Characterization of the Insect Assemblage and Associated Floral Volatiles of Black Cherry (Prunus serotina)
Abstract
:1. Introduction
2. Results
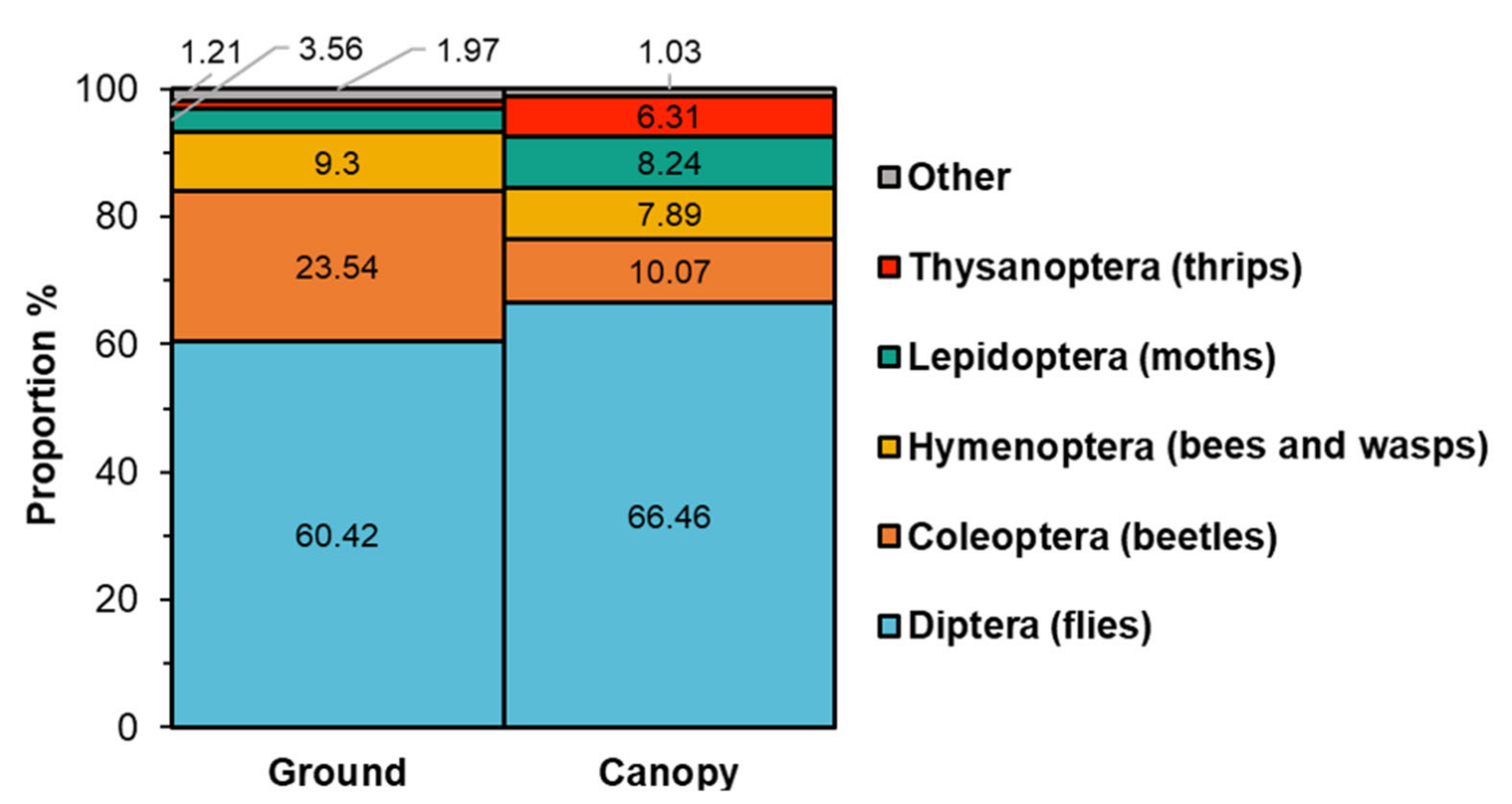
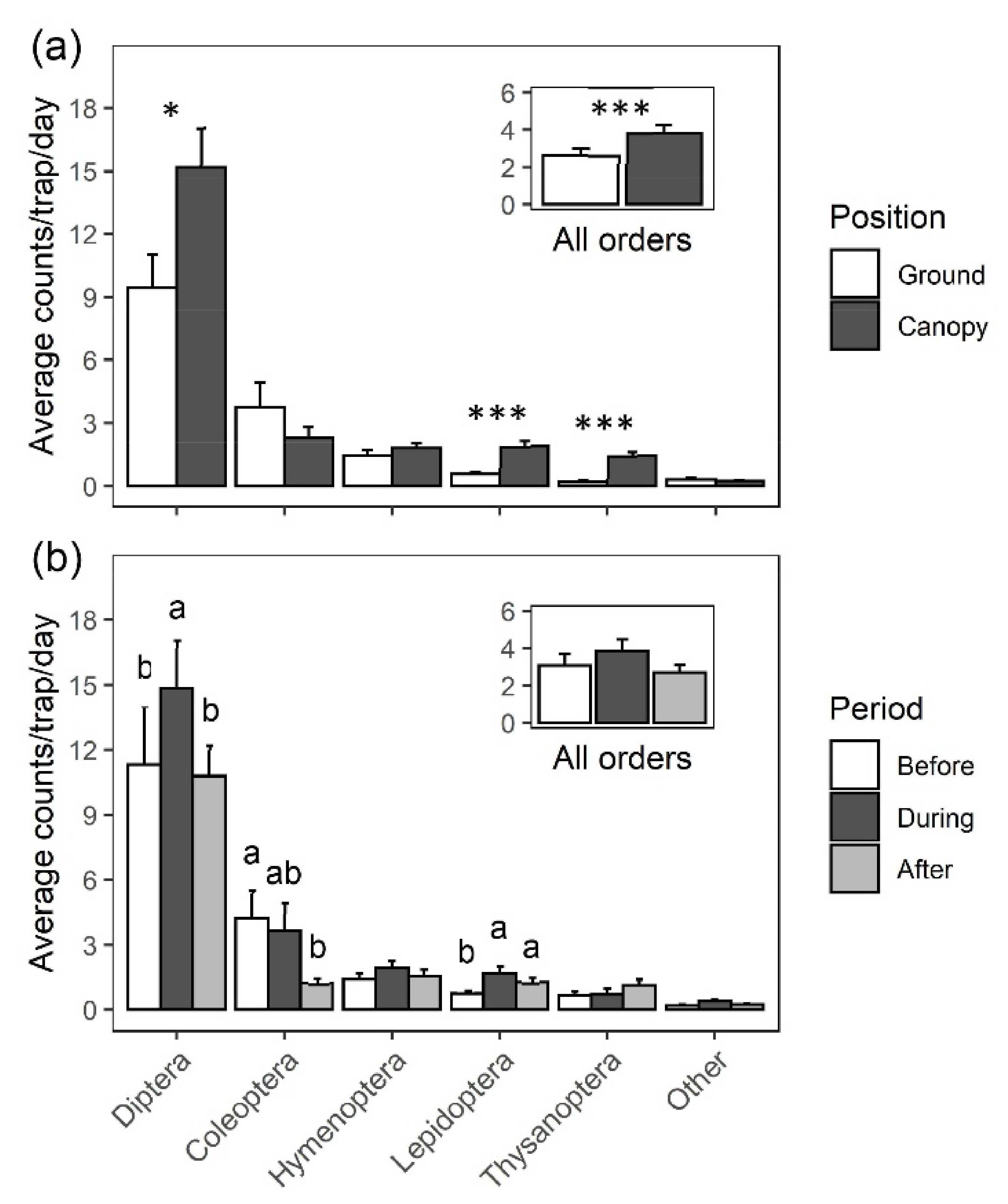
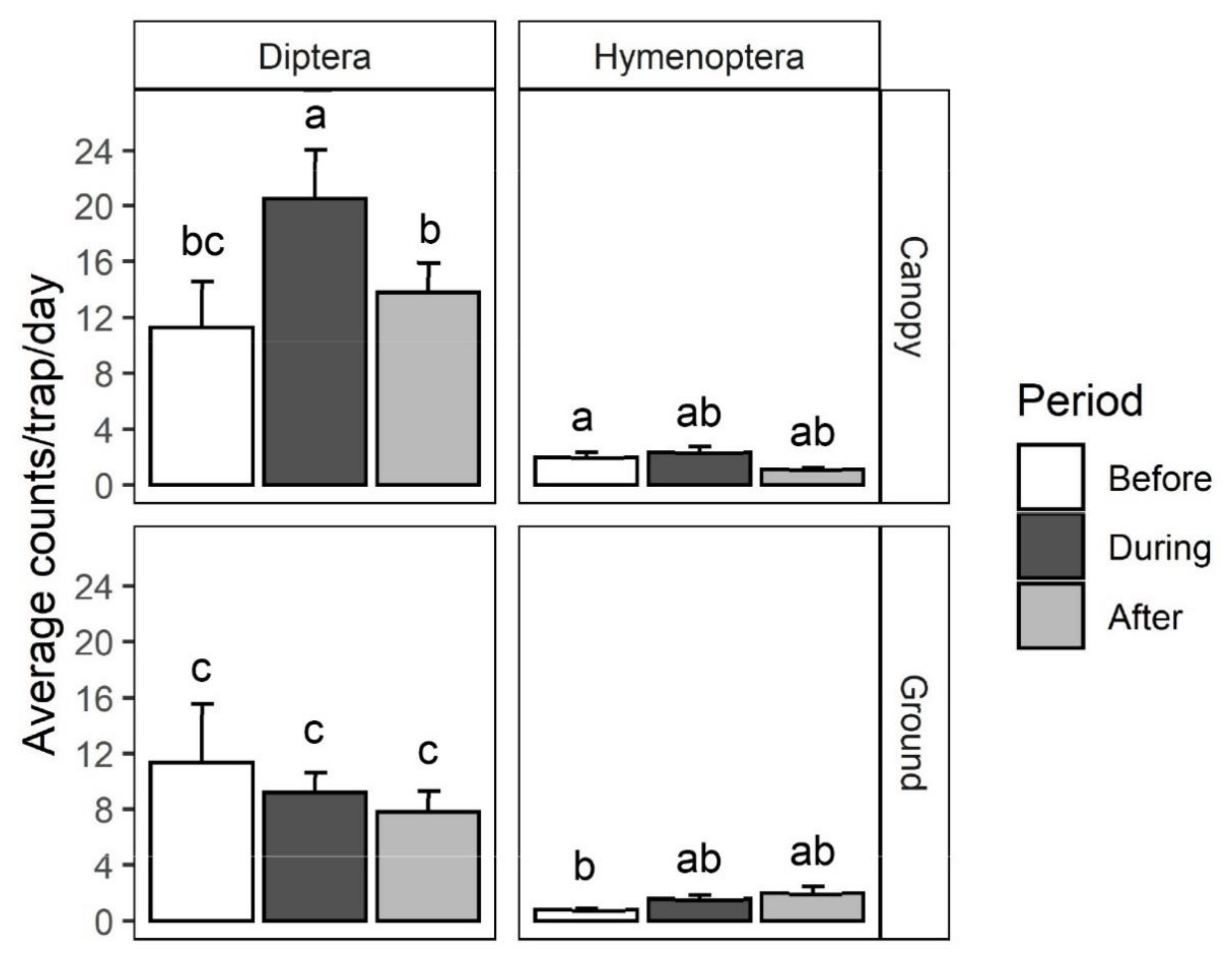
2.1. Survey and Identification of Insects Visiting Black Cherry
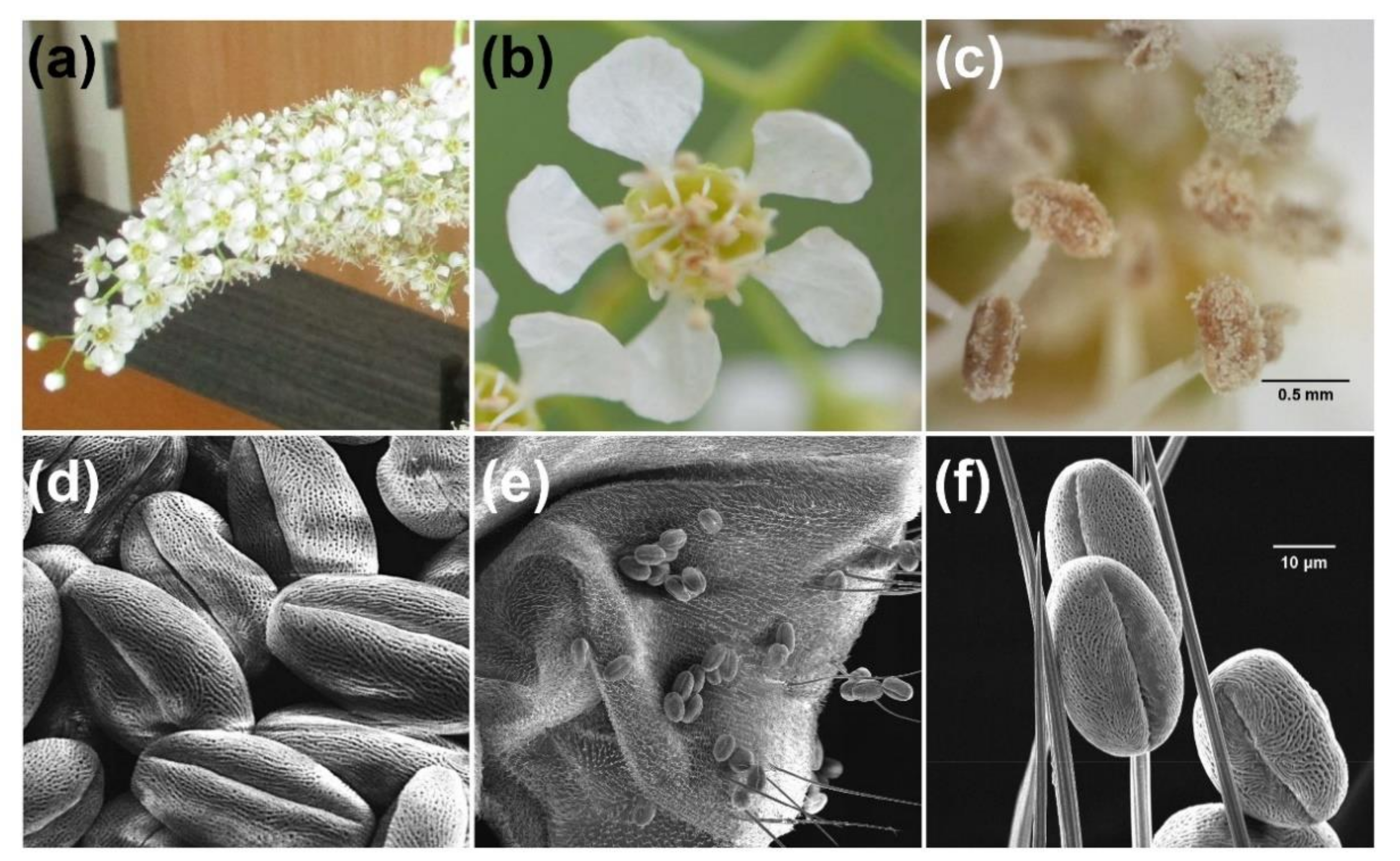
2.2. Characterization of Insects Carrying Black Cherry Pollen
2.3. Volatile Profile of Black Cherry Flowers
3. Discussion
4. Materials and Methods
4.1. Survey and Identification of Insects Visiting Black Cherry
4.2. Characterization of Insects Carrying Black Cherry Pollen
4.3. Collection and Analysis of Floral Volatiles
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hough, A.F. Silvical Characteristics of Black Cherry (Prunus serotina); Northeastern Forest Experiment Station, Station Paper No 139; USDA Forest Service: Upper Darby, PA, USA, 1960. [Google Scholar]
- McVaugh, R. A revision of the North American black cherries (Prunus serotina Ehrh and Relatives). Brittonia 1951, 7, 279–315. [Google Scholar] [CrossRef]
- Marquis, D.A. Prunus serotina Ehrh. black cherry. In Silvics of North America Vol. 2; Burns, R.M., Honkala, B.H., Eds.; USDA Forest Service: Washington, DC, USA, 1990; pp. 594–604. [Google Scholar]
- Litvaitis, J.A. Importance of early successional habitats to mammals in eastern forests. Wildlife Soc. Bull. 2001, 29, 466–473. [Google Scholar]
- Rodewald, P.G.; Brittingham, M.C. Stopover habitats of landbirds during fall: Use of edge-dominated and early-successional forests. Auk 2004, 121, 1040–1055. [Google Scholar] [CrossRef]
- Forbes, D.C. Self-and Cross-Incompatibility in Black Cherry (Prunus serotina). Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 1969. [Google Scholar]
- Muys, B.; Maddelein, D.; Lust, N. Ecology, practice and policy of black cherry (Prunus serotina Ehrh.) management in Belgium. Silva Gandavensis 1992, 57, 28–45. [Google Scholar] [CrossRef] [Green Version]
