Growth and Physiological Performance of a Coastal Species Trifolium fragiferum as Affected by a Coexistence with Trifolium repens, NaCl Treatment and Inoculation with Rhizobia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Measurements
2.2. Plant Material, Growth Conditions and Treatments
2.3. Assessment of Substrate Electrical Conductivity and Physiological Measurements
2.4. Plant Harvest
2.5. Measurement of Electrolyte Leakage and Biochemical Parameters
2.6. Data Analysis
3. Results
3.1. Field Measurements
3.2. Substrate Salinity
3.3. Morphological Parameters
3.4. Physiological Parameters
3.5. Ion Concentration
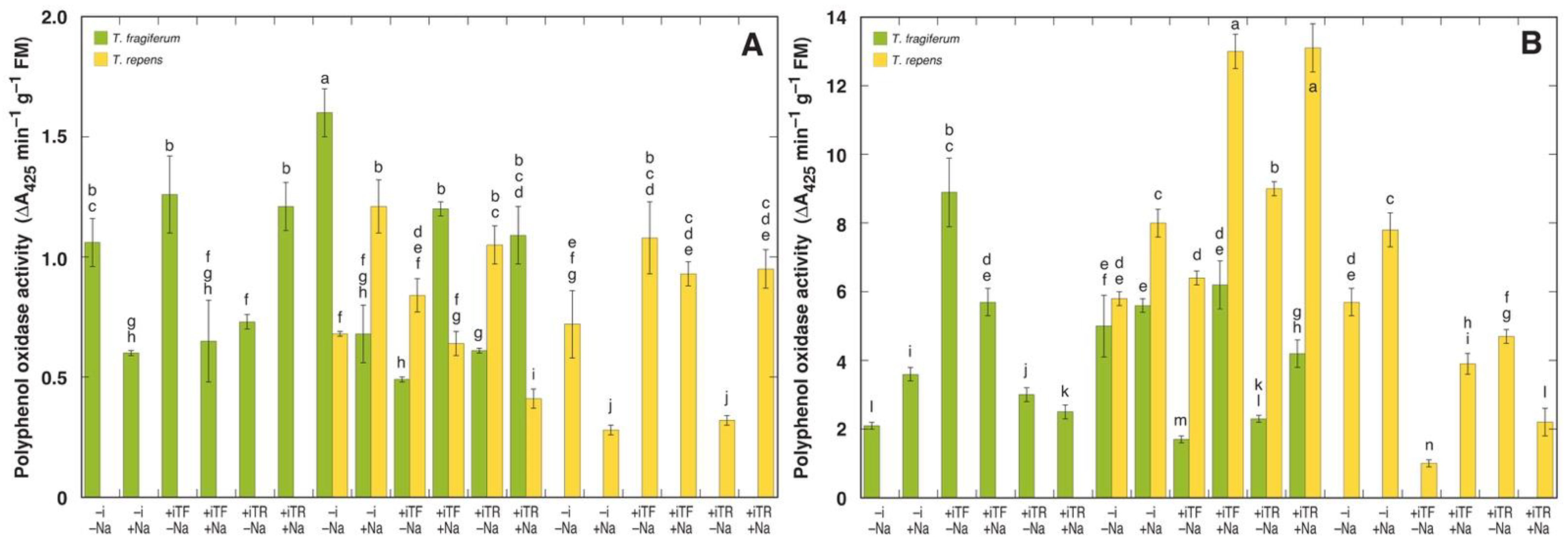
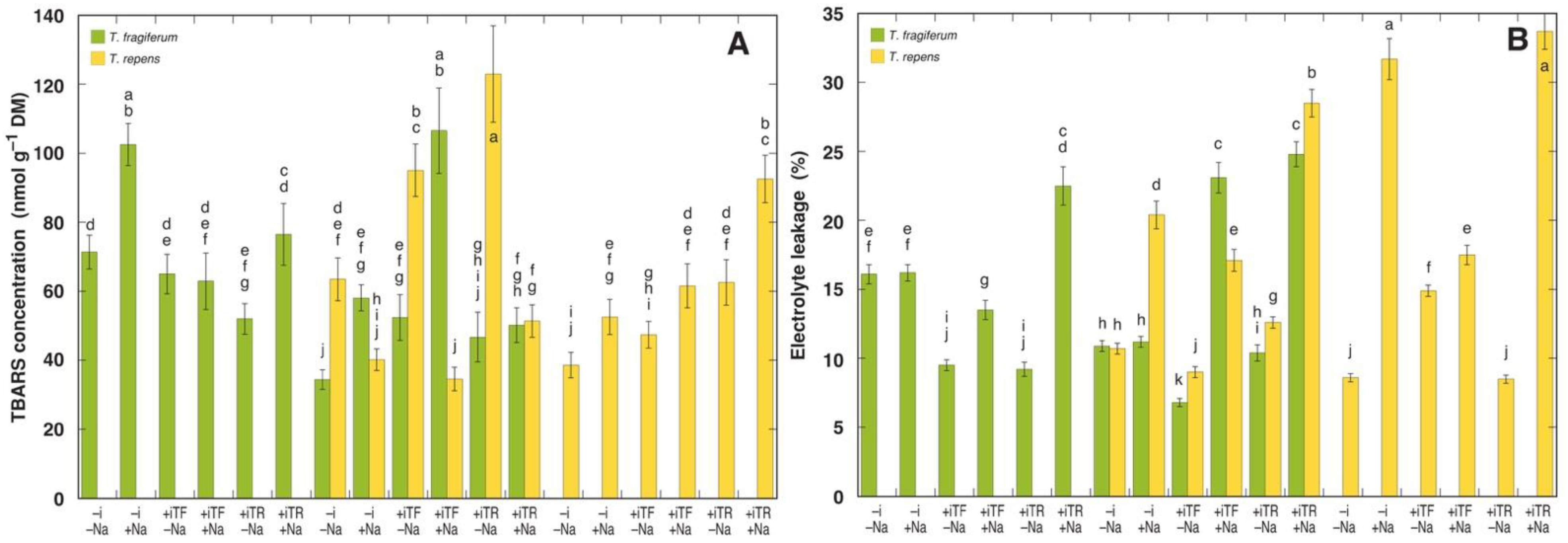
3.6. Oxidative Processes and Tissue Damage
4. Discussion
4.1. Experimental System
4.2. Species Coexistence and Response to Salinity
4.3. Bacterial Inoculation
4.4. Evaluation of Cellular Damage and Physiological Performance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cunningham, R.B.; Lindenmayer, D.B. Modeling count data of rare species: Some statistical issues. Ecology 2005, 86, 1135–1142. [Google Scholar] [CrossRef]
- Münzbergova, Z. Determinants of species rarity: Population growth rates of species sharing the same habitat. Am. J. Bot. 2005, 92, 1987–1994. [Google Scholar] [CrossRef]
- Ievinsh, G. Biological basis of biological diversity: Physiological adaptations of plants to heterogeneous habitats along a sea coast. Acta Univ. Latv. 2006, 710, 53–79. [Google Scholar]
- Townsend, C.E. Miscellaneous perennial clovers. In Clover Science and Technology; Taylor, J.L., Ed.; ASA/CSSA/SSSA: Madison, WI, USA, 1985; pp. 563–578. [Google Scholar]
- Ciocârlan, V.; Georgescu, M.I.; Sǎvulescu, E.; Anastasiu, P. Plopul salt marshes (Tulcea County)—An unique area for halophytes in Romania. Acta Horti Bot. Bucurest. 2013, 40, 27–32. [Google Scholar] [CrossRef]
- Janssen, J.A.M.; Rodwell, J.S. European Red List of Habitats: Part 2—Terrestrial and Freshwater Habitats; European Union: Brussels, Belgium, 2016. [Google Scholar]
- Cabinet of Ministers of Latvia. Provisions on the List of Specially Protected Species and Species of restricted Use. Regulations of the Cabinet of Ministers of Latvia No. 396. 2000. Available online: https://likumi.lv/ta/id/12821-noteikumi-par-ipasi-aizsargajamo-sugu-un-ierobezoti-izmantojamo-ipasi-aizsargajamo-sugu-sarakstu (accessed on 9 October 2021).
