Potential of Essential Oils from Anise, Dill and Fennel Seeds for the Gypsy Moth Control
Abstract
1. Introduction
2. Results
2.1. Essential Oils Chemical Composition
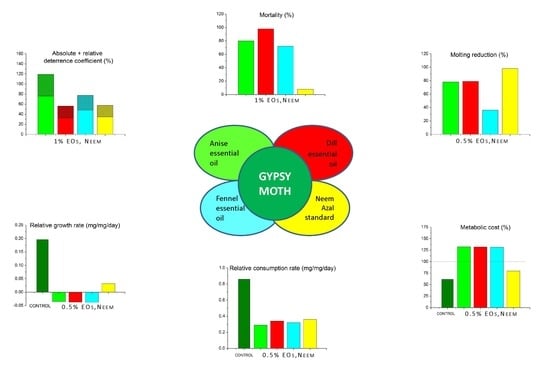
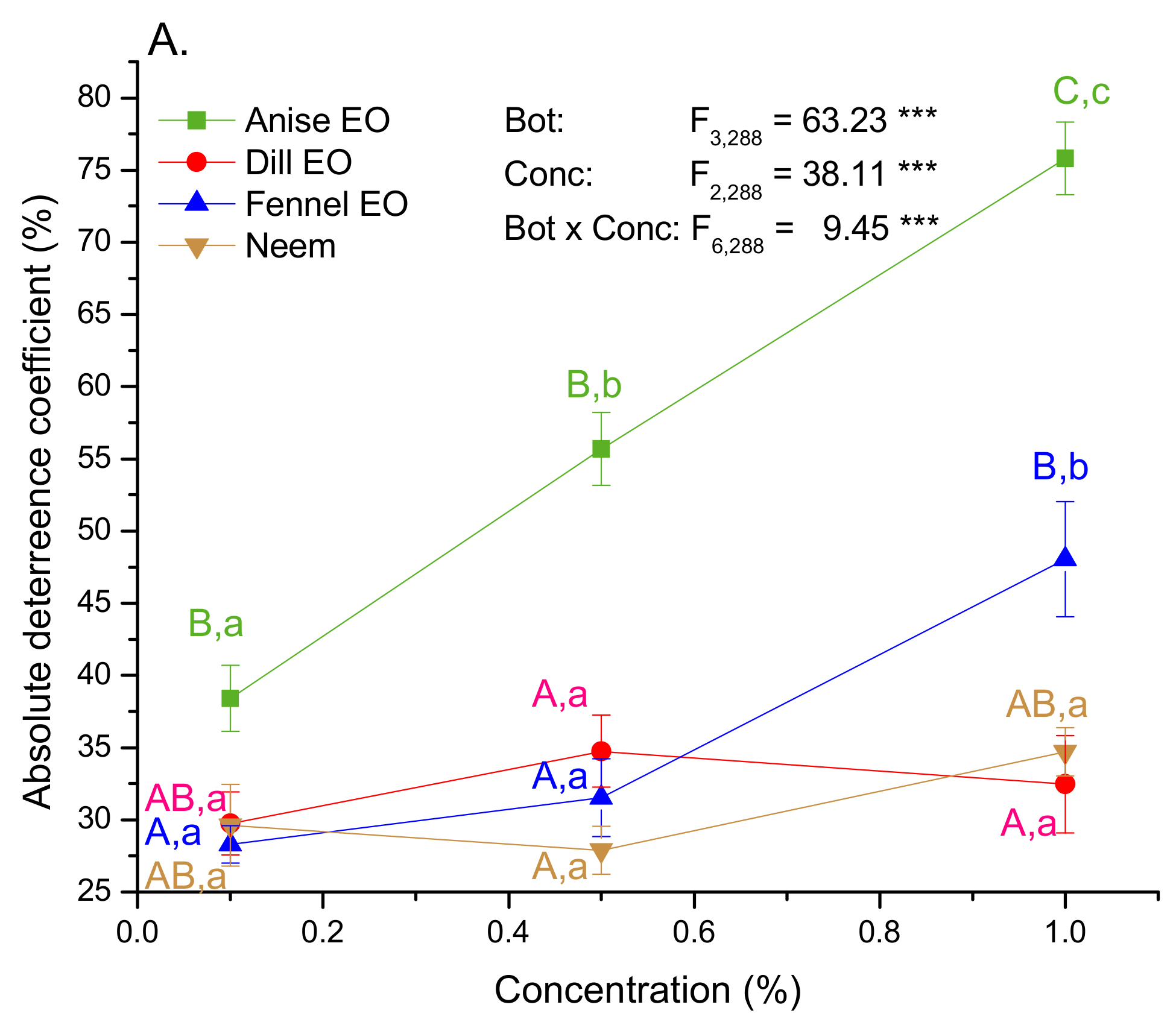
2.2. Antifeeding Activity of EOs
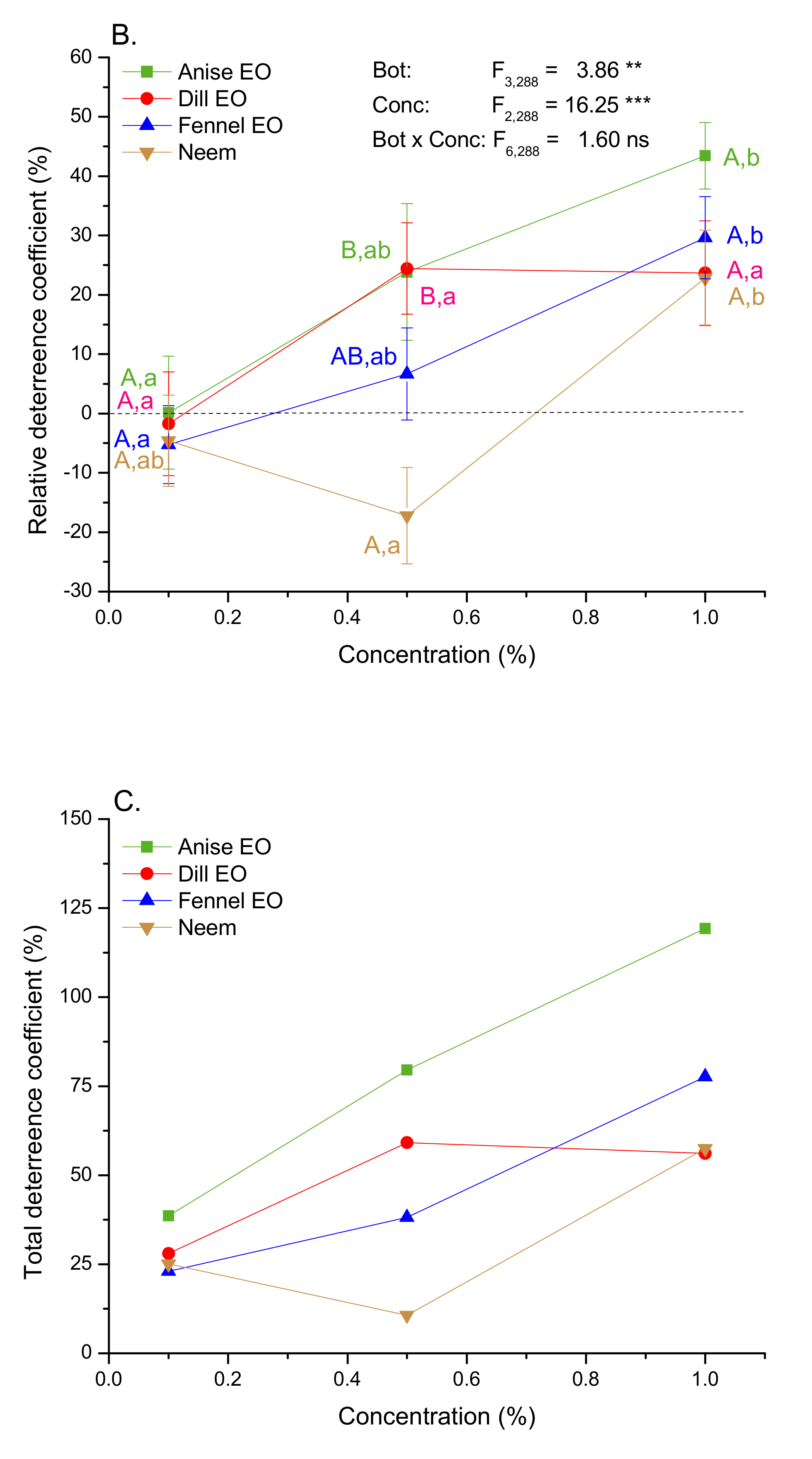
2.3. Digestive Toxicity and Molting Delay Effects of EOs
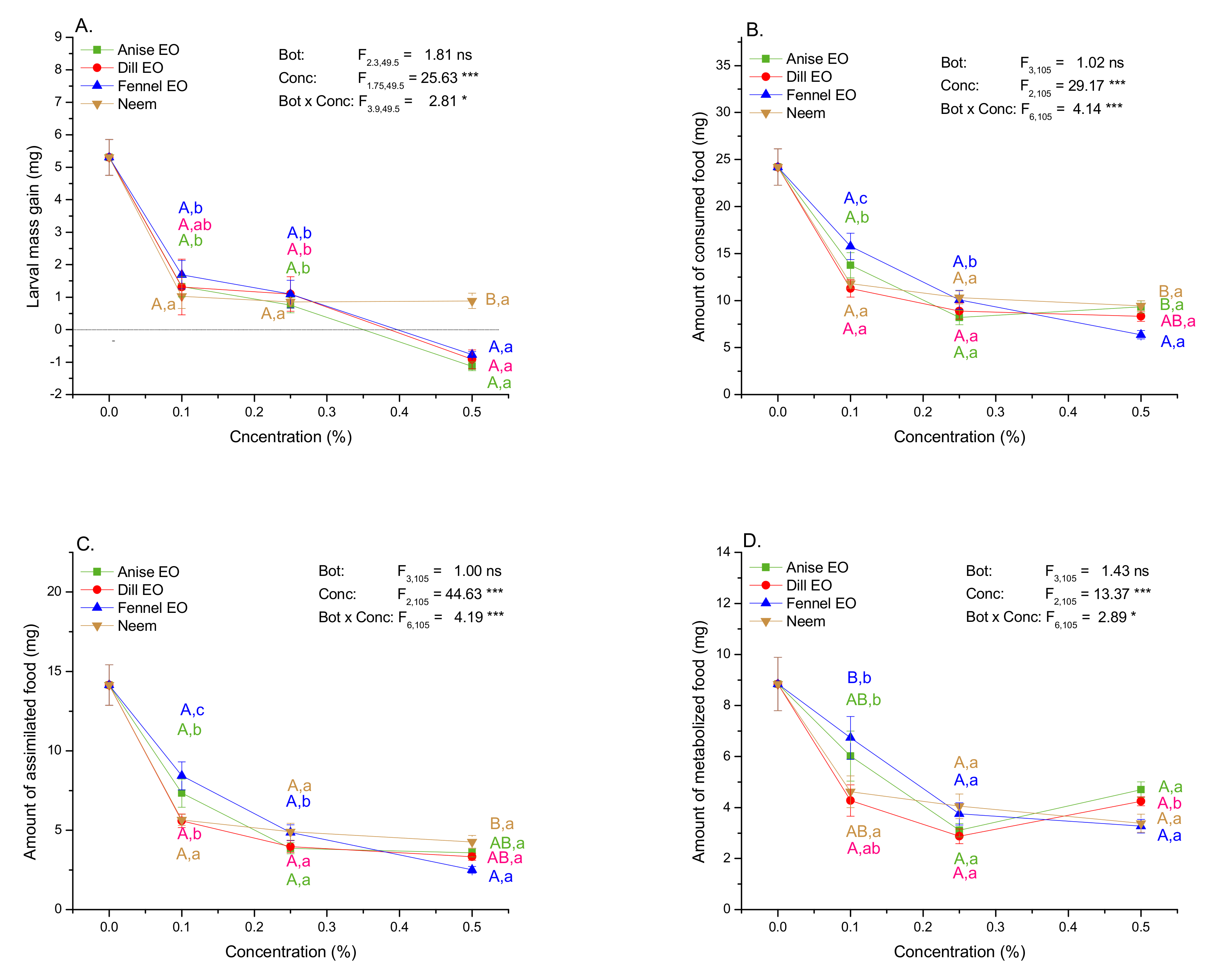
2.4. Impact of EOs and Neem on Mass Gain and Amounts of Consumed, Assimilated, and Metabolized Food
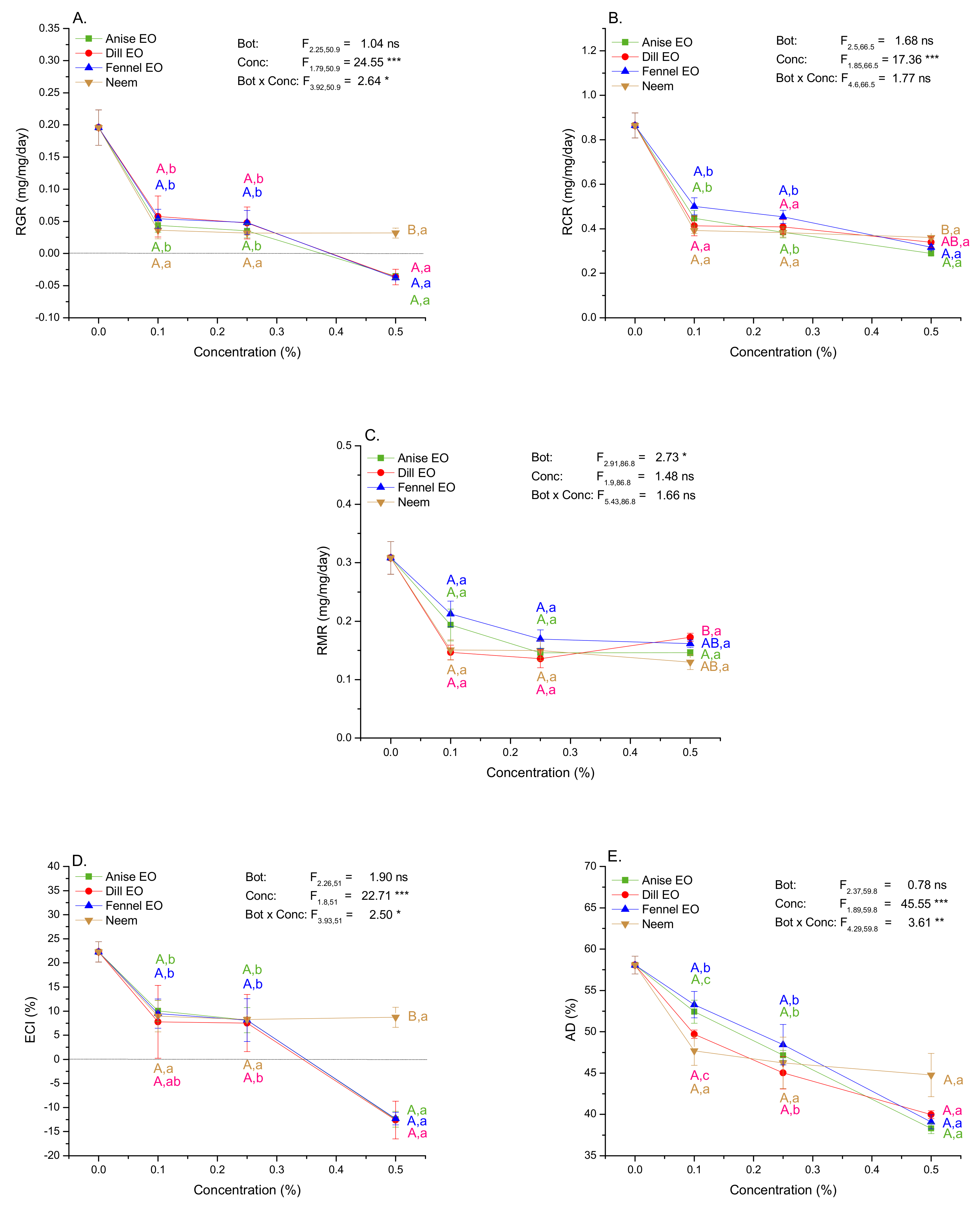
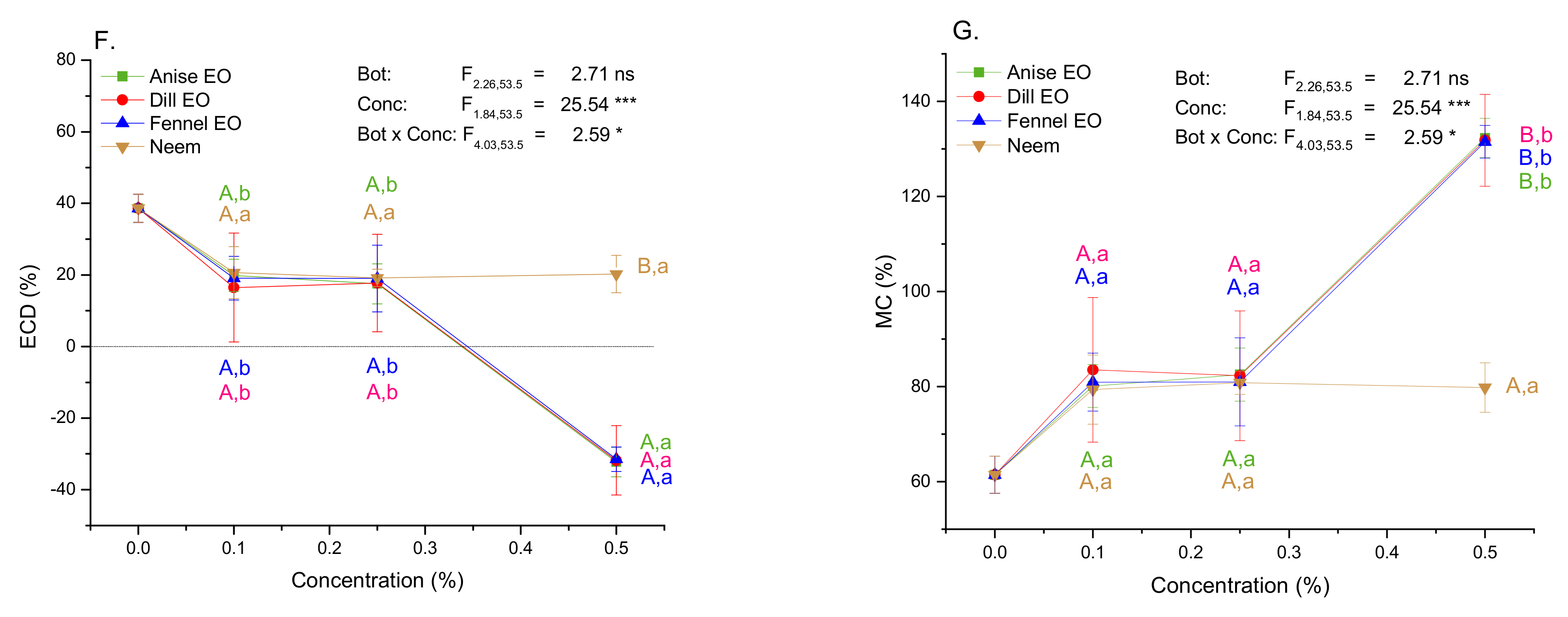
2.5. Impact of EOs and Neem on Growth and Nutritional Indices
3. Discussion
3.1. Apiaceae EOs Are Toxic, Deter Feeding and Delay Molting in GML
3.2. EO Composition Might Account for Differences in Their Biological Activity
3.3. Apiaceae EOs Reduce GML Growth through Pre- and Post-Ingestive Mechanisms
4. Materials and Methods
4.1. Plant Material and EOs Isolation
4.2. Chemical Characterization of EOs
4.3. GML Rearing
4.4. Antifeeding Activity
4.5. Digestive Toxicity and Molting
4.6. Growth and Nutritional Indices
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liebhold, A.M.; Gottschalk, K.W.; Muzika, R.M.; Montgomery, M.E.; Young, R.; O’Day, K.; Kelley, B. Suitability of North American Tree Species to the Gypsy Moth: A Summary of Field and Laboratory Tests; U.S. Department of Agriculture Forest Service NE Forest Experimental Station General Technical Bulletin NE-211; U.S. Department of Agriculture: Washington, DC, USA, 1995; p. 34.
- Milanović, S.; Lazarević, J.; Popović, Z.; Miletić, Z.; Kostić, M.; Radulović, Z.; Karadžić, D.; Vuleta, A. Preference and performance of the larvae of Lymantria dispar (Lepidoptera: Lymantriidae) on three species of European oaks. Eur. J. Entomol. 2014, 111, 371–378. [Google Scholar] [CrossRef]
- Naidoo, R.; Lechowicz, M.J. Effects of gypsy moth on radial growth of deciduous trees. For. Sci. 2001, 47, 338–348. [Google Scholar]
- Fajvan, M.A.; Rentch, J.; Gottschalk, K. The effects of thinning and gypsy moth defoliation on wood volume growth in oaks. Trees 2008, 22, 257–268. [Google Scholar] [CrossRef]
