Characterization of a Common S Haplotype BnS-6 in the Self-Incompatibility of Brassica napus
Abstract
:1. Introduction
2. Results
2.1. BnS-1/BnS-6 and BnS-7/BnS-6 Are the Main S Haplotypes in B. napus
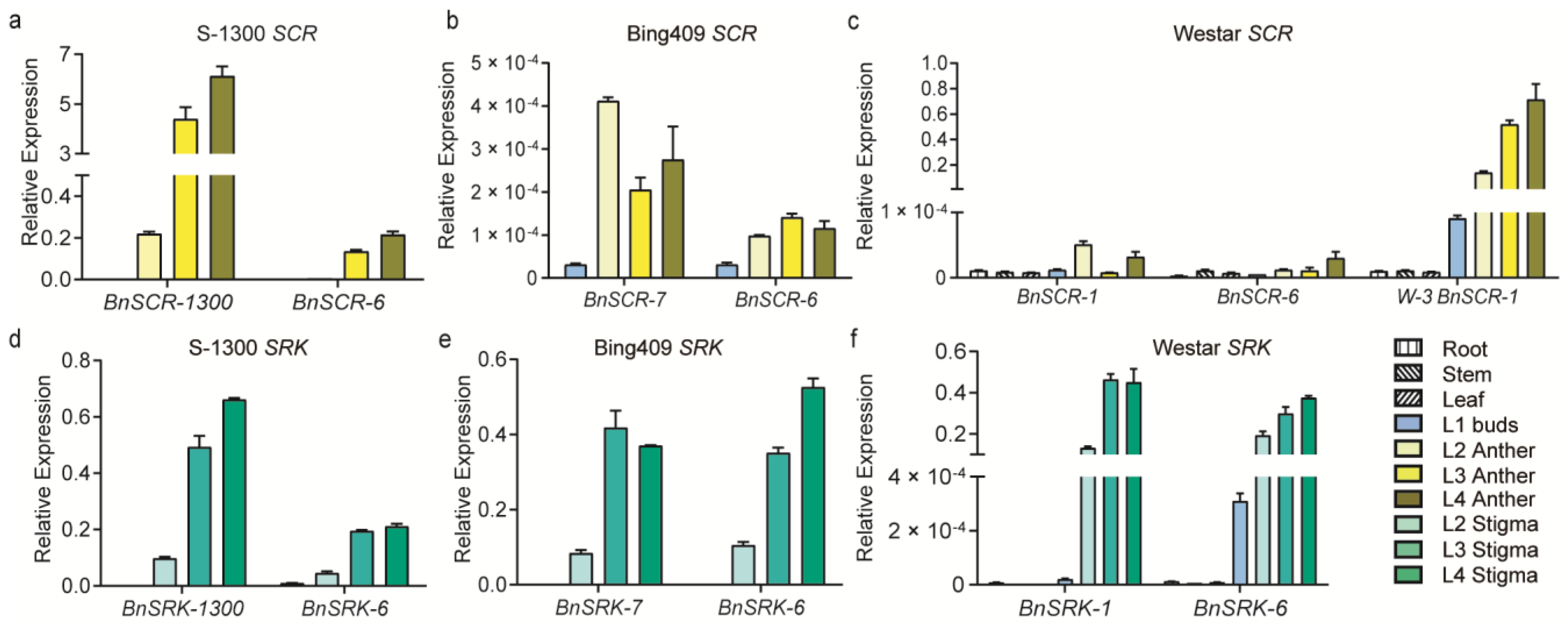
2.2. Gene Expression of SRK and SCR in Different S Haplotypes
2.3. Functional Validation of BnSCR-6
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. SI Phenotype Assay and Pollination Assay
4.3. Sequence Collinear Comparison and Primer Design
4.4. DNA Extraction and Genotyping Assay
4.5. RNA Extraction and Quantitative Real-Time PCR (Qrt-PCR)
4.6. Promoter Construct and GUS Assay
4.7. Vector Construction of Bnscr-6 Overexpression and Plant Transformation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nasrallah, J.B. Plant mating systems: Self-incompatibility and evolutionary transitions to self-fertility in the mustard family. Curr. Opin. Genet. Dev. 2017, 47, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, G.L. Flowering Plants: Evolution Above the Species Level; Belknap Press of Harvard University Press: Cambridge, MA, USA, 1974. [Google Scholar]
- Takayama, S.; Isogai, A. Self-Incompatibility in Plants. Annu. Rev. Plant Biol. 2005, 56, 467–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schopfer, C.R.; Nasrallah, M.E.; Nasrallah, J.B. The Male Determinant of Self-Incompatibility in Brassica. Science 1999, 286, 1697–1700. [Google Scholar] [CrossRef]
- Suzuki, G.; Kai, N.; Hirose, T.; Fukui, K.; Nishio, T.; Takayama, S.; Isogai, A.; Watanabe, M.; Hinata, K. Genomic organization of the S locus: Identification and characterization of genes in SLG/SRK region of S(9) haplotype of Brassica campestris (syn. rapa). Genetics 1999, 153, 391–400. [Google Scholar] [CrossRef]
- Stein, J.C.; Howlett, B.; Boyes, D.C.; Nasrallah, M.E.; Nasrallah, J.B. Molecular cloning of a putative receptor protein kinase gene encoded at the self-incompatibility locus of Brassica oleracea. Proc. Natl. Acad. Sci. USA 1991, 88, 8816–8820. [Google Scholar] [CrossRef] [Green Version]
- Takasaki, T.; Hatakeyama, K.; Suzuki, G.; Watanabe, M.; Isogai, A.; Hinata, K. The S receptor kinase determines self-incompatibility in Brassica stigma. Nature 2000, 403, 913–916. [Google Scholar] [CrossRef]
