Phytochemical, Pharmacological, and Biotechnological Study of Ageratina pichinchensis: A Native Species of Mexico
Abstract
:1. Introduction
2. Research Methodology
3. Botanical Description
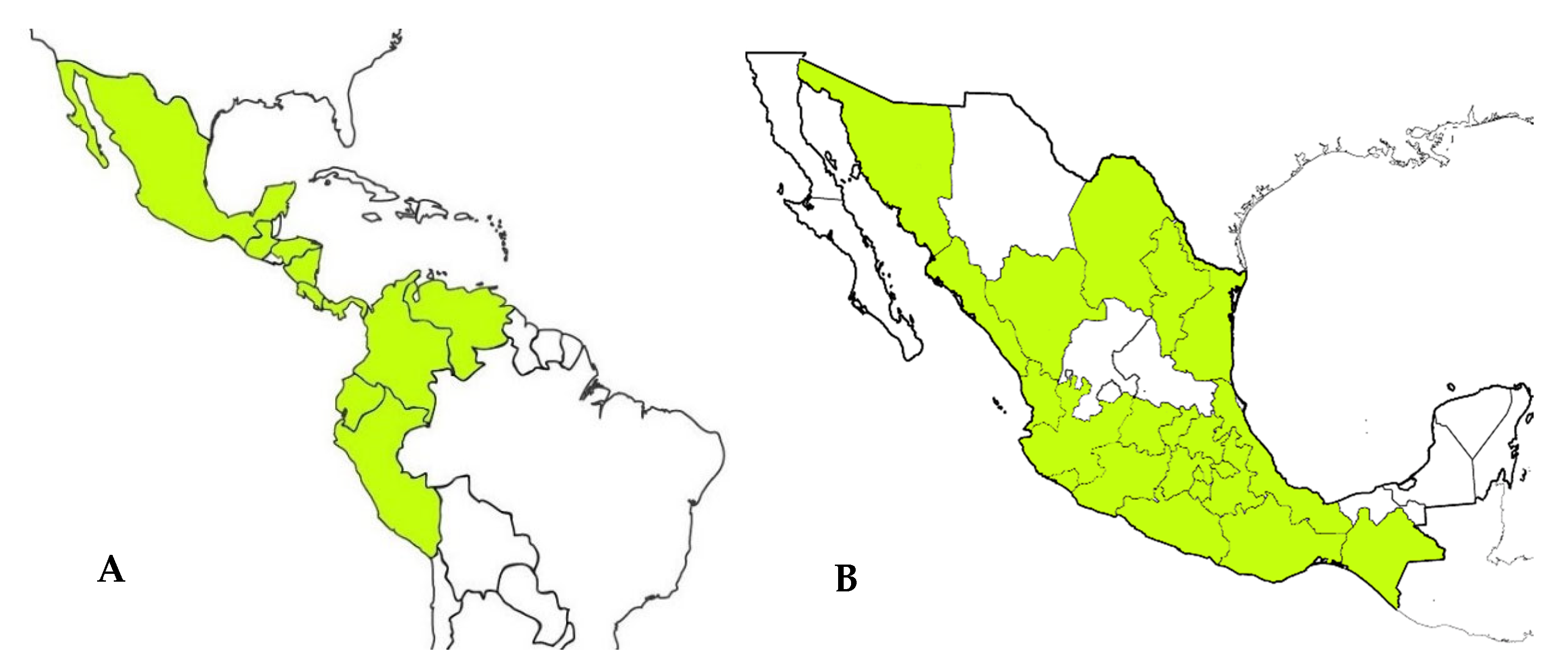
4. Geographic Distribution
5. Ethnomedicinal Uses
6. Phytochemistry
6.1. Benzochromenes and Benzofurans
6.2. Phenolic Compounds
6.3. Chemical Constituents of the Essential Oil
7. Pharmacological Activities
7.1. Antimicrobial Activity
7.1.1. In Vitro Assays
7.1.2. Clinical Trials
7.2. In Vivo Antiulcer Assay
7.3. Wound Healing Activity
7.3.1. In Vivo Assays
7.3.2. Clinical Trials
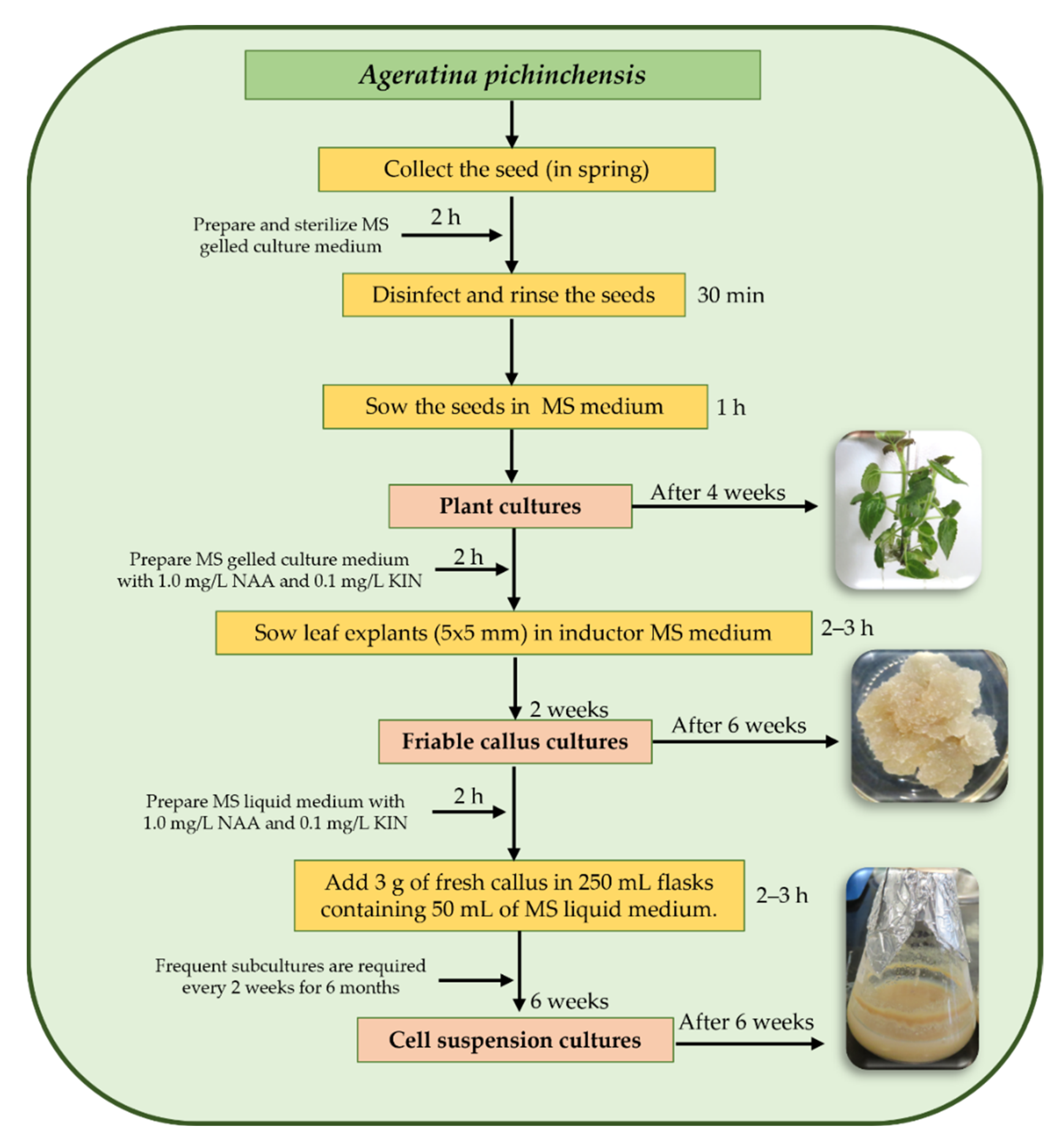
8. Plant Biotechnological Studies on A. pichinchensis
8.1. Callus Culture
8.2. Cell Suspension Culture
9. Discussion
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, N.C.C.; Fernandes Júnior, A. Biological properties of medicinal plants: A review of their antimicrobial activity. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 402–413. [Google Scholar] [CrossRef]
- The Plant List. Compositae. 2013. Available online: http://www.theplantlist.org/1.1/browse/A/Compositae/ (accessed on 1 January 2021).
- Rzedowski, J. Contribuciones a la fitogeografía florística e histórica de México III. Algunas tendencias en la distribución geográfica y ecológica de las Compositae mexicanas. Cienc. Mex. 1972, 27, 123–132. [Google Scholar]
- Villaseñor, J.L. Diversidad y distribución de la familia Asteraceae en México. Bot. Sci. 2018, 96, 332–358. [Google Scholar] [CrossRef] [Green Version]