- Burdon, J.J. Biological flora of the British Isles. Trifolium repens. L. J. Ecol. 1983, 71, 307–330. [Google Scholar] [CrossRef]
- Svenning, M.M.; Gudmundsson, J.; Fagerli, I.-L.; Leinonen, P. Competition for nodule occupancy between introduced strains of Rhizobium leguinnosarum biovar trifolii and its influence on plant production. Ann. Bot. 2001, 88, 781–787. [Google Scholar] [CrossRef]
- Harper, J.L.; Clatworthy, J.N. The comparative biology of closely related species: VI. Analysis of the growth of Trifolium repens and T. fragiferum in pure and mixed populations. J. Exp. Bot. 1963, 14, 172–190. [Google Scholar] [CrossRef]
- Huber, H.; During, H.J. No long-term costs of meristem allocation to flowering in stoloniferous Trifolium species. Evol. Ecol. 2001, 14, 731–748. [Google Scholar] [CrossRef]
- Baldwin, A.H.; Mendelssohn, I.A. Effects of salinity and water level on coastal marshes: An experimental test of disturbance as a catalyst for vegetation change. Aquat. Bot. 1998, 61, 255–268. [Google Scholar] [CrossRef]
- Burchill, W.; James, E.K.; Li, D.; Lanigan, G.J.; Williams, M.; Iannetta, P.P.M.; Humphreys, J. Comparisons of biological nitrogen fixation in association with white clover (Trifolium repens L.) under four fertiliser nitrogen inputs as measured using two 15N techniques. Plant Soil 2014, 385, 287–302. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A.; Bakker, R.; Verwaal, J.; Scheublin, T.R.; Rutten, M.; van Logtestijn, R.; Staehelin, C. Symbiotic bacteria as a determinant of plant community structure and plant productivity in dune grassland. FEMS Microbiol. Ecol. 2006, 56, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Cabot, C.; Sibole, J.V.; Barceló, J.; Poschenrieder, C. Lessons from crop plants struggling with salinity. Plant Sci. 2014, 226, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, M.; Wani, S.P. Rhizobacterial-plant interactions: Strategies ensuring plant growth promotion under drought and salinity stress. Agric. Ecosyst. Environ. 2016, 231, 68–78. [Google Scholar] [CrossRef]
- Touchette, B.W.; Adams, E.C.; Laimbeer, P. Age-specific responses to elevated salinity in the coastal marsh plant black needlerush (Juncus roemerianus Scheele) as determined through polyphasic chlorophyl a fluorescence transients (OJIP). Mar. Biol. 2012, 159, 2137–2147. [Google Scholar] [CrossRef]
- Badr, A.; Brüggemann, W. Comparative analysis of drought stress response of maize genotypes using chlorophyll fluorescence measurements and leaf relative water content. Photosynthetica 2020, 58, 638–645. [Google Scholar] [CrossRef] [Green Version]
- Carter, G.A.; Knapp, A.K. Leaf optical properties in higher plants: Linking spectral characteristics to stress and chlorophyll concentration. Am. J. Bot. 2001, 88, 677–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samsone, I.; Andersone, U.; Vikmane, M.; Ieviņa, B.; Pakarna, G.; Ievinsh, G. Nondestructive methods in plant biology: An accurate measurement of chlorophyll content by a chlorophyll meter. Acta Univ. Latv. 2007, 723, 145–154. [Google Scholar]
- Mayer, A.M. Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef] [PubMed]
- Cosio, C.; Dunand, C. Specific functions of individual class III peroxidase genes. J. Exp. Bot. 2009, 60, 391–408. [Google Scholar] [CrossRef]
- Mathé, C.; Barre, A.; Jourda, C.; Dunand, C. Evolution and expression of class III peroxidases. Arch. Biochem. Biophys. 2010, 500, 58–65. [Google Scholar] [CrossRef]
- Whitlow, T.H.; Bassuk, N.L.; Ranney, T.G.; Reichert, D.L. An improved method for using electrolyte leakage to assess membrane competence in plant tissues. Plant Physiol. 1992, 98, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Bajji, M.; Kinet, J.-M.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Reg. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2007, 133, 651–669. [Google Scholar] [CrossRef]
- Szczerba, M.W.; Britto, D.T.; Kronzucker, H.J. K+ transport in plants: Physiology and molecular biology. J. Plant Physiol. 2008, 166, 447–466. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Anschütz, U.; Becker, D.; Shabala, S. Going beyond nutrition: Regulation of potassium homeostasis as a common denominator of plant adaptive responses to environment. J. Plant Physiol. 2014, 171, 670–687. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, G.V.; Ito, O.; Berry, W.L.; Wheeler, R.M. Sodium—A functional plant nutrient. Crit. Rev. Plant Sci. 2003, 22, 391–416. [Google Scholar]
- Glenn, E.P.; Fister, R.P.; Brown, J.J.; Thompson, T.L.; O’Leary, J. Na and K accumulation and salt tolerance of Atriplex canescens (Chenopodiaceae) genotypes. Am. J. Bot. 1996, 83, 997–1005. [Google Scholar] [CrossRef]
- Levine, J.M.; Brewer, J.S.; Bertness, M.D. Nutrients, competition and plant zonation in a New England salt marsh. J. Ecol. 1998, 86, 285–292. [Google Scholar] [CrossRef]
- Trinder, C.J.; Brooker, R.W.; Davidson, H.; Robinson, D. Directly quantifying multiple interacting influences on plant competition. Plant Cell Environ. 2020, 144, 1268–1277. [Google Scholar] [CrossRef]
- Pennings, S.C.; Grant, M.-B.; Bertness, M.D. Plant zonation in low-latitude salt marshes: Disentangling the roles of flooding, salinity and competition. J. Ecol. 2005, 93, 139–167. [Google Scholar] [CrossRef]
- Schofield, E.J.; Rowntree, J.K.; Paterson, E.; Brewer, M.J.; Price, E.A.C.; Brearley, F.Q.; Brooker, R.W. Cultivar differences and impact on plant–plant competition on temporal patterns of nitrogen and biomass accumulation. Front. Plant Sci. 2019, 10, 215. [Google Scholar] [CrossRef] [Green Version]
- Hortal, S.; Lozano, Y.M.; Bastida, F.; Armas, C.; Moreno, J.L.; Garcia, C.; Pugnaire, F.I. Plant-plant competition outcomes are modulated by plant effects on the soil bacterial community. Sci. Rep. 2017, 7, 17756. [Google Scholar] [CrossRef]
- Xi, N.; Bloor, J.M.G.; Chu, C. Soil microbes alter seedling performance and biotic interactions under plant competition and contrasting light conditions. Ann. Bot. 2020, 126, 1089–1098. [Google Scholar] [CrossRef]
- Ke, P.-J.; Wan, J. Effects of soil microbes on plant competition: A perspective from modern coexistence theory. Ecol. Monogr. 2020, 90, e01391. [Google Scholar] [CrossRef]
- Vincent, J.M. The cultivation, isolation and maintenance of rhizobia. In A Manual for the Practical Study of the Root-Nodule Bacteria; Vincent, J.M., Ed.; Blackwell Scientific: Oxford, UK, 1970; pp. 1–13. [Google Scholar]
- Tsimilli-Michael, M. Revisiting JIP-test: An educative review on concepts, assumptions, approximations, definitions and terminology. Photosynthetica 2020, 58, 275–292. [Google Scholar] [CrossRef] [Green Version]
- Ievinsh, G.; Ieviņa, S.; Andersone-Ozola, U.; Samsone, I. Leaf sodium, potassium and electrolyte accumulation capacity of plant species from salt-affected coastal habitats of the Baltic Sea: Towards a definition of Na hyperaccumulation. Flora 2021, 274, 151748. [Google Scholar] [CrossRef]
- Aref, I.M.; Khan, P.R.; Khan, S.; El-Atta, H.; Ahmed, A.I.; Iqbal, M. Modulation of antioxidant enzymes in Juniperus procera needles in relation to habitat environment and dieback incidence. Trees 2016, 30, 1669–1681. [Google Scholar] [CrossRef]
- Luo, Q.; Yu, B.; Liu, Y. Differential sensitivity to chloride and sodium ions in seedlings of Glycine max and G. soja under NaCl stress. J. Plant Physiol. 2005, 162, 1003–1012. [Google Scholar] [CrossRef]
- Andersone, U.; Ievinsh, G. Changes of morphogenic competence in mature Pinus sylvestris L. in vitro. Ann. Bot. 2002, 90, 293–298. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 15 October 2021).
