Modulation of Cellular Redox Status and Antioxidant Defense System after Synergistic Application of Zinc Oxide Nanoparticles and Salicylic Acid in Rice (Oryza sativa) Plant under Arsenic Stress
Abstract
:1. Introduction
2. Results
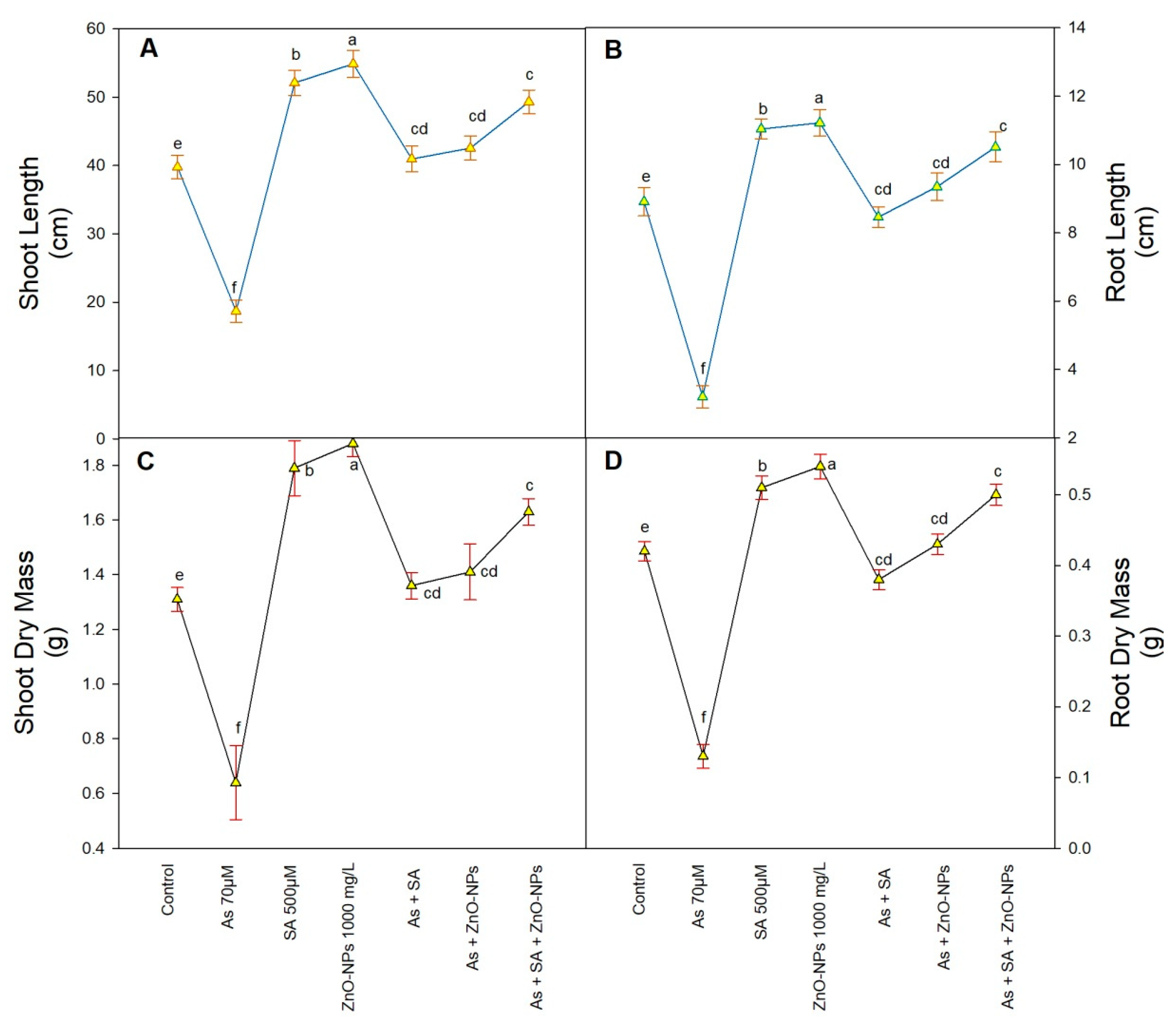
2.1. Effect of As Toxicity on Rice Growth
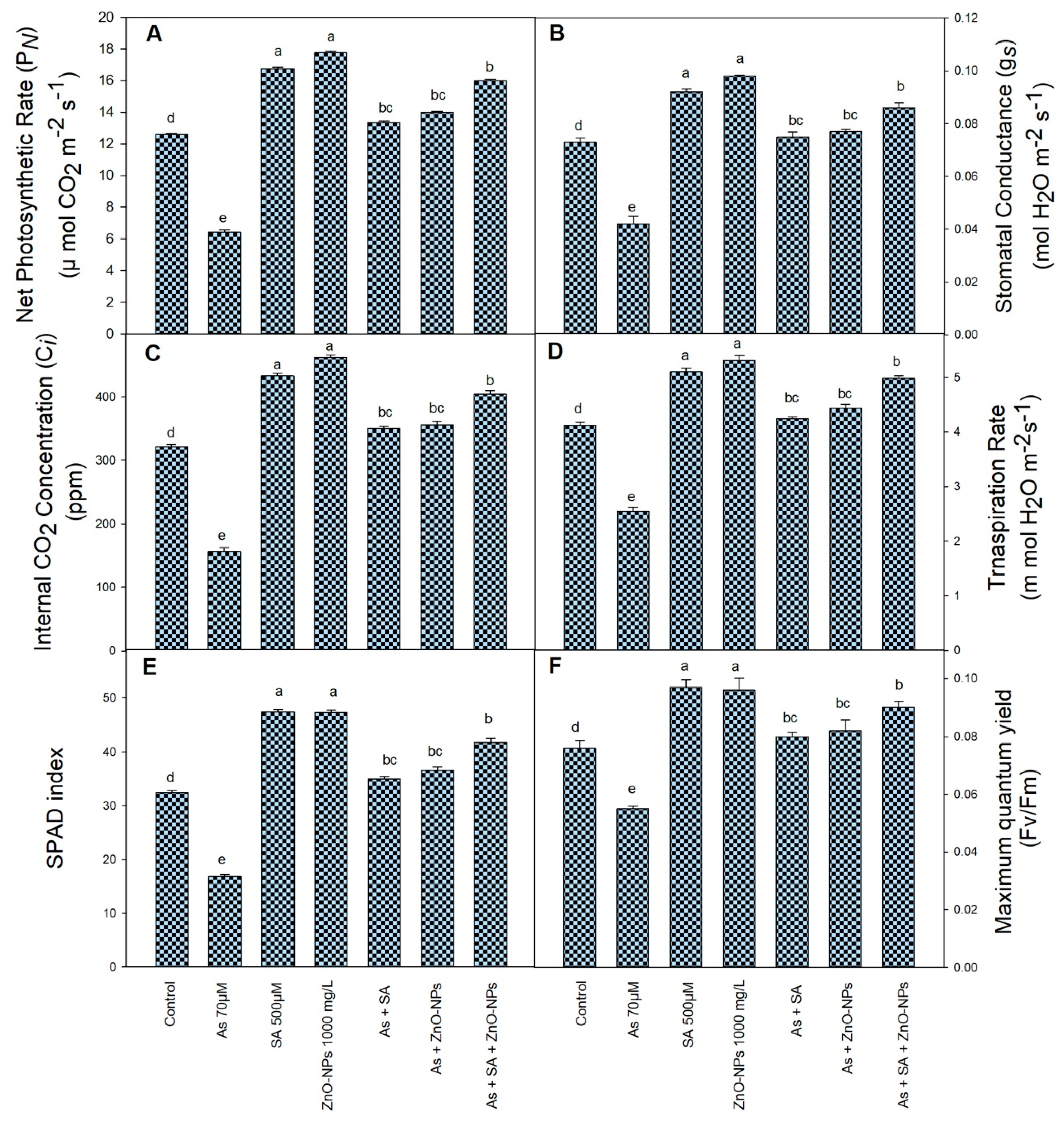
2.2. Effect of SA and ZnONPs on Physiological Indices under As Stress
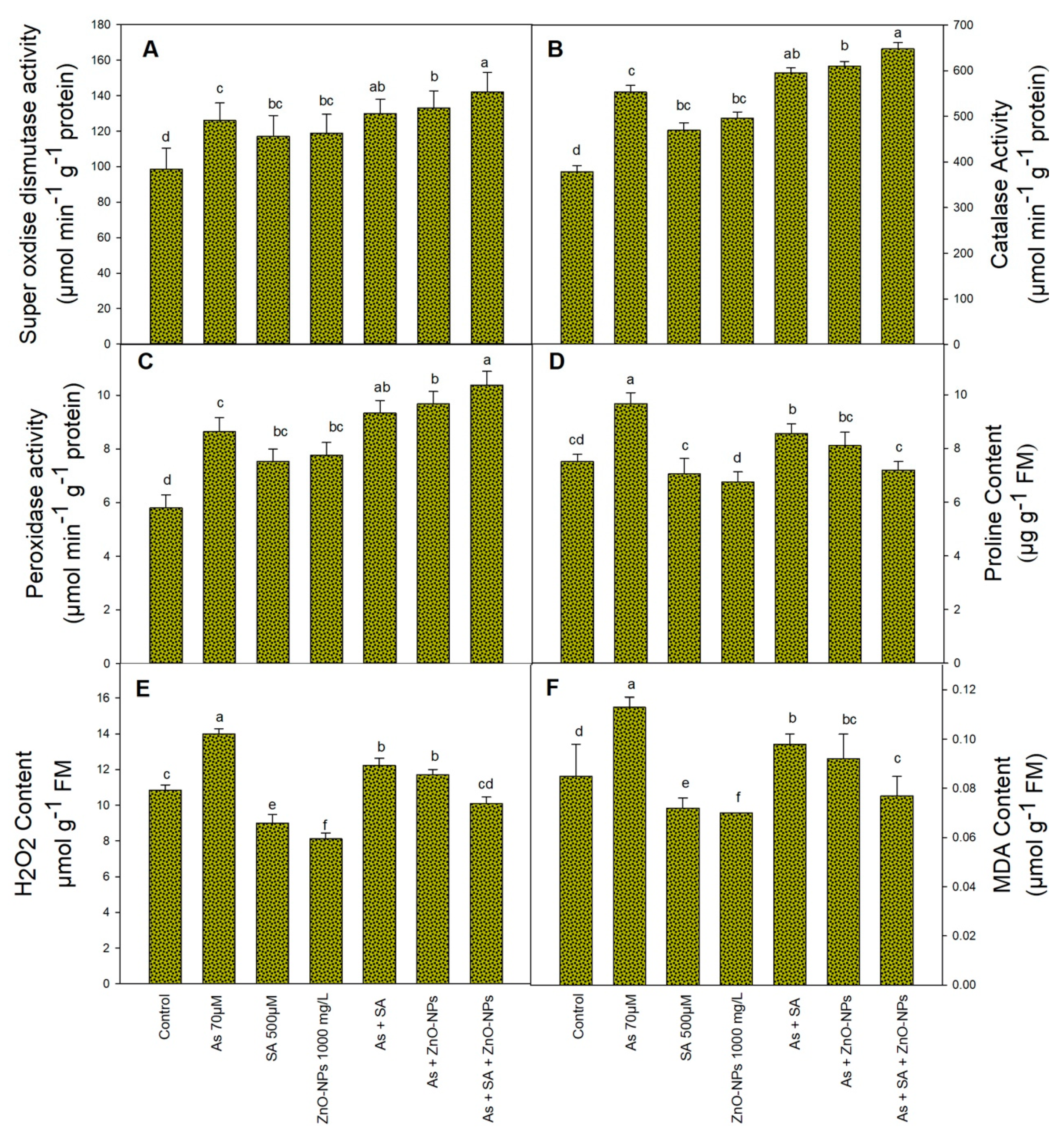
2.3. Combined Application of SA and ZnONPs on Antioxidant Enzyme Activity under As Stress
2.4. Effect of SA and ZnONPs As a Foliar Fertigation on H2O2, MDA and Proline Content under As
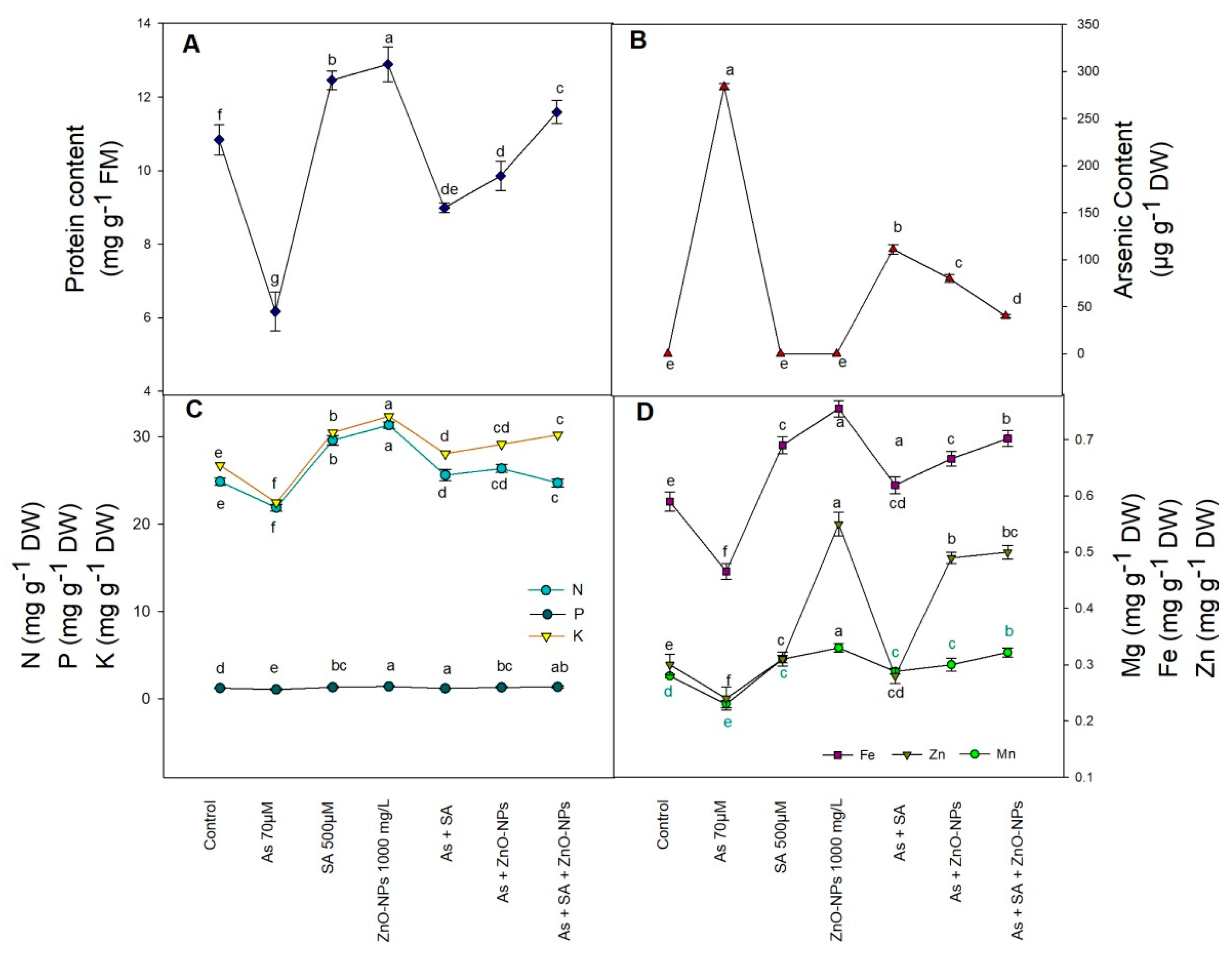
2.5. Total Soluble Protein and As Content
2.6. Arsenic Mediated Accumulation in Different Elements
3. Discussion
4. Materials and Methods
4.1. Characterization of Zinc Oxide Nanoparticles
4.2. Plant Material, Salicylic Acid and Zinc Oxide Nanoparticle Treatments and Sample Collection
4.3. Morphological Parameters
4.4. Physiological Measurement
4.5. Antioxidant Enzymes Analysis
4.6. Determination of Proline, Hydrogen Peroxide, Malondialdehyde and Total Soluble Protein
4.7. Accumulatin Analysis of Different Elements
4.8. Estimation of As
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, A.P.; Dixit, G.; Mishra, S.; Dwivedi, S.; Tiwari, M.; Mallick, S.; Pandey, V.; Trivedi, P.K.; Chakrabarty, D.; Tripathi, R.D. Salicylic acid modulates arsenic toxicity by reducing its root to shoot translocation in rice (Oryza sativa L.). Front. Plant Sci. 2015, 6, 340. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, R.D.; Srivastava, S.; Mishra, S.; Singh, N.; Tuli, R.; Gupta, D.K. Arsenic hazards: Strategies for tolerance and remediation by plants. Trends Biotechnol. 2007, 25, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dubey, R.S.; Tripathi, R.D.; Chakrabarty, D.; Trivedi, P.K. Omics and biotechnology of arsenic stress and detoxification in plants: Current updates and prospective. Environ. Int. 2015, 74, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, P.M.; Chen, W. Arsenic toxicity: The effects on plant metabolism. Front. Physiol. 2012, 3, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Hussain, I.; Singh, N.B.; Singh, H. Uptake, translocation and impact of green synthesized nanoceria on growth and antioxidant enzymes activity of Solanum lycopersicum L. Ecotoxicol. Environ. Saf. 2019, 182, 109410. [Google Scholar] [CrossRef]
- Xiao, C.; Li, H.; Zhao, Y.; Zhang, X.; Wang, X. Green synthesis of iron nanoparticle by tea extract (polyphenols) and its selective removal of cationic dyes. J. Environ. Manag. 2020, 275, 111262. [Google Scholar] [CrossRef]