- Godefroid, S.; Phartyal, S.S.; Weyembergh, G.; Koedam, N. Ecological factors controlling the abundance of non-native invasive black cherry (Prunus serotina) in deciduous forest understory in Belgium. Forest Ecol. Manag. 2005, 210, 91–105. [Google Scholar] [CrossRef]
- Deckers, B.; Verheyen, K.; Hermy, M.; Muys, B. Effects of landscape structure on the invasive spread of black cherry Prunus serotina in an agricultural landscape in Flanders, Belgium. Ecography 2005, 28, 99–109. [Google Scholar] [CrossRef]
- Schilthuizen, M.; Pimenta., L.P.; Lammers., Y.; Steenbergen., P.J.; Flohil, M.; Beveridge, N.G.; van Duijn, P.T.; Meulblok, M.M.; Sosef, N.; van de Ven, R.; et al. Incorporation of an invasive plant into a native insect herbivore food web. Peer J. 2016, 4, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Aerts, R.; Ewald, M.; Nicolas, M.; Piat, J.; Skowronek, S.; Lenoir, J.; Hattab, T.; Garzón-López, C.X.; Feilhauer, H.; Schmidtlein, S.; et al. Invasion by the alien tree Prunus serotina alters ecosystem functions in a temperate deciduous forest. Front. Plant Sci. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pairon, M.; Petitpierre, B.; Campbell, M.; Guisan, A.; Broennimann, O.; Baret, P.V.; Jacquemart, A.L.; Besnard, G. Multiple introductions boosted genetic diversity in the invasive range of black cherry (Prunus serotina; Rosaceae). Ann. Bot. 2010, 105, 881–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitney, G.G. The history and status of the hemlock-hardwood forests of the Allegheny Plateau. J. Ecol. 1990, 78, 443–458. [Google Scholar] [CrossRef]
- Long, R.P.; Ristau, T.E. Changes in Black Cherry Seed Production: Is Stand Age a Factor? Northeastern Naturalist 2020, 2, 281–298. [Google Scholar] [CrossRef]
- Stout, S.L.; Brose, P.H.; Cleveland, H.; Long, R.P.; McGuinness, B.; Peters, M.P.; Rebbeck, J.; Ristau, T.; Royo, A.A.; Stoleson, S.H.; et al. Fifty years of science-management cooperation from the SILVAH community of practice. In Proceedings of the Allegheny Society of American Foresters Training Session; Technical Report NRS-P-186; Stout, S.L., Ed.; USDA Forest Service: Newtown Square, PA, USA, 2019; pp. 8–25. [Google Scholar]