- Karlsons, A.; Druva-Lusite, I.; Necajeva, J.; Andersone-Ozola, U.; Samsone, I.; Osvalde, A.; Ievinsh, G. Adaptation strategies of rare plant species to heterogeneous soil conditions on a coast of a lagoon lake as revealed by analysis of mycorrhizal symbiosis and mineral constituent dynamics. Environ. Exp. Biol. 2017, 15, 113–126. [Google Scholar]
- Wright, A.; Schnitzer, S.A.; Reich, P.B. Daily environmental conditions determine the competition–facilitation balance for plant water status. J. Ecol. 2015, 103, 648–656. [Google Scholar] [CrossRef]
- Callaway, R.M.; Walker, L.R. Competition and facilitation: A synthetic approach to interactions in plant communities. Ecology 1997, 78, 1958–1965. [Google Scholar] [CrossRef]
- Brooker, R.W.; Maestre, F.T.; Callaway, R.M.; Lortie, C.L.; Lohengrin, A.; Cavieres, L.A.; Kunstler, G.; Liancourt, P.; Tielbörger, K.; Travis, J.M.J.; et al. Facilitation in plant communities: The past, the present and the future. J. Ecol. 2008, 96, 18–34. [Google Scholar] [CrossRef] [Green Version]
- Biedrzycki, M.L.; Bais, H.P. Kin recognition in plants: A mysterious behaviour unsolved. J. Exp. Bot. 2010, 61, 4123–4128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertness, M.D.; Shumway, S.W. Competition and facilitation in marsh plants. Am. Nat. 1993, 142, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Ungar, I.A. Are biotic factors significant in influencing the distribution of halophytes in saline habitats? Bot. Rev. 1998, 64, 176–199. [Google Scholar] [CrossRef]
- Craine, J.M.; Dybzinski, R. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 2013, 27, 833–840. [Google Scholar] [CrossRef]
- Rumbaugh, M.D.; Pendery, B.M.; James, D.W. Variation in the salinity tolerance of strawberry clover (Trifolium fragiferum L.). Plant Soil 1993, 153, 265–271. [Google Scholar] [CrossRef]
- Agarie, S.; Shimoda, T.; Shimizu, Y.; Baumann, K.; Sunagawa, H.; Kondo, A.; Ueno, O.; Nakahara, T.; Nose, A.; Cushman, J.C. Salt tolerance, salt accumulation, and ionic homeostasis in a epidermal bladder-cell-less mutant of the common ice plant Mesembryanthemum srystallinum. J. Exp. Bot. 2007, 58, 1957–1967. [Google Scholar] [CrossRef]
- White, A.C.; Colmer, T.D.; Cawthray, G.R.; Hanley, M.E. Variable response of three Trifolium repens ecotypes to soil flooding by seawater. Ann. Bot. 2014, 114, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Lankau, R.A. Coevolution between invasive and native plants driven by chemical competition and soil biota. Proc. Nat. Acad. Sci. USA 2012, 109, 11240–11245. [Google Scholar] [CrossRef] [Green Version]
- García-Abellan, J.O.; Egea, I.; Pineda, B.; Sanchez-Bel, P.; Belver, A.; Garcia-Sogo, B.; Flores, F.B.; Atares, A.; Moreno, V.; Bolarin, M.C. Heterologous expression of the yeast HAL5 gene in tomato enhances salt tolerance by reducing shoot Na+ accumulation in the long term. Physiol. Plant. 2014, 152, 700–713. [Google Scholar] [CrossRef]
- Staal, M.; Maathuis, F.J.; Elzenga, J.T.M.; Overbeek, J.H.M.; Prins, H. Na+/H+ antiport activity in tonoplast vesicles from roots of the salt-tolerant Plantago maritima and the salt-sensitive Plantago media. Physiol. Plant. 1991, 82, 179–184. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, W.; Ren, J.; Zhang, C. Selectivity of various types of salt-resistant plants for K+ over Na+. J. Arid Envrion. 2002, 52, 457–472. [Google Scholar] [CrossRef]
- Benzarti, M.; Rejeb, K.B.; Debez, A.; Messedi, D.; Abdelly, C. Photosynthetic activity and leaf antioxidative responses of Atriplex portulacoides subjected to extreme salinity. Acta Physiol. Plant. 2012, 34, 1679–1688. [Google Scholar] [CrossRef]
- Liu, S.; Dong, Y.; Xu, L.; Kong, J. Effects of foliar applications of nitric oxide and salicylic acid on salt-induced changes in photosynthesis and antioxidative metabolism of cotton seedlings. Plant Growth Reg. 2014, 73, 67–78. [Google Scholar] [CrossRef]
- Cekstere, G.; Karlsons, A.; Grauda, D. Salinity-induced responses and resistance in Trifolium repens L. Urban For. Urban Green. 2015, 14, 225–236. [Google Scholar] [CrossRef]
- Debez, A.; Koyro, H.-W.; Grignon, C.; Abdelly, C.; Huchzermeyer, B. Relationship between the photosynthetic activity and the performance of Cakile maritima after long-term salt treatment. Physiol. Plant. 2008, 133, 373–385. [Google Scholar] [CrossRef]
