Boosting of Antioxidants and Alkaloids in Catharanthus roseus Suspension Cultures Using Silver Nanoparticles with Expression of CrMPK3 and STR Genes
Abstract
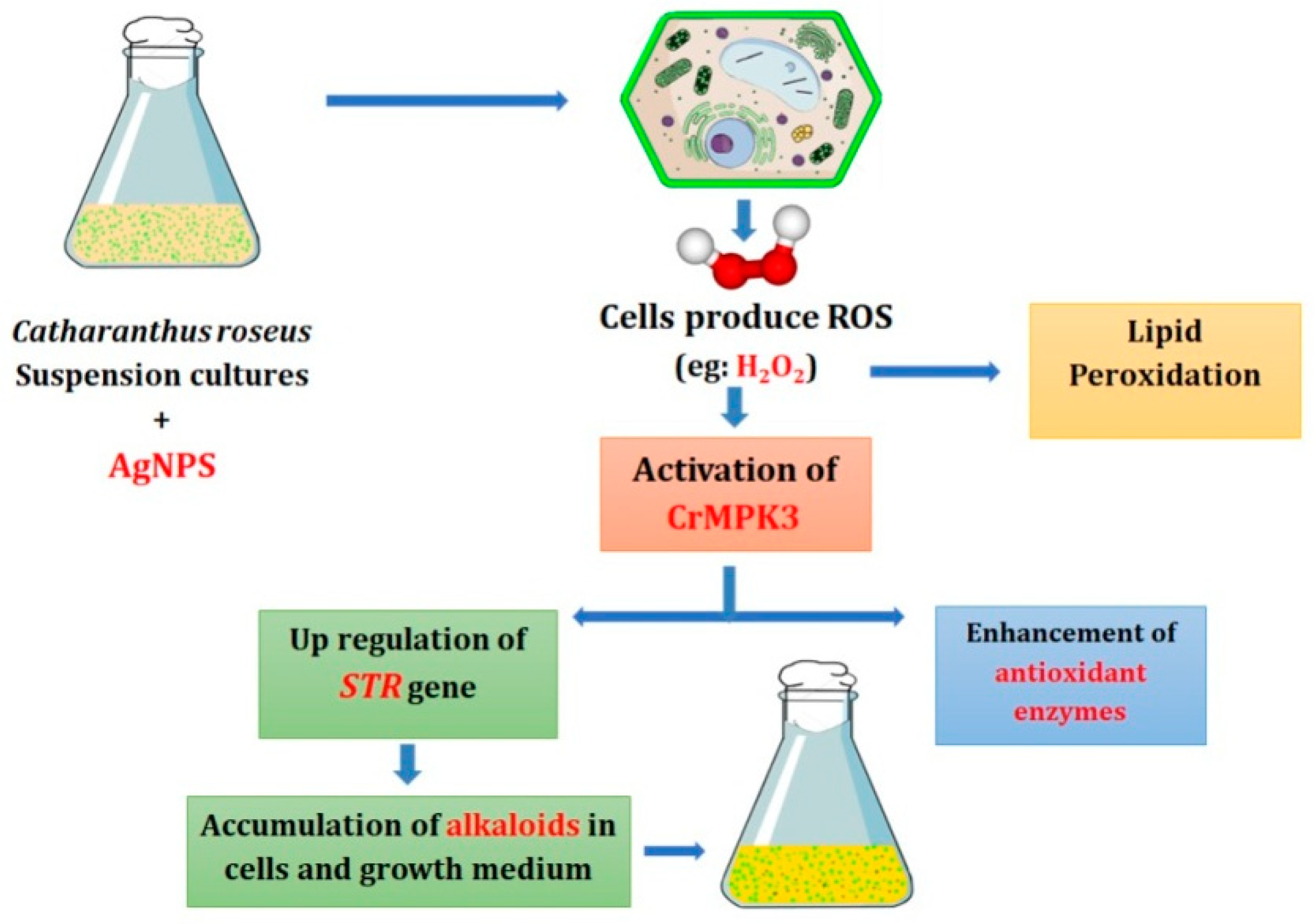
:1. Introduction
2. Results
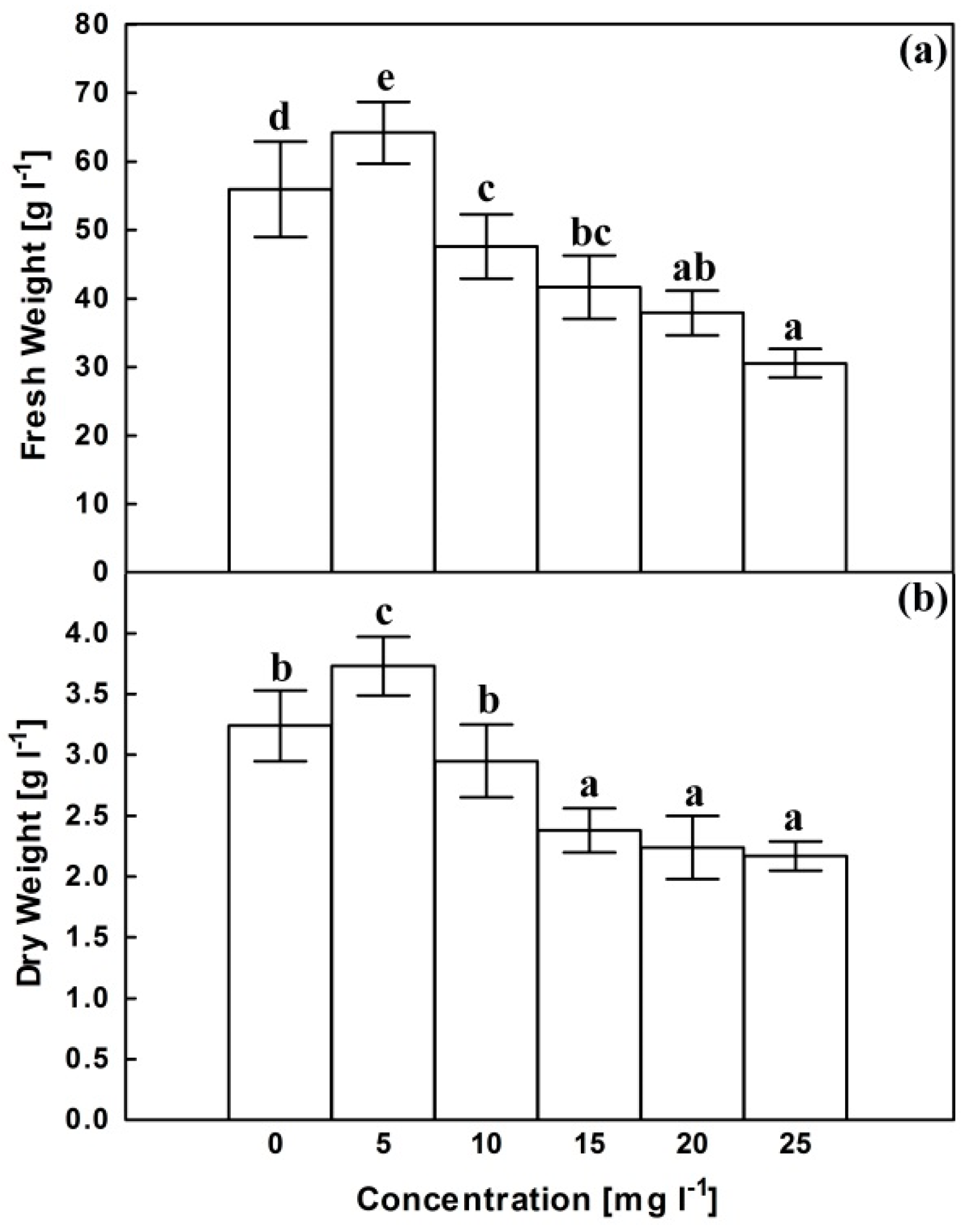
2.1. Growth Parameters
2.2. Hydrogen Peroxide (H2O2) Content and Lipid Peroxidation
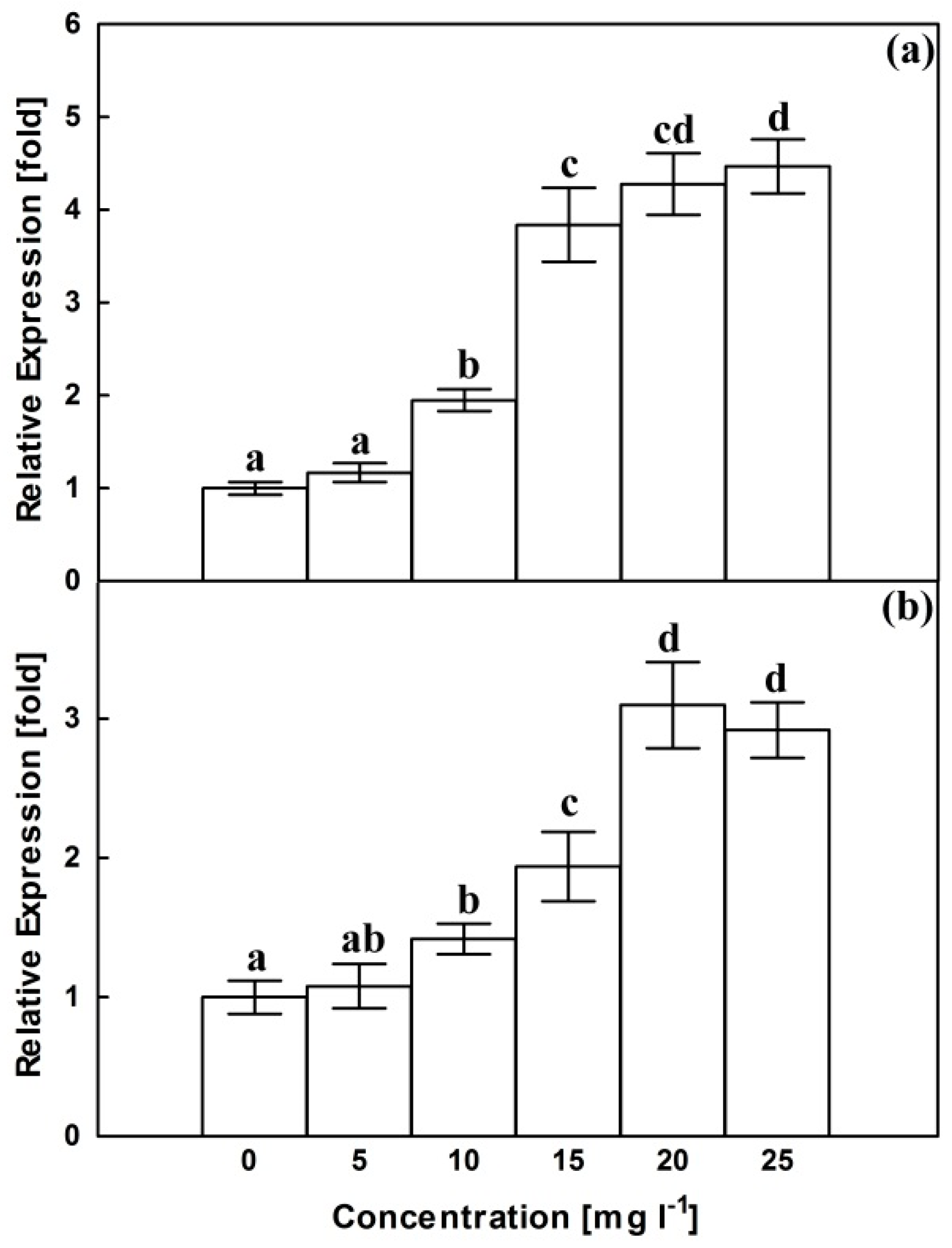
2.3. Relative Expression of CrMPK3 and STR Genes
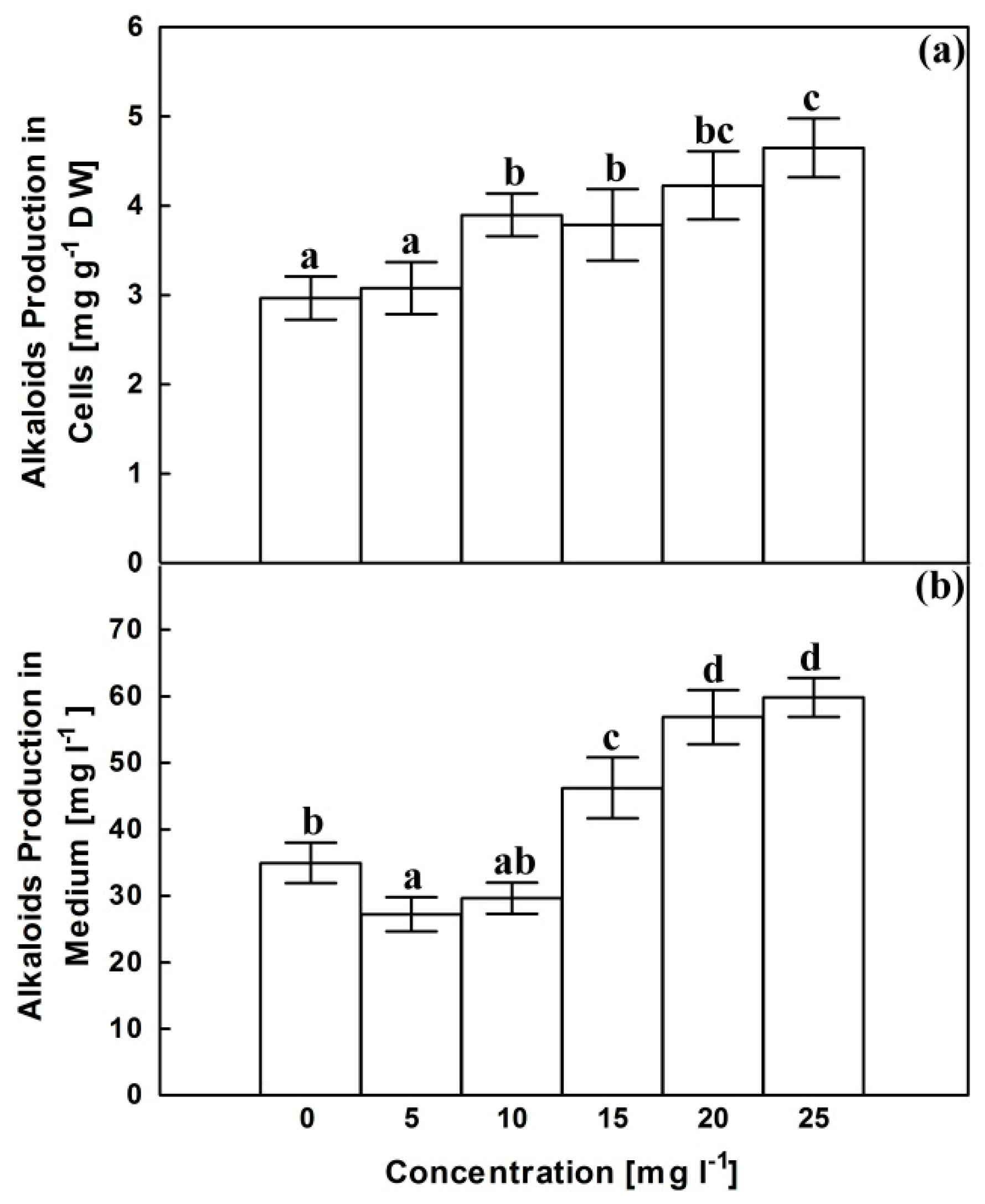
2.4. Alkaloids Content
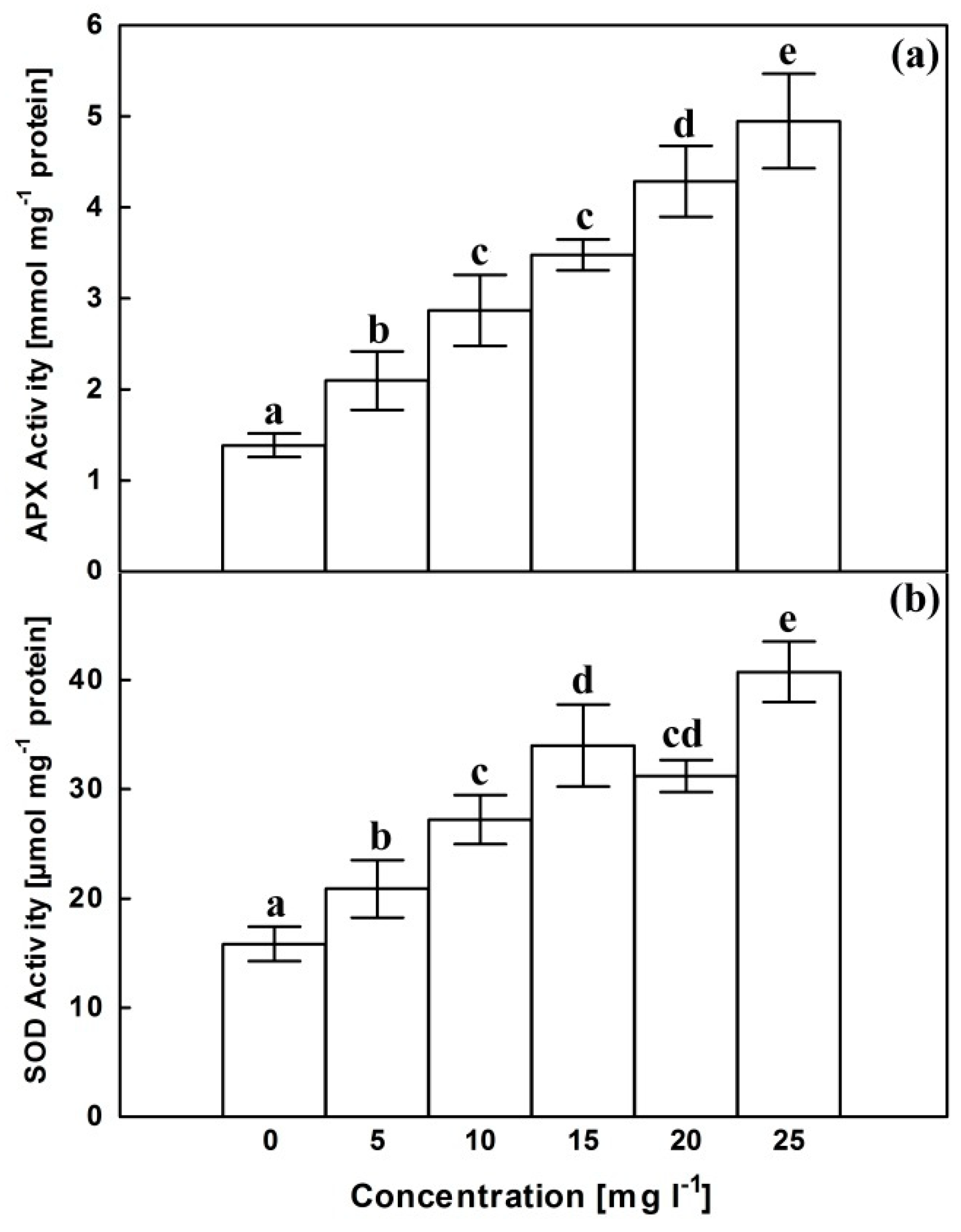
2.5. Antioxidant Enzymes
3. Discussion
4. Materials and Methods
4.1. Silver Nanoparticles Characterization and Dispersion
4.2. Plant Material and Explant Preparation
4.3. Tissue Cultures and Silver Treatments
4.4. H2O2
4.5. Lipid Peroxidation Assay
4.6. Antioxidant Enzymes and Soluble Proteins
4.7. Alkaloid Extraction and Determination
4.8. Real-Time Quantitative PCR
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, Q.; Mustafa, N.R.; Tang, K.; Choi, Y.H.; Verpoorte, R. Monoterpenoid Indole Alkaloids Biosynthesis and Its Regulation in Catharanthus roseus: A Literature Review from Genes to Metabolites. Phytochem. Rev. 2016, 15, 221–250. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.H.; Verma, A.K.; Choi, E.H. Biomedical Importance of Indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef]
- Pan, Y.-J.; Liu, J.; Guo, X.-R.; Zu, Y.-G.; Tang, Z.-H. Gene Transcript Profiles of the TIA Biosynthetic Pathway in Response to Ethylene and Copper Reveal Their Interactive Role in Modulating TIA Biosynthesis in Catharanthus roseus. Protoplasma 2015, 252, 813–824. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Hatami, M.; Hatami, M. Activating Antioxidant Enzymes, Hyoscyamine and Scopolamine Biosynthesis of Hyoscyamus Niger L. Plants with Nano-Sized Titanium Dioxide and Bulk Application. Acta Agric. Slov. 2015, 105, 23–32. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, B.; Komatsu, S.; Lu, X.; Li, X.; Tian, J. Binary Stress Induces an Increase in Indole Alkaloid Biosynthesis in Catharanthus roseus. Front. Plant Sci. 2015, 6, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austen, N.; Walker, H.J.; Lake, J.A.; Phoenix, G.K.; Cameron, D.D. The Regulation of Plant Secondary Metabolism in Response to Abiotic Stress: Interactions Between Heat Shock and Elevated CO2. Front. Plant Sci. 2019, 10, 1463. [Google Scholar] [CrossRef] [Green Version]