- Horsley, S.B. Role of allelopathy in hay-scented fern interference with black cherry regeneration. J. Chem. Ecol. 1993, 19, 2737–2755. [Google Scholar] [CrossRef]
- Lee, J.C.; Skelly, J.M.; Steiner, K.C.; Zhang, J.W.; Savage, J.E. Foliar response of black cherry (Prunus serotina) clones to ambient ozone exposure in central Pennsylvania. Environ. Pollut. 1999, 105, 325–331. [Google Scholar] [CrossRef]
- Marquis, D.A.; Gearhart, P. Cherry-maple: Silvicultural systems for the major forest types of the United States. Agricult. Handb. 1983, 445, 137–140. [Google Scholar]
- Packer, A.; Clay, K.J. Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature 2000, 404, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Packer, A.; Clay, K.J. Development of negative feedback during successive growth cycles of black cherry. R. Soc. 2004, 271, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C. Flowers and Insects: Rosaceae and Compositae; Academy of Science of St. Louis: St. Louis, MO, USA, 1894. [Google Scholar]
- Chaudhary, O.P. Influence of different colony placement distances on yield and quality parameters of peach (Prunus persica L.). Korean J. Apicult. 2008, 23, 89–95. [Google Scholar]
- Giurfa, M.; Vorobyev, M.; Kevan, P.; Menzel, R. Detection of coloured stimuli by honeybees: Minimum visual angles and receptor specific contrasts. J. Comparat. Physiol. A 1996, 178, 699–709. [Google Scholar] [CrossRef]
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef]
- Radulovic, N.S.; Dordevic, A.S.; Zlatkovic, B.K.; Palic, R.M. GC-MS analyses of flower ether extracts of Prunus domestica L. and Prunus padus L. (Rosaceae). Chem. Pap. 2009, 63, 377–384. [Google Scholar] [CrossRef]
- Hao, R.; Du, D.; Wang, T.; Yang, W.; Wang, J.; Zhang, Q. A comparative analysis of characteristic floral scent compounds in Prunus mume and related species. Biosci. Biotechnol. Biochem. 2014, 78, 1640–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sayed, A.M.; Sporle, A.; Colhoun, K.; Furlong, J.; White, R.; Suckling, D.M. Scents in orchards: Floral volatiles of four stone fruit crops and their attractiveness to pollinators. Chemoecology 2018, 28, 39–49. [Google Scholar] [CrossRef]