- Achika, J.I.; Arthur, D.E.; Gerald, I.; Adedayo, A. A review on the phytoconstituents and related medicinal properties of plants in the Asteraceae family. J. Appl. Chem. 2014, 7, 1–8. [Google Scholar] [CrossRef]
- WFO. Ageratina. 2021. Available online: http://www.worldfloraonline.org/taxon/wfo-4000000936 (accessed on 13 April 2021).
- WFO. Ageratina pichinchensis (Kunth) R.M.King & H.Rob. 2021. Available online: http://www.worldfloraonline.org/taxon/wfo-0000122234 (accessed on 22 April 2021).
- García, P.G.; García, S.E.; Martínez, G.I.; Scior, T.R.F.; Salvador, J.L.; Martínez, P.M.M.; del Río, R.E. Analgesic effect of leaf extract from Ageratina glabrata in the hot plate test. Rev. Bras. Farmacogn. 2011, 21, 928–938. [Google Scholar] [CrossRef] [Green Version]
- Arciniegas, A.; Pérez-Castorena, A.L.; Meléndez-Aguirre, M.; Ávila, J.G.; García-Bores, A.M.; Villaseñor, J.L.; Romo de Vivar, A. Chemical composition and antimicrobial activity of Ageratina deltoidea. Chem. Biodivers. 2018, 15, e1700529. [Google Scholar] [CrossRef] [PubMed]
- Poudel, R.; Neupane, N.P.; Mukeri, I.H.; Alok, S.; Verma, A. An updated review on invasive nature, phytochemical evaluation, & pharmacological activity of Ageratina adenophora. Int. J. Pharm. Sci. Res. 2020, 11, 2510–2520. [Google Scholar]
- BDMTM. Atlas de las Plantas de la Medicina Tradicional Mexicana. 1994. Available online: http://www.medicinatradicionalmexicana.unam.mx/index.html (accessed on 24 April 2021).