- Giranton, J.L.; Dumas, C.; Cock, J.M.; Gaude, T. The integral membrane S-locus receptor kinase of Brassica has serine/threonine kinase activity in a membranous environment and spontaneously forms oligomers in planta. Proc. Natl. Acad. Sci. USA 2000, 97, 3759–3764. [Google Scholar] [CrossRef]
- Kachroo, A.; Schopfer, C.R.; Nasrallah, M.E.; Nasrallah, J.B. Allele-specific receptor-ligand interactions in Brassica self-incompatibility. Science 2001, 293, 1824–1826. [Google Scholar] [CrossRef]
- Takayama, S.; Shimosato, H.; Shiba, H.; Funato, M.; Che, F.S.; Watanabe, M.; Iwano, M.; Isogai, A. Direct ligand–receptor complex interaction controls Brassica self-incompatibility. Nature 2001, 413, 534–538. [Google Scholar] [CrossRef]
- Ma, R.; Han, Z.; Hu, Z.; Lin, G.; Gong, X.; Zhang, H.; Nasrallah, J.B.; Chai, J. Structural basis for specific self-incompatibility response in Brassica. Cell Res. 2016, 26, 1320–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, N.; Goring, D. Mechanisms of self-incompatibility in flowering plants. Cell. Mol. Life Sci. 2001, 58, 1988–2007. [Google Scholar] [CrossRef] [PubMed]
- Kachroo, A.; Nasrallah, M.E.; Nasrallah, J.B. Self-Incompatibility in the Brassicaceae: Receptor–Ligand Signaling and Cell-to-Cell Communication. Plant Cell 2002, 14, S227–S238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nou, I.S.; Watanabe, M.; Isogai, A. Comparison of S-alleles and S-glycoproteins between two wild populations of Brassica campestris in Turkey and Japan. Sex. Plant Reprod. 1993, 6, 79–86. [Google Scholar] [CrossRef]
- Ockendon, D.J. Distribution of self-incompatibility alleles and breeding structure of open-pollinated cultivars of Brussels sprouts. Heredity 1974, 33, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Nasrallah, J.B.; Nishio, T.; Nasrallah, M.E. The self-incompatibility genes of Brassica: Expression and Use in genetic ablation of floral tissues. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 393–422. [Google Scholar] [CrossRef]
- Okamoto, S.; Odashima, M.; Fujimoto, R.; Sato, Y.; Kitashiba, H.; Nishio, T. Self-compatibility in Brassica napus is caused by independent mutations in S-locus genes. Plant J. 2007, 50, 391–400. [Google Scholar] [CrossRef]