- Reidel, R.V.B.; Cioni, P.L.; Pistelli, L. Volatile emission of different plant parts and fruit development from Italian cherry plums (Prunus cerasifera and P. cerasifera ‘Pissardii’). Biochem. Syst. Ecol. 2017, 75, 10–17. [Google Scholar] [CrossRef]
- Zhang, T.; Bao, F.; Yang, Y.; Hu, L.; Ding, A.; Ding, A.; Wang, J.; Cheng, T.; Zhang, Q. A comparative analysis of floral scent compounds in intraspecific cultivars of Prunus mume with different corolla colours. Molecules 2020, 25, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omura, H.; Honda, K.; Nakagawa, A.; Hayashi, N. The role of floral scent of the cherry tree, Prunus yedoensis, in the foraging behavior of Luehdorfia japonica (Lepidoptera: Papilionidae). Appl. Entomol. Zool. 1999, 34, 309–313. [Google Scholar] [CrossRef]
- Mastelic, J.; Jerkovic, I.; Mesic, M. Volatile constituents from flowers, leaves, bark and wood of Prunus mahaleb L. Flavour Fragr. J. 2006, 21, 306–313. [Google Scholar] [CrossRef]
- Kang, W.; Xu, Q. Rapid determination of volatile constituents from the buds and flowers of Prunus persica (L.) Batsch. f. duplex Rehd. In Proceedings of the 2nd International Conference on Bioinformatics and Biomedical Engineering, Shanghai, China, 27–29 December 2008; pp. 1024–1027. [Google Scholar]
- Gilani, S.A.; Qureshi, R.A.; Khan, A.M.; Potter, D. A molecular phylogeny of selected species of genus Prunus L. (Rosaceae) from Pakistan using the internal transcribed spacer (ITS) spacer DNA. Afr. J. Biotechnol. 2010, 9, 4867–4872. [Google Scholar]
- Ollerton, J.; Killick, A.; Lamborn, E.; Watts, S.; Whiston, M. Multiple meanings and modes: On the many ways to be a generalist flower. Taxon 2007, 56, 717–728. [Google Scholar] [CrossRef]
- Lee, S.; Wen, J. A phylogenetic analysis of Prunus and the Amygdaloideae (Rosaceae) using ITS sequences of nuclear ribosomal DNA. Am. J. Bot. 2001, 88, 150–160. [Google Scholar] [CrossRef]
- Sytsma, K.J. Rosaceae. Encyclopedia Britannica. 2016. Available online: https://www.britannica.com/plant/Rosaceae (accessed on 24 March 2021).