- Du, J.; Yu, F.-H.; Alpert, P.; Dong, M. Arbuscular mycorrhizal fungi reduce effects of physiological integration in Trifolium repens. Ann. Bot. 2009, 104, 335–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talaat, N.B.; Shawky, B.T. Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ. Exp. Bot. 2014, 98, 20–31. [Google Scholar] [CrossRef]
- Melino, V.J.; Drew, E.A.; Ballard, R.A.; Reeve, W.G.; Thomson, G.; White, R.G.; O’hara, G.W. Identifying abnormalities in symbiotic development between Trifolium spp. and Rhizobium leguminosarum bv. trifolii leading to sub-optimal and ineffective nodule phenotypes. Ann. Bot. 2012, 110, 1559–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, J.; Gerendás, J.; Härdter, R.; Sattelmacher, B. Effect of nitrogen form and root-zone pH on growth and nitrogen uptake of tea (Camellia sinensis) plants. Ann. Bot. 2007, 99, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Piwpuan, N.; Zhai, X.; Brix, H. Nitrogen nutrition of Cyperus laevigatus and Phormium tenax: Effects of ammonium versus nitrate on growth, nitrate reductase activity and N uptake kinetics. Aquat. Bot. 2013, 106, 42–51. [Google Scholar] [CrossRef]
- Crush, J.R.; Cosgrove, G.P.; Brougham, R.W. The effect of nitrogen fertiliser on clover nitrogen fixation in an intensively grazed Manawatu pasture. N. Z. J. Exp. Agric. 1982, 10, 395–399. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, N.; Umar, S.; Khan, N.A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant Physiol. 2015, 178, 84–91. [Google Scholar] [CrossRef]
- Chanway, C.P.; Holl, F.B.; Turkington, R. Effect of Rhizobium leguminosarum biovar trifolii genotype on specificity between Trifolium repens and Lolium perenne. J. Ecol. 1989, 77, 1150–1160. [Google Scholar] [CrossRef]
- Zahran, H.H. Rhizobum-legume symbiosis and nitrogen fixation under severe conditions and in arid climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [CrossRef] [Green Version]
- Cordovilla, M.P.; Ligero, F.; Lluch, C. The effect of salinity on N fixation and assimilation in Vicia faba. J. Exp. Bot. 1994, 45, 1483–1488. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, E.T.; Li, Y.; Chen, W.X. Diverse bacteria isolated from root nodules of Trifolium, Crotalaria and Mimosa grown in the subtropical regions of China. Arch. Microbiol. 2007, 188, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kim, M.-S.; Sun, Y.; Dowd, S.E.; Shi, H.; Paré, P.W. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol. Plant Microbe Interact. 2008, 21, 737–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.-L.; Aziz, M.; Qiao, Y.; Han, Q.-Q.; Li, J.; Wang, Y.-Q.; Shen, X.; Wang, S.-M.; Paré, P.W. Soil microbe Bacillus subtilis (GB03) induces biomass accumulation and salt tolerance with lower sodium accumulation in wheat. Crop Pasture Sci. 2014, 65, 423–427. [Google Scholar] [CrossRef]
- Khalid, M.; Bilal, M.; Hassani, D.; Iqbal, H.M.N.; Wang, H.; Huang, D. Mitigation of salt stress in white clover (Trifolium repens) by Azospirillum brasilense and its inoculation effect. Bot. Stud. 2017, 58, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamauchi, Y.; Furutera, A.; Seki, K.; Toyoda, Y.; Tanaka, K.; Sugimoto, Y. Malondialdehyde generated from peroxidized linolenic acid causes protein modification in heat-stressed plants. Plant Physiol. Biochem. 2008, 46, 786–793. [Google Scholar] [CrossRef]
- Demiral, T.; Türkan, I. Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ. Exp. Bot. 2005, 53, 247–257. [Google Scholar] [CrossRef]
- Bernstein, N.; Shoresh, M.; Xu, Y.; Huang, B. Involvement of the plant antioxidative response in the differential growth sensitivity to salinity of leaves vs roots during cell development. Free Radic. Biol. Med. 2010, 49, 1161–1171. [Google Scholar] [CrossRef]
- Rolny, N.; Costa, L.; Carrión, C.; Guiamet, J.J. Is the electrolyte leakage assay an unequivocal test of membrane deterioration during leaf senescence? Plant Physiol. Biochem. 2011, 49, 1220–1227. [Google Scholar] [CrossRef]
- Bor, M.; Ozdemir, F.; Türkan, I. The effect of salt stress on lipid peroxidation and antioxidants in leaves of sugar beet Beta vulgaris L. and wild beet Beta maritima L. Plant Sci. 2003, 164, 77–84. [Google Scholar] [CrossRef]