- Navarro, V.N.; González, A.; Fuentes, M.; Aviles, M.; Rios, M.Y.; Zepeda, G.; Rojas, M.G. Antifungal activities of nine traditional Mexican medicinal plants. J. Ethnopharmacol. 2003, 87, 85–88. [Google Scholar] [CrossRef]
- Rios, M.Y.; Aguilar-Guadarrama, A.B.; Navarro, V. Two new benzofuranes from Eupatorium aschenbornianum and their antimicrobial activity. Planta Med. 2003, 69, 967–970. [Google Scholar]
- Romero-Cerecero, O.; Rojas, G.; Navarro, V.; Herrera-Arellano, A.; Zamilpa-Alvarez, A.; Tortoriello, J. Effectiveness and tolerability of a standardized extract from Ageratina pichinchensis on patients with tinea pedis: An explorative pilot study controlled with ketoconazole. Planta Med. 2006, 72, 1257–1261. [Google Scholar] [CrossRef]
- Romero-Cerecero, O.; Zamilpa, A.; Jiménez-Ferrer, J.E.; Rojas-Bribiesca, G.; Román-Ramos, R.; Tortoriello, J. Double-blind clinical trial for evaluating the effectiveness and tolerability of Ageratina pichinchensis extract on patients with mild to moderate onychomycosis. A comparative study with ciclopirox. Planta Med. 2008, 74, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Guadarrama, B.; Navarro, V.; Leon-Rivera, I.; Rios, M.Y. Active compounds against tinea pedis dermatophytes from Ageratina pichinchensis var. bustamenta. Nat. Prod. Res. 2009, 23, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cerecero, O.; Román-Ramos, R.; Zamilpa, A.; Jiménez-Ferrer, J.E.; Rojas-Bribiesca, G.; Tortoriello, J. Clinical trial to compare the effectiveness of two concentrations of the Ageratina pichinchensis extract in the topical treatment of onychomycosis. J. Ethnopharmacol. 2009, 126, 74–78. [Google Scholar] [CrossRef]
- Romero-Cerecero, O.; Zamilpa, A.; Jiménez-Ferrer, E.; Tortoriello, J. Therapeutic effectiveness of Ageratina pichinchensis on the treatment of chronic interdigital Tinea Pedis: A randomized, double-blind clinical trial. J. Altern. Complement. Med. 2012, 18, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cerecero, O.; Islas-Garduño, A.L.; Zamilpa, A.; Tortoriello, J. Effectiveness of Ageratina pichinchensis extract in patients with vulvovaginal candidiasis. A randomized, double-blind, and controlled pilot study. Phytother. Res. 2017, 31, 885–890. [Google Scholar] [CrossRef]
- Sánchez-Mendoza, M.E.; Reyes-Trejo, B.; Sánchez-Gómez, P.; Rodríguez-Silverio, J.; Castillo-Henkel, C.; Cervantes-Cuevas, H.; Arrieta, J. Bioassay-guided isolation of an anti-ulcer chromene from Eupatorium aschenbornianum: Role of nitric oxide, prostaglandins and sulfydryls. Fitoterapia 2010, 81, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Mendoza, M.E.; Rodríguez-Silverio, J.; Rivero-Cruz, J.F.; Rocha-González, H.I.; Pineda-Farías, J.B.; Arrieta, J. Antinociceptive effect and gastroprotective mechanisms of 3, 5-diprenyl-4-hydroxyacetophenone from Ageratina pichinchensis. Fitoterapia 2013, 87, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cerecero, O.; Zamilpa, A.; Tortoriello, J. Effectiveness and tolerability of a standardized extract from Ageratina pichinchensis in patients with diabetic foot ulcer: A randomized, controlled pilot study. Planta Med. 2015, 81, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cerecero, O.; Zamilpa, A.; Tortoriello, J. Pilot study that evaluated the clinical effectiveness and safety of a phytopharmaceutical elaborated with an extract of Ageratina pichinchensis in patients with minor recurrent aphthous stomatitis. J. Ethnopharmacol. 2015, 173, 225–230. [Google Scholar] [CrossRef]
- Freitas, A.L.; Santos, C.A.; Souza, C.A.S.; Nunes, M.A.P.; Antoniolli, Â.R.; da Silva, W.B.; da Silva, F.A. The use of medicinal plants in venous ulcers: A systematic review with meta-analysis. Int. Wound J. 2017, 14, 1019–1024. [Google Scholar] [CrossRef]
- Romero-Cerecero, O.; Zamilpa-Álvarez, A.; Ramos-Mora, A.; Alonso-Cortés, D.; Jiménez-Ferrer, J.E.; Huerta-Reyes, M.E.; Tortoriello, J. Effect on the wound healing process and in vitro cell proliferation by the medicinal mexican plant Ageratina pichinchensis. Planta Med. 2011, 77, 979–983. [Google Scholar] [CrossRef]
- Romero-Cerecero, O.; Zamilpa, A.; González-Cortazar, M.; Alonso-Cortés, D.; Jiménez-Ferrer, E.; Nicasio-Torres, P.; Aguilar-Santamaría, L.; Tortoriello, J. Pharmacological and chemical study to identify wound-healing active compounds in Ageratina pichinchensis. Planta Med. 2013, 79, 622–627. [Google Scholar] [CrossRef]
- Romero-Cerecero, O.; Zamilpa, A.; Díaz-García, E.R.; Tortoriello, J. Pharmacological effect of Ageratina pichinchensis on wound healing in diabetic rats and genotoxicity evaluation. J. Ethnopharmacol. 2014, 156, 222–227. [Google Scholar] [CrossRef]
- Torres-Barajas, L.; Rojas-Vera, J.; Morales-Méndez, A.; Rojas-Fermín, L.; Lucena, M.; Buitrago, A. Chemical composition and evaluation of antibacterial activity of essential oils of Ageratina jahnii and Ageratina pichinchensis collected in Mérida, Venezuela. Bol. Latinoam. Caribe Plantas Med. Aromat. 2013, 12, 92–98. [Google Scholar]
- Sánchez-Ramos, M.; Bahena, S.M.; Romero-Estrada, A.; Bernabé-Antonio, A.; Cruz-Sosa, F.; González-Christen, J.; Acevedo-Fernández, J.J.; Perea-Arango, I.; Alvarez, L. Establishment and phytochemical analysis of a callus culture from Ageratina pichinchensis (Asteraceae) and its anti-inflammatory activity. Molecules 2018, 23, 1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Ramos, M.; Alvarez, L.; Romero-Estrada, A.; Bernabé-Antonio, A.; Marquina-Bahena, S.; Cruz-Sosa, F. Establishment of a cell suspension culture of Ageratina pichinchensis (Kunth) for the improved production of anti-inflammatory compounds. Plants 2020, 9, 1398. [Google Scholar] [CrossRef] [PubMed]
- King, R.M.; Robinson, H. Studies in the Eupatorieae (Compositae). XIX. New combinations in Ageratina. Phytologia 1970, 18, 208–229. [Google Scholar]
- Rzedowski, G.C.; de Rzedowski, J. Flora Fanerogámica del Valle de México, 2nd ed.; 1a reprint; Instituto de Ecología y Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. Pátzcuaro: Michoacán, México, 2005; p. 1406. [Google Scholar]
- Funk, V.A.; Susanna, A.; Stuessy, T.F.; Robinson, H.E. Classification of compositae. In Systematics, Evolution, and Biogeography of Compositae; Funk, V.A., Ed.; International Association for Plant Taxonomy: Berkeley, CA, USA, 2009; pp. 171–189. [Google Scholar]
- McVaugh, R. Compositae. Flora Novo-Galiciana. A Descriptive Account of the Vascular Plants of Western Mexico; The University of Michigan Press: Ann Arbor, MI, USA, 1984; Volume 12. [Google Scholar]
- Nash, D.L.; Williams, L.O. Flora of Guatemala, Compositae. Part XII. Fieldiana Bot. 1976, 24, 96–97. [Google Scholar]
- GBFI. Ageratina Pichinchensis. Available online: https://www.gbif.org/es/species/5400249 (accessed on 20 July 2021).