- Srivastava, S.; Shukla, A.; Rajput, V.D.; Kumar, K.; Minkina, T.; Mandzhieva, S.; Shmaraeva, A.; Suprasanna, P. Arsenic Remediation through Sustainable Phytoremediation Approaches. Minerals 2021, 11, 936. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Andrews, J.; Sanabria, J.; Bindraban, P.S.; Singh, U.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Interactive effects of drought, organic fertilizer, and zinc oxide nanoscale and bulk particles on wheat performance and grain nutrient accumulation. Sci Total Environ. 2020, 722, 137808. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Faraz, A.; Hayat, S. Effective use of zinc oxide nanoparticles through root dipping on the performance of growth, quality, photosynthesis and antioxidant system in tomato. J. Plant Biochem. Biotechn. 2019, 29, 553–567. [Google Scholar] [CrossRef]
- Vaghar, M.S.; Sayfzadeh, S.; Zakerin, H.R.; Kobraee, S.; Valadabadi, S.A. Foliar application of iron, zinc, and manganese nano-chelates improves physiological indicators and soybean yield under water deficit stress. J. Plant Nutr. 2020, 43, 2740–2756. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Wahid, A.; Ahmad, N.; Saleem, B.A. Improving the drought tolerance in rice (Oryza sativa L.) by exogenous application of salicylic acid. J. Agron. Crop Sci. 2009, 195, 237–246. [Google Scholar] [CrossRef]
- Boonyanitipong, P.; Kositsup, B.; Kumar, P.; Baruah, S.; Dutta, J. Toxicity of ZnO and TiO2 nanoparticles on germinating rice seed Oryza sativa L. Int. J. Biosci. Biochem. Bioinfo. 2011, 1, 282. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Chen, J.; Huang, Q.; Tang, S.; Wang, J.; Hu, P.; Shao, G. Can liming reduce cadmium (Cd) accumulation in rice (Oryza sativa) in slightly acidic soils? A contradictory dynamic equilibrium between Cd uptake capacity of roots and Cd immobilisation in soils. Chemosphere 2018, 193, 547–556. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.M.A.; Klessing, D. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, B.; Liang, Y.C.; Zhu, Y.G.; Zhao, F.J. Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environ. Pollut. 2007, 147, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Fariduddin, Q.; Varshney, P.; Ahmad, A. Salicylic acid minimizes nickel and/or salinity-induced toxicity in Indian mustard (Brassica juncea) through an improved antioxidant system. Environ. Sci. Pollut. Res. Int. 2012, 19, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.S.; Guo, K.; Elbaz, A.A.; Yang, Z.M. Salicylic acid alleviates mercury toxicity by preventing oxidative stress in roots of Medicago sativa. Environ. Exp. Bot. 2009, 65, 27–34. [Google Scholar] [CrossRef]