- Sekmen, A.H.; Türkan, I.; Takio, S. Differential responses of antioxidative enzymes and lipid peroxidation to salt stress in salt-tolerant Plantago maritima and salt-sensitive Plantago media. Physiol. Plant. 2007, 131, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Ellouzi, H.; Hamed, K.B.; Cela, J.; Munné-Bosch, S.; Abdelly, C. Early effects of salt stress on the physiological and oxidative sstatus of Cakile maritima (halophyte) and Arabidopsis thaliana (glycophyte). Physiol. Plant. 2011, 142, 128–143. [Google Scholar] [CrossRef]
- Lee, M.R.F.; Tweed, J.K.S.; Minchin, F.R.; Winters, A.L. Red clover polyphenol oxidase: Activation, activity and efficacy under grazing. Anim. Feed Sci. Technol. 2009, 149, 250–264. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Neto, V.P.; Mendes, J.B.S.; de Araújo, A.S.F.; de Alcântra Neto, F.; Bonifacio, A.; Rodrigues, A.C. Symbiotic performance, nitrogen flux and growth of lima bean (Phaseolus lunatus L.) varieties inoculated with different indigenous strains of rhizobia. Symbiosis 2017, 73, 117–124. [Google Scholar] [CrossRef]
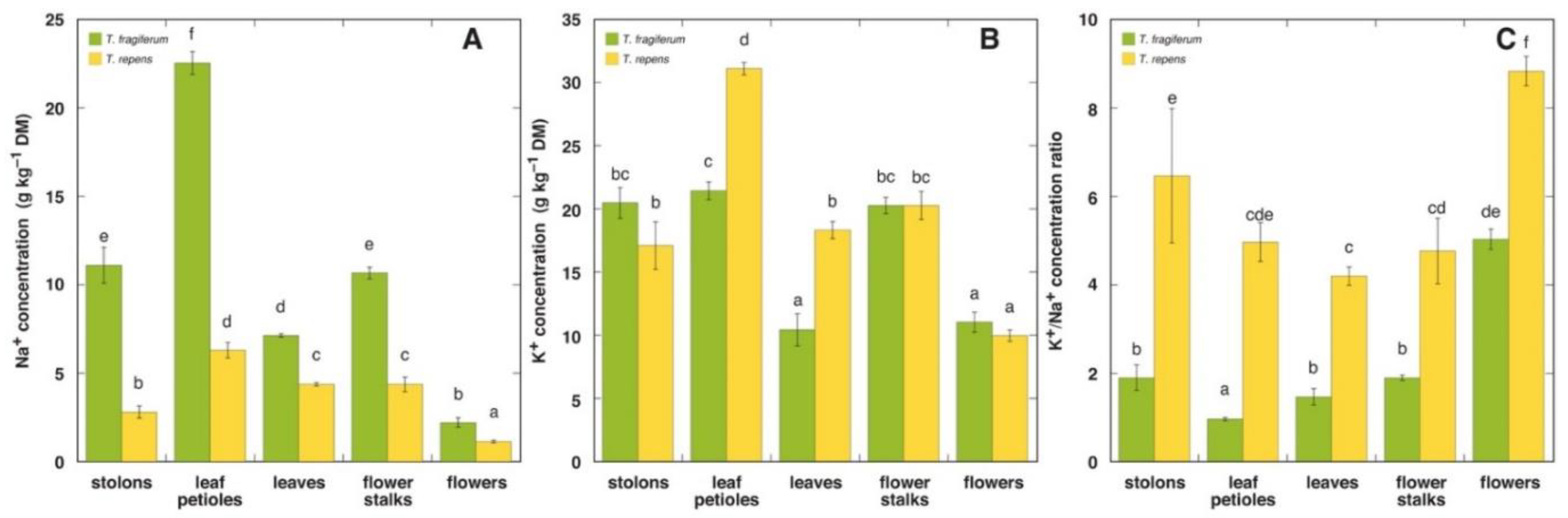
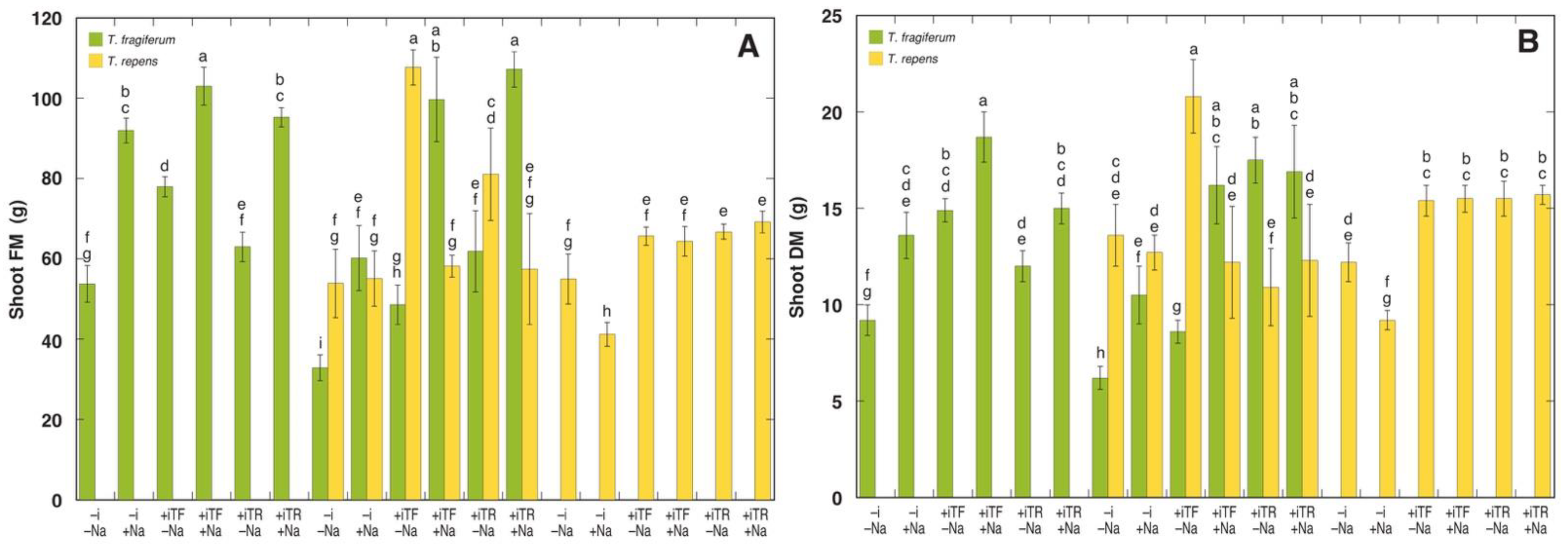

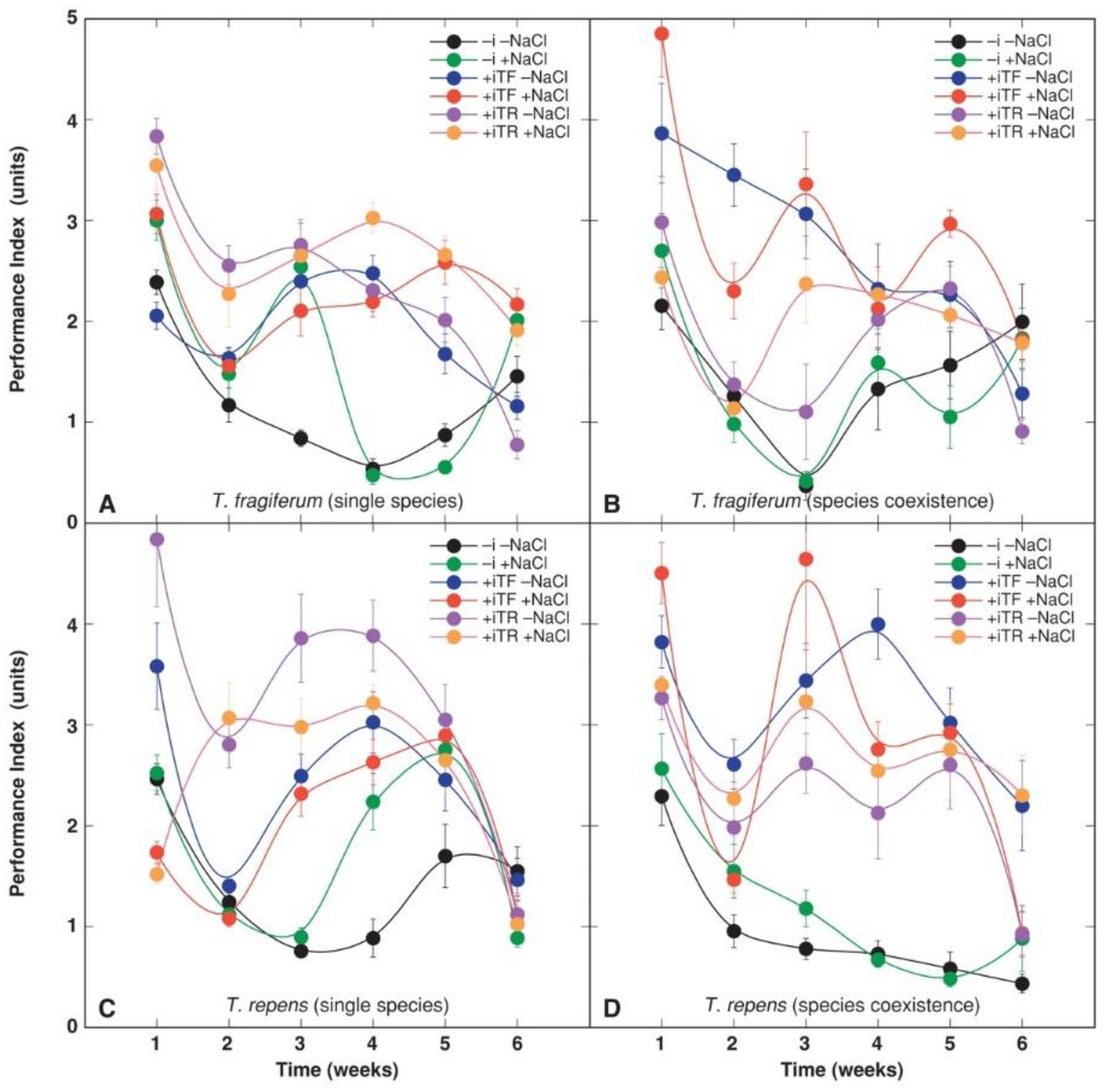
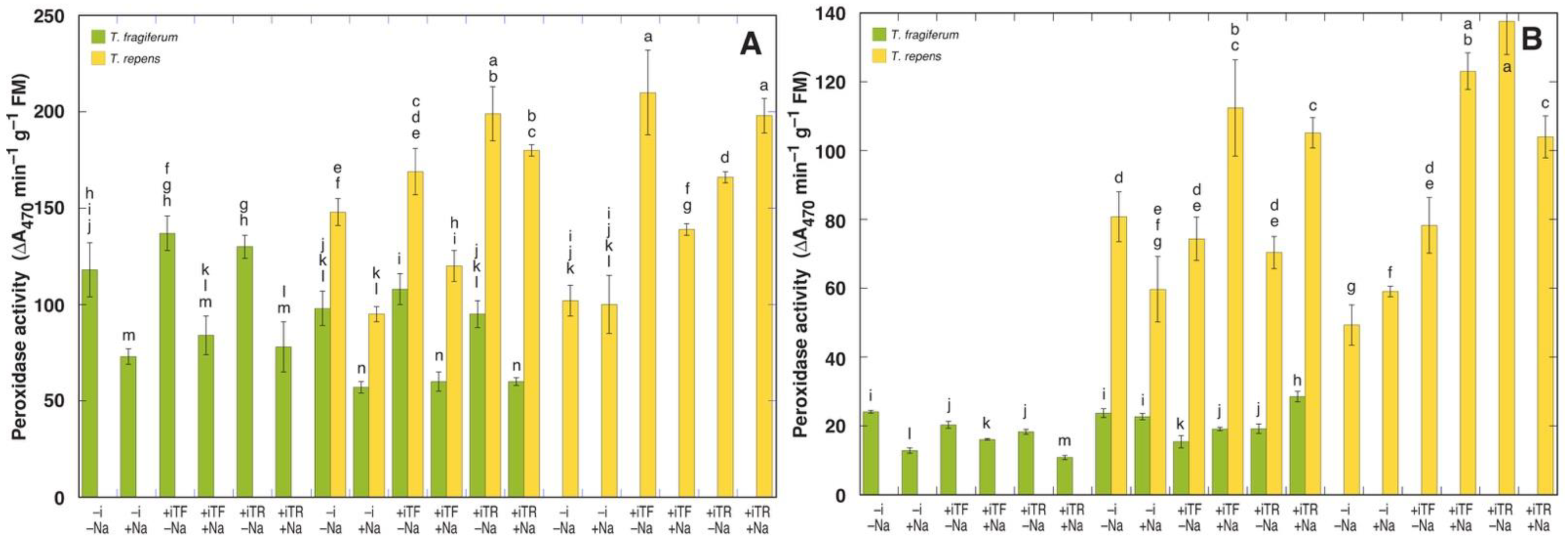
| Treatment | T. fragiferum | T. repens | iTF | iTR | NaCl |
|---|---|---|---|---|---|
| TFss–i–NaCl | +++ | – | – | – | – |
| TFss–i+NaCl | ++ | – | – | – | + |
| TFss+iTF–NaCl | ++ | – | + | – | – |
| TFss+iTF+NaCl | ++ | – | + | – | + |
| TFss+iTR–NaCl | ++ | – | – | + | – |
| TFss+iTR+NaCl | ++ | – | – | + | + |
| TFTR–i–NaCl | + | + | – | – | – |
| TFTR–i+NaCl | + | + | – | – | + |
| TFTR+iTF–NaCl | + | + | + | – | – |
| TFTR+iTF+NaCl | + | + | + | – | + |
| TFTR+iTR–NaCl | + | + | – | + | – |
| TFTR+iTR+NaCl | + | + | – | + | + |
| TRss–i–NaCl | – | ++ | – | – | – |
| TRss–i+NaCl | – | ++ | – | – | + |
| TRss+iTF–NaCl | – | ++ | + | – | – |
| TRss+iTF+NaCl | – | ++ | + | – | + |
| TRss+iTR–NaCl | – | ++ | – | + | – |
| TRss+iTR+NaCl | – | ++ | – | + | + |
| Treatment | Week 2 | Week 4 | Week 6 |
|---|---|---|---|
| TFss–i–NaCl | 89.5 ± 5.7 ef | 71.4 ± 2.7 e | 53.3 ± 3.6 gh |
| TFss–i+NaCl | 119.9 ± 10.3 abc | 163.9 ± 14.6 bcd | 252.4 ± 8.8 cd |
| TFss+iTF–NaCl | 107.0 ± 5.4 cd | 68.3 ± 4.9 ef | 53.5 ± 8.8 gh |
| TFss+iTF+NaCl | 132.6 ± 4.4 a | 148.1 ± 8.0 d | 235.7 ± 9.1 de |
| TFss+iTR–NaCl | 87.4 ± 6.8 ef | 70.1 ± 2.4 e | 50.1 ± 1.8 h |
| TFss+iTR+NaCl | 112.3 ± 5.5 bcd | 145.9 ± 8.9 d | 230.8 ± 9.2 e |
| TFTR–i–NaCl | 81.7 ± 6.6 f | 58.1 ± 2.3 g | 50.1 ± 2.1 h |
| TFTR–i+NaCl | 115.3 ± 3.0 bc | 183.6 ± 6.3 ab | 270.7 ± 8.1 abc |
| TFTR+iTF–NaCl | 129.8 ± 11.8 a | 76.6 ± 3.6 e | 71.4 ± 5.4 f |
| TFTR+iTF+NaCl | 121.5 ± 7.4 ab | 181.1 ± 5.3 ab | 255.7 ± 24.4 abcd |
| TFTR+iTR–NaCl | 94.9 ± 3.7 ef | 65.7 ± 3.3 efg | 58.2 ± 1.1 g |
| TFTR+iTR+NaCl | 105.7 ± 3.8 cd | 156.5 ± 7.4 cd | 262.7 ± 5.0 bc |
| TRss–i–NaCl | 93.8 ± 5.0 ef | 60.6 ± 2.3 fg | 55.2 ± 4.2 gh |
| TRss–i+NaCl | 103.9 ± 4.9 de | 190.7 ± 11.0 a | 289.5 ± 6.6 a |
| TRss+iTF–NaCl | 113.5 ± 6.1 bcd | 56.6 ± 2.9 g | 55.1 ± 1.5 gh |
| TRss+iTF+NaCl | 123.5 ± 6.4 ab | 173.5 ± 6.6 abc | 264.7 ± 12.7 abc |
| TRss+iTR–NaCl | 105.2 ± 8.2 cd | 61.3 ± 2.0 fg | 52.7 ± 2.5 h |
| TRss+iTR+NaCl | 133.5 ± 4.9 a | 177.5 ± 8.0 ab | 273.3 ± 7.4 ab |
| Source of Variation | df | Mean Square | F |
|---|---|---|---|
| Species coexistence | 2 | 3394 | 12.81 *** |
| Inoculant | 2 | 1046 | 3.95 * |
| NaCl | 1 | 797 795 | 3012.24 *** |
| Time | 2 | 59684 | 225.35 *** |
| Species coexistence × inoculant | 4 | 804 | 3.04 * |
| Species coexistence × NaCl | 2 | 3506 | 13.24 *** |
| Inoculant × NaCl | 2 | 2165 | 8.18 *** |
| Species coexistence × time | 4 | 489 | 1.85 |
| Inoculant × time | 4 | 1661 | 6.27 *** |
| NaCl × time | 2 | 193 783 | 731.67 *** |
| Species coexistence × inoculant × NaCl | 4 | 414 | 1.56 |
| Residuals | 240 | 265 |
| Treatment | H2O Content (g g−1 DM) | Number of Leaves | Number of Stolons | Total Stolon Length (mm) | Average Stolon Length (mm) |
|---|---|---|---|---|---|
| T. fragiferum | |||||
| TFss–i–NaCl | 4.9 | 736 ± 92 bcd | 44.3 ± 5.7 def | 1097 ± 136 ef | 25.0 ± 2.3 defg |
| TFss–i+NaCl | 5.8 | 829 ± 89 abc | 55.3 ± 6.4 cd | 1465 ± 122 b | 27.5 ± 2.3 cdef |
| TFss+iTF–NaCl | 4.2 | 607 ± 75 def | 52.0 ± 6.0 cde | 1516 ± 174 b | 30.0 ± 3.5 bcd |