- Tarutani, Y.; Shiba, H.; Iwano, M.; Kakizaki, T.; Suzuki, G.; Watanabe, M.; Isogai, A.; Takayama, S. Trans-acting small RNA determines dominance relationships in Brassica self-incompatibility. Nature 2010, 466, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Shiba, H.; Kakizaki, T.; Iwano, M.; Tarutani, Y.; Watanabe, M.; Isogai, A.; Takayama, S. Dominance relationships between self-incompatibility alleles controlled by DNA methylation. Nat. Genet. 2006, 38, 297–299. [Google Scholar] [CrossRef]
- Yasuda, S.; Wada, Y.; Kakizaki, T.; Tarutani, Y.; Miura-Uno, E.; Murase, K.; Fujii, S.; Hioki, T.; Shimoda, T.; Takada, Y.; et al. A complex dominance hierarchy is controlled by polymorphism of small RNAs and their targets. Nat. Plants 2016, 3, 16206. [Google Scholar] [CrossRef]
- Takahata, Y.; Hinata, K. A variation study of subtribe Brassicinae by principal component analysis. In Brassica Crops and Wild Allies; Japan Scientific Societies Press: Tokyo, Japan, 1980; pp. 33–49. [Google Scholar]
- Igic, B.; Lande, R.; Kohn, J.R. Loss of Self-Incompatibility and Its Evolutionary Consequences. Int. J. Plant Sci. 2008, 169, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Ma, C.; Zhang, X.; Li, F.; Zhang, J.; Zhai, W.; Wang, Y.; Tu, J.; Shen, J.; Fu, T. The genetic characterization of self-incompatibility in a Brassica napus line with promising breeding potential. Mol. Breed. 2013, 31, 485–493. [Google Scholar] [CrossRef]
- Zhai, W.; Zhang, J.F.; Yang, Y.; Ma, C.Z.; Liu, Z.Q.; Gao, C.B.; Zhou, G.L.; Tu, J.X.; Shen, J.X.; Fu, T.D. Gene expression and genetic analysis reveal diverse causes of recessive self-compatibility in Brassica napus L. BMC Genom. 2014, 15, 1037. [Google Scholar] [CrossRef] [Green Version]
- Tochigi, T.; Udagawa, H.; Li, F.; Kitashiba, H.; Nishio, T. The self-compatibility mechanism in Brassica napus L. is applicable to F1 hybrid breeding. Theor. Appl. Genet. 2011, 123, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhou, G.; Ma, C.; Zhai, W.; Zhang, T.; Liu, Z.; Yang, Y.; Wu, M.; Yue, Y.; Duan, Z.; et al. Helitron-like transposons contributed to the mating system transition from out-crossing to self-fertilizing in polyploid Brassica napus L. Sci. Rep. 2016, 6, 33785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.; Fu, T.; Yang, G.; Tu, J.; Yang, X.; Dan, F. Breeding for self-incompatibility lines with double-zero on Brassica napus L. J. Huazhong Agric. Univ. 1998, 17, 211–213. [Google Scholar]
- Zhang, X.; Ma, C.; Fu, T.; Li, Y.; Wang, T.; Chen, Q.; Tu, J.; Shen, J. Development of SCAR markers linked to self-incompatibility in Brassica napus L. Mol. Breed. 2008, 21, 305–315. [Google Scholar] [CrossRef]