- Isah, T. Stress and Defense Responses in Plant Secondary Metabolites Production. Biol. Res. 2019, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.-F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive Oxygen Species and Heavy Metal Stress in Plants: Impact on the Cell Wall and Secondary Metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Hatami, M.; Ghorbanpour, M. Retracted Chapter: Changes in Phytochemicals in Response to Rhizospheric Microorganism Infection. Microb. -Mediat. Induc. Syst. Resist. Plants 2016, 1–14. [Google Scholar] [CrossRef]
- Lajayer, B.A.; Ghorbanpour, M.; Nikabadi, S. Heavy Metals in Contaminated Environment: Destiny of Secondary Metabolite Biosynthesis, Oxidative Status and Phytoextraction in Medicinal Plants. Ecotoxicol. Environ. Saf. 2017, 145, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Srivastava, A. Influence of Some Heavy Metals on Growth, Alkaloid Content and Composition in Catharanthus roseus L. Indian J. Pharm. Sci. 2010, 72, 775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathalla, M.; Abd-El Kawy, A.; Taha, H. Effect of Heavy Metal (HgCl) on 2 Accumulation and Production of Total Indole Alkaloids, Vinblastine, and/or Vincristine from Egyptian Catharanthus roseus (L.) G. Don. Calli Cultures. J. Appl. Sci. Res. 2011, 7, 542–549. [Google Scholar]
- Chen, Q.; Wu, K.; Tang, Z.; Guo, Q.; Guo, X.; Wang, H. Exogenous Ethylene Enhanced the Cadmium Resistance and Changed the Alkaloid Biosynthesis in Catharanthus roseus Seedlings. Acta Physiol. Plant. 2017, 39, 1–12. [Google Scholar] [CrossRef]
- Paeizi, M.; Karimi, F.; Razavi, K. Changes in Medicinal Alkaloids Production and Expression of Related Regulatory and Biosynthetic Genes in Response to Silver Nitrate Combined with Methyl Jasmonate in Catharanthus roseus in Vitro Propagated Shoots. Plant Physiol. Biochem. 2018, 132, 623–632. [Google Scholar] [CrossRef]
- Fouad, A.S.; Hafez, R.M. Effect of Cobalt Nanoparticles and Cobalt Ions on Alkaloids Production and Expression of CrMPK3 Gene in Catharanthus roseus Suspension Cultures. Cell. Mol. Biol. 2018, 64, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khataee, E.; Karimi, F.; Razavi, K. Chromium-Induced Alkaloid Production in Catharanthus roseus (L.) G. Don in Vitro Cultured Shoots and Related Gene Expression Patterns Particularly for the Novel Gene GS. Acta Agric. Slov. 2019, 113, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Mi, X.; Chen, C.; Wang, H.; Guo, W. Identification on Mitogen-Activated Protein Kinase Signaling Cascades by Integrating Protein Interaction with Transcriptional Profiling Analysis in Cotton. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Khataee, E.; Karimi, F.; Razavi, K. Alkaloids Production and Antioxidant Properties in Catharanthus roseus (L.) G. Don. Shoots and Study of Alkaloid Biosynthesis-Related Gene Expression Levels in Response to Methyl Jasmonate and Putrescine Treatments as Eco-Friendly Elicitors. Biol. Futur. 2019, 70, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Schmid, G. Nanoparticles: From Theory to Application; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Yan, A.; Chen, Z. Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Azeem, A.; Nada, A.A.; O’donovan, A.; Thakur, V.K.; Elkelish, A. Mycogenic Silver Nanoparticles from Endophytic Trichoderma Atroviride with Antimicrobial Activity. J. Renew. Mater. 2020, 8, 171. [Google Scholar] [CrossRef]
- Salem, S.S.; El-Belely, E.F.; Niedba\la, G.; Alnoman, M.M.; Hassan, S.E.-D.; Eid, A.M.; Shaheen, T.I.; Elkelish, A.; Fouda, A. Bactericidal and In-Vitro Cytotoxic Efficacy of Silver Nanoparticles (Ag-NPs) Fabricated by Endophytic Actinomycetes and Their Use as Coating for the Textile Fabrics. Nanomaterials 2020, 10, 2082. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.; Qari, S.H.; Abu-Elsaoud, A.; El-Esawi, M.; Alhaithloul, H.; Elkelish, A. Rapid Green Synthesis of Silver Nanoparticles from Blue Gum Augment Growth and Performance of Maize, Fenugreek, and Onion by Modulating Plants Cellular Antioxidant Machinery and Genes Expression. Acta Physiol. Plant. 2020, 42, 1–16. [Google Scholar] [CrossRef]
- Jamshidi, M.; Ghanati, F. Taxanes Content and Cytotoxicity of Hazel Cells Extract after Elicitation with Silver Nanoparticles. Plant Physiol. Biochem. 2017, 110, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.-M.; Rajakumar, G.; Thiruvengadam, M. Effect of Silver Nanoparticles on Phenolic Compounds Production and Biological Activities in Hairy Root Cultures of Cucumis Anguria. Acta Biol. Hung. 2018, 69, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Mohammad, S.; Khan, M.A.; Raja, N.I.; Arif, M.; Kamil, A.; Mashwani, Z.-R. Silver Nanoparticles Elicited in Vitro Callus Cultures for Accumulation of Biomass and Secondary Metabolites in Caralluma Tuberculata. Artif. Cells Nanomed. Biotechnol. 2019, 47, 715–724. [Google Scholar] [CrossRef] [Green Version]