- Noriega, G.; Caggiano, E.; Lecube, M.L.; Santa Cruz, D.; Batlle, A.; Tomaro, M.; Balestrasse, K.B. The role of salicylic acid in the prevention of oxidative stress elicited by cadmium in soybean plants. Biometals 2012, 25, 1155–1165. [Google Scholar] [CrossRef]
- Metwally, A.; Finkemeier, I.; Georgi, M.; Dietz, K.J. Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol. 2003, 132, 272–281. [Google Scholar] [CrossRef] [Green Version]
- Afzal, S.; Sirohi, P.; Yadav, A.K.; Singh, M.P.; Kumar, A.; Singh, N.K. A comparative screening of abiotic stress tolerance in early flowering rice mutants. J. Biotech. 2019, 302, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Minamikawa, R.; Hattori, K.H.; Kurishima, K.; Kihou, N.; Yuita, K. Arsenic behaviour in paddy fields during the cycle of flooded and non-flooded periods. Environ. Sci. Technol. 2004, 38, 1038–1044. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Xu, X.Y.; Su, Y.H.; McGrath, S.P.; Zhao, F.J. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. USA 2008, 105, 9931–9935. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, S.; Tripathi, R.D.; Tripathi, P.; Kumar, A.; Dave, R.; Mishra, S.; Singh, R.; Sharma, D.; Rai, U.N.; Chakrabarty, D.; et al. Arsenate exposure affects amino acids, mineral nutrient status and antioxidants in rice (Oryza sativa L.) genotypes. Environ. Sci. Tech. 2010, 44, 9542–9549. [Google Scholar] [CrossRef]
- Faizan, M.; Yu, F.; Chen, C.; Faraz, A.; Hayat, S. Zinc oxide nanoparticles help to enhance plant growth and alleviate abiotic stress: A review. Curr. Prot. Pept. Sci. 2020, 21, 1–15. [Google Scholar] [CrossRef]
- Faizan, M.; Rajput, V.D.; Al-Khuraif, A.A.; Arshad, M.; Minkina, T.; Sushkova, S.; Yu, F. Effect of foliar fertigation of chitosan nanoparticles on cadmium accumulation and toxicity in Solanum lycopersicum. Biology 2021, 10, 666. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Almari, S.; Khan, M.N.; Corpas, F.J.; Al-Amri, A.A.; Alsubaie, Q.D.; Ali, H.M.; Kalaji, H.M.; Ahmad, P. Melatonin and calcium function synergistically to promote the resilience through ROS metabolism under arsenic-induced stress. J. Hazard. Mat. 2020, 398, 122882. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Ahmed, S.; Sehar, Z.; Gautam, H.; Gandhi, S.G.; Khan, N.A. Hydrogen peroxide modulates activity and expression of antioxidant enzymes and protects photosynthetic activity from arsenic damage in rice (Oryza sativa L.). J. Hazard. Mat. 2021, 401, 123365. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Chatterjee, S.; Moogouei, R.; Gupta, D.K. Arsenic accumulation in rice and probable mitigation approaches: A review. Agronomy 2017, 7, 67. [Google Scholar] [CrossRef]