- Calzoni, G.L.; Speranza, A. Insect controlled pollination in Japanese plum (Prunus salicina Lindl.). Sci. Hortic. 1998, 72, 227–237. [Google Scholar] [CrossRef]
- Gyan, K.Y.; Woodell, S.R.J. Analysis of insect pollen loads and pollination efficiency of some common insect visitors of four species of woody Rosaceae. Funct. Ecol. 1987, 1, 269–274. [Google Scholar] [CrossRef]
- Ryder, J.T.; Cherrill, A.; Prew, R.; Shaw, J.; Thorbek, P.; Walters, K.F. Impact of enhanced Osmia bicornis (Hymenoptera: Megachilidae) populations on pollination and fruit quality in commercial sweet cherry (Prunus avium L.) orchards. J. Apicult. Res. 2020, 59, 77–87. [Google Scholar] [CrossRef]
- Thapa, R.B. Honeybees and other insect pollinators of cultivated plants: A review. J. Inst. Agricult. Anim. Sci. 2006, 27, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Suarez-Gonzalez, A.; Good, S.V. Pollen limitation and reduced reproductive success are associated with local genetic effects in Prunus virginiana, a widely distributed self-incompatible shrub. Ann. Bot. 2013, 113, 595–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeCroy, K.A.; Shew, H.W.; van Zandt, P.A. Pollen presence on nocturnal moths in the Ketona Dolomite glades of Bibb County, Alabama. South. Lepid. News 2013, 35, 136–142. [Google Scholar]
- Frost, S.W. A preliminary study of North American insects associated with elderberry flowers. Fla. Entomol. 1979, 62, 341–355. [Google Scholar] [CrossRef]
- Kirk, W.D.J. Pollen-feeding in thrips (Insecta: Thysanoptera). J. Zool. 1984, 204, 107–117. [Google Scholar] [CrossRef]
- Bernays, E.A.; Chapman, R.F. Host-Plant Selection by Phytophagous Insects; Springer Science & Business Media: Berlin, Germany, 2007. [Google Scholar]
- Lewis, T. Feeding, Flight and Dispersal in Thrips. In Towards Understanding Thysanoptera; Technical Report NE-147; Parker, B.L., Skinner, M., Lewis, T., Eds.; USDA Northeastern Forest Experimental Station: Radnor, PA, USA, 1991; pp. 63–70. [Google Scholar]
- USDA NRCS. The PLANTS Database. 2021. Available online: http://plants.usda.gov (accessed on 24 March 2021).