- Villaseñor, R.; Espinosa, G.F.J. Catálogo de Malezas de México; Universidad Nacional Autónoma de México: México City, México, 1998; pp. 1–448. [Google Scholar]
- De Feo, V.; Soria, R.M.U. Medicinal plants and phytotherapy in traditional medicine of Paruro Province, Cusco Department, Peru. Pharmacologyonline 2012, 1, 154–219. [Google Scholar]
- Jamir, N.; Mazumder, M.U.; Khazeo, P.; Puro, K.N.; Jyrwa, R.; Sailo, L. Pharmacognostic study of the leaf of Ageratina adenophora. Adv. Eng. Res. 2018, 178, 155–158. [Google Scholar]
- INAH. Jardín Etnobotánico y Museo de la Medicina Tradicional. 2021. Available online: https://lugares.inah.gob.mx/es/museos-inah/colecciones/piezas/12915-12915-axihuitl.html?lugar_id=389 (accessed on 24 April 2021).
- Bhattarai, N.K. Traditional herbal medicines used to treat wounds and injuries in Nepal. Trop. Doct. 1997, 27, 43–47. [Google Scholar] [CrossRef]
- Gómez, F.; Quijano, L.; Calderón, J.S.; Perales, A.; Ríos, T. 2, 2-dimethylchromenes from Eupatorium aschembornianum. Phytochemistry 1982, 21, 2095–2097. [Google Scholar] [CrossRef]
- Reyes-Trejo, B.; Guerra-Ramírez, D.; Zuleta-Prada, H.; Santillán, R.; Sánchez-Mendoza, M.E.; Arrieta, J.; Reyes, L. Molecular disorder in (-)-Encecanescin. Molecules 2014, 19, 4695–4707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Barajas, L.; Rojas, V.R.; Buitrago, D.A.; Morales, M.A. Natural products and semisynthetic derivates obtained from Ageratina jahnii and Ageratina pichinchensis (Asteraceae) species. Rev. Cien. Ing. 2019, 40, 77–86. [Google Scholar]
- Kamthong, B.; Robertson, A. Furano compounds part V: The synthesis of tetrahydroeuparin and the structure of euparin. J. Chem. Soc. 1939, 933–936. [Google Scholar] [CrossRef]
- Zalkow, L.H.; Ekpo, B.A.; Harris, R.N.; Keinan, E.; Novak, J.R., Jr.; Ramming, C.T.; Van Derveer, D. The benzofurans of Isocoma wrightii structure and stereochemistry. J. Nat. Prod. 1979, 42, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Zachariah, S.M. Pharmacological activities of chromene derivates: An overview. Asian J. Pharm. Clin. Res. 2013, 6, 11–15. [Google Scholar]
- Kim, J.; Kim, C.S.; Lee, Y.M.; Sohn, E.; Jo, K.; Shin, S.D.; Kim, J.S. Scopoletin inhibits rat aldose reductase activity and cataractogenesis in galastores-fed rats. Complement. Altern. Med. 2013, 1, 1–8. [Google Scholar]