- Ma, C.; Jiang, Y.; Dan, F.; Dan, B.; Fu, T. Breeding for maintainer of self-incompatible lines and its potential in Brassica napus L. J. Huazhong Agric. Univ. 2003, 22, 13–17. [Google Scholar]
- Zhang, X.; Yin, D.; Zhu, W.; Ma, C.; Fu, T. Progress on characterization of self-incompatibility in Brassica napus L. Euphytica 2011, 182, 147–155. [Google Scholar] [CrossRef]
- Fujimoto, R.; Sugimura, T.; Fukai, E.; Nishio, T. Suppression of gene expression of a recessive SP11/SCR allele by an untranscribed SP11/SCR allele in Brassica self-incompatibility. Plant Mol. Biol. 2006, 61, 577–587. [Google Scholar] [CrossRef]
- Fukai, E.; Fujimoto, R.; Nishio, T. Genomic organization of the S core region and the S flanking regions of a class-II S haplotype in Brassica rapa. Mol. Genet. Genom. 2003, 269, 361–369. [Google Scholar] [CrossRef]
- Sato, Y.; Fujimoto, R.; Toriyama, K.; Nishio, T. Commonality of self-recognition specificity of S haplotypes between Brassica oleracea and Brassica rapa. Plant Mol. Biol. 2003, 52, 617–626. [Google Scholar] [CrossRef]
- Doucet, J.; Lee, H.K.; Goring, D.R. Pollen Acceptance or Rejection: A Tale of Two Pathways. Trends Plant Sci. 2016, 21, 1058–1067. [Google Scholar] [CrossRef]
- Luo, X.; Ma, C.; Yi, B.; Tu, J.; Shen, J.; Fu, T. Genetic distance revealed by genomic single nucleotide polymorphisms and their relationships with harvest index heterotic traits in rapeseed (Brassica napus L.). Euphytica 2016, 209, 41–47. [Google Scholar] [CrossRef]
- Fu, T.; Liu, H. Preliminary report on breeding of self-incompatible lines of Brassica napus. Oil Crop China 1975, 4, 77–85. [Google Scholar]
- Tang, J.; Zhang, J.; Ma, C.; Tang, W.; Gao, C.; Li, F.; Wang, X.; Liu, Y.; Fu, T. CAPS and SCAR markers linked to maintenance of self-incompatibility developed from SP11 in Brassica napus L. Mol. Breed. 2009, 24, 245–254. [Google Scholar] [CrossRef]
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Samans, B.; et al. Plant genetics. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, K.; Wei, L.; Li, X.; Wang, Y.; Wu, J.; Liu, M.; Zhang, C.; Chen, Z.; Xiao, Z.; Jian, H.; et al. Whole-genome resequencing reveals Brassica napus origin and genetic loci involved in its improvement. Nat. Commun. 2019, 10, 1154. [Google Scholar] [CrossRef] [Green Version]