- Shetty, R.; Vidya, C.S.N.; Vaculík, M. Comparison of the single and combined effects of arsenic and antimony on growth and physiology of giant reed (Arundo donax L.). Environ. Sci. Pollut. Res. 2021, 17, 1–10. [Google Scholar]
- Lebrun, M.; Miard, F.; Nandillon, R.; Morabito, D.; Bourgerie, S. Biochar application rate: Improving soil fertility and Linum usitatissimum growth on an arsenic and lead contaminated technosol. Int. J. Environ. Res. 2021, 15, 125–134. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Hussain, A.; Ali, Q.; Shakoor, M.B.; Zia-ur-Rehman, M.; Farid, M.; Asma, M. Effect of zinc-lysine on growth, yield and cadmium uptake in wheat (Triticum aestivum L.) and health risk assessment. Chemosphere 2017, 187, 35–42. [Google Scholar] [CrossRef]
- Ahmad, P.; Alyemeni, M.N.; Al-Huqail, A.A.; Alqahtani, M.A.; Wijaya, L.; Ashraf, M.; Kaya, C.; Bajguz, A. Zinc oxide nanoparticles application alleviates arsenic (As) toxicity in soybean plants by restricting the uptake of as and modulating key biochemical attributes, antioxidant enzymes, Ascorbate-Glutathione cycle and glyoxalase system. Plants 2020, 9, 825. [Google Scholar] [CrossRef]
- Ullaha, A.; Romdhaneb, L.; Rehmanc, A.; Farooq, M. Adequate zinc nutrition improves the tolerance against drought and heat stresses in chickpea. Plant Physiol. Biochem. 2020, 143, 11–18. [Google Scholar] [CrossRef]
- Yan, S.; Wu, F.; Zhou, S.; Yang, J.; Tang, X.; Ye, W. Zinc oxide nanoparticles alleviate the arsenic toxicity and decrease the accumulation of arsenic in rice (Oryza sativa L.). BMC Plant Biol. 2021, 21, 150. [Google Scholar] [CrossRef]
- Faizan, M.; Bhat, J.A.; Chen, C.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P.; Yu, F. Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol. Biochem. 2021, 161, 122–130. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Corpas, F.J.; Ahmad, P. Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J. Hazard. Mat. 2020, 399, 123020. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palma, F.; López-Gómez, M.; Tejera, N.A.; Lluch, C. Salicylic acid improves the salinity tolerance of Medicago sativa in symbiosis with Sinorhizobium meliloti by preventing nitrogen fixation inhibition. Plant Sci. 2013, 208, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D.; Laporte, M.M.; Kruger, E.L. Will increased photosynthetic efficiency lead to increased yield in rice. Stud. Plant Sci. 2000, 7, 73–86. [Google Scholar]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2010, 30, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Farooq, A.; Nadeem, M.; Abbas, G.; Shabbir, A.; Khalid, M.S.; Javeed, H.M.R.; Saeed, M.F.; Akram, A.; Younis, A.; Akhtar, G. Cadmium partitioning, physiological and oxidative stress responses in marigold (Calendula calypso) grown on contaminated soil: Implications for phytoremediation. Bull. Environ. Contam. Toxicol. 2020, 105, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Begum, M.; Mondal, S. Relative toxicity of arsenite and arsenate on early seedling growth and photosynthetic pigments of rice. Curr. J. Appl. Sci. Technol. 2019, 33, 1–5. [Google Scholar] [CrossRef]