- Meegapala, K.M.; Schrader, K.K.; Burandt, C.L.; Wedge, D.E.; Duke, S.O. New class of algicidal compounds and fungicidal activities derived from a chromene amide of Amyris texana. J. Agric. Food Chem. 2010, 58, 9476–9482. [Google Scholar]
- El-Seedi, H.R. Antimicrobial arylcoumarins from Asphodelus microcarpus. J. Nat. Prod. 2007, 70, 118–120. [Google Scholar] [CrossRef]
- Chen, J.J.; Cho, J.Y.; Hwang, T.L.; Chen, I.S. Benzoic acid derivates, acetophenones, and anti-inflammatory constituents from Melicope semacarpifolia. J. Nat. Prod. 2007, 71, 71–75. [Google Scholar] [CrossRef]
- Anand, P.; Singh, B.; Singh, N. A review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg. Med. Chem. 2012, 20, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Changwong, N.; Sabphon, C.; Ingkaninan, K.; Sawasdee, P. Acetyl- and butyryl-cholinesterase inhibitory activities of mansorins and mansonones. Phytother. Res. 2012, 26, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Nature, distribution, and function of plant flavonoids. In Plant Flavonoids in Biology and Medicine: Biochemical, Pharmacological, and Structure-Activity Relationship; Alan, R., Ed.; Liss Inc.: New York, NY, USA, 1986; pp. 15–24. [Google Scholar]
- Tomás-Barberán, F.A.; Wollenweber, E. Flavonoid aglycones from the leaf surfaces of some Labiatae species. Plant. Sys. Evol. 1990, 173, 109–118. [Google Scholar] [CrossRef]
- Merfort, I.; Wendisch, D. New flavonoid glycosides from Arnicae flos DAB 91. Planta Med. 1992, 58, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cerecero, O.; Islas-Garduño, A.L.; Zamilpa, A.; Tortoriello, J. Effectiveness of an encecalin standardized extract of Ageratina pichinchensis on the treatment of onychomycosis in patients with diabetes mellitus. Wiley. Phytother. Res. 2019, 1–9. [Google Scholar]
- Flores-Fernández, J.M.; Padilla-Camberos, E.P.; Fernández-Flores, O.; Díaz-Martínez, N.E.; Barragán-Álvarez, C.P.; Ramírez-Rodríguez, P.B. Gastroprotective activity and pharmacological safety evaluation of Eupatorium aschenbornianum. Exp. Ther. Med. 2019, 18, 4467–4472. [Google Scholar]
- Rauf, A.; Patel, S.; Uddin, G.; Siddiqui, B.S.; Ahmad, B.; Muhammad, N.; Mabkhot, Y.N.; Hadda, T.B. Phytochemical, ethnomedicinal uses and pharmacological profile of genus Pistacia. Biomed. Pharmacother. 2017, 86, 393–404. [Google Scholar] [CrossRef]
