripe rusts in wheat. Turk. J. Agric. For. 2005, 29, 121–127. [Google Scholar] [CrossRef]
- Ma, H. Contribution of Adult Plant Resistance Gene Yr18 in Protecting Wheat from Yellow Rust. Plant Dis. 1996, 80, 66–69. [Google Scholar] [CrossRef]
- Risk, J.M.; Selter, L.L.; Krattinger, S.; Viccars, L.A.; Richardson, T.M.; Buesing, G.; Herren, G.; Lagudah, E.S.; Keller, B. Functional variability of the Lr34 durable resistance gene in transgenic wheat. Plant Biotechnol. J. 2012, 10, 477–487. [Google Scholar] [CrossRef]
- Singh, D.B.; Park, R.; McIntosh, R.A. Characterisation of wheat leaf rust resistance gene Lr34 in Australian wheats using components of resistance and the linked molecular marker csLV34. Aust. J. Agric. Res. 2007, 58, 1106–1114. [Google Scholar] [CrossRef]
- Mccallum, B.D.; Somers, D.J.; Humphreys, D.G.; Cloutier, S. Molecular marker analysis of Lr34 in Canada Western Red Spring wheat cultivars. In Proceedings of the 11th International Wheat Genetics Symposium; Appels, R., Eastwood, R., Lagudah, E., Langridge, P., Mackay, M., McIntyre, L., Sharp, P., Eds.; Sydney University Press: Sydney, Australia, 2008; pp. 1–3. [Google Scholar]
- Wu, L.; Xia, X.; Rosewarne, G.M.; Zhu, H.; Li, S.; Zhang, Z.; He, Z. Stripe rust resistance gene Yr18 and its suppressor gene in Chinese wheat landraces. Plant Breed. 2015, 134, 634–640. [Google Scholar] [CrossRef]
- Bauer, B.E.; Wolfger, H.; Kuchler, K. Inventory and function of yeast ABC proteins: About sex, stress, pleiotropic drug and heavy metal resistance. Biochim. Biophys. Acta (BBA) Biomembr. 1999, 1461, 217–236. [Google Scholar] [CrossRef]
- Theodoulou, F.L. Plant ABC transporters. Biochim. Biophys. Acta (BBA) Biomembr. 2000, 1465, 79–103. [Google Scholar] [CrossRef]
- Dadrezaei, S.T.; Nazari, K.; Afshari, F.; Goltapeh, E.M. Phenotypic and Molecular Characterization of Wheat Leaf Rust Resistance Gene Lr34 in Iranian Wheat Cultivars and Advanced Lines. Am. J. Plant Sci. 2013, 04, 1821–1833. [Google Scholar] [CrossRef]
- Rinaldo, A.; Gilbert, B.; Boni, R.; Krattinger, S.G.; Singh, D.; Park, R.F.; Lagudah, E.; Ayliffe, M. The Lr34 adult plant rust resistance gene provides seedling resistance in durum wheat without senescence. Plant Biotechnol. J. 2017, 15, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Green, G.J. Stem rust of wheat, barley and rye in Canada in 1964. Can. Plant Dis. Surv. 1965, 45, 23–29. [Google Scholar]
- Omara, R.I.; Nehela, Y.; Mabrouk, O.I.; Elsharkawy, M.M. The Emergence of New Aggressive Leaf Rust Races with the Potential to Supplant the Resistance of Wheat Cultivars. Biology 2021, 10, 925. [Google Scholar] [CrossRef] [PubMed]
- Tervet, I.; Cassel, R.C. The use of cyclone in the race identification of microscopic particles. Phytopathology 1951, 41, 282–285. [Google Scholar]
- Parlevliet, J.E. Partial resistance of barley to leaf rust, Puccinia hordei. I. Effect of cultivar and development stage on latent period. Euphytica 1975, 24, 21–27. [Google Scholar] [CrossRef]
- Parlevliet, J.E.; Kuiper, H.J. Partial resistance of barley to leaf rust, Puccinia hordei. IV. Effect of cultivar and development stage on infection frequency. Euphytica 1977, 26, 249–255. [Google Scholar] [CrossRef]
- Johnston, C. Sixth revision of international register of physiologic races of the leaf rust of wheat Puccinia recondite. Rep. Suppl. 1961, 92, 19–30. [Google Scholar]
- Stakman, E.C.; Stewart, D.M.; Loegering, W.Q. Identification of Physiologic Races of Puccinia Graminis f. sp. Tritici; The Agricultural Research Service (ARS), United States Department of Agriculture (USDA): Washington, DC, USA, 1962; 54p.