- Zahir, A.; Nadeem, M.; Ahmad, W.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Chemogenic Silver Nanoparticles Enhance Lignans and Neolignans in Cell Suspension Cultures of Linum usitatissimum L. Plant Cell Tissue Organ Cult. 2019, 136, 589–596. [Google Scholar] [CrossRef]
- Naeem, M.; Aftab, T.; Khan, M.M.A. Catharanthus Roseus; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Hussain, M.S.; Fareed, S.; Saba Ansari, M.; Rahman, A.; Ahmad, I.Z.; Saeed, M. Current Approaches toward Production of Secondary Plant Metabolites. J. Pharm. Bioallied Sci. 2012, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Castro-González, C.G.; Sánchez-Segura, L.; Gómez-Merino, F.C.; Bello-Bello, J.J. Exposure of Stevia (Stevia Rebaudiana B.) to Silver Nanoparticles in Vitro: Transport and Accumulation. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Gupta, S.D.; Agarwal, A.; Pradhan, S. Phytostimulatory Effect of Silver Nanoparticles (AgNPs) on Rice Seedling Growth: An Insight from Antioxidative Enzyme Activities and Gene Expression Patterns. Ecotoxicol. Environ. Saf. 2018, 161, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.; Ali, E.; El-Deeb, B. Improvement of Postharvest Quality of Cut Rose Cv.‘First Red’by Biologically Synthesized Silver Nanoparticles. Sci. Hortic. 2014, 179, 340–348. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Wang, Y.; Zhang, Z.-H.; Zu, Y.-G.; Efferth, T.; Tang, Z.-H. The Combined Effects of Ethylene and MeJA on Metabolic Profiling of Phenolic Compounds in Catharanthus roseus Revealed by Metabolomics Analysis. Front. Physiol. 2016, 7, 217. [Google Scholar] [CrossRef] [Green Version]
- Thuesombat, P.; Hannongbua, S.; Ekgasit, S.; Chadchawan, S. Effects of Silver Nanoparticles on Hydrogen Peroxide Generation and Antioxidant Enzyme Responses in Rice. J. Nanosci. Nanotechnol. 2016, 16, 8030–8043. [Google Scholar] [CrossRef]