- Leather, S.R. Prunus Padus L. J. Ecol. 1996, 84, 125–132. [Google Scholar] [CrossRef]
- Dötterl, S.; Vereecken, N.J. The chemical ecology and evolution of bee-flower interactions: A review and perspectives. Can. J. Zool. 2010, 88, 668–697. [Google Scholar] [CrossRef]
- Klatt, B.K.; Burmeister, C.; Westphal, C.; Tscharntke, T.; von Fragstein, M. Flower volatiles, crop varieties and bee responses. PLoS ONE 2013, 8, e72724. [Google Scholar] [CrossRef]
- Konrade, L.; Shaw, J.; Beck, J. A rangewide herbarium-derived dataset indicates high levels of gene flow in black cherry (Prunus serotina). Ecol. Evol. 2019, 9, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.B.; Ferguson, C.J.; Mayfield, M.H.; Shaw, J. Reduced genetic variation in populations of black cherry (Prunus serotina subsp. serotina, Rosaceae) at its western range limit in Kansas. Northeastern Nat. 2014, 21, 472–478. [Google Scholar]
- Mozuraitis, R.; Hall, D.; Trandem, N.; Ralle, B.; Tunström, K.; Sigsgaard, L.; Baroffio, C.; Fountain, M.; Cross, J.; Wibe, A.; et al. Composition of strawberry floral volatiles and their effects on the behavior of strawberry blossom weevil, Anthonomus rubi. J. Chem. Ecol. 2020, 46, 1069–1081. [Google Scholar] [CrossRef]
- Schiestl, F.P. The evolution of floral scent and insect chemical communication. Ecol. Lett. 2010, 13, 643–656. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Tollsten, L. Trends in floral scent chemistry in pollination syndromes: Floral scent composition in moth-pollinated taxa. Bot. J. Linn. Soc. 1993, 113, 263–284. [Google Scholar] [CrossRef]
- Andersson, S.; Nilsson, L.A.; Groth, I.; Bergström, G. Floral scents in butterfly-pollinated plants: Possible convergence in chemical composition. Bot. J. Linn. Soc. 2002, 140, 129–153. [Google Scholar] [CrossRef] [Green Version]
- Dobson, H.E.M. Relationship between floral fragrance composition and type of pollinator. In Biology of Floral Scent; Dudareva, N., Pichersky, E., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 147–198. [Google Scholar]
- Honda, K.; Omura, H.; Hayashi, N. Identification of floral volatiles from Ligustrum japonicum that stimulate flower-visiting by cabbage butterfly, Pieris rapae. J. Chem. Ecol. 1998, 24, 2167–2180. [Google Scholar] [CrossRef]
- Woodcock, T.S.; Larson, B.M.H.; Kevan, P.G.; Imouye, D.W.; Lunau, K. Flies and flowers II: Floral attractants and rewards. J. Pollinat. Ecol. 2014, 12, 63–94. [Google Scholar] [CrossRef]
- Primate, C.; Dötterl, S. A syrphid fly uses olfactory cues to find a non-yellow flower. J. Chem. Ecol. 2010, 36, 1207–1210. [Google Scholar] [CrossRef]
- Braunschmid, H.; Műkisch, B.; Rupp, T.; Schäffler, I.; Zito, P.; Birtele, D.; Dötterl, S. Interpopulation variation in pollinators and floral scent of the lady’s-slipper orchid Cypripedium calceolus L. Arthropod Plant Interact. 2017, 11, 363–379. [Google Scholar] [CrossRef] [Green Version]
- Li, H.M.; Liu, W.B.; Yang, L.L.; Cao, H.Q.; Pelosi, P.; Wang, G.R.; Wang, B. Aromatic volatiles and odorant receptor 25 mediate attraction of Eupeodes corollae to flowers. J. Agric. Food Chem. 2020, 68, 12212–12220. [Google Scholar] [CrossRef]
- Zhu, J.; Park, K.C. Methyl salicylate, a soybean aphid-induced plant volatile attractive to the predator Coccinella septepunctata. J. Chem. Ecol. 2005, 31, 1733–1746. [Google Scholar] [CrossRef] [PubMed]
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohleműller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Powney, G.D.; Carvell, C.; Edwards, M.; Morris, R.K.A.; Roy, H.E.; Woodcock, B.A.; Nick, J.B.; Isaac, N.J.B. Widespread losses of pollinating insects in Britain. Nat. Commun. 2019, 10, 1018. [Google Scholar] [CrossRef]
- Baum, K.A.; Wallen, K.E. Potential bias in pan trapping as a function of floral abundance. J. Kansas Entomol. Soc. 2011, 84, 155–159. [Google Scholar] [CrossRef]
- Vrdoljak, S.M.; Samways, M.J. Optimising coloured pan traps to survey flower visiting insects. J. Insect Conserv. 2012, 16, 345–354. [Google Scholar] [CrossRef]
- Roulston, T.H.; Smith, S.S.; Brewster, A.L. A comparison of pan trap and intensive net sampling techniques for documenting a bee (Hymenoptera: Apiformes) fauna. J. Kansas Entomol. Soc. 2007, 80, 179–181. [Google Scholar] [CrossRef]
- Droege, S. Handy Bee Manual; USGS Native Bee Inventory and Monitoring Lab: Beltsville, MD, USA, 2012. [Google Scholar]
- Droege, S. Impact of Color and Size of Bowl Trap on Numbers of Bees Captured. 2006. Available online: http://online.sfsu.edu/beeplot/pdfs/color%20and%20size.pdf (accessed on 26 January 2021).