- Mandala, N.E.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A. Metabolomic insights into the bioconversion of isonitrosoacetophenone in Arabidopsis thaliana and its effects on defense-related pathways. Plant. Physiol. Biochem. 2014, 84, 87–95. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Jahn, L.; Lippert, A.; Püschel, J.; Walder, A. Improvement of hairy root cultures and plants by changing biosynthetic pathways leading to pharmaceutical metabolites: Strategies and applications. Biotechnol. Adv. 2014, 32, 1168–1179. [Google Scholar] [CrossRef]
- Vanisree, M.; Tsay, H.S. Plant cell cultures: Production of biologically important secondary metabolites from medicinal plants of Taiwan. In Medicinal Plant Biotechnology. From Basic Research to Industrial Application; Kayser, O., Quax, W., Eds.; WILEY-VCH Velag GmbH & Col. KGaA: Weinheim, Germany, 2007; pp. 267–285. [Google Scholar]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Mulabagal, V.; Tsay, H. Plant cell cultures as a source for the production of biologically important secondary metabolites. Int. J. App. Sci. 2004, 2, 29–48. [Google Scholar]
- Krüger, W.; Vielreicher, S.; Kapitan, M.; Jacobsen, I.D.; Niemiec, M.J. Fungal-bacterial interactions in health and disease. Pathogens 2019, 8, 70. [Google Scholar] [CrossRef] [Green Version]
- Flores, F.C.; Beck, R.C.; da Silva, C.D.B. Essential oils for treatment for onychomycosis: A mini-review. Mycopathologia 2016, 181, 9–15. [Google Scholar] [CrossRef]
- Hay, R. Therapy of skin, hair and nail fungal infections. J. Fungi 2018, 4, 99. [Google Scholar] [CrossRef] [Green Version]
- Yatoo, M.; Gopalakrishnan, A.; Saxena, A.; Parray, O.R.; Tufani, N.A.; Chakraborty, S.; Iqbal, H. Anti-inflammatory drugs and herbs with special emphasis on herbal medicines for countering inflammatory diseases and disorders—A review. Recent. Pat. Inflamm. Allergy Drug Discov. 2018, 12, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Smetanska, I. Production of secondary metabolites using plant cell cultures. Food Biotechnol. 2008, 111, 187–228. [Google Scholar]
- Verpoorte, R.; Contin, A.; Memelink, J. Biotechnology for the production of plant secondary metabolites. Phytochem. Rev. 2002, 1, 13–25. [Google Scholar] [CrossRef]

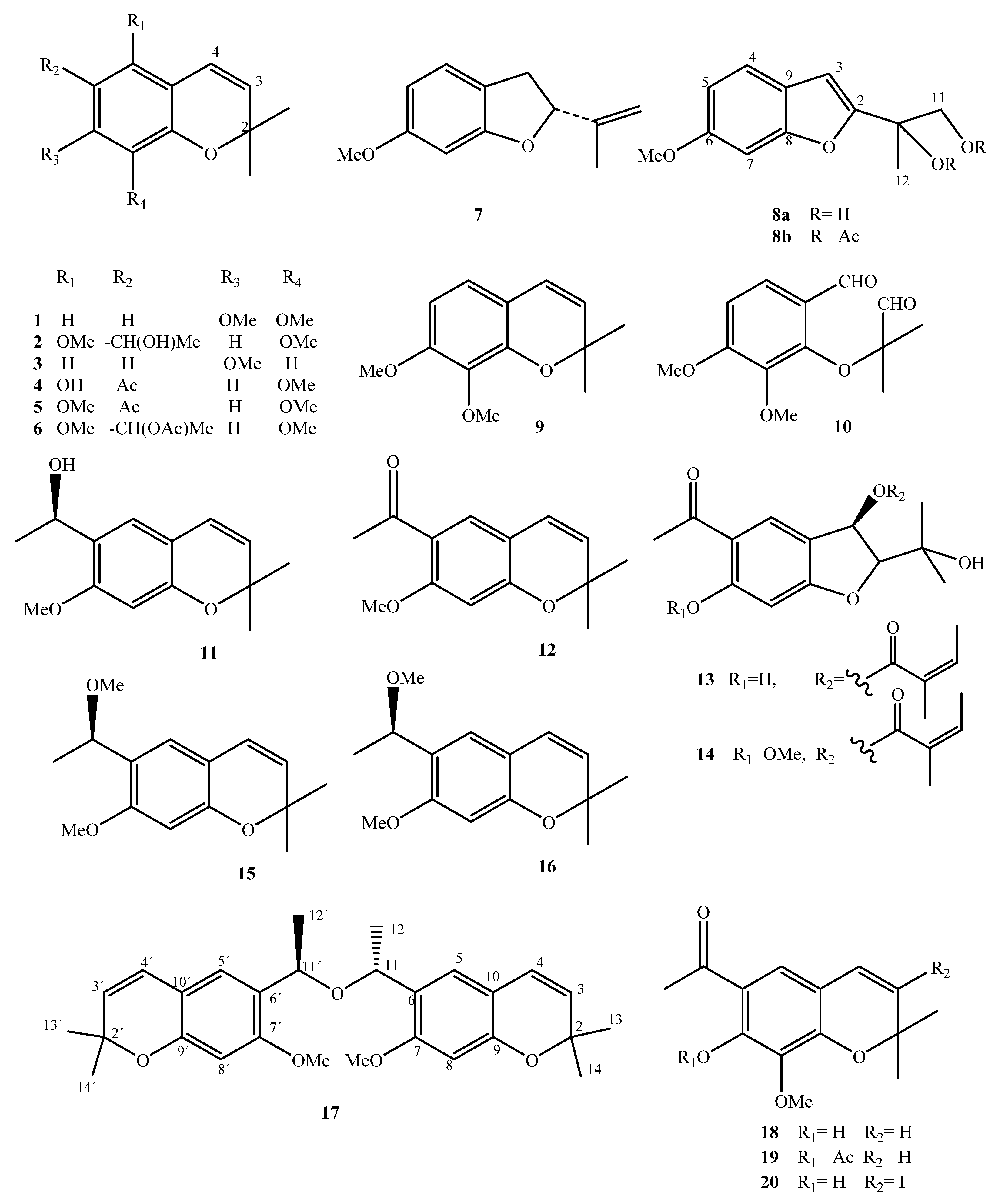
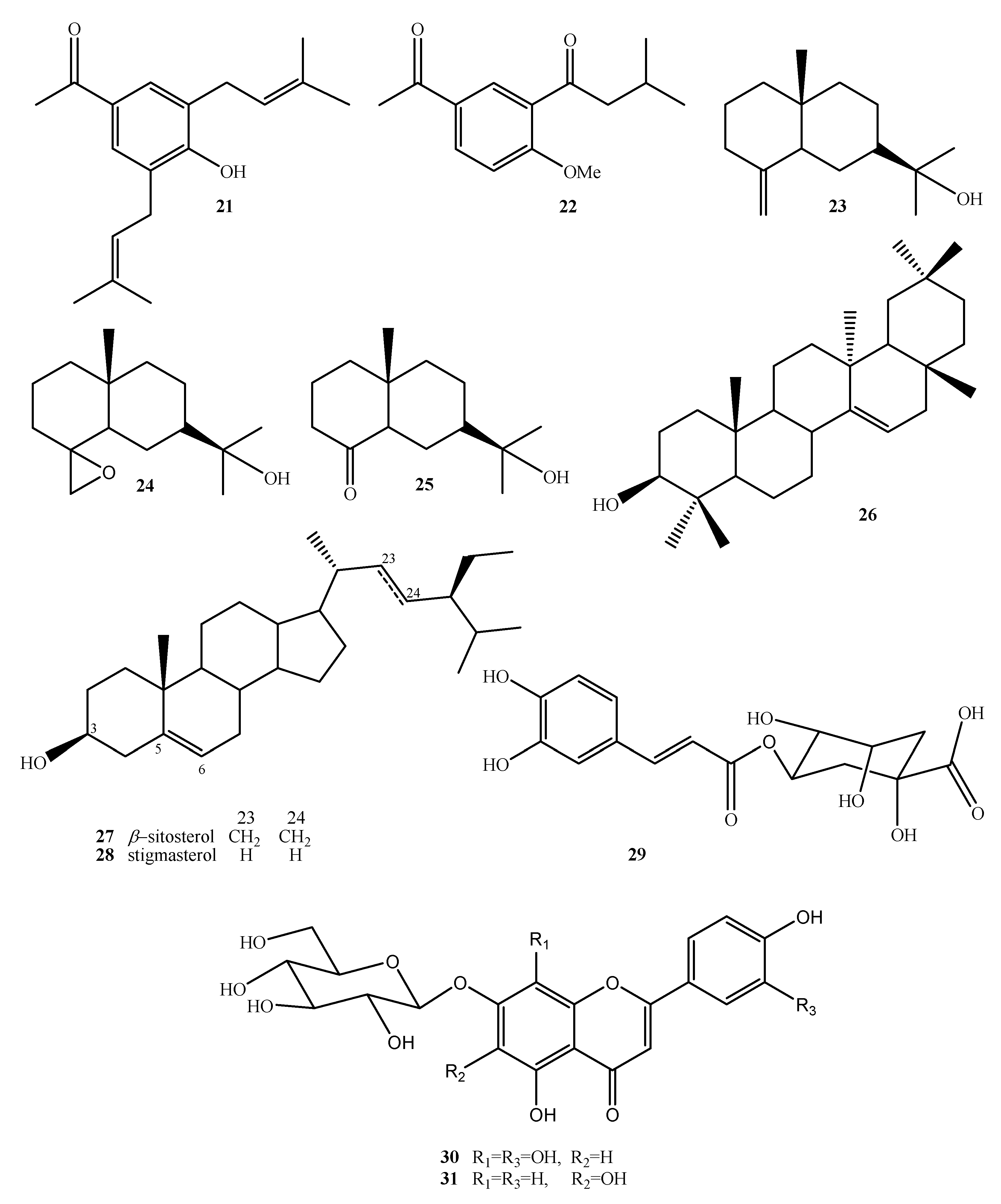
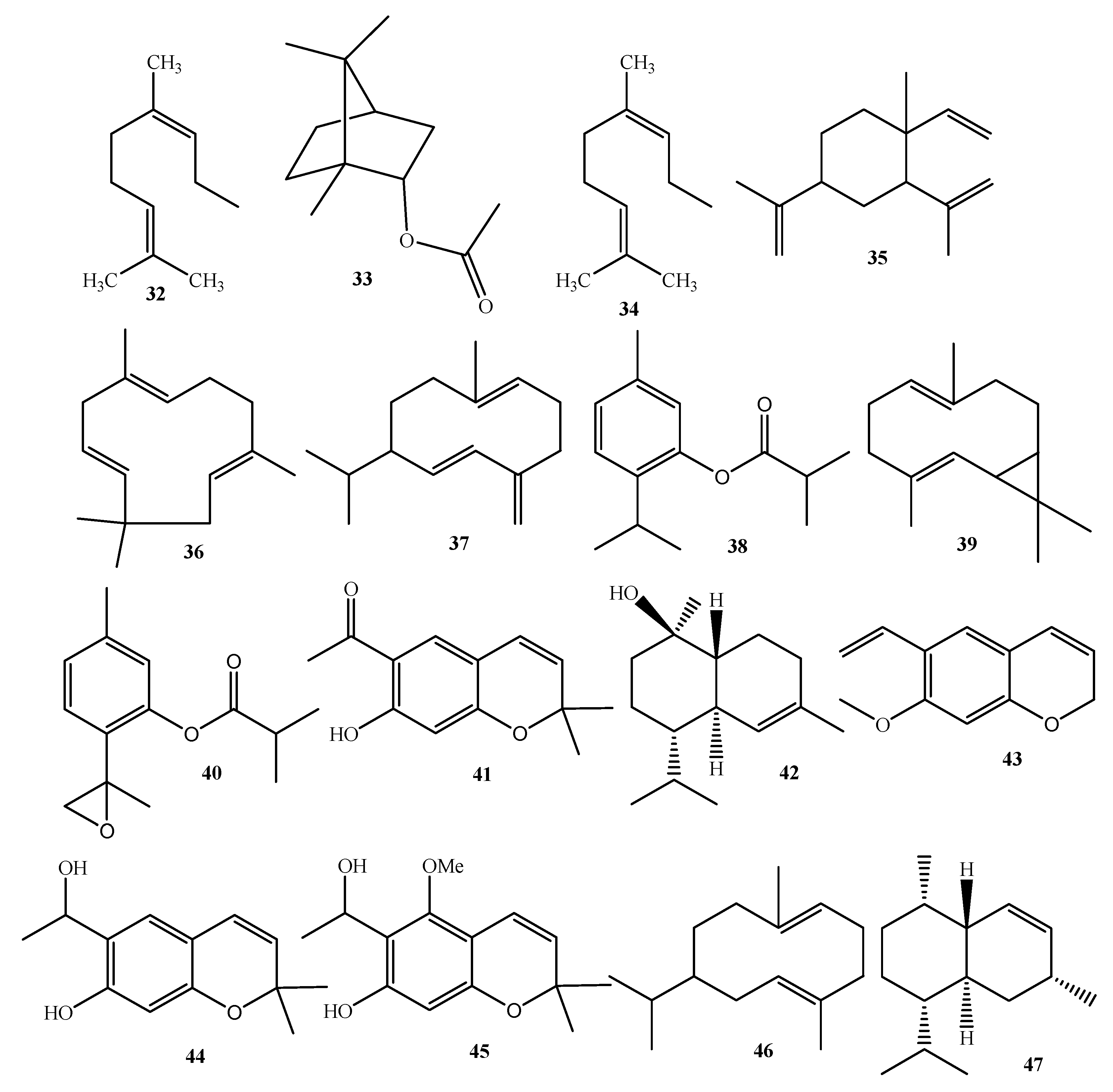