- Barbasz, A.; Kreczmer, B.; Oćwieja, M. Effects of Exposure of Callus Cells of Two Wheat Varieties to Silver Nanoparticles and Silver Salt (AgNO 3). Acta Physiol. Plant. 2016, 38, 76. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, D.K.; Singh, S.; Singh, S.; Srivastava, P.K.; Singh, V.P.; Singh, S.; Prasad, S.M.; Singh, P.K.; Dubey, N.K.; Pandey, A.C.; et al. Nitric Oxide Alleviates Silver Nanoparticles (AgNps)-Induced Phytotoxicity in Pisum Sativum Seedlings. Plant Physiol. Biochem. 2017, 110, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-Q.; Qing, T.; Li, C.-C.; Li, F.; Ge, F.; Fei, J.-J.; Peijnenburg, W.J. Integration of Subcellular Partitioning and Chemical Forms to Understand Silver Nanoparticles Toxicity to Lettuce (Lactuca Sativa L.) under Different Exposure Pathways. Chemosphere 2020, 258, 127349. [Google Scholar] [CrossRef] [PubMed]
- Moazzami Farida, S.H.; Karamian, R.; Albrectsen, B.R. Silver Nanoparticle Pollutants Activate Oxidative Stress Responses and Rosmarinic Acid Accumulation in Sage. Physiol. Plant. 2020, 170, 415–432. [Google Scholar] [CrossRef]
- Hafez, R.; Fouad, A. Mitigation of Genotoxic and Cytotoxic Effects of Silver Nanoparticles on Onion Root Tips Using Some Antioxidant Scavengers. Egypt. J. Bot. 2020, 60, 133–145. [Google Scholar] [CrossRef]
- Rivero-Montejo, S.d.J.; Vargas-Hernandez, M.; Torres-Pacheco, I. Nanoparticles as Novel Elicitors to Improve Bioactive Compounds in Plants. Agriculture 2021, 11, 134. [Google Scholar] [CrossRef]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles Alter Secondary Metabolism in Plants via ROS Burst. Front. Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef] [Green Version]
- Smirnoff, N.; Arnaud, D. Hydrogen Peroxide Metabolism and Functions in Plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef]
- Černỳ, M.; Habánová, H.; Berka, M.; Luklová, M.; Brzobohatỳ, B. Hydrogen Peroxide: Its Role in Plant Biology and Crosstalk with Signalling Networks. Int. J. Mol. Sci. 2018, 19, 2812. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Zhu, M.; Song, W.; Harmon, A.C.; Chen, S. Oxidation and Phosphorylation of MAP Kinase 4 Cause Protein Aggregation. Biochim. Et Biophys. Acta (BBA)-Proteins Proteom. 2015, 1854, 156–165. [Google Scholar] [CrossRef]
- Matern, S.; Peskan-Berghoefer, T.; Gromes, R.; Kiesel, R.V.; Rausch, T. Imposed Glutathione-Mediated Redox Switch Modulates the Tobacco Wound-Induced Protein Kinase and Salicylic Acid-Induced Protein Kinase Activation State and Impacts on Defence against Pseudomonas Syringae. J. Exp. Bot. 2015, 66, 1935–1950. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.-Y.; Zhang, C.; Li, Z.-C.; Wang, Z.-R.; Jiang, X.-X.; Shi, Y.-F.; Tian, S.