| TFss+iTF+NaCl | 4.5 | 835 ± 99 ab | 57.5 ± 5.0 bc | 2084 ± 189 a | 36.5 ± 3.8 ab |
| TFss+iTR–NaCl | 4.3 | 725 ± 13 cd | 42.3 ± 4.8 efg | 1087 ± 32 ef | 23.3 ± 1.8 fg |
| TFss+iTR+NaCl | 5.4 | 697 ± 77 cd | 37.0 ± 5.6 f | 1352 ± 151 bcd | 37.5 ± 2.5 a |
| TFsc–i–NaCl | 4.3 | 345 ± 37 i | 33.5 ± 5.5 g | 714 ± 31 g | 21.5 ± 2.5 gh |
| TFsc–i+NaCl | 4.7 | 585 ± 5 ef | 34.5 ± 3.5 f | 1111 ± 83 def | 33.0 ± 3.0 abc |
| TFsc+iTF–NaCl | 4.7 | 539 ± 20 fg | 54.5 ± 2.5 cd | 1456 ± 48 b | 26.5 ± 1.5 defg |
| TFsc+iTF+NaCl | 5.2 | 478 ± 11 gh | 42.0 ± 5.0 efg | 1363 ± 156 bcd | 32.5 ± 0.5 bc |
| TFsc+iTR–NaCl | 2.5 | 510 ± 75 fg | 32.5 ± 4.5 g | 1097 ± 95 ef | 34.0 ± 2.7 ab |
| TFsc+iTR+NaCl | 5.3 | 939 ± 130 ab | 77.0 ± 11.0 a | 2255 ± 182 a | 29.5 ± 0.5 cd |
| T. repens | |||||
| TRss–i–NaCl | 3.5 | 599 ± 48 def | 27.0 ± 3.4 h | 656 ± 75 g | 24.0 ± 2.2 efg |
| TRss–i+NaCl | 3.5 | 385 ± 16 hj | 31.8 ± 2.8 h | 775 ± 71 g | 25.0 ± 3.5 defg |
| TRss+iTF–NaCl | 3.3 | 652 ± 16 de | 26.8 ± 2.8 h | 1049 ± 98 ef | 40.5 ± 5.5 a |
| TRss+iTF+NaCl | 3.2 | 687 ± 69 cd | 40.3 ± 4.3 fg | 1106 ± 96 def | 27.5 ± 0.5 def |
| TRss+iTR–NaCl | 3.3 | 556 ± 49 efg | 45.0 ± 3.9 def | 1176 ± 102 cde | 26.0 ± 2.8 defg |
| TRss+iTR+NaCl | 3.4 | 861 ± 77 ab | 70.5 ± 6.9 ab | 1560 ± 112 b | 22.0 ± 2.9 fgh |
| TRsc–i–NaCl | 3.0 | 435 ± 78 ghi | 38.5 ± 4.5 fgh | 641 ± 78 g | 16.0 ± 3.0 hi |
| TRsc–i+NaCl | 3.3 | 486 ± 68 fgh | 27.5 ± 2.5 h | 776 ± 12 g | 29.0 ± 3.0 cde |
| TRsc+iTF–NaCl | 4.2 | 962 ± 108 a | 57.5 ± 6.5 bc | 1626 ± 178 b | 27.5 ± 3.8 cdef |
| TRsc+iTF+NaCl | 3.8 | 817 ± 94 abc | 63.5 ± 6.6 abc | 1478 ± 172 b | 22.0 ± 3.5 fgh |
| TRsc+iTR–NaCl | 6.4 | 773 ± 79 abc | 40.5 ± 3.5 fg | 957 ± 12 f | 24.0 ± 2.0 efg |
| TRsc+iTR+NaCl | 3.7 | 394 ± 31 hi | 39.0 ± 4.6 fgh | 516 ± 98 g | 14.0 ± 2.5 i |
| Source of Variation | df | Shoot Fresh Mass | Shoot Dry Mass | ||||
|---|---|---|---|---|---|---|---|
| Mean Square | F | ω2 | Mean Square | F | ω2 | ||
| Species coexistence Inoculant NaCl | 2 | 3053 | 13.58 *** | 0.078 | 8.22 | 0.73 | –0.002 |
| 2 | 6233 | 27.73 *** | 0.165 | 243.16 | 21.68 *** | 0.188 | |
| 1 | 3680 | 16.37 *** | 0.048 | 25.10 | 2.24 | 0.006 | |
| Species Species coexistence × inoculant | 1 | 6 | 0.03 | –0.003 | 72.27 | 6.44 * | 0.025 |
| 4 | 375 | 1.37 | 0.008 | 10.00 | 0.89 | –0.002 | |
| Species coexistence × NaCl Inoculant × NaCl Inoculant × species NaCl × species | 2 | 2385 | 10.61 *** | 0.059 | 43.39 | 3.87 * | 0.026 |
| 2 | 184 | 0.82 | –0.001 | 0.04 | 0.00 | –0.009 | |
| 2 | 916 | 4.08 * | 0.019 | 225.46 | 20.10 *** | 0.174 | |
| 1 | 16133 | 71.79 *** | 0.219 | 71.09 | 6.34 * | 0.024 | |
| Species coexistence × inoculant × NaCl | 4 | 173 | 0.77 | –0.03 | 5.35 | 0.48 | –0.009 |
| Inoculant × NaCl × species Residuals | 2 | 1718 | 7.64 *** | 0.041 | 52.35 | 4.67 * | 0.033 |
| 96 | 225 | 11.22 | |||||
| Treatment | Relative Mass (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| T. fragiferum | T. repens | |||||||
| Single Species | Species Coexistence | Single Species | Species Coexistence | |||||
| FM | DM | FM | DM | FM | DM | FM | ||
| –i | 171 ± 6 * | 147 ± 13 * | 183 ± 28 * | 168 ± 24 * | 75 ± 6 * | 75 ± 4 * | 102 ± 13 | 93 ± 6 |
| +iTF | 132 ± 6 * | 125 ± 9 * | 205 ± 22 * | 188 ± 23 * | 98 ± 6 | 100 ± 5 | 54 ± 3 * | 59 ± 4 * |
| +iTR | 151 ± 4 * | 125 ± 6 * | 176 ± 19 * | 96 ± 14 | 104 ± 4 | 101 ± 5 | 71 ± 17 * | 112 ± 27 |
| Treatment | Relative Mass (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| T. fragiferum | T. repens | |||||||
| Single Species | Species Coexistence | Single Species | Species Coexistence | |||||
| FM | DM | FM | DM | FM | DM | FM | ||
| –NaCl, iTF | 145 ± 5 * | 161 ± 7 * | 148 ± 15 * | 138 ± 10 * | 200 ± 8 * | 126 ± 7 * | 119 ± 4 | 207 ± 14 * |
| +NaCl, iTF | 114 ± 5 | 138 ± 10 * | 166 ± 17 * | 155 ± 19 * | 106 ± 5 | 168 ± 8 * | 156 ± 9 * | 96 ± 7 |
| –NaCl, iTR | 117 ± 7 | 130 ± 9 * | 188 ± 31 * | 280 ± 19 * | 151 ± 21 * | 127 ± 8 * | 121 ± 4 * | 81 ± 14 |
| Treatment | Relative Mass (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| T. fragiferum | T. repens | |||||||
| –NaCl | +NaCl | –NaCl | +NaCl | |||||
| FM | DM | FM | DM | FM | DM | FM | ||
| –i | 61 ± 6 * | 67 ± 6 * | 65 ± 9 * | 77 ± 11 * | 98 ± 15 | 111 ± 13 | 134 ± 17 * | 138 ± 9 * |
| +iTF | 62 ± 6 * | 58 ± 4 * | 97 ± 10 | 87 ± 11 | 164 ± 7 * | 134 ± 13 * | 91 ± 4 | 79 ± 6 * |