- Davidson, C.B.; Gottschalk, K.W.; Johnson, J.E. Tree mortality following defoliation by the European gypsy moth (Lymantria dispar L.) in the United States: A review. For. Sci. 1999, 45, 74–84. [Google Scholar]
- Milanović, S.; Mihajlović, L.; Karadžić, D.; Jankovsky, L.; Aleksić, P.; Janković-Tomanić, M.; Lazarević, J. Effects of pedunculate oak tree vitality on gypsy moth preference and performance. Arch. Biol. Sci. 2014, 66, 1659–1672. [Google Scholar] [CrossRef]
- Morin, R.S.; Liebhold, A.M. Invasive forest defoliator contributes to the impending downward trend of oak dominance in eastern North America. Forestry 2016, 89, 284–289. [Google Scholar] [CrossRef]
- Arai, T.; Yaginuma, K.; Toyoshima, S.; Ito, T.; Takanashi, M. Damage of Lymantria dispar and Lymantria mathura aurora in apple orchards. Annu. Rep. Soc. Plant Prot. North Jpn. 2010, 61, 220–224. [Google Scholar]
- Bigsby, K.M.; Ambrose, M.J.; Tobin, P.C.; Sills, E.O. The cost of gypsy moth sex in the city. Urban For. Urban Green. 2014, 13, 459–468. [Google Scholar] [CrossRef]
- Stenersen, J. Chemical Pesticides Mode of Action and Toxicology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Devine, G.J.; Furlong, M.J. Insecticide use: Contexts and ecological consequences. Agric. Hum. Values 2007, 24, 281–306. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Walse, S.S.; Throne, J.E. Sublethal exposure, insecticide resistance, and community stress. Curr. Opin. Insect Sci. 2017, 21, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Brevik, K.; Schoville, S.D.; Mota-Sanchez, D.; Chen, Y.H. Pesticide durability and the evolution of resistance: A novel application of survival analysis. Pest Manag. Sci. 2018, 74, 1953–1963. [Google Scholar] [CrossRef]
- Umina, P.A.; McDonald, G.; Maino, J.; Edwards, O.; Hoffmann, A.A. Escalating insecticide resistance in Australian grain pests: Contributing factors, industry trends and management opportunities. Pest Manag. Sci. 2019, 75, 1494–1506. [Google Scholar] [CrossRef]