-N.; Braun, E.; Mei, Y.; Qiu, W.-L.; et al. The MAPK Kinase Kinase GmMEKK1 Regulates Cell Death and Defense Responses. Plant Physiol. 2018, 178, 907–922. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, J.; Lee, S.; Wen, R. Copper-Caused Oxidative Stress Triggers the Activation of Antioxidant Enzymes via ZmMPK3 in Maize Leaves. PLoS ONE 2018, 13, e0203612. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, C. A Review of Redox Signaling and the Control of MAP Kinase Pathway in Plants. Redox Biol. 2017, 11, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Macáková, K.; Afonso, R.; Saso, L.; Mladěnka, P. The Influence of Alkaloids on Oxidative Stress and Related Signaling Pathways. Free Radic. Biol. Med. 2019, 134, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Heredia, A.A.; Marín-López, R.; Ponce-Noyola, T.; Cerda-García-Rojas, C.M.; Trejo-Tapia, G.; Ramos-Valdivia, A.C. Oxidative Stress Induces Alkaloid Production in Uncaria Tomentosa Root and Cell Cultures in Bioreactors. Eng. Life Sci. 2009, 9, 211–218. [Google Scholar] [CrossRef]
- Vera-Reyes, I.; Huerta-Heredia, A.A.; Ponce-Noyola, T.; Flores-Sanchez, I.J.; Esparza-García, F.; Cerda-García-Rojas, C.M.; Trejo-Tapia, G.; Ramos-Valdivia, A.C. Strictosidine-Related Enzymes Involved in the Alkaloid Biosynthesis of Uncaria Tomentosa Root Cultures Grown under Oxidative Stress. Biotechnol. Prog. 2013, 29, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Raina, S.K.; Wankhede, D.P.; Jaggi, M.; Singh, P.; Jalmi, S.K.; Raghuram, B.; Sheikh, A.H.; Sinha, A.K. CrMPK3, a Mitogen Activated Protein Kinase from Catharanthus roseus and Its Possible Role in Stress Induced Biosynthesis of Monoterpenoid Indole Alkaloids. BMC Plant Biol. 2012, 12, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Shakeran, Z.; Keyhanfar, M.; Asghari, G.; Ghanadian, M. Improvement of Atropine Production by Different Biotic and Abiotic Elicitors in Hairy Root Cultures of Datura Metel. Turk. J. Biol. 2015, 39, 111–118. [Google Scholar] [CrossRef]
- Karakaş, Ö. Effect of Silver Nanoparticles on Production of Indole Alkaloids in Isatis Constricta. Iran. J. Sci. Technol. Trans. A Sci. 2020, 44, 621–627. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Plant Defense against Herbivores: Chemical Aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nwaichi, E.; Anosike, E. Plant Response on Exposure to Ag Nanoparticles: A Study with Vigna Subterranean. Biochem. Anal. Biochem 2016, 5, 1–6. [Google Scholar] [CrossRef]