| +iTR | 98 ± 16 | 145 ± 10 * | 112 ± 13 | 112 ± 16 | 122 ± 17 | 71 ± 13 * | 83 ± 19 | 78 ± 19 |
| Source of Variation | df | Chlorophyll Concentration | Performance Index | ||
|---|---|---|---|---|---|
| Mean Square | F | Mean Square | F | ||
| Species coexistence | 2 | 186357 | 64.96 *** | 1.02 | 1.40 |
| Inoculant | 2 | 1594961 | 555.991 *** | 179.57 | 247.36 *** |
| NaCl | 1 | 1211 | 0.42 | 1.58 | 2.18 |
| Species | 1 | 89559 | 31.22 *** | 3.02 | 4.16 * |
| Time | 5 | 139103 | 48.49 *** | 64.53 | 88.89 *** |
| Species coexistence × inoculant | 4 | 116126 | 40.48 *** | 29.43 | 40.53 *** |
| Species coexistence × NaCl | 2 | 22439 | 7.82 *** | 18.63 | 25.66 *** |
| Inoculant × NaCl | 2 | 52265 | 18.22 *** | 2.86 | 3.94 * |
| Inoculant × species | 2 | 34570 | 12.05 *** | 12.85 | 17.70 *** |
| NaCl × species | 1 | 8278 | 2.89 | 0.02 | 0.03 |
| NaCl × time | 5 | 23032 | 8.03 *** | 2.65 | 3.66 ** |
| Species coexistence × time | 10 | 17178 | 5.99 *** | 2.61 | 3.59 *** |
| Inoculant × time | 10 | 153539 | 53.52 *** | 12.37 | 17.04 *** |
| Species × time | 5 | 5382 | 1.88 | 4.24 | 5.84 *** |
| Species coexistence × inoculant × NaCl | 4 | 31668 | 11.04 *** | 3.61 | 4.98 *** |
| Inoculant × NaCl × species | 2 | 23868 | 8.32 *** | 2.73 | 3.76 * |
| Residuals | 1237 | 2869 | 0.73 | ||
| Treatment | Stolons | Leaves | Petioles | Flowers | Flower Stalks |
|---|---|---|---|---|---|
| T. fragiferum | |||||
| TFss–i–NaCl | 0.93 ± 0.16 d | 0.49 ± 0.06 f | 0.71 ± 0.11 de | − | – |
| TFss–i+NaCl | 12.75 ± 0.25 a | 10.08 ± 0.08 a | 19.83 ± 1.33 b | − | – |
| TFss+iTF–NaCl | 0.78 ± 0.05 e | 0.56 ± 0.01 f | 0.61 ± 0.04 e | – | – |
| TFss+iTF+NaCl | 9.75 ± 1.42 b | 9.92 ± 0.92 ab | 19.17 ± 0.83 b | – | – |
| TFss+iTR–NaCl | 0.99 ± 0.33 d | 0.66 ± 0.06 e | 0.88 ± 0.15 d | – | – |
| TFss+iTR+NaCl | 10.25 ± 1.08 b | 8.58 ± 0.42 bc | 23.25 ± 0.42 a | – | – |
| TFsc–i–NaCl | 0.80 ± 0.35 e | 0.73 ± 0.23 e | 0.73 ± 0.21 de | – | – |
| TFsc–i+NaCl | 7.38 ± 0.71 c | 6.41 ± 1.36 d | 17.22 ± 0.94 c | – | – |
| TFsc+iTF–NaCl | 0.96 ± 0.24 d | 0.82 ± 0.19 e | 0.99 ± 0.17 d | – | – |
| TFsc+iTF+NaCl | 7.13 ± 1.70 c | 7.92 ± 0.25 cd | 17.17 ± 0.94 c | – | – |
| TFsc+iTR–NaCl | 1.04 ± 0.01 d | 0.67 ± 0.07 e | 0.83 ± 0.07 d | – | – |
| TFsc+iTR+NaCl | 9.58 ± 0.42 b | 7.08 ± 0.25 d | 19.08 ± 0.08 b | − | – |
| T. repens | |||||
| TRss–i–NaCl | 0.19 ± 0.02 e | 0.41 ± 0.08 f | 0.42 ± 0.10 d | 0.40 ± 0.07 d | 0.27 ± 0.08 g |
| TRss–i+NaCl | 3.63 ± 1.37 ab | 6.08 ± 0.58 a | 11.33 ± 1.83 a | 0.70 ± 0.12 c | 2.12 ± 0.74 d |
| TRss+iTF–NaCl | 0.25 ± 0.08 e | 0.49 ± 0.08 ef | 0.51 ± 0.01 d | 0.37 ± 0.06 d | 0.45 ± 0.05 f |
| TRss+iTF+NaCl | 4.32 ± 0.92 a | 3.09 ± 1.71 bc | 5.87 ± 1.24 b | 1.57 ± 0.88 b | 5.24 ± 0.74 b |
| TRss+iTR–NaCl | 0.32 ± 0.07 e | 0.45 ± 0.08 f | 0.44 ± 0.08 d | 0.32 ± 0.12 de | 0.37 ± 0.18 fg |
| TRss+iTR+NaCl | 4.97 ± 0.53 a | 7.02 ± 1.65 a | 14.92 ± 1.92 a | 1.83 ± 0.10 a | 7.83 ± 0.76 a |
| TRsc–i–NaCl | 0.65 ± 0.17 d | 1.09 ± 0.44 d | 0.25 ± 0.05 e | 0.20 ± 0.04 e | 0.27 ± 0.05 g |
| TRsc–i+NaCl | 1.47 ± 0.27 c | 0.69 ± 0.12 e | 2.08 ± 0.75 c | 0.49 ± 0.17 d | 1.31 ± 0.28 e |
| TRsc+iTF–NaCl | 0.27 ± 0.12 e | 0.41 ± 0.13 f | 0.41 ± 0.13 d | 0.34 ± 0.06 d | 0.52 ± 0.08 f |
| TRsc+iTF+NaCl | 2.72 ± 0.38 b | 2.56 ± 0.64 c | 6.03 ± 0.93 b | 0.80 ± 0.12 bc | 3.60 ± 0.44 c |
| TRsc+iTR–NaCl | 0.14 ± 0.03 f | 0.28 ± 0.02 g | 0.27 ± 0.07 e | 0.27 ± 0.05 e | 0.23 ± 0.04 g |
| TRsc+iTR+NaCl | 4.17 ± 0.30 ab | 5.19 ± 1.14 ab | 7.42 ± 0.92 b | 1.89 ± 0.21 a | 6.13 ± 0.53 b |
| Treatment | Stolons | Leaves | Petioles | Flowers | Flower Stalks | Leaf K+:Na+ Ratio |
|---|---|---|---|---|---|---|
| T. fragiferum | ||||||
| TFss–i–NaCl | 6.24 ± 1.93 a | 5.33 ± 0.17 c | 10.50 ± 1.00 ab | − | – | 10.88 |
| TFss–i+NaCl | 4.38 ± 0.41 ab | 6.00 ± 0.30 ab | 8.42 ± 0.25 d | – | – | 0.60 |
| TFss+iTF–NaCl | 2.33 ± 0.10 d | 5.83 ± 0.33 abc | 9.92 ± 0.92 bc | – | – | 10.41 |
| TFss+iTF+NaCl | 2.71 ± 0.51 cd | 5.23 ± 0.27 c | 8.33 ± 0.35 d | – | – | 0.53 |
| TFss+iTR–NaCl | 3.95 ± 1.22 ab | 6.67 ± 0.33 a | 11.08 ± 0.42 ab | – | – | 10.11 |
| TFss+iTR+NaCl | 3.46 ± 0.16 bc | 4.99 ± 0.18 cd | 7.92 ± 0.42 d | – | – | 0.58 |
| TFsc–i–NaCl | 4.11 ± 1.72 ab | 5.42 ± 0.75 bc | 8.22 ± 1.28 cd | – | – | 7.43 |
| TFsc–i+NaCl | 2.84 ± 0.34 cd | 4.20 ± 0.65 d | 6.02 ± 0.48 e | – | – | 0.66 |