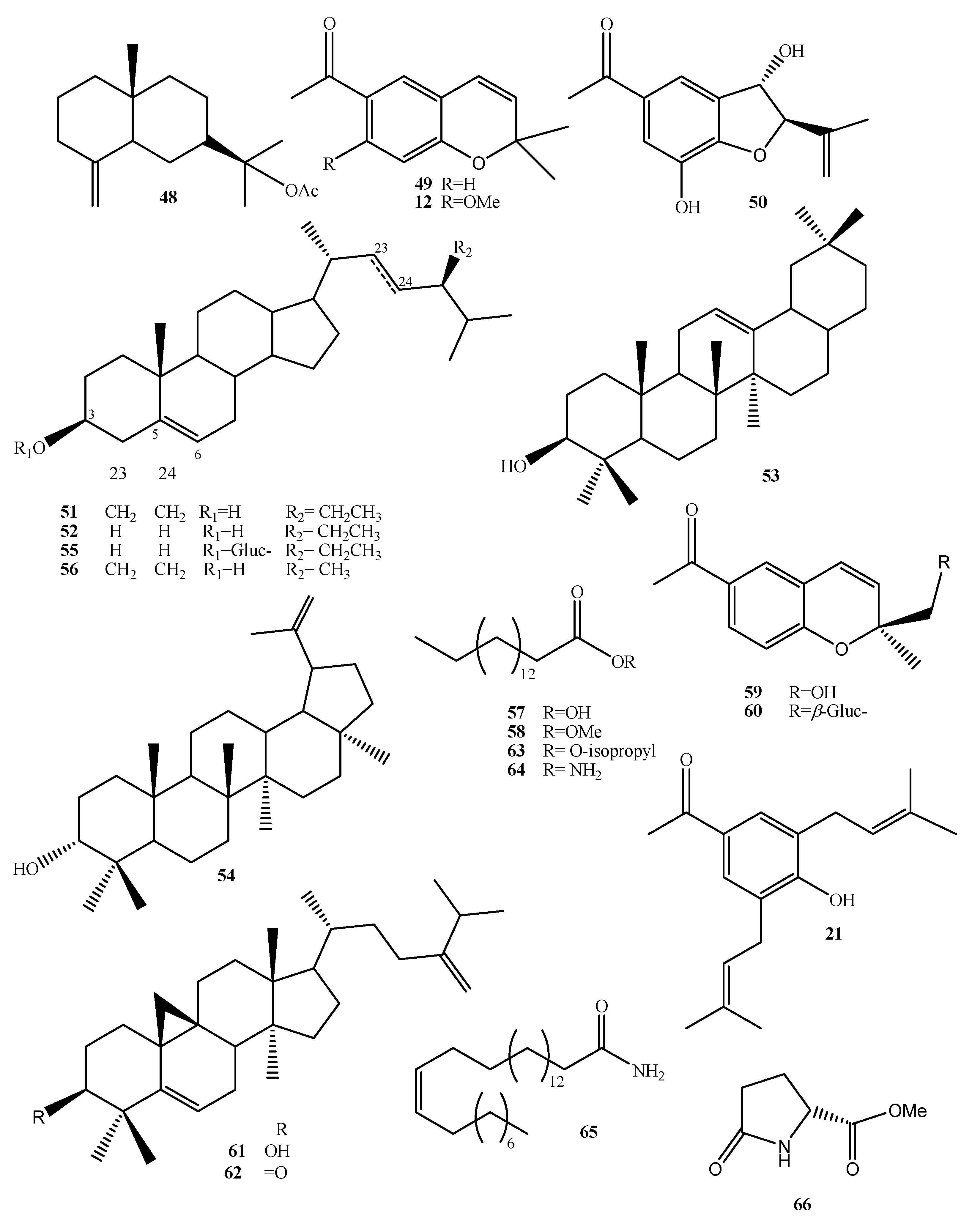
| Compound Name | No. | Plant Source | Extraction Method | Refs. |
|---|---|---|---|---|
| 7,8-Dimethoxy-2,2-dimethylchromene | 1 | Leaves and flowers | Isolation/CC | [42] |
| 6-[1-Hydroxyethyl]-5,8-dimethoxy-2,2-dimethylchromene | 2 | Leaves and flowers | Isolation/CC | [42] |
| 7-Methoxy-2,2-dimethylchromene | 3 | Leaves and flowers | Isolation/CC | [42] |
| 5-Hydroxy-6-acetyl-8-methoxy-2,2-dimethylchromene | 4 | Leaves and flowers | Isolation/CC | [42] |
| 5,8-Dimethoxy-6-acetyl-2,2-dimethylchromene | 5 | Leaves and flowers | Isolation/CC | [42] |
| 6-[Acetyl]-5,8-dimethoxy-2,2-dimethylchromene | 6 | N.A. | Acetylation of 2 | [42] |
| 2-Isopropenyl 6-methoxy-2,3-dihydrobenzofuran | 7 | Leaves and flowers | Isolation/CC | [42] |
| 6-Methoxy 2-[1,2-dihydroxy-2-propyl] benzofuran | 8a | Leaves and flowers | Isolation/CC | [42] |
| 6-Methoxy-2-[1-acetoxy-2-hydroxy-2-propyl] benzofuran | 8b | N.A. | Acetylation of 8a | [42] |
| 7,8-Dimethoxy-2,2-dimethylchromene | 9 | N.A. | Hydrogenation of 1 | [42] |
| 7,8-Mimethoxy-[2,2-dimethyloxacetalhyde] benzaldehyde | 10 | N.A. | Ozonolysis of 1 | [42] |
| Encecalinol | 11 | Aerial | HPLC Isolation/CC | [14] [16] |
| Encecalin | 12 | Aerial | HPLC Isolation/CC | [17] [16] |
| 5-Acetyl-3β-angeloyloxy-2β-(1-hydroxyisopropyl)-2,3-dihydrobenzofuran | 13 | Aerial | Isolation/CC | [17] |
| 5-Acetyl-3β-angeloyloxy-2β-(1-hydroxyisopropyl)-6-methoxy-2,3-dihydrobenzofurane | 14 | Aerial | Isolation/CC | [16] |
| O-Methylencecalinol | 15 | Aerial | Isolation/CC | [16] |
| Sonorol | 16 | Leaves | Isolation/CC | [16] |
| Encecanescin | 17 | Leaves | Isolation/CC | [20,21,43] |
| 6-Acetyl-7-hydroxy-8-methoxy-2,2-dimethylchromene | 18 | Leaves | Isolation/CC | [44] |
| 6-Acetyl-7-acetoxy-8-methoxy-2,2-dimethylchromene | 19 | N.A. | Acetylation of 18 | [44] |
| 6-Acetyl-7-hydroxy-8-methoxy-3-iodine-2,2-dimethyl-dimethylchromene | 20 | N.A. | Iodination of 18 | [44] |
| 3,5-Diprenyl-4-hydroxyacetophenone | 21 | Leaves | Isolation/CC | [21] |
| Espeleton | 22 | Aerial | Isolation/CC | [16] |
| (+)-β-eudesmol | 23 | Aerial | Isolation/CC | [16] |
| 4,15-Epoxy-(+)-β-eudesmol | 24 | N.A. | Epoxidation of 23 | [16] |
| 4-Acetoxy-(+)-β-eudesmol | 25 | N.A. | Oxidation of 23 | [16] |
| Taraxerol | 26 | Aerial | Isolation/CC | [16] |