- Jadczak, P.; Kulpa, D.; Drozd, R.; Przewodowski, W.; Przewodowska, A. Effect of AuNPs and AgNPs on the Antioxidant System and Antioxidant Activity of Lavender (Lavandula Angustifolia Mill.) from In Vitro Cultures. Molecules 2020, 25, 5511. [Google Scholar] [CrossRef] [PubMed]
- Dudziak, K.; Zapalska, M.; Börner, A.; Szczerba, H.; Kowalczyk, K.; Nowak, M. Analysis of Wheat Gene Expression Related to the Oxidative Stress Response and Signal Transduction under Short-Term Osmotic Stress. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fouad, A.; Hafez, R. The Effects of Silver Ions and Silver Nanoparticles on Cell Division and Expression of Cdc2 Gene in Allium Cepa Root Tips. Biol. Plant. 2018, 62, 166–172. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Fouad, A.S.; Hafez, R.M. Effects of Cobalt Ions and Cobalt Nanoparticles on Transient Expression of Gus Gene in Catharanthus roseus Suspension Cultures. J. Radiat. Res. Appl. Sci. 2020, 13, 765–775. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene Produced by Leaves Protects the Photosynthetic Apparatus against Ozone Damage, Quenches Ozone Products, and Reduces Lipid Peroxidation of Cellular Membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Stewart, R.R.; Bewley, J.D. Lipid Peroxidation Associated with Accelerated Aging of Soybean Axes. Plant Physiol. 1980, 65, 245–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, H.; Chen, G.; Li, F.; Wang, X.; Hu, Y.; Bi, Y. Involvement of G6PDH in Heat Stress Tolerance in the Calli from Przewalskia Tangutica and Nicotiana Tabacum. Biol. Plant. 2012, 56, 422–430. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Elkahoui, S.; Hernández, J.A.; Abdelly, C.; Ghrir, R.; Limam, F. Effects of Salt on Lipid Peroxidation and Antioxidant Enzyme Activities of Catharanthus roseus Suspension Cells. Plant Sci. 2005, 168, 607–613. [Google Scholar] [CrossRef] [Green Version]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Lee, S.; Cheng, K.; Scott, A. Effects of Bioregulators on Indole Alkaloid Biosynthesis in Catharanthus roseus Cell Culture. Phytochemistry 1981, 20, 1841–1843. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2- ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| AgNPs Conc. | Fresh Weight | Dry Weight | H2O2 Content | MDA Content | APX Activity | SOD Activity | CrMPK3 Expression | STR Expression | Alkaloids in Cells | |