- Nuttman, C.V.; Otieno, M.; Kwapong, P.K.; Combey, R.; Willmer, P.; Potts, S.G. The utility of aerial pan-trapping for assessing insect pollinators across vertical strata. J. Kansas Entomol. Soc. 2011, 4, 260–270. [Google Scholar] [CrossRef]
- Gbur, E.E. Analysis of Generalized Linear Mixed Models in the Agricultural and Natural Resources Sciences; American Society of Agronomy, Soil Science Society of America, Crop Science Society of America: Madison, WI, USA, 2012. [Google Scholar]
- SAS Institute Inc. SAS/STAT ®. 9.4 User’s. Guide; SAS Institute Inc.: Cary, NC, USA, 2019. [Google Scholar]
- Hafner, B. Scanning Electron Microscopy Primer; Characterization Facility, University of Minnesota: Twin Cities, MN, USA, 2007. [Google Scholar]
- Wang, F.; Park, Y.-L.; Gutensohn, M. Glandular trichome-derived sesquiterpenes of wild tomato accessions (Solanum habrochaites) affect aphid performance and feeding behavior. Phytochemistry 2020, 180, 112532. [Google Scholar] [CrossRef]
- Wang, F.; Park, Y.-L.; Gutensohn, M. Glandular trichome-derived mono-and sesquiterpenes of tomato have contrasting roles in the interaction with the potato aphid Macrosiphum euphorbiae. J. Chem. Ecol. 2021, 47, 204–214. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Order | Family | Species | Canopy | Ground | χ2 | p Value |
|---|---|---|---|---|---|---|
| Diptera | Hybotidae | Anthalia bulbosa | 109.7 ± 1.9 | 31.5 ± 1.6 | 7.2445 | 0.0071 ** |
| Thysanoptera | Thripidae | Frankliniella spp. | 40.2 ± 0.6 | 6.2 ± 0.4 | 4.083 | 0.0433 * |
| Diptera | Empididae | Rhamphomyia spp. | 30.4 ± 1.1 | 11.3 ± 0.7 | 1.899 | 0.1682 |
| Diptera | Ephydridae | Discocerina spp. | 20.8 ± 1.0 | 1.7 ± 0.3 | 1.547 | 0.2136 |
| Coleoptera | Elateridae | Melanotus hyslopi | 16.5 ± 0.4 | 1.4 ± 0.1 | 0.7259 | 0.3942 |
| Coleoptera | Staphylinidae | Eusphalerum convexum | 14.5 ± 1.3 | 0.9 ± 0.1 | 1.0891 | 0.2967 |
| Lepidoptera | Geometridae | Melanolophia canadaria | 9.14 ± 0.9 | 0.7 ± 0 | 3.3251 | 0.0682 |
| Coleoptera | Scraptiidae | Anaspis rufa | 8.4 ± 0.3 | 1 ± 0.1 | 1.1136 | 0.2913 |
| Hymenoptera | Halictidae | Auglochlora spp. | 5.7 ± 0.1 | 3.5 ± 0.2 | 1.7526 | 0.1855 |
| Coleoptera | Chrysomelidae | Crepidodera violacea | 4.3 ± 0.2 | 0.6 ± 0 | 1.3125 | 0.2519 |
| Diptera | Calliphoridae | Phormia regina | 3.4 ± 0.1 | 0.1 ± 0 | 0.3333 | 0.5637 |
| Compound 1 | CAS 2 | NIST RI 3 | Exp RI 4 | Chemotype 1 | Chemotype 2 | p Value 5 | |
|---|---|---|---|---|---|---|---|
| Pmol/Flower/Hr (Mean ± SE, n = 5) | |||||||
| 1 | α-Pinene | 80-56-8 | 937 | 935 | 0.24 ± 0.1 | 1.3 ± 0.69 | 0.1683 |
| 2 | Benzaldehyde | 100-52-7 | 962 | 962 | 161.21 ± 37.4 | 4.27 ± 0.62 | 0.0030 ** |
| 3 | α-Myrcene | 123-35-3 | 991 | 993 | 3.16 ± 0.38 | 2.23 ± 0.34 | 0.1011 |
| 4 | D-Limonene | 138-86-3 | 1030 | 1031 | 10.75 ± 2.41 | 3.19 ± 0.6 | 0.0159 * |
| 5 | (Z)-β-Ocimene | 3338-55-4 | 1038 | 1041 | 56.88 ± 8.41 | 31.23 ± 7.14 | 0.0486 * |
| 6 | Phenylacetaldehyde | 122-78-1 | 1045 | 1047 | 15.49 ± 3.19 | - | 0.0012 ** |
| 7 | (E)-β-Ocimene | 3779-61-1 | 1049 | 1054 | 415.91 ± 67.99 | 230.1 ± 43.54 | 0.0503 |
| 8 | (Z)-Linalool oxide | 5989-33-3 | 1074 | 1090 | 0.7 ± 0.16 | 1.54 ± 0.97 | 0.4139 |
| 9 | Methyl benzoate | 93-58-3 | 1094 | 1098 | 13.73 ± 2.25 | 3.5 ± 1.86 | 0.0080 ** |