- Dar, M.A.; Kaushik, G.; Chiu, J.F.V. Pollution status and biodegradation of organophosphate pesticides in the environment. In Abatement of Environmental Pollutants; Singh, P., Kumar, A., Borthakur, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 25–66. [Google Scholar]
- Senthil-Nathan, S. A review of bio pesticides and their mode of action against insect pests. In Environmental Sustainability—Role of Green Technologies; Thangavel, P., Sridevi, G., Eds.; Springer: New Delhi, India, 2015; pp. 49–63. [Google Scholar]
- Kumar, V. A review on efficacy of biopesticides to control the agricultural insect’s pest. Int. J. Agric. Sci. Res. 2015, 4, 168–179. [Google Scholar]
- Anwer, M.A. Biopesticides and Bioagents: Novel Tools for Pest Management, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Isman, M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2020, 19, 235–241. [Google Scholar] [CrossRef]
- Stanković, S.; Kostić, M.; Kostić, I.; Krnjajić, S. Practical approaches to pest control: The use of natural compounds. In Pests, Weeds and Diseases in Agricultural Crop and Animal Husbandry Production; Kontogiannatos, D., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Shahzad, K.; Manzoor, F. Nanoformulations and their mode of action in insects: A review of biological interactions. Drug Chem. Toxicol. 2021, 44, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liebhold, A.; McManus, M. The evolving use of insecticides in gypsy moth management. J. For. 1999, 97, 20–23. [Google Scholar]
- Sharov, A.A.; Leonard, D.; Liebhold, A.M.; Roberts, E.A.; Dickerson, W. “Slow the spread”: A national program to contain the gypsy moth. Forestry 2002, 100, 30–36. [Google Scholar]
- Helson, B. Naturally derived insecticides: Prospects for forestry use. For. Chron. 1992, 68, 349–354. [Google Scholar] [CrossRef]
- Norris, D.M.; Markovic, I. Ash Extractabels for Deterring Gypsy Moth. U.S. Patent No. 5,614,196, 25 March 1997. Available online: https://patentimages.storage.googleapis.com/1e/2d/2f/019e766c0d0db9/US5614196.pdf (accessed on 12 October 2021).