|---|---|---|---|---|---|---|---|---|---|---|
| Fresh Weight | −0.874 * | |||||||||
| Dry Weight | −0.839 * | 0.948 * | ||||||||
| H2O2 Content | 0.883 * | −0.848 * | −0.790 * | |||||||
| MDA Content | 0.928 * | −0.848 * | −0.852 * | 0.841 * | ||||||
| APX activity | 0.974 * | −0.813 * | −0.786 * | 0.898 * | 0.941 * | |||||
| SOD activity | 0.933 * | −0.808 * | −0.767 * | 0.753 * | 0.890 * | 0.907 * | ||||
| CrMPK3Expression | 0.950 * | −0.885 * | −0.883 * | 0.809 * | 0.904 * | 0.928 * | 0.883 * | |||
| STRExpression | 0.926 * | −0.819 * | −0.854 * | 0.828 * | 0.869 * | 0.908 * | 0.776 * | 0.904 * | ||
| Alkaloids in cells | 0.883 * | −0.802 * | −0.722 * | 0.787 * | 0.778 * | 0.853 * | 0.892 * | 0.821 * | 0.767 * | |
| Alkaloids in medium | 0.860 * | −0.814 * | −0.844 * | 0.685 * | 0.835 * | 0.834 * | 0.763 * | 0.902 * | 0.927 * | 0.751 * |
| Gene | Primers Sequence |
|---|---|
| Actin | 5′-CTATGTTCCCAGGTATTGCAGATAGA-3′ 5′-GCTGCTTGGAGCCAAAGC-3′ |
| CrMPK3 | 5′ACGAAATGAGGATGCAAAAAGATAC-3′ 5′-TGCTAACTGCTGACGAGGGAAT-3′ |
| STR | 5′-TGCTTCACTCCCATCATTTACAGT-3′ 5′-CTGCCATCATGGATTTAGATTCAG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouad, A.; Hegazy, A.E.; Azab, E.; Khojah, E.; Kapiel, T. Boosting of Antioxidants and Alkaloids in Catharanthus roseus Suspension Cultures Using Silver Nanoparticles with Expression of CrMPK3 and STR Genes. Plants 2021, 10, 2202. https://doi.org/10.3390/plants10102202
Fouad A, Hegazy AE, Azab E, Khojah E, Kapiel T. Boosting of Antioxidants and Alkaloids in Catharanthus roseus Suspension Cultures Using Silver Nanoparticles with Expression of CrMPK3 and STR Genes. Plants. 2021; 10(10):2202. https://doi.org/10.3390/plants10102202
Chicago/Turabian StyleFouad, Ahmed, Adel E. Hegazy, Ehab Azab, Ebtihal Khojah, and Tarek Kapiel. 2021. "Boosting of Antioxidants and Alkaloids in Catharanthus roseus Suspension Cultures Using Silver Nanoparticles with Expression of CrMPK3 and STR Genes" Plants 10, no. 10: 2202. https://doi.org/10.3390/plants10102202
APA StyleFouad, A., Hegazy, A. E., Azab, E., Khojah, E., & Kapiel, T. (2021). Boosting of Antioxidants and Alkaloids in Catharanthus roseus Suspension Cultures Using Silver Nanoparticles with Expression of CrMPK3 and STR Genes. Plants, 10(10), 2202. https://doi.org/10.3390/plants10102202