- Hadj-Arab, H.; Chevre, A.M.; Gaude, T.; Chable, V. Variability of the self-incompatibility reaction in Brassica oleracea L. with S 15 haplotype. Sex. Plant Reprod. 2010, 23, 141–151. [Google Scholar] [CrossRef]
- Kucera, V.; Chytilová, V.; Vyvadilová, M.; Klíma, M. Hybrid breeding of cauliflower using self-incompatibility and cytoplasmic male sterility. Hortic. Sci. 2006, 33, 148–152. [Google Scholar] [CrossRef] [Green Version]
- Sr, S.; Vinod. Breeding for cytoplasmic male sterility in broccoli (Brassica oleracea L.var. italica Plenck). Indian J. Genet. Plant Breed. 2002, 62, 165–166. [Google Scholar]
- Chen, B.Y.; Heneen, W.K.; Jönsson, R. Resynthesis of Brassies napus L. through Interspecific Hybridization between B. alboglabra Bailey and B. campestris L. with Special Emphasis on Seed Colour. Plant Breed. 1988, 101, 52–59. [Google Scholar] [CrossRef]
- Singh, J.N.; Murty, B.R. Combining ability and maternal effects in Brassica campestris L. var. ‘yellow sarson’. Theor. Appl. Genet. 1980, 56, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hu, K.; Zhang, Z.; Guan, C.; Chen, S.; Hua, W.; Li, J.; Wen, J.; Yi, B.; Shen, J.; et al. Genome-wide association study reveals the genetic architecture of flowering time in rapeseed (Brassica napus L.). DNA Res. 2016, 23, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Liu, Z.; Zhang, T.; Zhou, G.; Duan, Z.; Li, B.; Dou, S.; Liang, X.; Tu, J.; Shen, J.; et al. Mechanism of Salt-Induced Self-Compatibility Dissected by Comparative Proteomic Analysis in Brassica napus L. Int. J. Mol. Sci. 2018, 19, 1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Malley, R.C.; Barragan, C.C.; Ecker, J.R. A user’s guide to the Arabidopsis T-DNA insertion mutant collections. Methods Mol. Biol. 2015, 1284, 323–342. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Willemsen, V.; Wolkenfelt, H.; de Vrieze, G.; Weisbeek, P.; Scheres, B. The HOBBIT gene is required for formation of the root meristem in the Arabidopsis embryo. Development 1998, 125, 521–531. [Google Scholar] [CrossRef]
- Dun, X.; Zhou, Z.; Xia, S.; Wen, J.; Yi, B.; Shen, J.; Ma, C.; Tu, J.; Fu, T. BnaC.Tic40, a plastid inner membrane translocon originating from Brassica oleracea, is essential for tapetal function and microspore development in Brassica napus. Plant J. 2011, 68, 532–545. [Google Scholar] [CrossRef] [PubMed]