- Peterson, R.F.; Campbell, A.B.; Hannah, A.E. A Diagrammatic Scale For Estimating Rust Intensity On Leaves And Stems of Cereals. Can. J. Res. 1948, 26, 496–500. [Google Scholar] [CrossRef]
- Jeffers, J.N.R.; Van Der Plank, J.E. Plant Diseases: Epidemics and Control. J. R. Stat. Soc. Ser. D 1965, 15, 90. [Google Scholar] [CrossRef]
- Pandey, H.N.; Menon, T.C.M.; Rao, M.V. A simple formula for calculating area under disease progress curve. Barley Wheat Newsl. 1989, 8, 38–39. [Google Scholar]
- Akhtar, M.A. Evaluation of Candidate Lines Against Stripe and Leaf Rusts Under National Uniform Wheat and Barley Yield Trial 2000–2001. Asian J. Plant Sci. 2002, 1, 450–453. [Google Scholar] [CrossRef]
- Ullah, N.; Bashir, T.; Asif, M.; Badshah, H.; Samad Mumtaz, A. Characterization of Durable Resistance gene Yr18/Lr34 against Stripe Rust (Puccinia striiformis f. sp. tritici) in different Pakistani Wheat Cultivars by Using Molecular (STS) and Morphological (LTN) Markers. Int. J. Sci. Eng. Res. 2015, 6, 63–74. [Google Scholar]
- Chen, X. Relationship Between Virulence Variation and DNA Polymorphism in Puccinia striiformis. Phytopathology 1993, 83, 1489–1497. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple Range and Multiple F Tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, G.E.; Mazrou, Y.S.A.; EL-Kazzaz, M.K.; Ghoniem, K.E.; Ashmawy, M.A.; Emeran, A.A.; Mabrouk, O.I.; Nehela, Y. Durability of Adult Plant Resistance Gene Yr18 in Partial Resistance Behavior of Wheat (Triticum aestivum) Genotypes with Different Degrees of Tolerance to Stripe Rust Disease, Caused by Puccinia striiformis f. sp. tritici: A Five-Year Study. Plants 2021, 10, 2262. https://doi.org/10.3390/plants10112262
Omar GE, Mazrou YSA, EL-Kazzaz MK, Ghoniem KE, Ashmawy MA, Emeran AA, Mabrouk OI, Nehela Y. Durability of Adult Plant Resistance Gene Yr18 in Partial Resistance Behavior of Wheat (Triticum aestivum) Genotypes with Different Degrees of Tolerance to Stripe Rust Disease, Caused by Puccinia striiformis f. sp. tritici: A Five-Year Study. Plants. 2021; 10(11):2262. https://doi.org/10.3390/plants10112262
Chicago/Turabian StyleOmar, Ghady E., Yasser S. A. Mazrou, Mohammad K. EL-Kazzaz, Kamal E. Ghoniem, Mammduh A. Ashmawy, Amero A. Emeran, Ola I. Mabrouk, and Yasser Nehela. 2021. "Durability of Adult Plant Resistance Gene Yr18 in Partial Resistance Behavior of Wheat (Triticum aestivum) Genotypes with Different Degrees of Tolerance to Stripe Rust Disease, Caused by Puccinia striiformis f. sp. tritici: A Five-Year Study" Plants 10, no. 11: 2262. https://doi.org/10.3390/plants10112262
APA StyleOmar, G. E., Mazrou, Y. S. A., EL-Kazzaz, M. K., Ghoniem, K. E., Ashmawy, M. A., Emeran, A. A., Mabrouk, O. I., & Nehela, Y. (2021). Durability of Adult Plant Resistance Gene Yr18 in Partial Resistance Behavior of Wheat (Triticum aestivum) Genotypes with Different Degrees of Tolerance to Stripe Rust Disease, Caused by Puccinia striiformis f. sp. tritici: A Five-Year Study. Plants, 10(11), 2262. https://doi.org/10.3390/plants10112262