- Zengin, F. Effects of exogenous salicylic acid on growth characteristics and biochemical content of wheat seeds under arsenic stress. J. Environ. Biol. 2015, 36, 249. [Google Scholar] [PubMed]
- Kumari, A.; Pandey, N.; Pandey-Rai, S. Exogenous salicylic acid-mediated modulation of arsenic stress tolerance with enhanced accumulation of secondary metabolites and improved size of glandular trichomes in Artemisia annua L. Protoplasma 2018, 255, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Ali, S.; Rizwan, M.; Rehman, M.Z.U.; Javed, M.R.; Imran, M.; Chatha, S.A.S.; Nazir, R. Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ. Pollut. 2018, 242, 1518–1526. [Google Scholar] [CrossRef]
- Ahmad, B.; Jaleel, H.; Sadiq, Y.; Khan, M.M.A.; Shabbir, A. Response of exogenous salicylic acid on cadmium induced photosynthetic damage, antioxidant metabolism and essential oil production in peppermint. Plant Growth Regul. 2018, 86, 273–286. [Google Scholar] [CrossRef]
- Faraz, A.; Faizan, M.; Sami, F.; Siddiqui, H.; Hayat, S. Supplementation of salicylic acid and citric acid for alleviation of cadmium toxicity to Brassica Juncea. J. Plant Growth Regul. 2019, 39, 641–655. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Talukdar, D. Arsenic-induced oxidative stress in the common bean legume, Phaseolus vulgaris L. seedlings and its amelioration by exogenous nitric oxide. Physiol. Mol. Biol. Plants 2013, 19, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Yadav, R.K.; Srivastava, S.K. Effect of arsenite and arsenate on lipid peroxidation, enzymatic and non-enzymatic antioxidants in Zea mays Linn. Biochem. Physiol. 2015, 4, 4. [Google Scholar]
- Siddiqui, F.; Tandon, P.K.; Srivastava, S. Arsenite and arsenate impact the oxidative status and antioxidant responses in Ocimum tenuiflorum L. Physiol Mol Bil Plants 2015, 21, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, M.; Ali, S.; Zia Ur Rehman, M.; Adrees, M.; Arshad, M.; Qayyum, M.F.; Ali, L.; Hussain, A.; Chatha, S.; Imran, M. Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ. Pollut. 2019, 248, 358–367. [Google Scholar] [CrossRef]
- Singh, N.; Singh, S.P.; Gupta, V.; Yadav, H.K.; Ahuja, T.; Tripathy, S.S.; Rashmi. A process for the selective removal of arsenic from contaminated water using acetate functionalized zinc oxide nanomaterials. Environ. Prog Sustain Energy 2013, 32, 1023–1029. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Mir, A.R.; Hayat, S. Role of zinc oxide nanoparticles in countering negative effects generated by cadmium in Lycopersicon esculentum. J. Plant Growth Regul. 2020, 40, 101–115. [Google Scholar] [CrossRef]