- Hussein, R.A.; El-Anssary, A.A. Plants secondary metabolites: The key drivers of the pharmacological actions of medicinal plants. In Herbal Medicine; Builders, P.H., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Khare, S.; Singh, N.B.; Singh, A.; Hussain, I.; Niharika, K.; Yadav, V.; Bano, C.; Yadav, R.K.; Amist, N. Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J. Plant Biol. 2020, 63, 203–216. [Google Scholar] [CrossRef]
- Pavela, R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects—A review. Plant Protect. Sci. 2016, 52, 229–241. [Google Scholar]
- Isman, M.B.; Machial, C.M. Pesticides based on plant essential oils: From traditional practice to commercialization. In Advances in Phytomedicine; Rai, M., Carpinella, M.C., Eds.; Elsevier: Amsterdam, The Netherland, 2006; Volume 3, pp. 29–44. [Google Scholar]
- Tripathi, A.K.; Upadhyay, S.; Bhuiyan, M.; Bhattacharya, P.R. A review on prospects of essential oils as biopesticide in insect-pest management. J. Pharmacogn. Phytother. 2009, 1, 052–063. [Google Scholar]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Zibaee, A. Botanical insecticides and their effects on insect biochemistry and immunity. In Pesticides in the Modern World—Pests Control and Pesticides Exposure and Toxicity Assessment; Stoytcheva, M., Ed.; IntechOpen: London, UK, 2011; pp. 55–68. [Google Scholar]
- Isman, M.B.; Tak, J.H. Commercialization of insecticides based on plant essential oils: Past, present, and future. In Green Pesticides Handbook; Nollet, L.M.L., Rathore, H.S., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 27–40. [Google Scholar]
- De Souza, M.A.; Da Silva, L.; Macêdo, M.J.F.; Lacerda-Neto, L.J.; dos Santos, M.A.C.; Coutinho, H.D.M.; Cunha, F.A.B. Adulticide and repellent activity of essential oils against Aedes aegypti (Diptera: Culicidae)–A review. S. Afr. J. Bot. 2019, 124, 160–165. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Proença, P.L.F.; Oliveira, J.L.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Use of botanical insecticides for sustainable agriculture: Future perspectives. Ecol. Indic. 2019, 105, 483–495. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides in the twenty-first century—Fulfilling their promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef]
- Benelli, G. On a magical mystery tour of green insecticide research: Current issues and challenges. Molecules 2020, 25, 5014. [Google Scholar] [CrossRef]
- Spinozzi, E.; Maggi, F.; Bonacucina, G.; Pavela, R.; Boukouvala, M.C.; Kavallieratos, N.G.; Canale, A.; Romano, D.; Desneux, N.; Wilke, A.B.B.; et al. Apiaceae essential oils and their constituents as insecticides against mosquitoes—A review. Ind. Crops Prod. 2021, 171, 113892. [Google Scholar] [CrossRef]
- Bruce, T.J.; Pickett, J.A. Perception of plant volatile blends by herbivorous insects–finding the right mix. Phytochemistry 2011, 72, 1605–1611. [Google Scholar] [CrossRef]
- Deletre, E.; Schatz, B.; Bourguet, D.; Chandre, F.; Williams, L.; Ratnadass, A.; Martin, T. Prospects for repellent in pest control: Current developments and future challenges. Chemoecology 2016, 26, 127–142. [Google Scholar] [CrossRef]
- Da Cunha, F.A.B.; Wallau, G.L.; Pinho, A.I.; Nunes, M.E.M.; Leite, N.F.; Tintino, S.R.; da Costa, G.M.; Athayde, M.L.; Boligon, A.A.; Coutinho, H.D.M.; et al. Eugenia uniflora leaves essential oil induces toxicity in Drosophila melanogaster: Involvement of oxidative stress mechanisms. Toxicol. Res. 2015, 4, 634–644. [Google Scholar] [CrossRef]
- Shahriari, M.; Sahebzadeh, N.; Zibaee, A. Effect of Teucrium polium (Lamiaceae) essential oil on digestive enzyme activities and energy reserves of Ephestia kuehniella (Lepidoptera: Pyralidae). Invertebr. Surviv. J. 2017, 14, 182–189. [Google Scholar]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Canale, A.; Senthil-Nathan, S.; Maggi, F. Not just popular spices! Essential oils from Cuminum cyminum and Pimpinella anisum are toxic to insect pests and vectors without affecting non-target invertebrates. Ind. Crops Prod. 2018, 124, 236–243. [Google Scholar] [CrossRef]
- Hashem, A.S.; Awadalla, S.S.; Zayed, G.M.; Maggi, F.; Benelli, G. Pimpinella anisum essential oil nanoemulsions against Tribolium castaneum—Insecticidal activity and mode of action. Environ. Sci. Pollut. Res. 2018, 25, 18802–18812. [Google Scholar] [CrossRef]
- Hashem, A.S.; Ramadan, M.M.; Abdel-Hady, A.A.; Sut, S.; Maggi, F.; Dall’Acqua, S. Pimpinella anisum essential oil nanoemulsion toxicity against Tribolium castaneum? Shedding light on its interactions with aspartate aminotransferase and alanine aminotransferase by molecular docking. Molecules 2020, 25, 4841. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Morales, R.M.; Otero, A.L.C.; Mendez-Sanchez, S.C.; Da Silva, M.A.N.; Stashenko, E.E.; Duque, J.E. Mitochondrial affectation, DNA damage and AChE inhibition induced by Salvia officinalis essential oil on Aedes aegypti larvae. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 221, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Fergani, Y.A.; Elbanna, H.M.; Hamama, H.M. Genotoxicity of some plant essential oils in cotton leafworm, Spodopteralittoralis (Lepidoptera: Noctuidae): The potential role of detoxification enzymes. Egypt. J. Zool. 2020, 73, 53–66. [Google Scholar] [CrossRef]
- Milanović, S.D.; Popović, M.M.; Dobrosavljević, J.N.; Kostić, I.M.; Lazarević, J.M. Desperate times call for desperate measures: Short-term use of the common ash tree by gypsy moth larvae (Lepidoptera: Erebidae) under density and starvation stress. Arch. Biol. Sci. 2020, 72, 63–69. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Zhang, B.W.; Yang, J.; Zou, C.S.; Li, T.; Zhang, G.C.; Chen, G.S. Detoxification, antioxidant, and digestive enzyme activities and gene expression analysis of Lymantria dispar larvae under carvacrol. J. Asia-Pac. Entomol. 2021, 24, 208–216. [Google Scholar] [CrossRef]
- Nasr, E.E.; Teleb, S.S.; Abou-Saty, A.I. Nutritional responses of the black cutworm, Agrotis ipsilon (Hufn.), larvae under toxicity effects of five wild botanical extracts from Sinai, Egypt. Annu. Res. Rev. Biol. 2021, 36, 30–46. [Google Scholar] [CrossRef]
- Haddi, K.; Turchen, L.M.; Viteri Jumbo, L.O.; Guedes, R.N.; Pereira, E.J.; Aguiar, R.W.; Oliveira, E.E. Rethinking biorational insecticides for pest management: Unintended effects and consequences. Pest Manag. Sci. 2020, 76, 2286–2293. [Google Scholar] [CrossRef]
- Evergetis, E.; Michaelakis, A.N.; Haroutounian, S.A. Essential oils of Umbelliferae (Apiaceae) family taxa as emerging potent agents for mosquito control. In Integrated Pest Management and Pest Control—Currentand Future Tactics; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen: Rijeka, Croatia, 2012; Chapter 26; pp. 613–638. [Google Scholar]