| TFsc+iTF–NaCl | 3.11 ± 0.15 bcd | 6.89 ± 0.95 ab | 8.61 ± 0.39 cd | – | – | 7.18 |
| TFsc+iTF+NaCl | 3.22 ± 0.92 bcd | 4.96 ± 0.04 cd | 8.58 ± 0.08 d | – | – | 0.63 |
| TFsc+iTR–NaCl | 3.83 ± 1.06 ab | 6.25 ± 0.25 ab | 11.67 ± 0.50 a | – | – | 9.33 |
| TFsc+iTR+NaCl | 3.34 ± 0.18 bc | 5.00 ± 0.83 cd | 8.58 ± 0.92 cd | − | – | 0.71 |
| T. repens | ||||||
| TRss–i–NaCl | 4.07 ± 0.35 bc | 6.92 ± 0.79 ab | 7.75 ± 0.08 c | 9.50 ± 0.56 ab | 4.15 ± 0.10 e | 16.88 |
| TRss–i+NaCl | 3.73 ± 0.58 bc | 7.67 ± 0.67 a | 10.08 ± 0.92 a | 9.00 ± 0.68 bc | 7.00 ± 0.14 c | 1.26 |
| TRss+iTF–NaCl | 2.58 ± 0.48 def | 6.17 ± 0.17 c | 8.33 ± 0.33 b | 10.42 ± 0.42 a | 4.88 ± 0.34 d | 12.59 |
| TRss+iTF+NaCl | 1.74 ± 0.40 f | 6.89 ± 0.49 abc | 9.39 ± 0.78 ab | 8.25 ± 0.08 bcd | 6.83 ± 0.67 c | 2.23 |
| TRss+iTR–NaCl | 2.48 ± 0.27 def | 6.75 ± 0.58 abc | 6.92 ± 0.25 d | 8.67 ± 0.67 bcd | 5.15 ± 0.68 d | 15.00 |
| TRss+iTR+NaCl | 2.37 ± 0.27 def | 7.58 ± 0.08 a | 6.67 ± 0.67 d | 7.67 ± 0.43 d | 4.88 ± 0.67 d | 1.08 |
| TRsc–i–NaCl | 4.87 ± 0.81 ab | 7.26 ± 0.71 ab | 6.71 ± 0.63 d | 8.17 ± 0.56 cd | 8.00 ± 7.25 b | 6.67 |
| TRsc–i+NaCl | 2.72 ± 0.30 de | 6.51 ± 0.58 bc | 9.00 ± 0.67ab | 9.35 ± 0.60 bc | 10.29 ± 1.48 ab | 9.44 |
| TRsc+iTF–NaCl | 2.20 ± 0.40 ef | 6.69 ± 0.50 abc | 6.29 ± 0.44 d | 8.08 ± 0.96 cd | 3.18 ± 0.35 f | 16.32 |
| TRsc+iTF+NaCl | 3.30 ± 0.51 cd | 6.50 ± 0.38 bc | 10.33 ± 0.89 a | 7.67 ± 0.44 d | 4.98 ± 0.22 d | 2.54 |
| TRsc+iTR–NaCl | 2.08 ± 0.36 ef | 6.17 ± 0.50 c | 5.05 ± 0.28 e | 8.00 ± 0.65 cd | 4.49 ± 0.06 de | 22.04 |
| TRsc+iTR+NaCl | 4.92 ± 0.22 a | 7.36 ± 0.14 a | 9.75 ± 0.58 a | 7.83 ± 0.42 d | 10.00 ± 0.77 a | 1.42 |
| Source of Variation | df | Na+ Concentration | K+ Concentration | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Stolons | Leaves | Petioles | Leaves | ||||||
| Mean Square | F | Mean Square | F | Mean Square | F | Mean Square | F | ||
| Species Coexistence | 2 | 1.91 | 13.07 *** | 0.40 | 1.33 | 0.81 | 8.17 ** | 0.072 | 5.04 * |
| Inoculant | 2 | 0.02 | 0.17 | 0.04 | 0.13 | 0.17 | 1.68 | 0.003 | 0.20 |
| NaCl | 1 | 34.93 | 239.22 *** | 30.00 *** | 100.78 *** | 55.90 | 564.71 *** | 0.021 | 1.50 |
| Species | 1 | 3.94 | 26.99 *** | 1.95 * | 6.55 * | 4.31 | 43.52 *** | 0.15 | 10.56 ** |
| Residuals | 17 | 0.15 | 0.30 | 0.10 | 0.014 | ||||
| Peroxidase Activity | Polyphenol Oxidase Activity | Electrolyte Leakage | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Leaves | Petioles | Petioles | Leaves | ||||||
| Mean Square | F | Mean Square | F | Mean Square | F | Mean Square | F | ||
| Species Coexistence | 2 | 4090 | 6.53 ** | 4.14 | 42.71 *** | 17.88 | 2.71 | 38.0 | 1.44 |
| Inoculant | 2 | 3343 | 5.34 * | 0.09 | 0.89 | 1.12 | 0.17 | 48.1 | 1.82 |
| NaCl | 1 | 7995 | 12.77 ** | 0.00 | 0.00 | 17.02 | 2.61 | 737.0 | 27.87 *** |
| Species | 1 | 15586 | 24.89 *** | 5.54 | 57.20 *** | 76.60 | 11.74 ** | 10.3 | 0.39 |
| Residuals | 17 | 626 | 0.10 | 6.53 | 26.4 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dūmiņš, K.; Andersone-Ozola, U.; Samsone, I.; Elferts, D.; Ievinsh, G. Growth and Physiological Performance of a Coastal Species Trifolium fragiferum as Affected by a Coexistence with Trifolium repens, NaCl Treatment and Inoculation with Rhizobia. Plants 2021, 10, 2196. https://doi.org/10.3390/plants10102196
Dūmiņš K, Andersone-Ozola U, Samsone I, Elferts D, Ievinsh G. Growth and Physiological Performance of a Coastal Species Trifolium fragiferum as Affected by a Coexistence with Trifolium repens, NaCl Treatment and Inoculation with Rhizobia. Plants. 2021; 10(10):2196. https://doi.org/10.3390/plants10102196
Chicago/Turabian StyleDūmiņš, Kārlis, Una Andersone-Ozola, Ineta Samsone, Didzis Elferts, and Gederts Ievinsh. 2021. "Growth and Physiological Performance of a Coastal Species Trifolium fragiferum as Affected by a Coexistence with Trifolium repens, NaCl Treatment and Inoculation with Rhizobia" Plants 10, no. 10: 2196. https://doi.org/10.3390/plants10102196
APA StyleDūmiņš, K., Andersone-Ozola, U., Samsone, I., Elferts, D., & Ievinsh, G. (2021). Growth and Physiological Performance of a Coastal Species Trifolium fragiferum as Affected by a Coexistence with Trifolium repens, NaCl Treatment and Inoculation with Rhizobia. Plants, 10(10), 2196. https://doi.org/10.3390/plants10102196