- Shemi, R.; Wang, R.; Gheith, E.M.S.; Hussain, H.A.; Hussain, S.; Irfan, M.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Sci. Rep. 2021, 11, 3195. [Google Scholar] [CrossRef]
- Mahmoud El-Badri, A.; Batool, M.; Wang, C.; Hashem, A.M.; Tabl, K.M.; Nishawy, E.; Kuai, J.; Zhou, G.; Wang, B. Selenium and zinc oxide nanoparticles modulate the molecular and morpho-physiological processes during seed germination of Brassica napus under salt stress. Ecotox. Environ. Saf. 2021, 225, 112695. [Google Scholar] [CrossRef]
- Dianata, M.; Saharkhiza, M.J.; Tavassolian, I. Salicylic acid mitigates drought stress in Lippia citriodora L. Biocatal. Agric. Biotechnol. 2016, 8, 286–293. [Google Scholar] [CrossRef]
- Tripathi, P.; Mishra, A.; Dwivedi, S.; Chakrabarty, D.; Trivedi, P.K.; Singh, R.P. Differential response of oxidative stress and thiol metabolism in contrasting rice genotypes for arsenic tolerance. Ecotoxicol. Environ. Saf. 2012, 79, 189–198. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Srivastava, S.; Mishra, S.; D’Souza, S.F.; Suprasanna, P. Identification of redox-regulated components of arsenate (As V) tolerance through thiourea supplementation in rice. Metallomics 2014, 6, 1718–1730. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.; Singh, N.B.; Singh, A.; Hussain, I. Exogenous application of salicylic acid to alleviate glyphosate stress in Solanum lycopersicum. Int. J. Veg. Sci. 2017, 23, 552–566. [Google Scholar] [CrossRef]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Lutts, S.; Lefevre, I.; Delperee, C.; Kivits, S.; Dechamps, C.; Robledo, A.; Correal, E. Heavy metal accumulation by the halophyte species mediterranean saltbush. J. Environ. Qual. 2004, 33, 1271–1279. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Bhat, J.A.; Hayat, S.; Yu, F. Zinc oxide nanoparticles and epibrassinolide enhance growth through the modulation of antioxidant enzymes activity and photosynthetic performance in Lycopersicon esculentum. BIOCELL 2021, 45, 1081–1093. [Google Scholar] [CrossRef]
- Lata, S.; Samadder, S.R. Removal of arsenic from water using nano adsorbents and challenges: A review. J. Environ. Manag. 2016, 166, 387–406. [Google Scholar] [CrossRef]
- Wang, X.; Sun, W.; Ma, X. Differential impacts of copper oxide nanoparticles and copper(II) ions on the uptake and accumulation of arsenic in rice (Oryza sativa). Environ. Pollut. 2019, 252, 967–973. [Google Scholar] [CrossRef]