- Yeom, H.J.; Kang, J.S.; Kim, G.H.; Park, I.K. Insecticidal and acetylcholine esterase inhibition activity of Apiaceae plant essential oils and their constituents against adults of German cockroach (Blattella germanica). J. Agric. Food Chem. 2012, 60, 7194–7203. [Google Scholar] [CrossRef]
- Ebadollahi, A. Plant essential oils from Apiaceae family as alternatives to conventional insecticides. Ecol. Balk. 2013, 5, 149–172. [Google Scholar]
- Sousa, R.M.O.; Rosa, J.S.; Oliveira, L.; Cunha, A.; Fernandes-Ferreira, M. Activities of Apiaceae essential oils and volatile compounds on hatchability, development, reproduction and nutrition of Pseudaletia unipuncta (Lepidoptera: Noctuidae). Ind. Crops Prod. 2015, 63, 226–237. [Google Scholar] [CrossRef]
- Camilo, C.J.; Alves Nonato, C.D.F.; Galvão-Rodrigues, F.F.; Costa, W.D.; Clemente, G.G.; Sobreira Macedo, M.A.C.; Galvão Rodrigues, F.F.; da Costa, J.G.M. Acaricidal activity of essential oils: A review. Trends Phytochem. Res. 2017, 1, 183–198. [Google Scholar]
- Benelli, G.; Pavela, R. Repellence of essential oils and selected compounds against ticks—A systematic review. Acta Trop. 2018, 179, 47–54. [Google Scholar] [CrossRef]
- Chaubey, M.K. Essential oils as green pesticides of stored grain insects. Eur. J. Biol. Res. 2019, 9, 202–244. [Google Scholar]
- Ikbal, C.; Pavela, R. Essential oils as active ingredients of botanical insecticides against aphids. J. Pest Sci. 2019, 92, 971–986. [Google Scholar] [CrossRef]
- Pavela, R.; Morshedloo, M.R.; Mumivand, H.; Khorsand, G.J.; Karami, A.; Maggi, F.; Desneux, N.; Benelli, G. Phenolic monoterpene-rich essential oils from Apiaceae and Lamiaceae species: Insecticidal activity and safety evaluation on non-target earthworms. Entomol. Gen. 2020, 40, 421–435. [Google Scholar] [CrossRef]
- Sousa, R.M.O.; Cunha, A.C.; Fernandes-Ferreira, M. The potential of Apiaceae species as sources of singular phytochemicals and plant-based pesticides. Phytochemistry 2021, 187, 112714. [Google Scholar] [CrossRef] [PubMed]
- Muturi, E.J.; Doll, K.; Ramirez, J.L.; Rooney, A.P. Bioactivity of wild carrot (Daucus carota, Apiaceae) essential oil against mosquito larvae. J. Med. Entomol. 2019, 56, 784–789. [Google Scholar] [CrossRef]
- Kostić, I.; Petrović, O.; Milanović, S.; Popović, Z.; Stanković, S.; Todorović, G.; Kostić, M. Biological activity of essential oils of Athamanta haynaldii and Myristica fragrans to gypsy moth larvae. Ind. Crops Prod. 2013, 41, 17–20. [Google Scholar] [CrossRef]
- Mossa, A.T.H. Green pesticides: Essential oils as biopesticides in insect-pest management. J. Environ. Sci. Technol. 2016, 9, 354. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Rocha, D.K.; Matos, O.; Novo, M.T.; Figueiredo, A.C.; Delgado, M.; Moiteiro, C. Larvicidal activity against Aedes aegypti of Foeniculum vulgare essential oils from Portugal and Cape Verde. Nat. Prod. Commun. 2015, 10, 677–682. [Google Scholar] [CrossRef]
- Pavela, R.; Žabka, M.; Bednář, J.; Tříska, J.; Vrchotová, N. New knowledge for yield, composition and insecticidal activity of essential oils obtained from the aerial parts or seeds of fennel (Foeniculum vulgare Mill.). Ind. Crops Prod. 2016, 83, 275–282. [Google Scholar] [CrossRef]
- Skuhrovec, J.; Douda, O.; Zouhar, M.; Maňasová, M.; Božik, M.; Klouček, P. Insecticidal and behavioral effect of microparticles of Pimpinella anisum essential oil on larvae of Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). J. Econom. Entomol. 2020, 113, 255–262. [Google Scholar] [CrossRef]
- Lazarević, J.; Jevremović, S.; Kostić, I.; Kostić, M.; Vuleta, A.; Manitašević Jovanović, S.; Šešlija Jovanović, D. Toxic, oviposition deterrent and oxidative stress effects of Thymus vulgaris essential oil against Acanthoscelides obtectus. Insects 2020, 11, 563. [Google Scholar] [CrossRef]
- Moretti, M.D.; Sanna-Passino, G.; Demontis, S.; Bazzoni, E. Essential oil formulations useful as a new tool for insect pest control. AAPS PharmSciTech 2002, 3, 64–74. [Google Scholar] [CrossRef]
- Cetin, H.; Erler, F.; Yanikoglu, A. A comparative evaluation of Origanum onites essential oil and its four major components as larvicides against the pine processionary moth, Thaumetopoea wilkinsoni Tams. Pest Manag. Sci. 2007, 63, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sharma, S.; Naik, S.N. Biopesticidal value of selected essential oils against pathogenic fungus, termites, and nematodes. Int. Biodeter. Biodegr. 2011, 65, 703–707. [Google Scholar] [CrossRef]
- Ezzine, O.; Dhahri, S.; Akkari, H.; Ben Jamâa, M.L. Larvicidal activity of essential oil of Mentha pulegium on larvae of Orgyia trigotephras Boisduval, 1829 (Lepidoptera, Erebidae). J. New Sci. 2018, 20, 3423–3428. [Google Scholar]
- Devrnja, N.; Kostić, I.; Lazarević, J.; Savić, J.; Ćalić, D. Evaluation of tansy essential oil as a potential “green” alternative for gypsy moth control. Environ. Sci. Pollut. Res. 2020, 27, 11958–11967. [Google Scholar] [CrossRef] [PubMed]
- Kostić, M.; Popović, Z.; Brkić, D.; Milanović, S.; Sivčev, I.; Stanković, S. Larvicidal and antifeedant activity of some plant-derived compounds to Lymantria dispar L. (Lepidoptera: Limantriidae). Bioresour. Technol. 2008, 99, 7897–7901. [Google Scholar] [CrossRef] [PubMed]
- Popović, Z.; Kostić, M.; Stanković, S.; Milanović, S.; Sivčev, I.; Kostić, I.; Kljajić, P. Ecologically acceptable usage of derivatives of essential oil of sweet basil, Ocimum basilicum, as antifeedants against larvae of the gypsy moth, Lymantria dispar. J. Insect Sci. 2013, 13, 161. [Google Scholar] [CrossRef] [PubMed]
- Gvozdenac, S.M.; Inđić, D.V.; Vuković, S.M.; Grahovac, M.S.; Tanasković, S.T. Antifeeding activity of several plant extracts against Lymantria dispar L. (Lepidoptera: Lymantriidae) larvae. Pestic. Phytomed. 2012, 27, 305–311. [Google Scholar] [CrossRef]
- Işıkber, A.A.; Özder, N.; Sağlam, Ö. Susceptibility of eggs of Tribolium confusum, Ephestia kuehniella and Plodia interpunctella to four essential oil vapors. Phytoparasitica 2009, 37, 231. [Google Scholar] [CrossRef]
- Karahroodi, Z.R.; Moharramipour, S.; Rahbarpour, A. Investigated repellency effect of some essential oils of 17 native medicinal plants on adults Plodia interpunctella. Am.-Eurasian J. Sustain. Agric. 2009, 3, 181–185. [Google Scholar]
- Elumalai, K.; Krishnappa, K.; Anandan, A.; Govindarajan, M.; Mathivanan, T. Larvicidal and ovicidal activity of seven essential oil against lepidopteran pest S. litura (Lepidoptera: Noctuidae). Int. J. Recent Sci. Res. 2010, 1, 8–14. [Google Scholar]