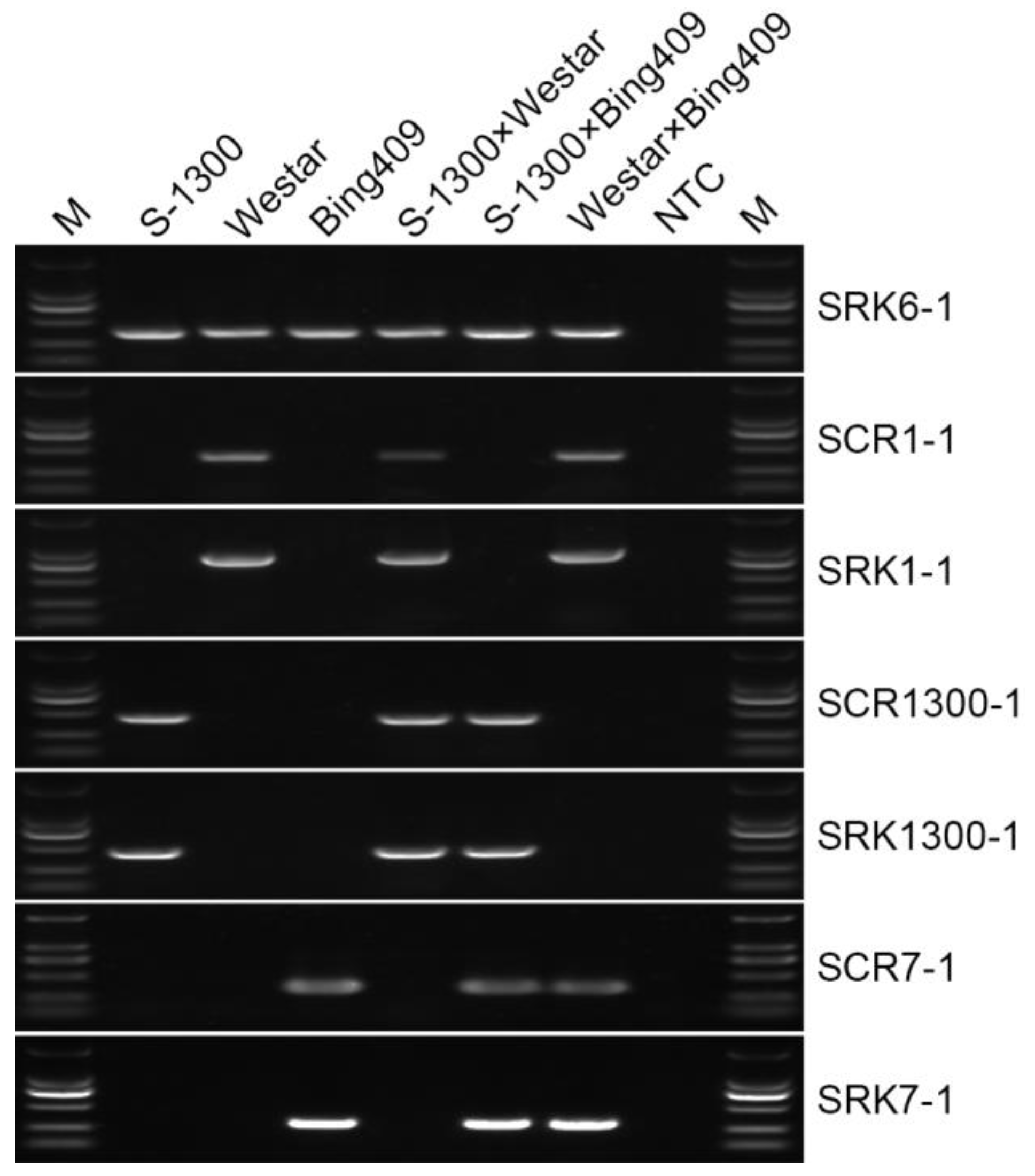



| Material | Origin | SRK1300-1/ SCR1300-1 | SRK1-1/ SCR1-1 | SRK7-1/ SCR7-1 | SRK6-1 | SI Phenotype 1 | |
|---|---|---|---|---|---|---|---|
| SCI | SC/SI | ||||||
| S-1300 | China | + | − | − | + | 0.01 | SI |
| Huashuang2 | China | − | + | − | + | 21.06 | SC |
| Westar | Canada | − | + | − | + | 16.95 | SC |
| Tapidor | Europe | − | + | − | + | 20.60 | SC |
| 89008 | China | − | + | − | + | 17.68 | SC |
| Ningyou-7 | China | − | − | + | + | 0.02 | SI |
| 326 | China | − | − | + | + | 0.31 | SI |
| 614 | China | − | − | + | + | 0.50 | SI |
| 1728 | China | − | − | + | + | 0.15 | SI |
| C32 | China | − | − | + | + | 0.23 | SI |
| Bing409 | China | − | − | + | + | 0.41 | SI |
| 242 | China | − | − | + | + | 0.64 | SI |
| 198 | China | − | − | + | + | 0.34 | SI |
| 1745 | China | − | − | + | + | 0.21 | SI |
| 230 | China | − | − | + | + | 0.05 | SI |
| Genotypes of the Lines | Number of Lines | Proportion | |||
|---|---|---|---|---|---|
| SRK1300-1 | SRK1-1 | SRK7-1 | SRK6-1 | ||
| + | − | − | + | 3 | 0.57% |
| − | + | − | + | 239 | 45.70% |
| − | − | + | + | 226 | 43.21% |
| − | + | + | + | 20 | 3.82% |
| − | − | − | + | 35 | 6.69% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Li, B.; Yang, Y.; Gao, C.; Yi, B.; Wen, J.; Shen, J.; Tu, J.; Fu, T.; Dai, C.; et al. Characterization of a Common S Haplotype BnS-6 in the Self-Incompatibility of Brassica napus. Plants 2021, 10, 2186. https://doi.org/10.3390/plants10102186
Liu Z, Li B, Yang Y, Gao C, Yi B, Wen J, Shen J, Tu J, Fu T, Dai C, et al. Characterization of a Common S Haplotype BnS-6 in the Self-Incompatibility of Brassica napus. Plants. 2021; 10(10):2186. https://doi.org/10.3390/plants10102186
Chicago/Turabian StyleLiu, Zhiquan, Bing Li, Yong Yang, Changbin Gao, Bin Yi, Jing Wen, Jinxiong Shen, Jinxing Tu, Tingdong Fu, Cheng Dai, and et al. 2021. "Characterization of a Common S Haplotype BnS-6 in the Self-Incompatibility of Brassica napus" Plants 10, no. 10: 2186. https://doi.org/10.3390/plants10102186
APA StyleLiu, Z., Li, B., Yang, Y., Gao, C., Yi, B., Wen, J., Shen, J., Tu, J., Fu, T., Dai, C., & Ma, C. (2021). Characterization of a Common S Haplotype BnS-6 in the Self-Incompatibility of Brassica napus. Plants, 10(10), 2186. https://doi.org/10.3390/plants10102186