- Conway, J.R.; Adeleye, A.S.; Gardea-Torresdey, J.; Keller, A.A. Aggregation, dissolution, and transformation of copper nanoparticles in natural waters. Environ. Sci. Technol. 2015, 49, 2749–2756. [Google Scholar] [CrossRef] [Green Version]
- Sharifan, H.; Wang, X.X.; Guo, B.L.; Ma, X.M. Investigation on the modification of physicochemical properties of cerium oxide nanoparticles through adsorption of Cd and As(III)/As(V). ACS Sustain. Chem. Eng. 2018, 6, 13454–13461. [Google Scholar] [CrossRef]
- Doganlar, Z.B.; Demir, K.; Basak, H.; Gul, I. Effects of salt stress on pigment and total soluble protein contents of three different tomato cultivars. Afric. J. Agric. Res. 2010, 5, 2056–2065. [Google Scholar]
- Popova, L.P.; Maslenkova, L.T.; Yordanova, R.Y.; Ivanova, A.P.; Krantev, A.P.; Szalai, G.; Janda, T. Exogenous treatment with salicylic acid attenuates cadmium toxicity in pea seedlings. Plant Physiol. Biochem. 2009, 47, 224–231. [Google Scholar] [CrossRef]
- Orcutt, D.M.; Nilsen, E.T. The Physiology of Plants under Stress; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Yang, Y.; Qi, M.; Mei, C. Endogenous salicylic acid protect rice plants from oxidative damage caused by ageing as well as biotic and abiotic stress. Plant J. 2004, 40, 909–919. [Google Scholar] [CrossRef]
- Lamhamdi, M.; El-Galiou, O.; Bakrim, A.; No´voa-Muñoz, J.C.; Arias-Este´vez, M.; Aarab, A.; Lafont, R. Effect of lead stress on mineral content and growth of wheat (Triticum aestivum) and spinach (Spinacia oleracea) seedlings. Saudi J. Biol. Sci. 2013, 20, 29–36. [Google Scholar] [CrossRef]
- Nabila, M.I.; Kannabiran, K. Biosynthesis, characterization and antibacterial activity of copper oxide nanoparticles (CuO NPs) from actinomycetes. Biocatal. Agric. Biotechnol. 2018, 15, 56–62. [Google Scholar] [CrossRef]
- Abboud, Y.; Saffaj, T.; Chagraoui, A.; El Bouari, A.; Brouzi, K.; Tanane, O.; Ihssane, B. Biosynthesis, characterization and antimicrobial activity of copper oxide nanoparticles (CuNPs) produced using brown alga extract (Bifurcaria bifurcata). Appl. Nanosci. 2014, 4, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Faizan, M.; Bhat, J.A.; Hessini, K.; Yu, F.; Ahmad, P. Zinc oxide nanoparticles alleviates the adverse effects of cadmium stress on Oryza sativa via modulation of the photosynthesis and antioxidant defense system. Ecotoxic. Environ. Saf. 2021, 220, 112401. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Bhat, J.A.; Noureldeen, A.; Ahmad, P.; Yu, F. Zinc oxide nanoparticles and 24-Epibrassinolide alleviates Cu toxicity in tomato by regulating ROS scavenging, stomatal movement and photosynthesis. Ecotoxic. Environ. Saf. 2021, 218, 112293. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Hayat, S. Effect of foliar spray of ZnO-NPs on the physiological parameters efficiency and antioxidant systems of Lycopersicon Esculentum. Pol. J. Nat. Sci. 2019, 34, 87–105. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Sci. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Patterson, B.D.; MacRae, E.A.; Ferguson, I.B. Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal. Biochem. 1984, 139, 487–492. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Yusuf, M.; Khan, S.T.; Hayat, S. Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 2018, 56, 678–686. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis: Advanced Course; UW-Madison Libraries Parallel Press: Madison, WI, USA, 2005. [Google Scholar]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis for Soil, Plants and Water; Division of Agricultural Science, University of California: Berkeley, CA, USA, 1961. [Google Scholar]
- Nassar, M.A.; El-Sahhar, K.F. Botanical Preparations and Microscopy (Micro Technique); Academic Bookshop: Giza, Egypt, 1998. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faizan, M.; Sehar, S.; Rajput, V.D.; Faraz, A.; Afzal, S.; Minkina, T.; Sushkova, S.; Adil, M.F.; Yu, F.; Alatar, A.A.; et al. Modulation of Cellular Redox Status and Antioxidant Defense System after Synergistic Application of Zinc Oxide Nanoparticles and Salicylic Acid in Rice (Oryza sativa) Plant under Arsenic Stress. Plants 2021, 10, 2254. https://doi.org/10.3390/plants10112254
Faizan M, Sehar S, Rajput VD, Faraz A, Afzal S, Minkina T, Sushkova S, Adil MF, Yu F, Alatar AA, et al. Modulation of Cellular Redox Status and Antioxidant Defense System after Synergistic Application of Zinc Oxide Nanoparticles and Salicylic Acid in Rice (Oryza sativa) Plant under Arsenic Stress. Plants. 2021; 10(11):2254. https://doi.org/10.3390/plants10112254
Chicago/Turabian StyleFaizan, Mohammad, Shafaque Sehar, Vishnu D. Rajput, Ahmad Faraz, Shadma Afzal, Tatiana Minkina, Svetlana Sushkova, Muhammad Faheem Adil, Fangyuan Yu, Abdulrahman A. Alatar, and et al. 2021. "Modulation of Cellular Redox Status and Antioxidant Defense System after Synergistic Application of Zinc Oxide Nanoparticles and Salicylic Acid in Rice (Oryza sativa) Plant under Arsenic Stress" Plants 10, no. 11: 2254. https://doi.org/10.3390/plants10112254
APA StyleFaizan, M., Sehar, S., Rajput, V. D., Faraz, A., Afzal, S., Minkina, T., Sushkova, S., Adil, M. F., Yu, F., Alatar, A. A., Akhter, F., & Faisal, M. (2021). Modulation of Cellular Redox Status and Antioxidant Defense System after Synergistic Application of Zinc Oxide Nanoparticles and Salicylic Acid in Rice (Oryza sativa) Plant under Arsenic Stress. Plants, 10(11), 2254. https://doi.org/10.3390/plants10112254