- Sousa, R.M.O.; Rosa, J.S.; Oliveira, L.; Cunha, A.; Fernandes-Ferreira, M. Activities of Apiaceae essential oils against armyworm, Pseudaletia unipuncta (Lepidoptera: Noctuidae). J. Agric. Food Chem. 2013, 61, 7661–7672. [Google Scholar] [CrossRef] [PubMed]
- Chantawee, A.; Soonwera, M. Larvicidal, pupicidal and oviposition deterrent activities of essential oils from Umbelliferae plants against house fly Musca domestica. Asian Pac. J. Trop. Med. 2018, 11, 621. [Google Scholar] [CrossRef]
- Pavela, R. Screening of Eurasian plants for insecticidal and growth inhibition activity against Spodoptera littoralis larvae. Afr. J. Agric. Res. 2011, 6, 2895–2907. [Google Scholar]
- Oviedo-Sarmiento, J.S.; Cortes, J.J.B.; Ávila, W.A.D.; Suárez, L.E.C.; Daza, E.H.; Patiño-Ladino, O.J.; Prieto-Rodríguez, J.A. Fumigant toxicity and biochemical effects of selected essential oils toward the red flour beetle, Tribolium castaneum (Coleoptera: Tenebrionidae). Pestic. Biochem. Physiol. 2021, 104941. [Google Scholar] [CrossRef]
- Szczepanik, M.; Dams, I.; Wawrzeńczyk, C. Feeding deterrent activity of terpenoid lactones with the p-menthane system against the Colorado potato beetle (Coleoptera: Chrysomelidae). Environ. Entomol. 2005, 34, 1433–1440. [Google Scholar] [CrossRef]
- Nawrot, J.; Dams, I.; Wawrzeńczyk, C. Feeding deterrent activity of terpenoid lactones with a p-menthane system against stored-product pests. J. Stored Prod. Res. 2009, 45, 221–225. [Google Scholar] [CrossRef]
- Kanda, D.; Kaur, S.; Koul, O. A comparative study of monoterpenoids and phenylpropanoids from essential oils against stored grain insects: Acute toxins or feeding deterrents. J. Pest Sci. 2017, 90, 531–545. [Google Scholar] [CrossRef]
- Morgan, E.D. Azadirachtin, a scientific gold mine. Bioorg. Med. Chem. 2009, 17, 4096–4105. [Google Scholar] [CrossRef]
- Mello, C.B.; Uzeda, C.D.; Bernardino, M.V.; Mendonça-Lopes, D.; Kelecom, A.; Fevereiro, P.C.; Guerra, M.S.; Oliveira, A.P.; Rocha, L.M.; Gonzalez, M.S. Effects of the essential oil obtained from Pilocarpus spicatus Saint-Hilaire (Rutaceae) on the development of Rhodnius prolixus nymphae. Rev. Bras. Farmacogn. 2007, 17, 514–520. [Google Scholar] [CrossRef][Green Version]
- Oliveira, A.P.; Rodrigo, A.S.; Botas, G.S.; Gonzalez, M.S.; Santos, M.G.; Teixeira, L.A.; Rocha, L.M. Chemical and biological investigations of Pilocarpus spicatus essential oils. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2010, 9, 206–211. [Google Scholar]
- Qin, W.; Huang, S.; Li, C.; Chen, S.; Peng, Z. Biological activity of the essential oil from the leaves of Piper sarmentosum Roxb. (Piperaceae) and its chemical constituents on Brontispa longissima (Gestro) (Coleoptera: Hispidae). Pestic. Biochem. Physiol. 2010, 96, 132–139. [Google Scholar] [CrossRef]
- Ghoneim, K.; Amer, M.; Al-Daly, A.; Mohammad, A.; Khadrawy, F.; Mahmoud, M.A. Disturbed acid and alkaline phosphatase activities in desert locust Schistocerca gregaria (Forskal) (Orthoptera: Acrididae) by extracts from the khella plant Ammi visnaga L. (Apiaceae). Int. J. Adv. Res. 2014, 2, 584–596. [Google Scholar]
- Hummelbrunner, L.A.; Isman, M.B. Acute, sublethal, antifeedant, and synergistic effects of monoterpenoid essential oil compounds on the tobacco cutworm, Spodoptera litura (Lep., Noctuidae). J. Agric. Food Chem. 2001, 49, 715–720. [Google Scholar] [CrossRef]
- Pavela, R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culexquinquefasciatus Say larvae. Parasitol. Res. 2015, 114, 3835–3853. [Google Scholar] [CrossRef] [PubMed]
- Cruz, G.S.; Wanderley-Teixeira, V.; da Silva, L.M.; Dutra, K.A.; Guedes, C.A.; de Oliveira, J.V.; Navarro, D.M.A.F.; Araújo, B.C.; Teixeira, Á.A.C. Chemical composition and insecticidal activity of the essential oils of Foeniculum vulgare Mill., Ocimum basilicum L., Eucalyptus staigeriana F. Muell. ex Bailey, Eucalyptus citriodora Hook and Ocimum gratissimum L. and their major components on Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Essent. Oil-Bear. Plants 2017, 20, 1360–1369. [Google Scholar]
- Zahran, H.E.D.M.; Abdelgaleil, S.A. Insecticidal and developmental inhibitory properties of monoterpenes on Culex pipiens L. (Diptera: Culicidae). J. Asia-Pac. Entomol. 2011, 14, 46–51. [Google Scholar] [CrossRef]
- Al-Nagar, N.M.; Abou-Taleb, H.K.; Shawir, M.S.; Abdelgaleil, S.A. Comparative toxicity, growth inhibitory and biochemical effects of terpenes and phenylpropenes on Spodoptera littoralis (Boisd.). J. Asia-Pac. Entomol. 2020, 23, 67–75. [Google Scholar] [CrossRef]
- Sohail, M.; Aqueel, M.A.; Dai, P.; Ellis, J.D. The larvicidal and adulticidal effects of selected plant essential oil constituents on greater wax moths. J. Econom. Entomol. 2021, 114, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Regnault-Roger, C.; Hamraoui, A. Fumigant toxic activity and reproductive inhibition induced by monoterpenes on Acanthoscelides obtectus (Say) (Coleoptera), a bruchid of kidney bean (Phaseolus vulgaris L.). J. Stored Prod. Res. 1995, 31, 291–299. [Google Scholar] [CrossRef]
- Céspedes, C.L.; Ortega, C.; Alarcon, J.; Salazar, J.R. Antifeedant, feeding deterrent and insect growth regulatory effects of Calceolaria integrifolia sensu lato complex: C. integrifolia x talcana (Scrophulariaceae). Rev. Latinoamer. Quím. 2014, 42, 113–132. [Google Scholar]
- Céspedes, C.L.; Alarcon, J.E.; Aqueveque, P.; Seigler, D.S.; Kubo, I. In the search for new secondary metabolites with biopesticidal properties. Isr. J. Plant Sci. 2015, 62, 216–228. [Google Scholar] [CrossRef]
- Mann, R.S.; Kaufman, P.E. Natural product pesticides: Their development, delivery and use against insect vectors. Mini-Rev. Org. Chem. 2012, 9, 185–202. [Google Scholar] [CrossRef]
- Pavela, R. Acute, synergistic and antagonistic effects of some aromatic compounds on the Spodoptera littoralis Boisd. (Lep., Noctuidae) larvae. Ind. Crops Prod. 2014, 60, 247–258. [Google Scholar] [CrossRef]
- Gaire, S.; Scharf, M.E.; Gondhalekar, A.D. Synergistic toxicity interactions between plant essential oil components against the common bed bug (Cimex lectularius L.). Insects 2020, 11, 133. [Google Scholar] [CrossRef]
- Aungtikun, J.; Soonwera, M.; Sittichok, S. Insecticidal synergy of essential oils from Cymbopogon citratus (Stapf.), Myristica fragrans (Houtt.), and Illicium verum Hook. f. and their major active constituents. Ind. Crops Prod. 2021, 164, 113386. [Google Scholar] [CrossRef]