| β-sitosterol | 27 | Aerial | Isolation/CC | [16] |
| Stigmasterol | 28 | Aerial | Isolation/CC | [16] |
| Chlorogenic acid | 29 | Leaves | HPLC | [26] |
| 7-O-(β-D-glucopyranosyl)-gossypetin | 30 | Leaves | HPLC | [44] |
| 7-O-(β-D-glucopyranosyl)-galactin | 31 | Leaves | HPLC | [44] |
| Nerol | 32 | Leaves | HydD/GC-MS | [28] |
| Bornyl acetate | 33 | Leaves | HydD/GC-MS | [28] |
| Thymol | 34 | Leaves | HydD/GC-MS | [28] |
| β-Elemene | 35 | Leaves | HydD/GC-MS | [28] |
| α-Humulene | 36 | Leaves | HydD/GC-MS | [28] |
| Germacrene-D | 37 | Leaves | HydD/GC-MS | [28] |
| Thymylisobutyrate | 38 | Leaves | HydD/GC-MS | [28] |
| Bicyclogermacrene | 39 | Leaves | HydD/GC-MS | [28] |
| 8,9-Epoxithymylisobutyrate | 40 | Leaves | HydD/GC-MS | [28] |
| Eupatoriochromene | 41 | Leaves | HydD/GC-MS | [28] |
| α-Cadinol | 42 | Leaves | HydD/GC-MS | [28] |
| Androencecalinol | 43 | Leaves | HydD/GC-MS | [28] |
| Encecalol | 44 | Leaves | HydD/GC-MS | [28] |
| Ripariochromene-A | 45 | Leaves | HydD/GC-MS | [28] |
| Germacrene-A | 46 | Leaves | HydD/GC-MS | [28] |
| Cadinene | 47 | Leaves | HydD/GC-MS | [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Ramos, M.; Marquina-Bahena, S.; Alvarez, L.; Román-Guerrero, A.; Bernabé-Antonio, A.; Cruz-Sosa, F. Phytochemical, Pharmacological, and Biotechnological Study of Ageratina pichinchensis: A Native Species of Mexico. Plants 2021, 10, 2225. https://doi.org/10.3390/plants10102225
Sánchez-Ramos M, Marquina-Bahena S, Alvarez L, Román-Guerrero A, Bernabé-Antonio A, Cruz-Sosa F. Phytochemical, Pharmacological, and Biotechnological Study of Ageratina pichinchensis: A Native Species of Mexico. Plants. 2021; 10(10):2225. https://doi.org/10.3390/plants10102225
Chicago/Turabian StyleSánchez-Ramos, Mariana, Silvia Marquina-Bahena, Laura Alvarez, Angélica Román-Guerrero, Antonio Bernabé-Antonio, and Francisco Cruz-Sosa. 2021. "Phytochemical, Pharmacological, and Biotechnological Study of Ageratina pichinchensis: A Native Species of Mexico" Plants 10, no. 10: 2225. https://doi.org/10.3390/plants10102225
APA StyleSánchez-Ramos, M., Marquina-Bahena, S., Alvarez, L., Román-Guerrero, A., Bernabé-Antonio, A., & Cruz-Sosa, F. (2021). Phytochemical, Pharmacological, and Biotechnological Study of Ageratina pichinchensis: A Native Species of Mexico. Plants, 10(10), 2225. https://doi.org/10.3390/plants10102225