| 10 | α-Linalool | 78-70-6 | 1099 | 1104 | 4.59 ± 0.52 | 3.36 ± 1.08 | 0.3327 |
| 11 | Nonanal | 124-19-6 | 1104 | 1107 | 1.46 ± 0.35 | 2.65 ± 1.26 | 0.3857 |
| 12 | Phenylethanol | 60-12-8 | 1116 | 1119 | 71.51 ± 10.02 | 15.66 ± 3.47 | 0.0007 *** |
| 13 | 3,4-Dimethyl-2,4,6-octatriene | 57396-75-5 | 1121 | 1132 | 0.98 ± 0.23 | 0.11 ± 0.11 | 0.0092 ** |
| 14 | Methyl nicotinate | 93-60-7 | 1139 | 1142 | 3.33 ± 0.78 | 13.31 ± 2.69 | 0.0073 ** |
| 15 | Ethyl benzoate | 93-89-0 | 1171 | 1175 | 2.91 ± 0.41 | 1.18 ± 0.33 | 0.0104 * |
| 16 | Methyl salicylate | 119-36-8 | 1192 | 1199 | 2.75 ± 0.2 | 0.12 ± 0.12 | <0.001 *** |
| 17 | Dodecane | 112-40-3 | 1200 | 1200 | 0.02 ± 0.02 | 2.07 ± 1.05 | 0.0853 |
| 18 | Decanal | 112-31-2 | 1206 | 1209 | 0.71 ± 0.17 | 0.86 ± 0.3 | 0.6788 |
| 19 | N-Phenylformamide | 103-70-8 | 1221 | 1225 | 6.1 ± 2.13 | 6.56 ± 1.36 | 0.8585 |
| 20 | Benzothiazole | 95-16-9 | 1229 | 1232 | 4.79 ± 1.53 | 6.55 ± 1.47 | 0.4303 |
| 21 | p-Anisaldehyde | 123-11-5 | 1250 | 1264 | - | 14.73 ± 3.23 | 0.0019 ** |
| 22 | p-Anisyl alcohol | 105-13-5 | 1290 | 1293 | - | 6.89 ± 1.92 | 0.0070 ** |
| 23 | Tridecane | 629-50-5 | 1300 | 1300 | 0.39 ± 0.09 | 1.66 ± 0.86 | 0.1839 |
| 24 | N,N-Dibutylformamide | 761-65-9 | 1310 | 1308 | 0.54 ± 0.08 | 0.56 ± 0.13 | 0.8589 |
| 25 | Texanol | 77-68-9 | 1380 | 1381 | 0.76 ± 0.15 | 1.82 ± 0.97 | 0.3075 |
| 26 | Methyl p-anisate | 121-98-2 | 1373 | 1383 | - | 3.36 ± 0.64 | 0.0007 *** |
| 27 | Tetradecane | 629-59-4 | 1400 | 1400 | 0.52 ± 0.12 | 0.78 ± 0.3 | 0.4336 |
| 28 | (Z)-Jasmone | 488-10-8 | 1394 | 1406 | 7.93 ± 1.42 | 3.18 ± 0.82 | 0.0199 * |
| 29 | Pentadecane | 629-62-9 | 1500 | 1500 | 0.7 ± 0.22 | 0.35 ± 0.05 | 0.1648 |
| 30 | (E,E)-α-Farnesene | 502-61-4 | 1508 | 1514 | 4.97 ± 0.53 | 0.95 ± 0.22 | <0.001 *** |
| 31 | Hexadecane | 544-76-3 | 1600 | 1600 | 1.21 ± 0.37 | 1.94 ± 0.62 | 0.3388 |
| 32 | 4-sec-Butyl-2,6-di-tert-butylphenol | 17540-75-9 | 1640 | 1650 | 6.91 ± 3.06 | 1.32 ± 0.9 | 0.1175 |
| 33 | Heptadecane | 629-78-7 | 1700 | 1700 | 0.98 ± 0.24 | 0.62 ± 0.12 | 0.2151 |
| 34 | Benzyl benzoate | 120-51-4 | 1762 | 1789 | 2.24 ± 0.45 | 0.16 ± 0.13 | 0.0022 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larcenaire, C.; Wang, F.; Holásková, I.; Turcotte, R.; Gutensohn, M.; Park, Y.-L. Characterization of the Insect Assemblage and Associated Floral Volatiles of Black Cherry (Prunus serotina). Plants 2021, 10, 2195. https://doi.org/10.3390/plants10102195
Larcenaire C, Wang F, Holásková I, Turcotte R, Gutensohn M, Park Y-L. Characterization of the Insect Assemblage and Associated Floral Volatiles of Black Cherry (Prunus serotina). Plants. 2021; 10(10):2195. https://doi.org/10.3390/plants10102195
Chicago/Turabian StyleLarcenaire, Craig, Fumin Wang, Ida Holásková, Richard Turcotte, Michael Gutensohn, and Yong-Lak Park. 2021. "Characterization of the Insect Assemblage and Associated Floral Volatiles of Black Cherry (Prunus serotina)" Plants 10, no. 10: 2195. https://doi.org/10.3390/plants10102195
APA StyleLarcenaire, C., Wang, F., Holásková, I., Turcotte, R., Gutensohn, M., & Park, Y.-L. (2021). Characterization of the Insect Assemblage and Associated Floral Volatiles of Black Cherry (Prunus serotina). Plants, 10(10), 2195. https://doi.org/10.3390/plants10102195