- Kostyukovsky, M.; Rafaeli, A.; Gileadi, C.; Demchenko, N.; Shaaya, E. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: Possible mode of action against insect pests. Pest Manag. Sci. 2002, 58, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- López, M.D.; Pascual-Villalobos, M.J. Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind. Crops Prod. 2010, 31, 284–288. [Google Scholar] [CrossRef]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular targets for components of essential oils in the insect nervous system—A review. Molecules 2018, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, M.; Zibaee, A.; Sahebzadeh, N.; Shamakhi, L. Effects of α-pinene, trans-anethole, and thymol as the essential oil constituents on antioxidant system and acetylcholine esterase of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). Pestic. Biochem. Physiol. 2018, 150, 40–47. [Google Scholar] [CrossRef]
- Bloomquist, J.R.; Boina, D.R.; Chow, E.; Carlier, P.R.; Reina, M.; Gonzalez-Coloma, A. Mode of action of the plant-derived silphinenes on insect and mammalian GABAA receptor/chloride channel complex. Pestic. Biochem. Physiol. 2008, 91, 17–23. [Google Scholar] [CrossRef]
- Drijfhout, F.P.; Morgan, E.D.; Liu, H.W.; Mander, L. Terrestrial natural products as antifeedants. In Comprehensive Natural Products II; Liu, H.-W., Mander, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 457–501. [Google Scholar]
- Santana, O.; Andres, M.F.; Sanz, J.; Errahmani, N.; Abdeslam, L.; González-Coloma, A. Valorization of essential oils from Moroccan aromatic plants. Nat. Prod. Commun. 2014, 9, 1109–1114. [Google Scholar] [CrossRef]
- Petrović, M.; Popović, A.; Kojić, D.; Šućur, J.; Bursić, V.; Aćimović, M.; Malenčić, Đ.; Stojanović, T.; Vuković, G. Assessment of toxicity and biochemical response of Tenebrio molitor and Tribolium confusum exposed to Carum carvi essential oil. Entomol. Gen. 2019, 38, 333–348. [Google Scholar] [CrossRef]
- Shahriari, M.; Zibaee, A.; Shamakhi, L.; Sahebzadeh, N.; Naseri, D.; Hoda, H. Bio-efficacy and physiological effects of Eucalyptus globulus and Allium sativum essential oils against Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). Toxin Rev. 2019, 39, 422–433. [Google Scholar] [CrossRef]
- El-Naggar, S.A.F.; Doskotch, R.W.; ODell, T.M.; Girard, L. Antifeedant diterpenes for the gypsy moth larvae from Kalmia latifolia: Isolation and characterization of ten grayanoids. J. Nat. Prod. 1980, 43, 617–631. [Google Scholar] [CrossRef]
- Powell, J.S.; Raffa, K.F. Effects of selected Larix laricina terpenoids on Lymantria dispar (Lepidoptera: Lymantriidae) development and behavior. Environ. Entomol. 1999, 28, 148–154. [Google Scholar] [CrossRef]
- Rharrabe, K.; Amri, H.; Bouayad, N.; Sayah, F. Effects of azadirachtin on post-embryonic development, energy reserves and α-amylase activity of Plodia interpunctella Hübner (Lepidoptera: Pyralidae). J. Stored Prod. Res. 2008, 44, 290–294. [Google Scholar] [CrossRef]
- Khosravi, R.; Sendi, J.J. Effect of neem pesticide (Achook) on midgut enzymatic activities and selected biochemical compounds in the hemolymph of lesser mulberry pyralid, Glyphodes pyloalis Walker (Lepidoptera: Pyralidae). J. Plant Prot. Res. 2013, 53, 238–247. [Google Scholar] [CrossRef]
- Bezzar-Bendjazia, R.; Kilani-Morakchi, S.; Maroua, F.; Aribi, N. Azadirachtin induced larval avoidance and antifeeding by disruption of food intake and digestive enzymes in Drosophila melanogaster (Diptera: Drosophilidae). Pestic. Biochem. Physiol. 2017, 143, 135–140. [Google Scholar] [CrossRef]
- Shu, B.; Zhang, J.; Cui, G.; Sun, R.; Yi, X.; Zhong, G. Azadirachtin affects the growth of Spodoptera litura Fabricius by inducing apoptosis in larval midgut. Front. Physiol. 2018, 9, 137. [Google Scholar] [CrossRef]
- Kaur, M.; Saraf, I.; Kumar, R.; Singh, I.P.; Kaur, S. Bioefficacy of hexane extract of Inula racemosa (Asteraceae) against Spodoptera litura (Lepidoptera: Noctuidae). Gesunde Pflanz. 2019, 71, 165–174. [Google Scholar] [CrossRef]
- Mojarab-Mahboubkar, M.; Sendi, J.J.; Aliakbar, A. Effect of Artemisia annua L. essential oil on toxicity, enzyme activities, and energy reserves of cotton bollworm Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). J. Plant Prot. Res. 2015, 55, 371–377. [Google Scholar] [CrossRef]
- Kiran, S.; Kujur, A.; Patel, L.; Ramalakshmi, K.; Prakash, B. Assessment of toxicity and biochemical mechanisms underlying the insecticidal activity of chemically characterized Boswellia carterii essential oil against insect pest of legume seeds. Pestic. Biochem. Physiol. 2017, 139, 17–23. [Google Scholar]
- De Souza Alves, M.; Campos, I.M.; de Brito, D.D.M.C.; Cardoso, C.M.; Pontes, E.G.; de Souza, M.A.A. Efficacy of lemongrass essential oil and citral in controlling Callosobruchus maculatus (Coleoptera: Chrysomelidae), a post-harvest cowpea insect pest. Crop. Protect. 2019, 119, 191–196. [Google Scholar] [CrossRef]
- Chintalchere, J.M.; Dar, M.A.; Shaha, C.; Pandit, R.S. Impact of essential oils on Musca domestica larvae: Oxidative stress and antioxidant responses. Int. J. Trop. Insect Sci. 2021, 41, 821–830. [Google Scholar] [CrossRef]
- Vahabi Mashhoor, M.; Mikani, A.; Mehrabadi, M.; Moharramipour, S. Antifeedant activity of nanoemulsion formulation of arugula Eruca sativa oil on elm leaf beetle Xanthogaleruca luteola (Coleoptera: Chrysomelidae). J. Agric. Sci. Technol. 2021, 23, 125–136. [Google Scholar]
- Valizadeh, B.; Sendi, J.J.; Zibaee, A.; Oftadeh, M. Effect of Neem based insecticide Achook® on mortality, biological and biochemical parameters of elm leaf beetle Xanthogaleruca luteola (Col.: Chrysomelidae). J. Crop. Protect. 2013, 2, 319–330. [Google Scholar]
- Zhang, J.; Sun, T.; Sun, Z.; Li, H.; Qi, X.; Zhong, G.; Yi, X. Azadirachtin acting as a hazardous compound to induce multiple detrimental effects in Drosophila melanogaster. J. Hazard. Mater. 2018, 359, 338–347. [Google Scholar] [CrossRef]
- Shahriari, M.; Sahbzadeh, N.; Zibaee, A.; Khani, A.; Senthil-Nathan, S. Metabolic response of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) to essential oil of Ajwain and thymol. Toxin. Rev. 2017, 36, 204–209. [Google Scholar] [CrossRef]
- Jayakumar, M.; Ramachandran, M.; Krishnaveni, T.; Nattudurai, G. Toxicity and biochemical effects of essential oils of Anethumgraveolens L. and Melaleuca cajuputi Powell against Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae). Int. J. Trop. Insect. Sci 2021, 41, 945–951. [Google Scholar] [CrossRef]
- Piri, A.; Sahebzadeh, N.; Zibaee, A.; Sendi, J.J.; Shamakhi, L.; Shahriari, M. Toxicity and physiological effects of ajwain (Carum copticum, Apiaceae) essential oil and its major constituents against Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Chemosphere 2020, 256, 127103. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J. Microsomal oxidation of allelochemicals in generalist (Spodoptera frugiperda) and semispecialist (Anticarsiagemmatalis) insect. J. Chem. Ecol. 1987, 13, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Moldenke, A.F.; Berry, R.E.; Miller, J.C.; Kelsey, R.G.; Wernz, J.G.; Venkateswaran, S. Carbaryl susceptibility and detoxication enzymes in gypsy moth (Lepidoptera: Lymantriidae): Influence of host plant. J. Econ. Entomol. 1992, 85, 1628–1635. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines. Determination of essential oils in vegetable drugs. In Europea Pharmacopoea, 4th ed.; Council of Europe Editions; European Directorate for the Quality of Medicines: Strasbourg, France, 2002; pp. 183–184. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Compounds by Gas Chromatography and Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2009. [Google Scholar]
- Markovic, I.; Norris, D.M.; Nordheim, E.V. Gypsy moth (Lymantria dispar) larval development and survival to pupation on diet plus extractables from green ash foliage. Entomol. Exp. Appl. 1997, 84, 247–254. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Shim, J.K.; Hwang, H.S.; Bunch, H.; Lee, K.Y. Acaricidal effects of methyl benzoate against Tetranychus urticae Koch (Acari: Tetranychidae) on common crop plants. Pest Manag. Sci. 2020, 76, 2347–2354. [Google Scholar] [CrossRef]
- Wilcox, D.; Dove, B.; McDavid, D.; Greer, D. Image Tool Copyright UTHSCSA 1996–2002; University of Texas Health Science Center (UTHSCSA): San Antonio, TX, USA, 1996. [Google Scholar]
- Szczepanik, M.; Szumny, A.; Wawrzenczyk, C. The effect of α-methylene lactone group on the feeding deterrent activity of natural and synthetic alkenes against Colorado Potato Beetle, Leptinotarsa decemlineata Say. Pol. J. Environ. Stud. 2009, 18, 1107–1112. [Google Scholar]
- Waldbauer, G.P. The consumption and utilization of food by insects. Adv. Insect Phys. 1968, 5, 229–288. [Google Scholar]
- Scriber, J.M.; Slansky Jr, F. The nutritional ecology of immature insects. Annu. Rev. Entomol. 1981, 26, 183–211. [Google Scholar] [CrossRef]
- Farrar, R.R.; Barbour, J.D.; Kennedy, G.G. Quantifying food consumption and growth in insects. Ann. Entomol. Soc. Am. 1989, 82, 593–598. [Google Scholar] [CrossRef]
- McDonald, J.H. Handbook of Biological Statistics; Sparky House Publishing: Baltimore, MD, USA, 2014. [Google Scholar]
- Brunner, E.; Puri, M.L. Nonparametric methods in factorial designs. Stat. Pap. 2001, 42, 1–52. [Google Scholar] [CrossRef]
- Lichtenstein, E.P.; Liang, T.T.; Schulz, K.R.; Schnoes, H.K.; Carter, G.T. Insecticidal and synergistic components isolated from dill plants. J. Agric. Food Chem. 1974, 22, 658–664. [Google Scholar] [CrossRef]
- He, W.; Huang, B. A review of chemistry and bioactivities of a medicinal spice: Foeniculum vulgare. J. Med. Plants Res. 2011, 5, 3595–3600. [Google Scholar]
- Pavela, R. Essential oils from Foeniculum vulgare Miller as a safe environmental insecticide against the aphid Myzus persicae Sulzer. Environ. Sci. Pollut. Res. 2018, 25, 10904–10910. [Google Scholar] [CrossRef]
- Kaur, V.; Kaur, R.; Bhardwaj, U. A review on dill essential oil and its chief compounds as natural biocide. Flavour Fragr. J. 2021, 36, 412–431. [Google Scholar] [CrossRef]
| RIlit | RIexp | Compound | Contribution to Essential Oil (% m/m) | ||
|---|---|---|---|---|---|
| Anise | Dill | Fennel | |||
| Monoterpene hydrocarbons | 0.61 | 45.50 | 4.12 | ||
| 921 | 919 | Tricyclene | - | 0.13 | - |
| 924 | 924 | α-Thujene | 0.61 | 0.44 | - |
| 932 | 924 | α-Pinene | - | - | 1.53 |
| 946 | 938 | Camphene | - | - | 0.16 |
| 969 | 965 | Sabinene | - | - | tr |
| 974 | 967 | β-Pinene | - | - | 0.13 |
| 988 | 986 | β-Myrcene | - | 0.51 | tr |
| 1002 | 996 | α-Phellandrene | - | 13.12 | 0.38 |
| 1008 | 1003 | δ-3-Carene | - | - | tr |
| 1020 | 1018 | p-Cymene | - | 2.26 | 0.28 |
| 1024 | 1021 | Limonene | - | 29.04 | 1.32 |
| 1054 | 1052 | γ-Terpinene | - | - | 0.32 |
| Oxygenated monoterpenes | 4.75 | 52.79 | 26.38 | ||
| 1026 | 1024 | 1,8-cineole | 2.35 | - | 0.17 |
| 1083 | 1080 | Fenchone | - | - | 25.56 |
| 1095 | 1097 | Linalool | 0.43 | - | - |
| 1141 | 1135 | Camphor | - | - | 0.65 |
| 1148 | 1146 | Menthone | - | 0.42 | - |
| 1158 | 1156 | iso-Menthone | - | 0.17 | - |
| 1161 | 1166 | neo-Menthol | - | 0.28 | - |
| 1174 | 1172 | Terpinen-4-ol | 0.05 | - | - |
| 1184 | 1176 | Dill ether | - | 6.52 | - |
| 1186 | 1185 | α-Terpineol | 0.22 | - | - |
| 1191 | 1189 | cis-Dihydrocarvone | - | 1.52 | - |
| 1200 | 1196 | trans-Dihydrocarvone | - | 0.81 | - |
| 1212 | 1210 | iso-Dihydrocarveol | - | 0.14 | - |
| 1226 | 1223 | neoiso-Dihydrocarveol | - | 0.46 | - |
| 1239 | 1238 | Carvone | - | 42.47 | - |
| 1380 | 1388 | Anisyl methyl ketone | 1.70 | - | - |
| Phenylpropanoids | 92.91 | 0.00 | 69.28 | ||
| 1195 | 1193 | Methyl chavicol (estragole) | 5.32 | - | 3.44 |
| 1249 | 1248 | cis-Anethol | 0.11 | - | 0.79 |
| 1282 | 1280 | trans-Anethol | 87.48 | - | 65.05 |
| Sesquiterpene hydrocarbons | 1.41 | 0.24 | 0 | ||
| 1374 | 1366 | α-Copaene | tr | - | - |
| 1400 | 1397 | β-Longipinene | 0.10 | - | - |
| 1471 | 1470 | Dauca-5,8-diene | - | 0.24 | - |
| 1500 | 1480 | γ-Himachalene | 1.31 | - | - |
| Total identified | 99.68 | 98.53 | 99.78 | ||
| Indices | Formula |
|---|---|
| Relative growth rate | RGR = (m2 − m0)/(2 × m0) |
| Relative consumption rate | RCR = mc/(2 × m0) |
| Relative metabolic rate | RMR = [(mc − me) − (m2 − m0)]/(2 × m0) |
| The efficiency of conversion of ingested food (gross growth efficiency) | ECI = (m2 − m0)/mc × 100 |
| Approximate digestibility (assimilation efficiency) | AD= (mc − me)/mc × 100 |
| The efficiency of conversion of digested food (net growth efficiency) | ECD = (m2 − m0)/(mc − me) × 100 |
| Metabolic cost | MC = 100 − ECD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostić, I.; Lazarević, J.; Šešlija Jovanović, D.; Kostić, M.; Marković, T.; Milanović, S. Potential of Essential Oils from Anise, Dill and Fennel Seeds for the Gypsy Moth Control. Plants 2021, 10, 2194. https://doi.org/10.3390/plants10102194
Kostić I, Lazarević J, Šešlija Jovanović D, Kostić M, Marković T, Milanović S. Potential of Essential Oils from Anise, Dill and Fennel Seeds for the Gypsy Moth Control. Plants. 2021; 10(10):2194. https://doi.org/10.3390/plants10102194
Chicago/Turabian StyleKostić, Igor, Jelica Lazarević, Darka Šešlija Jovanović, Miroslav Kostić, Tatjana Marković, and Slobodan Milanović. 2021. "Potential of Essential Oils from Anise, Dill and Fennel Seeds for the Gypsy Moth Control" Plants 10, no. 10: 2194. https://doi.org/10.3390/plants10102194
APA StyleKostić, I., Lazarević, J., Šešlija Jovanović, D., Kostić, M., Marković, T., & Milanović, S. (2021). Potential of Essential Oils from Anise, Dill and Fennel Seeds for the Gypsy Moth Control. Plants, 10(10), 2194. https://doi.org/10.3390/plants10102194